Abstract
Background
Consumer electronics and Web-enabled mobile devices are playing an increasing role in patient care, and their use in the oncologic sector opens up promising possibilities in the fields of supportive cancer care and systematic patient follow-up.
Objective
The objective of our study was to assess the acceptance and possible benefits of a mobile app–based concept for supportive care of cancer patients undergoing radiotherapy.
Methods
In total, 975 patients presenting for radiotherapy due to breast or prostate cancer were screened; of them, 200 owned a smartphone and consented to participate in the survey. Patients were requested to complete a questionnaire at 2 time points: prior to the initiation (T0) and after the completion (T1) of radiotherapy. The questionnaire included questions about the habits of smartphone usage, technical knowledge and abilities of the participants, readiness to use a mobile app within the context of radiotherapy, possible features of the mobile app, and general attitude toward the different aspects of oncologic treatments. For quantitative analysis, sum scores were calculated for all areas of interest, and results were correlated with patient characteristics. Additionally, answers were quantitatively compared between time points T0 and T1.
Results
Median patient age was 57 (range 27-78) years. Of the 200 participants, 131 (66.2%) reported having the ability to use their smartphones with minimal to no help and 75.8% (150/200) had not used their smartphones in a medical context before. However, 73.3% (146/200) and 83.4% (166/200) of patients showed a strong interest in using a mobile app for supportive care during radiotherapy and as part of the clinical follow-up, respectively. Patients most commonly requested functionalities regarding appointment scheduling in the clinic (176/200, 88.0%) and the collection of patient-reported outcome data regarding their illness, therapy, and general well-being (130/200, 65.0%). Age was identified as the most influential factor regarding patient attitude, with patients aged <55 years being significantly more inclined toward and versed in smartphone use (P<.001). The acceptance of mobile apps was significantly higher in patients exhibiting a Karnofsky performance index <80% (P=.01). Support in the context of therapy-related side effects was judged most important by patients with poor clinical performance (P=.006). The overall acceptance of mobile apps in the context of radiotherapy surveillance was high at a median item sum score of 71.4/100 and was not significantly influenced by tumor stage, age, gender, treatment setting, or previous radiotherapies.
Conclusions
The acceptance of mobile apps for the surveillance and follow-up of cancer patients undergoing radiotherapy is high; this high acceptance level will serve as a basis for future clinical trials investigating the clinical benefits of mobile app–based treatment support. Introduction of mobile apps into the clinical routine should be considered as an opportunity to improve and intensify supportive treatment for cancer patients.
Keywords: mHealth, radiotherapy, mobile app, quality of life, surveillance, patient-reported outcome, acceptance, smartphone, mobile phone
Introduction
The usage of consumer electronics and Web-enabled mobile devices is steadily increasing in the medical sector. Mobile health care apps are summarized by the World Health Organization under the term “mHealth” (mobile health) and have recently shown a significant rise in availability and market share [1,2]. Far from being but a response to the increased availability of smartphones and similar devices, the increased role of mHealth can be interpreted as a reaction to structural and demographic challenges faced by health care providers in today’s society. As patient empowerment and shared decision making become increasingly valued in health care, mHealth can provide the means of incorporating those values into modern treatments, for example, facilitating the collection of patient-reported outcomes or providing information about disease management and prevention [3]. The main arguments that favor mHealth approaches include the possibility to overcome geographic distances and language barriers or selectively address special needs of patient subgroups, such as children or ethnic minorities [4,5].
Mobile apps have been well implemented for the management of highly prevalent conditions such as diabetes, obesity, or cardiovascular diseases [4,6,7]. Cancer, with generally improved long-term survival rates, is developing into a chronic illness with similar requirements such as close patient monitoring and extensive and long-term supportive care [3]. Few mobile apps have been established for supportive cancer care, and the areas of use are still limited [8]. Furthermore, cancer-related mHealth apps and online resources often lack clinical validation. Several reviews examining the clinical benefits of available mobile apps have shown that the overall accuracy, actuality, and systematic validity of the provided information have rarely been confirmed in clinical studies [9-11]. To date, there are no validated mobile apps specifically for the management of patients receiving radiotherapy, a treatment modality with its very own subset of possible side effects and requirements regarding surveillance and supportive care [12].
The acceptance of mobile apps and Web-based medical resources by cancer patients remains largely unclear, especially because patient satisfaction in this context is rarely assessed systematically [11]. This is even more critical as this patient cohort is extremely heterogeneous regarding patient age, technological affinity and skills, income status, and individual burden and distress attributable to this often severe illness.
This prospective study aimed to systematically examine the acceptance of mobile apps by cancer patients undergoing radiotherapy by conducting a systematic survey at the Departments of Radiation Oncology of the National Center for Tumor Diseases, Heidelberg University Hospital and the German Cancer Research Center in Heidelberg. It specifically addresses the patients’ readiness and inclination toward the usage of a mHealth-based approach for additional supportive care in the context of radiotherapy. Furthermore, patient-side infrastructure, such as technical skills, reachability, or mobile data availability, was assessed. The possible functionalities and features of a supportive mobile app were systematically evaluated, and the potential influences of radiotherapy and other predictive and clinical factors on patients’ attitude were investigated.
Methods
Patient Characteristics
A total of 975 cancer patients presenting for radiotherapy at the above-mentioned institutions for breast or prostate cancer were screened for participation in this survey. Of them, 200 patients owned a smartphone and consented to participate. All the participants were asked to complete a survey prior to the initiation of radiotherapy (T0) and again upon the completion of the treatment (T1). Patient characteristics are presented in Table 1, and a flowchart of the recruitment workflow is illustrated in Figure 1. This prospective survey was approved by the Heidelberg University Independent Ethics Committee on February 15, 2017 (approval #S-007/2017).
Table 1.
Patient characteristics before radiotherapy (N=200).
| Characteristics | Value | |
| Age (years) | ||
|
|
Mean (SD) | 57.2 (11.08) |
|
|
Median (range) | 57 (27-78) |
|
|
Q1-Q3 | 50-65 |
| Gender, n (%) | ||
|
|
Male | 85 (42.5) |
|
|
Female | 115 (57.5) |
| Diagnosis, n (%) | ||
|
|
Breast cancer | 115 (57.5) |
|
|
Prostate cancer | 85 (42.5) |
| Treatment setting, n (%) | ||
|
|
Curative | 169 (84.5) |
|
|
Palliative | 31 (15.5) |
| T status, n (%) | ||
|
|
is | 4 (2.0) |
|
|
1 | 88 (44.0) |
|
|
2 | 65 (32.5) |
| 3 | 36 (18.0) | |
|
|
4 | 4 (2.0) |
|
|
X | 1 (0.5) |
|
|
Unknown | 2 (1.0) |
| N status, n (%) | ||
|
|
0 | 132 (66.0) |
|
|
+ | 19 (9.5) |
|
|
1 | 38 (19.0) |
|
|
2 | 4 (2.0) |
|
|
3 | 4 (2.0) |
|
|
X | 3 (1.5) |
| M status, n (%) | ||
|
|
0 | 159 (79.5) |
|
|
1 | 34 (17.0) |
|
|
X | 7 (3.5) |
| Initial Karnofsky performance index, n (%) | ||
|
|
60 | 3 (1.5) |
|
|
70 | 19 (9.5) |
|
|
80 | 43 (21.5) |
|
|
90 | 68 (34.0) |
|
|
100 | 67 (33.5) |
| Previous radiotherapy, n (%) | ||
|
|
No | 144 (72.0) |
|
|
Yes | 56 (28.0) |
| Tumor stagea, n (%) | ||
|
|
Early | 102 (51.0) |
|
|
Advanced | 98 (49.0) |
aEarly tumor stage: Tis/1/2, N0, M0; advanced tumor stage: T3 or above, N1 or above, M1.
Figure 1.
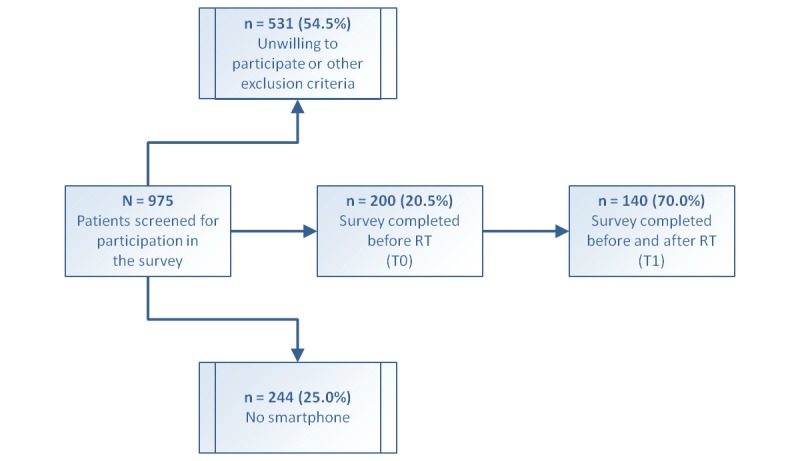
Flowchart illustrating patient screening and recruitment workflow. RT: radiotherapy.
Survey Methods
The survey form consisted of a standardized paper questionnaire containing 27 items (Q1-Q27) about smartphone use. The questionnaire was developed by experienced radiation oncologists with the help of a biomedical informatist and biostatistician and was tested on a small group of patients to allow room for clarifications and corrections before its distribution within this survey. The types of questions included multiple-choice questions, requiring the patients to choose one or several answers out of 2, 4, or 5 provided options. Two were polar questions, requiring the patients to choose either “yes” or “no.” Five questions prompted the patients to additionally fill in optional free text. Items assessed the habits of smartphone usage (Q3, Q7, and Q8), assessed technical knowledge and abilities in smartphone usage (Q4-Q6), assessed readiness to use a smartphone app within the context of cancer and radiotherapy (Q9, Q14, Q20-22, and Q27), suggested features for a potential radiotherapy-related mobile app (Q10-Q13), suggested the timeframe of reachability for smartphone notifications (Q23-Q25), and assessed the general attitude toward the different aspects of radiotherapy (Q15-Q19 and Q26). Additionally, patient- and disease-related information was collected. An English version of the survey questionnaire is provided in Multimedia Appendix 1.
Statistical Analysis
To allow for quantitative comparison, a simple scoring method was devised in which the aforementioned areas of interest (AOI) were considered as the subscales of the questionnaire. Within each subscale, every question was weighted by the number of possible answers, and the points were divided equally between the provided answers. Questions Q5, Q10, and Q13 were taken out of the score because they only provided qualitative information. The sum for every subscale was calculated and used for the comparison. Detailed information about the scoring system is provided in Multimedia Appendix 2.
For descriptive analyses, continuous variables were presented as mean (SD) and median (IQR and min and max) and categorical variables as absolute and relative frequencies. Group comparisons were made according to tumor stage, age (below and above 55 years), Karnofsky performance scale index (KPI), previous courses of radiotherapy, treatment setting, and gender [13]. Wilcoxon rank sum test for ordinal scaled variables and chi-square test for categorical variables were used to evaluate potential differences between patients in the mentioned groups. Group differences were assessed for all subscales and, additionally, for all questions included in the calculation for one of the subscales. To evaluate the differences at time points T0 and T1, Wilcoxon signed-rank test for paired ordinal scaled data and McNemar test for categorical variables were used. All statistical analyses were performed using R software (version 3.4.3, The R Foundation for Statistical Computing, Vienna, Austria).
Results
Descriptive Analysis
Of all patients, 73.2% (146/200) indicated that they were using mobile data on their smartphones, and the common usage included social media apps (45/200, 22.5%), picture taking and Web browsing (86/200, 43.0%), or further apps (48/200, 24.0%); 10.5% (21/200) of the patients used their smartphones for voice calls and instant messaging. Only 24.2% (48/200) of the patients indicated having used their smartphones in a health-related context before; 66.2% (131/200) of the patients stated that they never or rarely required assistance in using their smartphones and 63.9% (124/200) estimated their technical skills in this regard to be solid or advanced.
Patients showed a high overall readiness to use a mobile app in the context of radiotherapy: 73.3% (146/200) of all patients judged using a dedicated mobile app for additional supportive care during their treatment as helpful or very helpful. Mobile apps usage was judged especially helpful in providing support for the occurrences of treatment-associated toxicities (163/200, 81.8%, helpful or very helpful). The favored frequencies at which patients would be willing to answer short app-based queries regarding their well-being or general symptoms were weekly (98/100, 50.8%) and as required (37/200, 19.2%). Thirty-two out of 200 patients (16.6%) wished to do this only at the beginning and end of therapy. Concerns regarding data security were voiced by 12.2% (24/200) of patients. These concerns were somewhat more frequent in patients older than 55 years, although the difference was not significant compared with patients younger than 55 years (13.7% vs 10.0%, P=.16).
The most requested feature of a mobile app was assistance in appointment making for radiotherapy and consultations (176/200, 88.0%), followed by general or specific questions regarding patient well-being during radiotherapy (130/200, 65%). Additionally, patients requested the option to receive answers to questions and information material about diagnosis and therapy. Of all patients, 83.4% (166/200) welcomed the idea of continually using the app during follow-up to stay in touch with the treating physicians, describing this option as helpful or very helpful. The same was true for being contacted by the treating physician if medical warning signs were detected (182/200, 91.6%, helpful or very helpful). A reminder feature for scheduled follow-up examinations was favored by 81.5% (163/200) of patients.
Of the 200 patients, 21.8% (43/200) indicated their timeframe for reachability via smartphone between 7 am and 11 pm to be at least 12 hours; 40.1% (79/200) of patients indicated it to be between 2 and 12 hours. Regarding smartphone notifications about missed calls, instant messages, or push notifications, 75.4% (147/200) of patients stated that they would review those within a maximum timeframe of 2 hours or shorter; 21.5% (42/200) of patients answered “within 12 hours,” and only 3.1% (6/200) would need “2 days or longer.” The same was true for app-specific notifications regarding radiotherapy: the percentages were 67.8% (132/200) for 2 hours or less, 26.2% (51/200) for 12 hours, and 6.2% (12/200) for 2 days or longer.
In addition to the above-mentioned aspects of smartphone and app usage, the survey enquired about the general and organizational aspects of radiotherapy that could be improved using a smartphone app. Regarding the desired frequency of consultations with a supervising physician during radiotherapy, 34.4% (67/200) of patients favored weekly appointments, followed by 29.2% (57/200) favoring appointments “only as required.” Regarding consultations, a waiting period of up to 30 minutes was considered acceptable by 53.4% (106/200) of patients. However, regarding daily radiotherapy, the acceptable waiting period was shorter: 25.7% (50/200) of patients opted for 15 minutes or less and 6.2% (12/200) for even 10 minutes or less. Detailed information about the answers provided to all survey items is illustrated in Figures 2 and 3 and in Multimedia Appendices 3 and 4.
Figure 2.
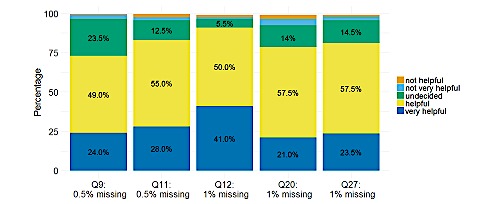
Distribution of the answers provided to selected questions regarding the helpfulness of mobile app–based therapy support in different situations.
Figure 3.
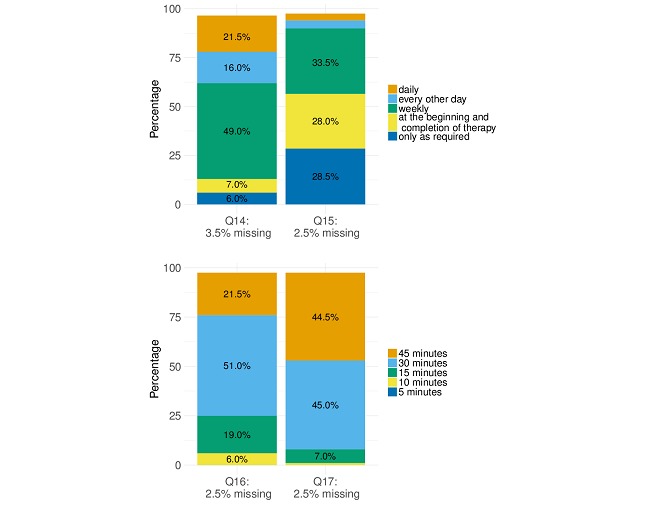
Distribution of the answers provided to selected questions regarding the favored frequencies of consulting a physician (Q14) or answering app-based health-related queries (Q15) as well as maximum acceptable waiting times for daily radiotherapy (Q16) or for a spontaneous medical consultation, if required (Q17).
In Figure 2, Q9 corresponds to “Use of a dedicated smartphone app for support during radiotherapy”; Q11 to “Staying in touch via smartphone app during follow-up”; Q12 to “Being contacted about medical warning signs via a smartphone app”; Q20 to “App collecting relevant medical information prior to consultation”; and Q27 to “App-based supportive care in the context of treatment side effects.”
Areas of Interest
For quantitative evaluation, the items in the questionnaire were grouped according to subject to calculate the scores for AOI, as described above. The scores were transformed to represent a value between 0 and 100, where a higher score represents a higher inclination or acceptance toward the use of a smartphone app [14-16]. The scores for AOI as calculated from the analysis of all returned questionnaires filled out at T0 are indicated in Table 2 and Figure 4. The highest scores were achieved for AOI 3 (“readiness to use a dedicated app within the context of radiotherapy”) and AOI 4 (“suggested features of a mobile app”), achieving median values of 71.4 (Q1-Q3=61.9-76.2) and 75.0 (Q1-Q3=75.0-87.5), respectively. AOI 3 and 4 translate most directly into a high acceptance for the presented app-based model of therapy support. A similar median value of 71.4 (Q1-Q3=42.9-85.7) was calculated for AOI 2 (“technical knowledge and abilities”). AOI 5 (“timeframe of reachability”) and 6 (“general attitude”) provided mostly qualitative information regarding reachability and the setting and frequency of medical consultations during treatment. The median score of 58.3 (Q1-Q3=41.7-66.7) for AOI 5 translates into an average reachability within 2 hours during a daily timeframe of 2 to 12 hours for the majority of patients. The score of 33.3 (Q1-Q3=25.0-50.0) for AOI 6 shows a general acceptance for waiting periods of up to 30 minutes for a medical consultation.
Table 2.
Scores for the areas of interest (AOI) as calculated from the analysis of all returned questionnaires filled out at T0.
| Scale | Description | Items | Mean (SD) | Median | Q1 | Q3 | Min | Max |
| AOI 1 | Habits of smartphone use | Q3 + Q7 + Q8 | 52.2 (27.22) | 50 | 40 | 80 | 0 | 100 |
| AOI 2 | Technical knowledge and abilities | Q4 + Q6 | 66.2 (21.39) | 71.4 | 42.9 | 85.7 | 0 | 100 |
| AOI 3 | Readiness to use an app | Q9 + Q14 + Q20 + Q21 + Q22+ Q27 | 68.6 (14.44) | 71.4 | 61.9 | 76.2 | 19 | 95.2 |
| AOI 4 | Possible features of a mobile app | Q11 + Q12 | 79.4 (17.01) | 75 | 75 | 87.5 | 0 | 100 |
| AOI 5 | Timeframe of reachability | Q23 + Q24 + Q25 | 58.8 (18.58) | 58.3 | 41.7 | 66.7 | 16.7 | 100 |
| AOI 6 | General attitude | Q15 + Q16 + Q17 | 37.5 (13.47) | 33.3 | 25 | 50 | 0 | 75 |
Figure 4.
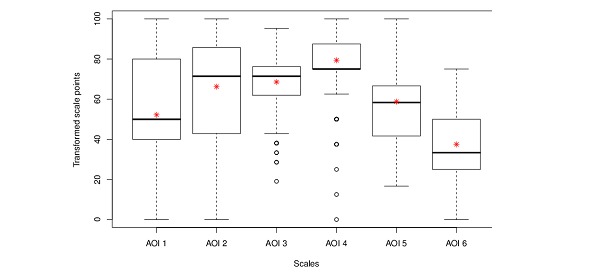
Scores for the different areas of interest (AOI) covered by the questionnaires. The asterisks indicate the mean value of the corresponding AOI.
Influence of Radiotherapy
For 140 patients, survey data before (T0) as well as after the completion (T1) of radiotherapy were available and were compared to evaluate whether having undergone radiotherapy influenced the attitude of patients. A small but statistically significant decrease of 4.7 points in the median transformed score for readiness to use an app within the context of radiotherapy (AOI 3) was detected at T1 (mean 68.6 vs 65.5, P<.001, Wilcoxon signed-rank test). The question within AOI 3 leading to this difference addressed the favored frequency of answering symptom-related questions posed by the app (Q14). At T1, patients selected “at therapy start and completion” and “only as required” more frequently instead of “weekly” and “every other day.” The helpfulness of app-based support in the context of toxicity was judged slightly lower at T1 (mean 4.04 before radiotherapy vs 3.88 after radiotherapy, P=.03). Regarding the median score for Q12, addressing the helpfulness of being contacted by a physician in case of medical warning signs, the mean/median score was 4.29/4 at T0 and 4.11/4 at T1 (P=.03). This question translated into a small but significant difference of 4.4 points in the transformed score for AOI 4 (mean 79.4 before radiotherapy vs 75.0 after radiotherapy, P=.04, Wilcoxon signed-rank test). For the other AOIs (AOI 1, 2 and 5, 6), as well as for individual questions, no significant difference was observed between the time points T0 and T1. A comparison of AOI scores between the time points T0 and T1 is illustrated in Figure 5. Detailed information regarding the quantitative comparison between answers provided at T0 and T1 for different survey items is provided in Table 3.
Figure 5.
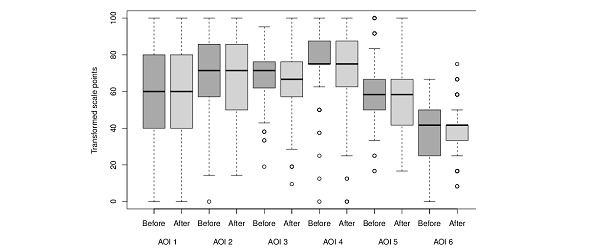
Comparison between the area of interest (AOI) scores of 140 patients who answered the survey at time points T0 and T1.
Table 3.
Quantitative comparison between answers provided at time points T0 and T1 for different survey items for 140 patients who filled out the survey questionnaire twice.
| Area of interest (AOI) | P value | |
| AOI 1 | .27 | |
|
|
Q3 | .81 |
|
|
Q7 | .94 |
|
|
Q8 | .005 |
| AOI 2 | .497 | |
|
|
Q4 | .98 |
|
|
Q6 | .23 |
| AOI 3 | .001a,b | |
|
|
Q9 | .17 |
|
|
Q14 | <.001a,b |
|
|
Q20 | .23 |
|
|
Q21 | .98 |
|
|
Q22 | <.001 |
|
|
Q27 | .03b |
| AOI 4 | .04a | |
|
|
Q11 | .09 |
|
|
Q12 | .03a,b |
| AOI5 | .76 | |
|
|
Q23 | .68 |
|
|
Q24 | .14 |
|
|
Q25 | .88 |
| AOI 6 | .21 | |
|
|
Q15 | .45 |
|
|
Q16 | .39 |
|
|
Q17 | .13 |
|
|
Q18 | .56 |
|
|
Q19 | .81 |
|
|
Q26 | .77 |
aLower score after the completion of radiotherapy.
bSignificant P values.
Predictive Factors
To determine possible factors that influenced patients’ attitude toward the usage of a mobile app, we tested several patient characteristics for their impact on survey results. The tested factors were age (<55 years vs ≥55 years), tumor stage (early [defined as tumor stage ≤T2, N0, M0/X] vs advanced), gender, previous radiotherapies, treatment setting (curative vs palliative), and initial KPI (<80% vs ≥80%). Age appeared to have the most sizable impact on the answers provided in the questionnaire, leading to significant differences in usage habits and technical skills as well as reachability. In all cases, younger patients were found to be more inclined toward and versed in more intensive smartphone use (AOI 2, P<.001). Interestingly, the favored frequency of seeing a physician during therapy was higher in patients younger than 55 years (P=.009). An overview of the survey items significantly impacted by the analyzed patient characteristics is displayed in Table 4.
Table 4.
P values for the influence of tumor stage, age, gender, initial Karnofsky performance scale index (KPI), treatment setting (curative vs palliative), and previous radiotherapy on the answers provided to the survey items and area of interest (AOI) scores.
| AOI | Tumor stage | Age 55 years | Gender | KPI | Treatment setting | Previous radiotherapy | |||||||
| AOI 1 | .45 | .005a,b | .78 | .51 | .006a,c | .26 | |||||||
|
|
Q3 | .63 | .19 | .57 | .56 | .16 | .09 | ||||||
|
|
Q7 | .74 | .01a,b | .64 | .81 | .002a,c | .48 | ||||||
|
|
Q8 | .047a,d | .004a,b | .12 | .83 | >.99 | .73 | ||||||
| AOI 2 | .66 | .001a,b | .72 | .24 | .495 | .93 | |||||||
|
|
Q4 | .73 | .003a,b | .64 | .35 | .25 | .88 | ||||||
|
|
Q6 | .70 | .004a,b | .92 | .28 | .88 | .74 | ||||||
| AOI 3 | .08 | .26 | .67 | .01a,e | .32 | .89 | |||||||
|
|
Q9 | .45 | .58 | .17 | .58 | .12 | .35 | ||||||
|
|
Q14 | .09 | .12 | .26 | .06 | .003a,f | .22 | ||||||
|
|
Q20 | .20 | .75 | .39 | .04a,e | .94 | .77 | ||||||
|
|
Q21 | .29 | .16 | .67 | .15 | .91 | .84 | ||||||
|
|
Q22 | .67 | .88 | >.99 | .64 | .76 | .83 | ||||||
|
|
Q27 | .18 | .67 | .13 | .006a,e | .63 | .99 | ||||||
| AOI 4 | .09 | .86 | .203 | .12 | .92 | .90 | |||||||
|
|
Q11 | .19 | .74 | .146 | .20 | .95 | .99 | ||||||
|
|
Q12 | .07 | .68 | .542 | .18 | .72 | .99 | ||||||
| AOI 5 | .07 | .003a,b | .058 | .54 | .55 | .29 | |||||||
|
|
Q23 | .94 | .06 | .106 | .30 | .77 | .19 | ||||||
|
|
Q24 | .07 | .002a,b | .484 | .87 | .41 | .30 | ||||||
|
|
Q25 | .26 | .12 | .641 | .84 | .75 | .98 | ||||||
| AOI 6 | .96 | .05 | .041a,g | .67 | .99 | .84 | |||||||
|
|
Q15 | .71 | .009a,h | .091 | .78 | .82 | .58 | ||||||
|
|
Q16 | .50 | .53 | .186 | .17 | .87 | .87 | ||||||
|
|
Q17 | .78 | .20 | .116 | .49 | .63 | .36 | ||||||
aSignificant P values.
bHigher score for age <55 years.
cHigher score for curative treatment setting.
dHigher score for advanced tumor stage.
eHigher score for KPI <80%.
fHigher score for palliative treatment setting.
gHigher score for gender (male).
hHigher score for age ≥55 years.
Discussion
Interpretation of Survey Results
We conducted a prospective and systematic survey regarding the habits and skills in smartphone usage, readiness to use a supportive mobile app during and after radiotherapy, and opinions on suggested functionality for such an app among cancer patients undergoing radiotherapy. Our results showed high overall acceptance levels for the usage of mobile technology in the context of radiotherapy, and the proposed functionality and features were considered as helpful by a large majority of patients.
Moreover, our survey showed that the ability to use a dedicated mobile app for additional supportive care (eg, mobile data on smartphone, reachability, and technical skills) on the patient side were present in almost three quarters of the survey population, and the obtained numbers were consistent across the different subareas of this survey. These results suggest a promising potential for an mHealth approach in the field of radiotherapy, although they also outline the need for careful patient selection to avoid the unnecessary burdening of subgroups that are either not willing or not capable and, thus, will likely not benefit. As the ownership of a smartphone and informed consent were prerequisites to participating in the survey, 975 patients were screened, and a total of 200 patients participated. The possible selection bias introduced by this approach has to be considered when generalizing the survey results to all patients undergoing radiotherapy. On the other hand, the results can be valuable in identifying and describing patient subgroups that are most likely to benefit from additional mobile app–based support.
The quantitative analysis of the survey data allowed us to identify age as the most influential factor for patient willingness with younger patients being considerably more inclined toward the use of mobile technology in the context of radiotherapy. Oncological diagnoses are typically associated with certain age groups (eg, prostate cancer, lung cancer, and different subgroups of head and neck cancer). Thus, a degree of patient selection based on age and age-associated diagnosis seams feasible when offering mHealth-based support. On the other hand, prospective clinical evidence exists, showing that patients with unfavorable characteristics regarding age and diagnosis, such as lung cancer patients, can also benefit. This especially holds true if the design of the electronic health (eHealth) or mHealth app integrates the patient’s next of kin to assist in the usage [17,18].
Several studies have shown that patient compliance plays a special role in the successful implementation of mHealth initiatives [19]. Clinical experience in oncology shows patient compliance to be worse in subgroups with lifestyle-related risk factors (eg, heavy smoking and alcohol abuse), potentially making such patients less eligible for mHealth-based support [20,21]. However, evidence exists that offering mHealth support in addition to close clinical surveillance may improve patient compliance, particularly in the abovementioned subgroups, by providing regular prompts and reminders and facilitating adherence to prescribed exercises or supportive regimens [22-24].
The perceived needs of cancer patients for supportive measures are manifold; they may strongly vary depending on culture, diagnosis, prognosis, and associated symptoms and may, hence, influence the acceptance of such measures [25-27]. Our survey showed that, disregarding few minor points, the acceptance of the proposed mobile app approach was high among patients, irrespective of tumor stage, treatment setting, potential previous radiotherapies, and initial clinical performance. Regarding toxicity-related surveillance, acceptance was significantly higher in patients with a reduced performance status. Nevertheless, as poor-performing patients require a different form of intensified personal care, the use of a mobile app alone may show limitations, particularly in the palliative setting [28].
It can be argued that by limiting the present survey to include only prostate and breast cancer patients, a selection bias in favor of patients with favorable prognosis and good clinical performance is introduced, and the generalizability of the results for other cancer patients undergoing radiotherapy could be limited. This potential limitation was accepted to achieve a more homogeneous dataset and ensure timely and systematic survey completion and analysis. However, it should be noted that we included 49.0% of patients with advanced tumor stages, 17.0% of patients with a metastatic disease, and 28.0% of patients who had undergone previous radiotherapy. These patient subgroups feature a different clinical profile characterized by unfavorable prognosis, usually a rapid decline in clinical performance, and a high need for supportive care [29]. The described clinical profile is shared by a majority of patients undergoing radiotherapy for different diagnoses, and the survey results regarding the acceptance of mobile app–-based support did not differ significantly in this subgroup [30-32]. Furthermore, it is of interest that irrespective of age, no further diagnosis-specific influencing factors regarding mobile app acceptance were identified for either breast or prostate cancer patients. This finding, in turn, supports the approach of cautiously extrapolating our results to patients with differing diagnoses.
The helpfulness of app-based supportive care in the context of monitoring potential radiotherapy-induced side effects was judged minimally higher by patients before the beginning of therapy, as was the favored frequency of interaction with the app. These results highlight the importance of providing sufficient information and support before and during the early stages of radiotherapy to address potential fears and worries. Such fears and worries and, thus, the need for additional support are less likely to be observed when the therapy is successfully completed, and the results of our survey accurately mirror this constellation.
Review of the Literature
The management of cancer patients is an area of special interest for the development of new eHealth and mHealth initiatives because they show promising potential, particularly in the context of supportive care and follow-up [7].
Only one other survey focusing on the acceptance of a dedicated mobile app among cancer patients has been published [33]. Overall results showed convincing similarity in both surveys. However, good or very good technical skills and the willingness to send data to the treating clinic via an app were slightly less frequent in the previous survey than in our survey (54.1% vs 63.9% and 48.5% vs 71.1%, respectively). Moreover, younger patients were generally more willing to use mobile technology in a disease-related context. In comparison, our survey described a more homogeneous and precisely selected cohort, focusing exclusively on patients receiving radiotherapy. Consequently, the specific requirements and circumstances related to this course of treatment are more comprehensively examined.
Ruland and colleagues have reported results similar to those of our survey, testing the usefulness and acceptance of a Web-based, multicomponent eHealth app with special focus on patient self-management among breast and prostate cancer patients [34]. The tested app did not focus exclusively on radiotherapy, and it provided supportive care in a more general manner, featuring message boards and general information sections, among other functionalities. Active usage among the regarded cohort was 64%, which is close to the results of our survey, and in this context, age and diagnosis were reported to significantly impact patient usage [34].
The value of an eHealth or mHealth resource is particularly high when tailored to fit the needs of the target patient cohort because patients are more likely to appreciate the immediately relevant and personalized support [34]. Depending on the diagnosis and therapy regimen, the wide heterogeneity among cancer patients and the very specific needs of different subgroups make them a challenging collective to address as a whole. Consequently, existing mHealth projects have typically been addressing either a specific question or a specific subgroup of cancer patients.
A recent randomized controlled trial by Denis and colleagues has shown a significant median overall survival benefit of 5 months for lung cancer patients who were systematically telemonitored using a mobile app based on patient-reported data and using the dynamics of patients’ clinical symptoms for risk stratification and individualized follow-up [18,35]. Similar approaches have successfully been used in the management of toxicities related to head and neck cancer treatment: computerized screening could facilitate the identification of treatment-related toxicities, and telepractice apps or videoconferencing could assist in the delivery of intensive home-based dysphagia therapy [24,36]. Regarding general supportive care, considerable advances have been made in the development of mHealth interventions to address the common issue of fatigue among cancer survivors. A recent meta-analysis identified 9 completed eHealth studies that revealed a significant beneficial effect of eHealth interventions on fatigued patients with improvements in health-related quality of life and depression [37].
Patients undergoing radiotherapy represent a distinct subgroup of cancer patients with special requirements in terms of supportive care. The nature of radiotherapy results in a specific set of therapy-related side effects and medical issues that require surveillance and potential support. Of the symptoms, the most common are dermatitis, nausea, fatigue, and localized toxicities within the respective treatment region [12]. Furthermore, depending on the diagnosis, patients undergoing radiotherapy vary in terms of age and characteristic profiles regarding individual risk constellations and comorbidities. Based on the data reported here, the usefulness and clinical implementation of a mobile app will be evaluated in a prospective trial (OPTIMISE-1; ClinicalTrials.gov identifier #NCT03168048) for which the dedicated mobile app has been designed according to the requirements of the patients who were assessed [38].
Conclusion
To the best of our knowledge, this study is the first to prospectively evaluate and demonstrate a high acceptance of and distinct patient requirements for the use of a supportive mobile app in a large homogeneous cohort of cancer patients undergoing radiotherapy. The reported patient acceptance will serve as a basis for future clinical trials that prospectively investigate the benefits of mobile app–based treatment support in routine clinical settings. The introduction of mobile apps into the clinical routine should be regarded as an opportunity to improve and intensify supportive treatment for cancer patients.
Acknowledgments
We thank our study nurses Renate Haselmann, Karen Lossner, and Alexandros Gioules for their support during this trial.
Abbreviations
- AOI
area of interest
- eHealth
electronic health
- KPI
Karnofsky performance scale index
- mHealth
mobile health
Survey questionnaire.
Scoring system.
Descriptive statistics before radiotherapy.
Descriptive statistics after radiotherapy.
Footnotes
Authors' Contributions: RAES, JD, and NHN developed and planned this survey. NB and DO helped devising survey items for the usage of mobile app and electronic data management. DW is the trial biostatistician, and she performed data analysis. RAES and TS performed patient recruitment and data collection. RAES and NHN drafted the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of Interest: None declared.
References
- 1.Carroll Jennifer K, Moorhead Anne, Bond Raymond, LeBlanc William G, Petrella Robert J, Fiscella Kevin. Who Uses Mobile Phone Health Apps and Does Use Matter? A Secondary Data Analytics Approach. J Med Internet Res. 2017 Apr 19;19(4):e125. doi: 10.2196/jmir.5604. http://www.jmir.org/2017/4/e125/ v19i4e125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. [2018-08-10]. mHealth: New horizons for health through mobile technologies http://www.who.int/goe/publications/goe_mhealth_web.pdf .
- 3.Nasi Greta, Cucciniello Maria, Guerrazzi Claudia. The role of mobile technologies in health care processes: the case of cancer supportive care. J Med Internet Res. 2015 Feb 12;17(2):e26. doi: 10.2196/jmir.3757. http://www.jmir.org/2015/2/e26/ v17i2e26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St George Sara M, Delamater Alan M, Pulgaron Elizabeth R, Daigre Amber, Sanchez Janine. Access to and Interest in Using Smartphone Technology for the Management of Type 1 Diabetes in Ethnic Minority Adolescents and Their Parents. Diabetes Technol Ther. 2016 Feb;18(2):104–9. doi: 10.1089/dia.2015.0086. [DOI] [PubMed] [Google Scholar]
- 5.Baranowski Tom, Blumberg Fran, Buday Richard, DeSmet Ann, Fiellin Lynn E, Green C Shawn, Kato Pamela M, Lu Amy Shirong, Maloney Ann E, Mellecker Robin, Morrill Brooke A, Peng Wei, Shegog Ross, Simons Monique, Staiano Amanda E, Thompson Debbe, Young Kimberly. Games for Health for Children-Current Status and Needed Research. Games Health J. 2016 Feb;5(1):1–12. doi: 10.1089/g4h.2015.0026. http://europepmc.org/abstract/MED/26262772 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonoto Bráulio Cezar, de Araújo Vânia Eloisa, Godói Isabella Piassi, de Lemos Lívia Lovato Pires, Godman Brian, Bennie Marion, Diniz Leonardo Mauricio, Junior Augusto Afonso Guerra. Efficacy of Mobile Apps to Support the Care of Patients With Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JMIR Mhealth Uhealth. 2017 Mar 01;5(3):e4. doi: 10.2196/mhealth.6309. http://mhealth.jmir.org/2017/3/e4/ v5i3e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis J, Ray P, Liaw S-t. Recent Worldwide Developments in eHealth and mHealth to more Effectively Manage Cancer and other Chronic Diseases - A Systematic Review. Yearb Med Inform. 2016 Nov 10;(1):93–108. doi: 10.15265/IY-2016-020. http://europepmc.org/abstract/MED/27830236 .me2016-020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouard Benoit, Bardo Pascale, Bonnet Clément, Mounier Nicolas, Vignot Marina, Vignot Stéphane. Mobile applications in oncology: is it possible for patients and healthcare professionals to easily identify relevant tools? Ann Med. 2016 Dec;48(7):509–515. doi: 10.1080/07853890.2016.1195010. [DOI] [PubMed] [Google Scholar]
- 9.Bender Jacqueline Lorene, Yue Rossini Ying Kwan, To Matthew Jason, Deacken Laetitia, Jadad Alejandro R. A lot of action, but not in the right direction: systematic review and content analysis of smartphone applications for the prevention, detection, and management of cancer. J Med Internet Res. 2013 Dec 23;15(12):e287. doi: 10.2196/jmir.2661. http://www.jmir.org/2013/12/e287/ v15i12e287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BinDhim Nasser F, Hawkey Alexandra, Trevena Lyndal. A systematic review of quality assessment methods for smartphone health apps. Telemed J E Health. 2015 Feb;21(2):97–104. doi: 10.1089/tmj.2014.0088. [DOI] [PubMed] [Google Scholar]
- 11.Hickson Ryan, Talbert Jeffery, Thornbury William C, Perin Nathan R, Goodin Amie J. Online medical care: the current state of “eVisits” in acute primary care delivery. Telemed J E Health. 2015 Feb;21(2):90–6. doi: 10.1089/tmj.2014.0022. [DOI] [PubMed] [Google Scholar]
- 12.Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider J E, Shank B, Solin L J, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991 May 15;21(1):109–22. doi: 10.1016/0360-3016(91)90171-y.0360-3016(91)90171-Y [DOI] [PubMed] [Google Scholar]
- 13.Karnofsky, DA, Burchenal, JH . The Clinical Evaluation of Chemotherapeutic Agents in Cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. New York City: Columbia University Press; 1949. p. 196. [Google Scholar]
- 14.McHorney C A, Ware J E, Lu J F, Sherbourne C D. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994 Jan;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 15.McHorney C A, Ware J E, Raczek A E. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993 Mar;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 16.McHorney C A, Ware J E, Rogers W, Raczek A E, Lu J F. The validity and relative precision of MOS short- and long-form health status scales and Dartmouth COOP charts. Results from the Medical Outcomes Study. Med Care. 1992 May;30(5 Suppl):MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 17.Global Burden of Disease Cancer Collaboration. Fitzmaurice Christina, Akinyemiju Tomi F, Hasan Al Lami F, Alam Tahiya, Alizadeh-Navaei Reza, Allen Christine, Alsharif Ubai, Alvis-Guzman Nelson, Amini Erfan, Anderson Benjamin O, Aremu Olatunde, Artaman Al, Asgedom Solomon Weldegebreal, Assadi Reza, Atey Tesfay Mehari, Avila-Burgos Leticia, Awasthi Ashish, Ba Saleem Huda Omer, Barac Aleksandra, Bennett James R, Bensenor Isabela M, Bhakta Nickhill, Brenner Hermann, Cahuana-Hurtado Lucero, Castañeda-Orjuela Carlos A, Catalá-López Ferrán, Choi Jee-Young Jasmine, Christopher Devasahayam Jesudas, Chung Sheng-Chia, Curado Maria Paula, Dandona Lalit, Dandona Rakhi, das Neves José, Dey Subhojit, Dharmaratne Samath D, Doku David Teye, Driscoll Tim R, Dubey Manisha, Ebrahimi Hedyeh, Edessa Dumessa, El-Khatib Ziad, Endries Aman Yesuf, Fischer Florian, Force Lisa M, Foreman Kyle J, Gebrehiwot Solomon Weldemariam, Gopalani Sameer Vali, Grosso Giuseppe, Gupta Rahul, Gyawali Bishal, Hamadeh Randah Ribhi, Hamidi Samer, Harvey James, Hassen Hamid Yimam, Hay Roderick J, Hay Simon I, Heibati Behzad, Hiluf Molla Kahssay, Horita Nobuyuki, Hosgood H Dean, Ilesanmi Olayinka S, Innos Kaire, Islami Farhad, Jakovljevic Mihajlo B, Johnson Sarah Charlotte, Jonas Jost B, Kasaeian Amir, Kassa Tesfaye Dessale, Khader Yousef Saleh, Khan Ejaz Ahmad, Khan Gulfaraz, Khang Young-Ho, Khosravi Mohammad Hossein, Khubchandani Jagdish, Kopec Jacek A, Kumar G Anil, Kutz Michael, Lad Deepesh Pravinkumar, Lafranconi Alessandra, Lan Qing, Legesse Yirga, Leigh James, Linn Shai, Lunevicius Raimundas, Majeed Azeem, Malekzadeh Reza, Malta Deborah Carvalho, Mantovani Lorenzo G, McMahon Brian J, Meier Toni, Melaku Yohannes Adama, Melku Mulugeta, Memiah Peter, Mendoza Walter, Meretoja Tuomo J, Mezgebe Haftay Berhane, Miller Ted R, Mohammed Shafiu, Mokdad Ali H, Moosazadeh Mahmood, Moraga Paula, Mousavi Seyyed Meysam, Nangia Vinay, Nguyen Cuong Tat, Nong Vuong Minh, Ogbo Felix Akpojene, Olagunju Andrew Toyin, Pa Mahesh, Park Eun-Kee, Patel Tejas, Pereira David M, Pishgar Farhad, Postma Maarten J, Pourmalek Farshad, Qorbani Mostafa, Rafay Anwar, Rawaf Salman, Rawaf David Laith, Roshandel Gholamreza, Safiri Saeid, Salimzadeh Hamideh, Sanabria Juan Ramon, Santric Milicevic Milena M, Sartorius Benn, Satpathy Maheswar, Sepanlou Sadaf G, Shackelford Katya Anne, Shaikh Masood Ali, Sharif-Alhoseini Mahdi, She Jun, Shin Min-Jeong, Shiue Ivy, Shrime Mark G, Sinke Abiy Hiruye, Sisay Mekonnen, Sligar Amber, Sufiyan Muawiyyah Babale, Sykes Bryan L, Tabarés-Seisdedos Rafael, Tessema Gizachew Assefa, Topor-Madry Roman, Tran Tung Thanh, Tran Bach Xuan, Ukwaja Kingsley Nnanna, Vlassov Vasiliy Victorovich, Vollset Stein Emil, Weiderpass Elisabete, Williams Hywel C, Yimer Nigus Bililign, Yonemoto Naohiro, Younis Mustafa Z, Murray Christopher J L, Naghavi Mohsen. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018 Jun 02; doi: 10.1001/jamaoncol.2018.2706.2683251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denis Fabrice, Lethrosne Claire, Pourel Nicolas, Molinier Olivier, Pointreau Yoann, Domont Julien, Bourgeois Hugues, Senellart Hélène, Trémolières Pierre, Lizée Thibaut, Bennouna Jaafar, Urban Thierry, El Khouri Claude, Charron Alexandre, Septans Anne-Lise, Balavoine Magali, Landry Sébastien, Solal-Céligny Philippe, Letellier Christophe. Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients. J Natl Cancer Inst. 2017 Dec 01;109(9) doi: 10.1093/jnci/djx029.3573360 [DOI] [PubMed] [Google Scholar]
- 19.Darlow Susan, Wen Kuang-Yi. Development testing of mobile health interventions for cancer patient self-management: A review. Health Informatics J. 2016 Dec;22(3):633–50. doi: 10.1177/1460458215577994.1460458215577994 [DOI] [PubMed] [Google Scholar]
- 20.Shiraev Timothy P, Durur Elif, Robinson David A. Factors Predicting Noncompliance with Follow-up after Endovascular Aneurysm Repair. Ann Vasc Surg. 2018 May 21; doi: 10.1016/j.avsg.2018.03.037.S0890-5096(18)30378-9 [DOI] [PubMed] [Google Scholar]
- 21.Han Summer S, Erdogan S Ayca, Toumazis Iakovos, Leung Ann, Plevritis Sylvia K. Evaluating the impact of varied compliance to lung cancer screening recommendations using a microsimulation model. Cancer Causes Control. 2017 Sep;28(9):947–958. doi: 10.1007/s10552-017-0907-x. http://europepmc.org/abstract/MED/28702814 .10.1007/s10552-017-0907-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Argent Rob, Daly Ailish, Caulfield Brian. Patient Involvement With Home-Based Exercise Programs: Can Connected Health Interventions Influence Adherence? JMIR Mhealth Uhealth. 2018 Mar 01;6(3):e47. doi: 10.2196/mhealth.8518. http://mhealth.jmir.org/2018/3/e47/ v6i3e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short Camille E, Finlay Amy, Sanders Ilea, Maher Carol. Development and pilot evaluation of a clinic-based mHealth app referral service to support adult cancer survivors increase their participation in physical activity using publicly available mobile apps. BMC Health Serv Res. 2018 Dec 16;18(1):27. doi: 10.1186/s12913-017-2818-7. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-017-2818-7 .10.1186/s12913-017-2818-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall Laurelie R, Ward Elizabeth C, Cartmill Bena, Hill Anne J, Porceddu Sandro V. Examining user perceptions of SwallowIT: A pilot study of a new telepractice application for delivering intensive swallowing therapy to head and neck cancer patients. J Telemed Telecare. 2017 Jan;23(1):53–59. doi: 10.1177/1357633X15617887.1357633X15617887 [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto Nobuhiro, Takiguchi Shuji, Komatsu Hirokazu, Okuyama Toru, Nakaguchi Tomohiro, Kubota Yosuke, Ito Yoshinori, Sugano Koji, Wada Makoto, Akechi Tatsuo. Supportive care needs and psychological distress and/or quality of life in ambulatory advanced colorectal cancer patients receiving chemotherapy: a cross-sectional study. Jpn J Clin Oncol. 2017 Dec 01;47(12):1157–1161. doi: 10.1093/jjco/hyx152.4564462 [DOI] [PubMed] [Google Scholar]
- 26.Molassiotis A, Yates P, Li Q, So W K W, Pongthavornkamol K, Pittayapan P, Komatsu H, Thandar M, Li M-s, Titus Chacko S, Lopez V, Butcon J, Wyld D, Chan R J, STEP Study Collaborators Mapping unmet supportive care needs, quality-of-life perceptions and current symptoms in cancer survivors across the Asia-Pacific region: results from the International STEP Study. Ann Oncol. 2017 Oct 01;28(10):2552–2558. doi: 10.1093/annonc/mdx350.3965267 [DOI] [PubMed] [Google Scholar]
- 27.Okediji Paul T, Salako Omolola, Fatiregun Olamijulo O. Pattern and Predictors of Unmet Supportive Care Needs in Cancer Patients. Cureus. 2017 May 09;9(5):e1234. doi: 10.7759/cureus.1234. http://europepmc.org/abstract/MED/28620565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicolay Nils Henrik. [A close web-based patient follow-up improves overall survival in lung cancer patients] Strahlenther Onkol. 2018 Dec;194(6):604–606. doi: 10.1007/s00066-018-1297-z.10.1007/s00066-018-1297-z [DOI] [PubMed] [Google Scholar]
- 29.Nieder Carsten, Kämpe Thomas A, Pawinski Adam, Dalhaug Astrid. Patient-reported symptoms before palliative radiotherapy predict survival differences. Strahlenther Onkol. 2018 Jun;194(6):533–538. doi: 10.1007/s00066-018-1259-5.10.1007/s00066-018-1259-5 [DOI] [PubMed] [Google Scholar]
- 30.Sprave Tanja, Verma Vivek, Förster Robert, Schlampp Ingmar, Bruckner Thomas, Bostel Tilman, Welte Stefan Ezechiel, Tonndorf-Martini Eric, Nicolay Nils Henrik, Debus Jürgen, Rief Harald. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018 Dec;128(2):274–282. doi: 10.1016/j.radonc.2018.04.030. https://linkinghub.elsevier.com/retrieve/pii/S0167-8140(18)30222-6 .S0167-8140(18)30222-6 [DOI] [PubMed] [Google Scholar]
- 31.Nieder Carsten, Tollåli Terje, Haukland Ellinor, Reigstad Anne, Flatøy Liv Randi, Dalhaug Astrid. External Validation of a Prognostic Score for Patients Receiving Palliative Thoracic Radiotherapy for Lung Cancer. Clin Lung Cancer. 2017 Dec;18(4):e297–e301. doi: 10.1016/j.cllc.2017.01.006.S1525-7304(17)30021-9 [DOI] [PubMed] [Google Scholar]
- 32.Chow Selina, Ding Keyue, Wan Bo Angela, Brundage Michael, Meyer Ralph M, Nabid Abdenour, Chabot Pierre, Coulombe Genevieve, Ahmed Shahida, Kuk Joda, Dar A Rashid, Mahmud Aamer, Fairchild Alysa, Wilson Carolyn F, Wu Jackson S Y, Dennis Kristopher, DeAngelis Carlo, Wong Rebecca K S, Zhu Liting, Chow Edward. Patient Reported Outcomes After Radiation Therapy for Bone Metastases as a Function of Age: A Secondary Analysis of the NCIC CTG SC-Twenty-Three Randomized Trial. Am J Hosp Palliat Care. 2018 Apr;35(4):718–723. doi: 10.1177/1049909117733435. [DOI] [PubMed] [Google Scholar]
- 33.Kessel Kerstin Anne, Vogel Marco Me, Kessel Carmen, Bier Henning, Biedermann Tilo, Friess Helmut, Herschbach Peter, von Eisenhart-Rothe Rüdiger, Meyer Bernhard, Kiechle Marion, Keller Ulrich, Peschel Christian, Schmid Roland M, Combs Stephanie E. Mobile Health in Oncology: A Patient Survey About App-Assisted Cancer Care. JMIR Mhealth Uhealth. 2017 Jun 14;5(6):e81. doi: 10.2196/mhealth.7689. http://mhealth.jmir.org/2017/6/e81/ v5i6e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruland Cornelia M, Maffei Roxana M, Børøsund Elin, Krahn Astrid, Andersen Trine, Grimsbø Gro H. Evaluation of different features of an eHealth application for personalized illness management support: cancer patients' use and appraisal of usefulness. Int J Med Inform. 2013 Jul;82(7):593–603. doi: 10.1016/j.ijmedinf.2013.02.007.S1386-5056(13)00041-5 [DOI] [PubMed] [Google Scholar]
- 35.Denis Fabrice, Yossi Senna, Septans Anne-Lise, Charron Alexandre, Voog Eric, Dupuis Olivier, Ganem Gérard, Pointreau Yoann, Letellier Christophe. Improving Survival in Patients Treated for a Lung Cancer Using Self-Evaluated Symptoms Reported Through a Web Application. Am J Clin Oncol. 2017 Oct;40(5):464–469. doi: 10.1097/COC.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 36.Ward Elizabeth C, Wall Laurelie R, Burns Clare L, Cartmill Bena, Hill Anne J. Application of telepractice for head and neck cancer management: a review of speech language pathology service models. Curr Opin Otolaryngol Head Neck Surg. 2017 Jun;25(3):169–174. doi: 10.1097/MOO.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 37.Seiler Annina, Klaas Vanessa, Tröster Gerhard, Fagundes Christopher P. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: A systematic review and meta-analysis. Psychooncology. 2017 Sep;26(9):1239–1253. doi: 10.1002/pon.4489. [DOI] [PubMed] [Google Scholar]
- 38.El Shafie RA, Bougatf Nina, Sprave Tanja, Weber Dorothea, Oetzel Dieter, Machmer Timo, Huber Peter E, Debus Jürgen, Nicolay Nils H. Oncologic Therapy Support Via Means of a Dedicated Mobile App (OPTIMISE-1): Protocol for a Prospective Pilot Trial. JMIR Res Protoc. 2018 Mar 06;7(3):e70. doi: 10.2196/resprot.8915. http://www.researchprotocols.org/2018/3/e70/ v7i3e70 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey questionnaire.
Scoring system.
Descriptive statistics before radiotherapy.
Descriptive statistics after radiotherapy.


