Summary
Despite extensive research on the canonical Wnt signaling pathway, the mechanism by which this signal downregulates the activity of destruction complexes and inhibits β-catenin degradation remains controversial. In particular, recent attention has focused on two main competing mechanisms—inhibition of phosphorylation and inhibition of ubiquitination. Our combined experimental and theoretical analysis demonstrates that the disassembly of a fraction of the intracellular destruction complexes results in the partial inhibition of both β-catenin phosphorylation and ubiquitination. This inhibition is spatially patterned, consistent with the relocalization of some destruction complexes to the cellular membrane upon Wnt stimulation. Moreover, in contrast to the generally accepted view that the destruction complex is highly processive, our analysis supports a distributive model, in which β-catenin can dissociate from the complex between sequential phosphorylation events. Understanding the fundamental mechanism by which Wnt signaling is regulated provides a rational basis for tuning the pathway for scientific and therapeutic purposes.
Subject Areas: Molecular Biology, Molecular Mechanism of Gene Regulation, Bioinformatics
Graphical Abstract
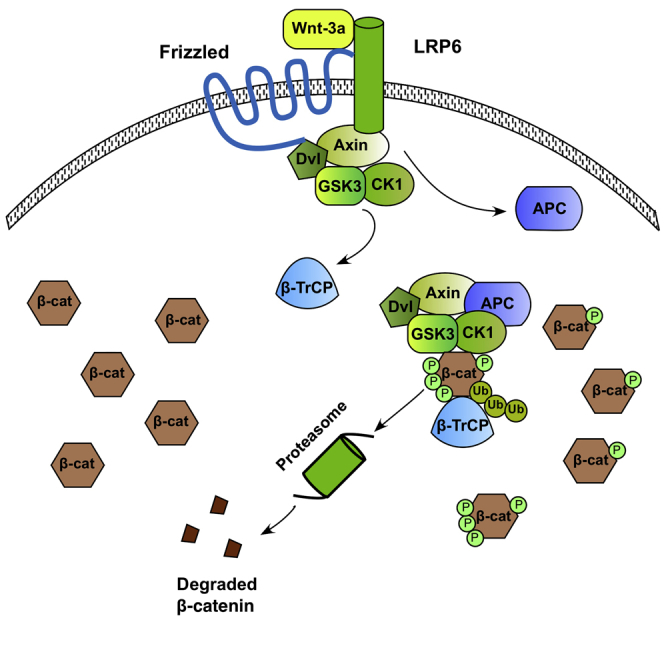
Highlights
-
•
Wnt signaling partially inhibits both β-catenin phosphorylation and ubiquitination
-
•
Some destruction complexes relocalize to the cellular membrane upon Wnt stimulation
-
•
Analysis supports a distributive mechanism for destruction complex activity
Molecular Biology; Molecular Mechanism of Gene Regulation; Bioinformatics
Introduction
The canonical Wnt signaling pathway has been the subject of extensive research given its fundamental role in biological processes ranging from development to controlling stem cell fate and because of the association of abnormal Wnt signaling with cancers (Azzolin et al., 2014, Bilic et al., 2007, Clevers, 2006, Cong et al., 2004, Hernandez et al., 2012, Kim et al., 2013, Li et al., 2012, Nelson and Nusse, 2004, Nusse and Clevers, 2017, Nusse and Varmus, 2012, Peifer and Polakis, 2000). In the absence of a Wnt signal, β-catenin binds to the destruction complex and is sequentially phosphorylated by CK1 (at Ser45) and GSK3 (at Ser33/37/Thr41), then ubiquitinated by the β-TrCP ubiquitin E3 ligase complex, and finally degraded by the proteasome (Nusse and Clevers, 2017). The continuous synthesis and degradation of β-catenin collectively result in a low steady-state level of this Wnt effector (Hernandez et al., 2012). The canonical Wnt signaling pathway is activated by the binding of Wnt to its cellular receptors Frizzled (Fzd) and LRP5/6 (Cong et al., 2004, Nusse and Clevers, 2017). Wnt stimulation inhibits the destruction complex-mediated degradation of β-catenin by a mechanism that remains controversial, but results in an increase in the cytosolic concentration of β-catenin. The accumulated β-catenin translocates to the nucleus and, together with the T-cell factor (TCF) family of transcription factors, activates transcription (Cong et al., 2004).
Although numerous mechanisms have been proposed to explain the Wnt-mediated increase in levels of β-catenin, recent attention has focused on two main competing mechanisms—inhibition of ubiquitination and inhibition of phosphorylation (Figure 1A) (Azzolin et al., 2014, Hernandez et al., 2012, Kim et al., 2013, Li et al., 2012). Li et al. (2012) observed that the cytosolic accumulation of β-catenin was detectable as early as 30 min following Wnt treatment. They concluded that this initial activation of the Wnt pathway could not be caused by Axin1 degradation, since significant degradation of Axin1 was not observed until 4 hr post-Wnt stimulation (Li et al., 2012). To elucidate the mechanism of Wnt activation, Li et al. (2012) immunoprecipitated Axin1 and thus pulled down the other proteins associated with the destruction complex. Their experiments suggested that the destruction complex remained intact following Wnt stimulation. They reported that the concentrations of CK1-phosphorylated β-catenin (CK1-p-β-cat) and GSK3-phosphorylated β-catenin (GSK3-p-β-cat) associated with Axin1 increased, whereas the amount of ubiquitinated GSK3-p-β-cat associated with Axin1 decreased after Wnt stimulation. Li et al. (2012) concluded that Wnt stimulation led to a temporary inactivation-by-saturation of the destruction complex with GSK3-p-β-cat. Azzolin et al. (2014) also suggested a primary role for the inhibition of ubiquitination. They reported that the Hippo transducer proteins YAP/TAZ formed a part of the destruction complex and were essential for the docking of the β-TrCP ubiquitin E3 ligase. They suggested that Wnt stimulation caused YAP/TAZ to be released from the destruction complex, preventing the docking of the E3 ligase and thus inhibiting ubiquitination.
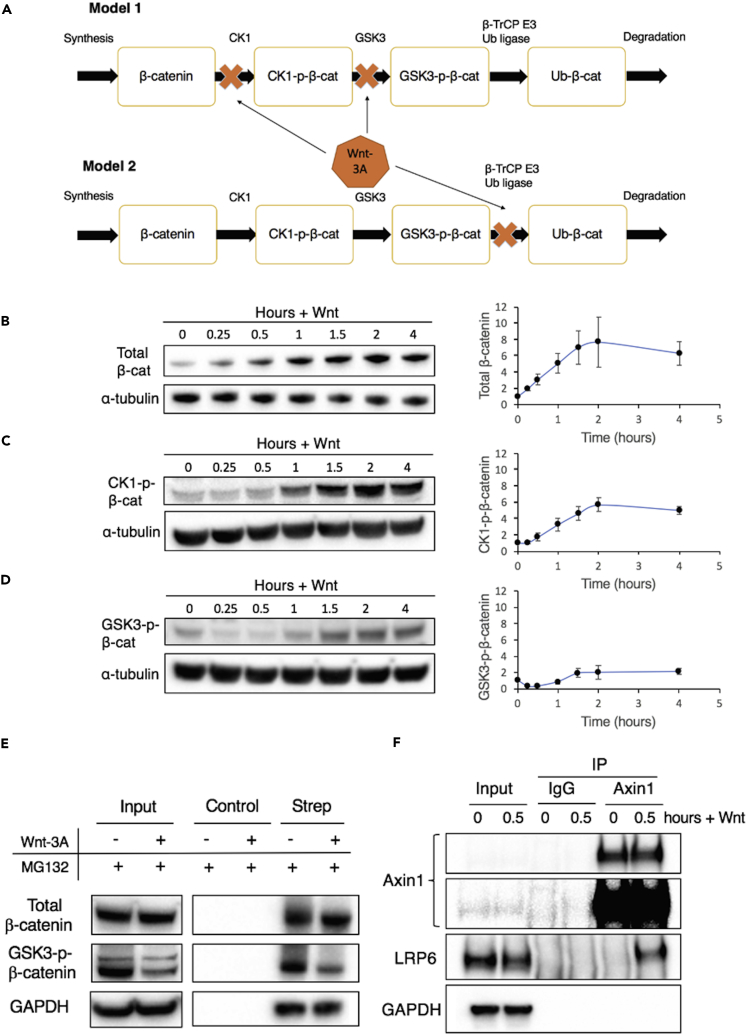
Figure 1.
Characterizing β-Catenin Dynamics in Response to Wnt-3A Stimulation
(A) Schematic showing two models that have been proposed to explain Wnt-induced increases in levels of β-catenin. Model 1 (top) suggests that the inhibition of phosphorylation by CK1 and/or GSK3 causes β-catenin to accumulate in the cell, whereas Model 2 (bottom) is based on the inhibition of ubiquitination.
(B–D) Characterization of changes in the concentration of (B) non-membrane-associated β-catenin, (C) whole-cell CK1-phosphorylated-β-catenin, and (D) GSK3-phosphorylated-β-catenin with time upon Wnt stimulation through immunoblotting. Plots show mean ±1 SD (n = 3 replicates).
(E) Characterization of concentrations of newly synthesized azidohomoalanine-tagged proteins before (−) and after (+) Wnt stimulation after pull down with streptavidin-agarose beads (Strep) or agarose beads (control). Input lanes correspond to samples before pull down with streptavidin-agarose beads and therefore represent total protein, not newly synthesized AHA-tagged proteins. The input lanes were imaged separately.
(F) Characterization of changes in protein-protein interactions upon Wnt stimulation via co-immunoprecipitation (IP) using an anti-Axin1 antibody.
See also Figure S1.
In contrast, Hernandez et al. (2012) suggested a primary role for the inhibition of the CK1 and GSK3 phosphorylation steps. They stimulated mammalian cells with Wnt-3a and measured time-dependent changes in the concentrations of total and phosphorylated β-catenin. The total cytosolic β-catenin concentration initially increased with time and then reached a higher steady-state level, whereas the concentration of GSK3-p-β-cat decreased initially but subsequently recovered. Moreover, the maximum in the rate of accumulation of total β-catenin coincided in time with the minimum in the concentration of GSK3-p-β-cat. Kim et al. (2013) also reported that the ratio of the concentration of GSK3-p-β-cat to that of total β-catenin decreased significantly upon Wnt stimulation and never recovered over time and suggested that this decrease indicated a key role for the inhibition of phosphorylation. Hernandez et al. (2012) reasoned that these dynamics were inconsistent with inhibition at a step downstream of phosphorylation (e.g., ubiquitination) and also noted that their results were consistent with the inhibition of phosphorylation due to the disassembly of the destruction complex. Moreover, they argued that if Wnt caused complete inhibition of destruction complex activity by its saturation, then the degradation flux would not recover to its original level, resulting in an uncontrolled accumulation of β-catenin. Thus, a mechanistic model of Wnt signaling must also explain how β-catenin is maintained at an elevated steady-state level upon Wnt stimulation and what prevents it from accumulating indefinitely (Hernandez et al., 2012).
Results and Discussion
To clarify this issue, we conducted experiments using HEK293T cells, which have been used in many of the seminal mechanistic investigations of Wnt signaling (Azzolin et al., 2014, Hernandez et al., 2012, Kim et al., 2013, Li et al., 2012). We also stress that for the reasons noted by Li et al (Li et al., 2012), we did not use overexpression analyses and instead characterized endogenous destruction complexes.
We stimulated cultured HEK293T cells with Wnt-3a and first monitored changes in the levels of total β-catenin over time. A significant amount of β-catenin in these cells is reported to remain associated with membrane-bound E-cadherin complexes, a highly stable pool not involved in canonical Wnt signaling (Li et al., 2012). As a result, in keeping with prior studies, we removed the membrane-associated β-catenin fraction by incubating whole-cell lysates with concanavalin A-Sepharose 4B beads (Hernandez et al., 2012) and monitored changes in the level of free (cytoplasmic and nuclear) β-catenin over time (Figures 1B and S1). Consistent with the results of Hernandez et al. (2012), we observed that the concentration of total β-catenin increased 15 min after Wnt stimulation and reached an elevated steady-state concentration in approximately 2 hr (Figure 1B). Since the rate of synthesis of β-catenin does not change following Wnt stimulation (Hernandez et al., 2012), the observed accumulation of β-catenin suggests that its rate of degradation must initially decrease. However, 2 hr after Wnt stimulation, the rate of β-catenin degradation must recover to its original value to offset synthesis and thereby help maintain the new, elevated steady-state concentration of β-catenin. We also monitored changes in the levels of CK1-p-β-catenin in whole-cell lysates upon Wnt stimulation. Levels of CK1-p-β-catenin increased with time and also attained a higher steady-state value approximately 2 hr after Wnt stimulation (Figure 1C).
We next measured the changes in the levels of GSK3-p-β-cat in whole-cell lysates. We found that this level decreased initially upon Wnt stimulation and then recovered, reaching a steady state after 2 hr (Figure 1D). This observation is consistent with the inhibition of phosphorylation being responsible for the inhibition of β-catenin degradation, but is inconsistent with inhibition solely occurring downstream of phosphorylation (e.g., in the ubiquitination step). That is, as noted by Hernandez et al. (2012), if inhibition were to occur downstream of phosphorylation, then GSK3-p-β-cat would continue to accumulate, which would in turn restore the degradation flux at a higher steady-state level of this species.
Next, we compared the GSK3 phosphorylation of newly synthesized β-catenin with and without Wnt stimulation using bioorthogonal noncanonical amino acid tagging (BONCAT) (Dieterich et al., 2007). In BONCAT, we tagged the newly synthesized proteins with L-azidohomoalanine, a non-canonical amino acid that contains an azide moiety. Using azide-alkyne “click” cycloaddition, the newly synthesized proteins can then be selectively labeled with biotin and pulled down using streptavidin beads (Debets et al., 2010). As seen in Figure 1E, newly synthesized β-catenin was GSK3-phosphorylated even after Wnt addition, indicating that some destruction complex activity was retained even after Wnt stimulation. The amount of newly synthesized GSK3-p-β-cat was, however, lower in the presence of Wnt than in its absence (Figure 1E), consistent with the partial inhibition of β-catenin phosphorylation upon Wnt stimulation. We next sought to understand the reason for this partial inhibition. It is well known that the binding of Wnt to its cellular receptors results in the relocalization of some destruction complexes to the cellular membrane (Bilic et al., 2007, Hendriksen et al., 2008, Li et al., 2012). Indeed, we immunoprecipitated Axin1 from whole-cell lysates and confirmed that LRP6 co-immunoprecipitated with Axin1 upon Wnt stimulation (Figure 1F). We hypothesized that the partial relocalization of destruction complexes to the cellular membrane may be responsible for the partial loss of destruction complex activity upon Wnt stimulation.
To further probe the mechanism of phosphorylation inhibition, we monitored changes in the levels of non-membrane-associated GSK3-p-β-cat and CK1-p-β-cat (i.e., the cytoplasmic and nuclear fraction) following Wnt stimulation (Figures 2A and S2). Importantly, the similarity in the levels of non-membrane-associated and whole-cell GSK3-p-β-cat at different time points after Wnt stimulation (Figure 2B) indicates that most of the GSK3-p-β-cat is not membrane associated. Similarly, the data in Figure 2B indicate that most CK1-p-β-cat is also not membrane associated. These results suggest that relocalization of destruction complexes to the membrane results in inhibition of their phosphorylation activity, with residual activity after Wnt stimulation primarily coming from non-membrane-associated destruction complexes.
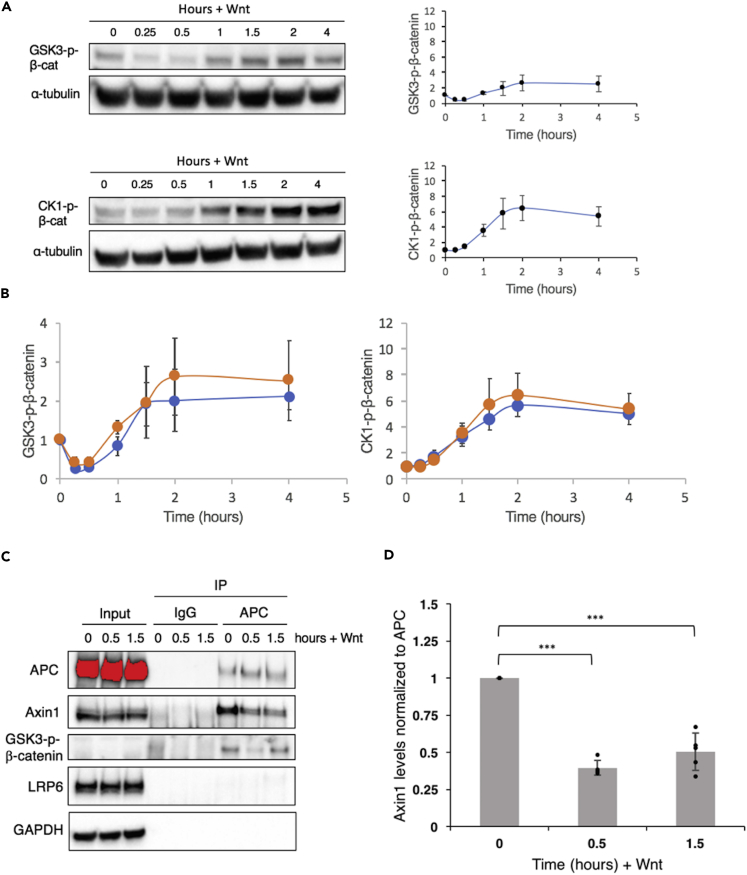
Figure 2.
Characterization of Destruction Complex Components upon Wnt Stimulation
(A) Characterization of changes in concentration of non-membrane-associated GSK3-phosphorylated-β-catenin (top) and CK1-phosphorylated-β-catenin (bottom) with time upon Wnt stimulation through immunoblotting. Plots show mean ±1 SD (n = 3 replicates).
(B) Comparison of whole-cell (blue) and non-membrane associated (orange) concentrations of GSK3-phosphorylated-β-catenin (left) and CK1-phosphorylated-β-catenin (right). Plots show mean ±1 SD (n = 3 replicates).
(C) Monitoring changes in interactions of various proteins with APC upon Wnt signaling via co-immunoprecipitation (IP) using an anti-APC antibody.
(D) Quantifying changes in Axin1-APC interactions upon Wnt stimulation. Plot shows mean ±1 SD (n = 5 replicates), ***p < 0.001 by a two-tailed t test.
See also Figure S2.
Next, to probe the mechanism responsible for inhibition, we immunoprecipitated adenomatous polyposis coli (APC) and measured the changes in concentrations of Axin1 and GSK3-p-β-catenin being pulled down along with APC. We chose to immunoprecipitate APC in light of recent reports that APC levels are significantly lower than Axin1 levels in HEK293T cells (Kitazawa et al., 2017, Tan et al., 2012). We found that there was a significant decrease in Axin1-APC interactions upon Wnt stimulation, consistent with a partial disassembly of destruction complexes (Figures 2C and 2D). Valvezan et al. (2012) observed a similar decrease in Axin1-APC interactions in L cells upon Wnt stimulation. We also observed an initial decrease in the concentration of co-immunoprecipitated GSK3-p-β-cat (Figure 2C). These results indicate a partial disassembly of destruction complexes upon Wnt stimulation, resulting in an initial decrease in the rate of GSK3 phosphorylation and degradation of β-catenin. Levels of co-immunoprecipitated GSK3-p-β-cat had recovered substantially by 1.5 hr (Figure 2), consistent with β-catenin levels approaching a new steady state.
Collectively, the results from Figures 1 and 2 indicate that upon Wnt signaling, a fraction of the destruction complexes partitions to the membrane, which inhibits their activity and leads to the inhibition of β-catenin phosphorylation. The fraction of the destruction complexes in the cytosol, however, remains structurally intact and active. The decrease in the total number of active destruction complexes results in an initial decrease in the rate of β-catenin degradation and thus an increase in the intracellular concentration of β-catenin. Mass action then causes an increase in the rate of β-catenin phosphorylation by the remaining, active destruction complexes. At the new steady state following Wnt stimulation, the rate of degradation of β-catenin would once again equal its rate of synthesis, and the flux through the destruction complexes would be expected to return to its original value.
Figure 3A depicts the sequence of intracellular β-catenin modifications. The rate of phosphorylation by CK1 is governed by Michaelis-Menten kinetics, and for low substrate concentrations relative to the Michaelis constant (KM1), it is given by (kcat1/KM1)[DC][B0], where kcat1 is the catalytic rate of CK1α on the destruction complex, [DC] is the destruction complex concentration, and [B0] is the concentration of unphosphorylated β-catenin (Hernandez et al., 2012). In Figure 3A, the rate constant k1=(kcat1/KM1)[DC] (Hernandez et al., 2012). When [DC] decreases in response to Wnt stimulation, then an increase in [B0] would be required for the rate of CK1 phosphorylation, (kcat1/KM1)[DC][B0], to recover (Figure 1B).
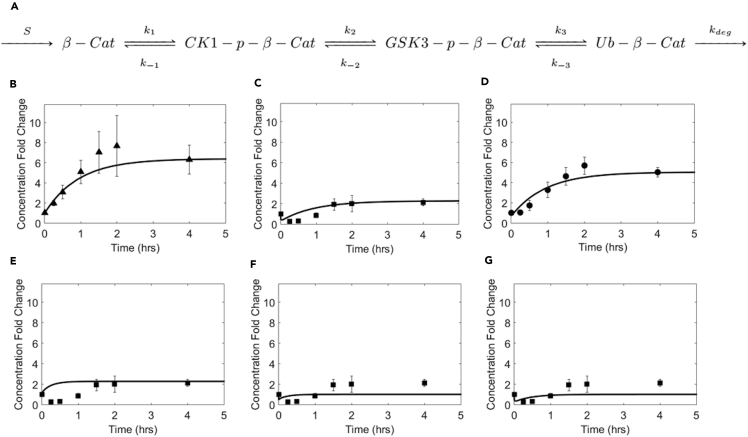
Figure 3.
Modeling β-Catenin Dynamics in Response to Wnt Stimulation
(A) Cartoon showing sequential enzymatic modifications of β-catenin.
(B–D) Comparison of results from numerical integration of rate equations with experimental data for changes in concentrations of (B) total β-catenin, (C) GSK3-p-β-cat, and (D) CK1-p-β-cat. Values of the rate constants (k1, k2, and k3) were each assumed to decrease to 0.44 times their original value, to reflect the decrease in the number of active destruction complexes.
(E–G) Comparison of results from numerical integration of rate equations with experimental data for changes in concentrations of GSK3-p-β-cat for a decrease in the value of (E) only k3, (F) only k2, and (G) k1 and k2, but not k3. Experimental data in plots (B–G) show mean ±1 SD (n = 3 replicates).
See also Figure S3.
We next used our results to analyze whether the destruction complex activity was processive or distributive. The generally accepted view is that the destruction complex is processive, with β-catenin undergoing a series of phosphorylations before being released from the complex. Our results—suggesting inhibition of phosphorylation due to partial disassembly that causes a decrease in the number of active destruction complexes—are inconsistent with this view. As explained by Hernandez et al. (2012), in the “processive” case, CK1-p-β-cat would remain bound to the destruction complex before its GSK3 phosphorylation, and the rate of GSK3 phosphorylation would be (k2)[CK1-p-β-cat]; the rate constant for GSK3 phosphorylation, k2 (Figure 3A), would then be independent of the destruction complex concentration and depend only on the intrinsic catalytic activity of GSK3 in the complex (k2 = kcat2). The concentration of CK1-p-β-cat at the new steady state should therefore return to its original value, so that the rate of GSK3 phosphorylation (k2*[CK1-p-β-cat]) also recovers. In contrast, we see a significant (∼5-fold) increase in the concentration of CK1-p-β-catenin upon Wnt stimulation (Figure 1D), similar to the significant increase in concentration of total β-catenin. This significant increase is inconsistent with a processive model and supports a distributive model for destruction complex activity, with the increase in CK1-p-β-cat concentration being necessary to compensate for the decrease in [DC]. CK1-p-β-cat would therefore be present in both free and destruction-complex-bound states.
As seen in Figure 1C, the concentration of GSK3-p-β-cat at the new steady state (following Wnt stimulation) is also greater than its initial value. Our co-immunoprecipitation results confirm that levels of destruction-complex-bound GSK3-p-β-cat recover to their original steady-state value upon Wnt stimulation. Collectively, these results suggest that GSK3-p-β-cat is also present in both free and destruction-complex-bound states. Moreover, these results are consistent with ubiquitination occurring primarily in an intact destruction complex as suggested by Li et al. (2012). An increase in the total concentration of GSK3-p-β-cat would be required if ubiquitination were occurring primarily in the destruction complex, to compensate for the decrease in the number of active destruction complexes upon Wnt stimulation. In contrast, if ubiquitination were occurring primarily outside the destruction complex, we would not expect to see an increase in total levels of GSK3-p-β-cat at the new steady state.
We used our results to predict β-catenin dynamics in response to Wnt signaling (Figures 3B–3D and Supplemental Information). In our proposed distributive model, the response to a Wnt signal is caused primarily by a decrease in the number of active destruction complexes, which causes a decrease in magnitudes of the rate constants for phosphorylation and ubiquitination—k1, k2, and k3 (Figure 3A). The lines in Figures 3B–3D represent β-catenin concentrations obtained by numerical integration of the dynamical equations (see Supplemental Information, equations S1–S4) with the value of each of the rate constants k1, k2, and k3 being decreased to 0.44 times their value before Wnt stimulation. The choice of 0.44 was guided by experimental data. The average Axin1/APC ratio upon Wnt stimulation ranged from ca. 0.39 to 0.5 times its value before Wnt stimulation (Figure 2D), and 0.44 lies in this range and is close to the average of these two values. As seen in Figures 3B–3D, the predicted changes in steady-state concentrations and the dynamical behaviors match the experimental results. In contrast, predictions based on inhibition of only ubiquitination (Figure 3E) or only phosphorylation (Figures 3F and 3G) do not explain the experimental results for β-catenin dynamics. If only ubiquitination is inhibited, results obtained by numerical integration of the dynamical equations do not show a dip in the concentration of GSK3-p-β-cat (Figure 3E). If only phosphorylation is inhibited, but not ubiquitination (Figures 3F and 3G), then GSK3-p-β-cat levels at the new steady state would recover to their initial value, but not exceed it, in contrast to experimental results. We note that whereas our experimental data are most consistent with a mechanism based on the inhibition of both phosphorylation and ubiquitination (Figures 3 and S3), our simulations do not perfectly match the experimental data for recovery of GSK3-p-β-cat concentrations. This difference could be due to factors not accounted for in our simple model. For instance, the inhibition could occur with a slight lag, and at different rates in different cells, due to heterogeneity in the time taken for Wnt to diffuse and bind to receptors on different cells and in the time required for complex disassembly.
In summation, our results support the following mechanistic model for Wnt signaling (Figure 4). Following Wnt stimulation, some of the destruction complexes relocate to the membrane, where they are associated with LRP6, and this subsequently leads to the dissociation of APC from the destruction complexes and the inhibition of GSK3 phosphorylation of β-catenin. The remaining destruction complexes remain intact and active in the cytoplasm. However, the initial decrease in the number of active destruction complexes results in an initial decrease in the rate of degradation of β-catenin. Consequently, free β-catenin is able to accumulate, until mass action results in an increase in the levels of destruction-complex-bound GSK3-p-β-cat and a recovery of the rate of degradation of β-catenin, but at an elevated steady-state level of β-catenin. We note that although it is possible that there are also free kinases in the cell, numerous published reports have confirmed a primary role for destruction complexes in mediating β-catenin phosphorylation and degradation (Li et al., 2012). Destruction complex-mediated degradation of β-catenin may in fact facilitate the regulation of intracellular levels of β-catenin in response to external signals, allowing the binding of an “external” Wnt ligand to induce the relocation of destruction complexes to the membrane, their disassembly, and a resulting increase in levels of β-catenin. Recent reports have suggested a role for a spatially localized Wnt signal in asymmetric stem cell division (Habib et al., 2013). It is intriguing that the spatial patterning of destruction complex activity is itself central to the core Wnt signal transduction mechanism. Understanding this fundamental mechanism provides a rational basis for tuning the pathway for scientific and therapeutic purposes (Kahn, 2014, Nusse and Clevers, 2017).
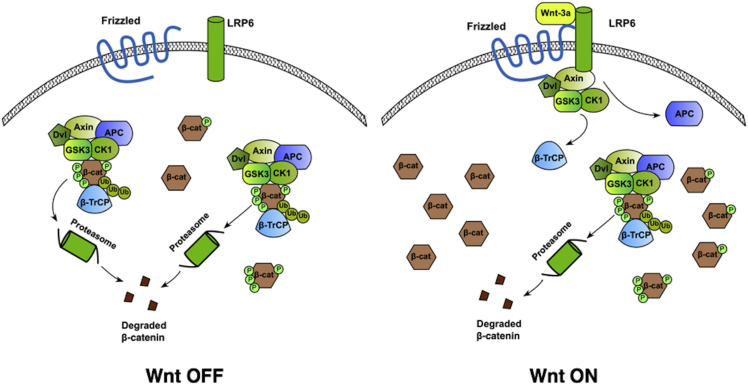
Figure 4.
Proposed Mechanism for Wnt Signaling
(Left) In the absence of Wnt, all destruction complexes target cytosolic β-catenin for degradation. (Right) Wnt stimulation causes the disassembly of a fraction of the intracellular destruction complexes, inhibiting their ability to phosphorylate and ubiquitinate cytosolic β-catenin. Levels of free β-catenin increase until mass action results in a recovery of the rate of degradation of β-catenin by the active destruction complexes.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We acknowledge support from NIH grants R01 NS087253 and R01 NS083856.
Author Contributions
R.S.K., A.M., N.D., and D.V.S. designed experiments; A.M., N.D., and M.S. performed experiments and analyzed data; R.S.K. and A.M. wrote the manuscript with revision and editing from D.S., N.D., and M.S.
Declaration of Interests
The authors declare no competing interests.
Published: August 31, 2018
Footnotes
Supplemental Information includes Transparent Methods and three figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.07.007.
Contributor Information
David V. Schaffer, Email: schaffer@berkeley.edu.
Ravi S. Kane, Email: ravi.kane@chbe.gatech.edu.
Supplemental Information
References
- Azzolin L., Panciera T., Soligo S., Enzo E., Bicciato S., Dupont S., Bresolin S., Frasson C., Basso G., Guzzardo V. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Bilic J., Huang Y.L., Davidson G., Zimmermann T., Cruciat C.M., Bienz M., Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Cong F., Schweizer L., Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- Debets M.F., van Berkel S.S., Schoffelen S., Rutjes F., van Hest J.C.M., van Delft F.L. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem. Commun. 2010;46:97–99. doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- Dieterich D.C., Lee J.J., Link A.J., Graumann J., Tirrell D.A., Schuman E.M. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat. Protoc. 2007;2:532–540. doi: 10.1038/nprot.2007.52. [DOI] [PubMed] [Google Scholar]
- Habib S.J., Chen B.C., Tsai F.C., Anastassiadis K., Meyer T., Betzig E., Nusse R. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J., Jansen M., Brown C.M., van der Velde H., van Ham M., Galjart N., Offerhaus G.J., Fagotto F., Fornerod M. Plasma membrane recruitment of dephosphorylated beta-catenin upon activation of the Wnt pathway. J. Cell Sci. 2008;121:1793–1802. doi: 10.1242/jcs.025536. [DOI] [PubMed] [Google Scholar]
- Hernandez A.R., Klein A.M., Kirschner M.W. Kinetic responses of beta-catenin specify the sites of Wnt control. Science. 2012;338:1337–1340. doi: 10.1126/science.1228734. [DOI] [PubMed] [Google Scholar]
- Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13:513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.E., Huang H., Zhao M., Zhang X., Zhang A., Semonov M.V., MacDonald B.T., Zhang X., Garcia Abreu J., Peng L., He X. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867–870. doi: 10.1126/science.1232389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M., Hatta T., Ogawa K., Fukuda E., Goshima N., Natsume T. Determination of rate-limiting factor for formation of beta-catenin destruction complexes using absolute protein quantification. J. Proteome Res. 2017;16:3576–3584. doi: 10.1021/acs.jproteome.7b00305. [DOI] [PubMed] [Google Scholar]
- Li V.S., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., Mohammed S., Heck A.J., Maurice M.M., Mahmoudi T., Clevers H. Wnt signaling through inhibition of beta-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Nelson W.J., Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M., Polakis P. Wnt signaling in oncogenesis and embryogenesis–a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- Tan C.W., Gardiner B.S., Hirokawa Y., Layton M.J., Smith D.W., Burgess A.W. Wnt signalling pathway parameters for mammalian cells. PLoS One. 2012;7:e31882. doi: 10.1371/journal.pone.0031882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvezan A.J., Zhang F., Diehl J.A., Klein P.S. Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J. Biol. Chem. 2012;287:3823–3832. doi: 10.1074/jbc.M111.323337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.