Abstract
Background
About 5% of breast cancer cases are metastatic at diagnosis, and 20%-30% of localized breast cancer cases become secondarily metastatic. Patients frequently report many detrimental symptoms related to metastasis and treatments. The physical, biological, psychological, and clinical benefits of physical activity during treatment in patients with localized breast cancer have been demonstrated; however, limited literature exists regarding physical activity and physical activity behavior change in patients with metastatic breast cancer.
Objective
The primary objective of this study is to assess the feasibility of a 6-month physical activity intervention with activity trackers in patients with metastatic breast cancer (the Advanced stage Breast cancer and Lifestyle Exercise, ABLE Trial). Secondary objectives are to examine the effects of physical activity on physical, psychological, anthropometrics, clinical, and biological parameters.
Methods
We plan to conduct a single-center, single-arm trial with 60 patients who are newly diagnosed with metastatic breast cancer. Patients will receive an unsupervised and personalized 6-month physical activity program that includes an activity tracker Nokia Go and is based on the physical activity recommendation. Patients will be encouraged to accumulate at least 150 minutes per week of moderate-to-vigorous intensity physical activity. Baseline and 6-month assessments will include anthropometric measures, functional tests (eg, 6-minute walk test and upper and lower limb strength), blood draws, patient-reported surveys (eg, quality of life and fatigue), and clinical markers of tumor progression (eg, Response Evaluation Criteria In Solid Tumors criteria).
Results
Data collection occurred between October 2016 and January 2018, and the results are expected in August 2018.
Conclusions
The ABLE Trial will be the first study to assess the feasibility and effectiveness of an unsupervised and personalized physical activity intervention performed under real-life conditions with activity trackers in patients with metastatic breast cancer.
Trial Registration
ClinicalTrials.gov NCT03148886; https://clinicaltrials.gov/ct2/show/NCT03148886 (Accessed by WebCite at http://www.webcitation.org/71yabi0la)
Registered Report Identifier
RR1-10.2196/10487
Keywords: metastatic breast cancer, physical activity, oxidative stress, activity trackers, feasibility
Introduction
Breast cancer is the most common cancer among women worldwide with >1.6 million new cases diagnosed annually and 54,062 incident cases in France in 2015 [1]. About 5% of breast cancer cases are metastatic at diagnosis, and 20%-30% of localized breast cancer cases become secondarily metastatic [2-4]. Metastatic breast cancer is considered incurable, and treatments are proposed to improve the quality of life and overall survival.
Fatigue and reduced quality of life are frequent in patients with metastatic breast cancer related to the site of metastasis and cancer treatment [5,6]. Evidence from meta-analyses and systematic reviews in patients with localized breast cancer has demonstrated the benefits of physical activity on multiple health outcomes [7-12]. However, only five studies have focused on the investigation of physical activity interventions in patients with metastatic breast cancer [13-17] despite the need, desire, and ability of these patients to engage in physical activity [14,15,18]. Some studies have already investigated the association among metastatic cancer, fatigue, and physical activity; however, the results are mixed and warrant confirmation, specifically in patients with metastatic breast cancer [13-16].
In the cancer context, activity trackers with step pedometers are increasingly being used to measure physical activity and promote physical activity behaviors [19-21]. The benefits of mobile eHealth apps and pedometers on physical fitness, physical activity, and quality of life of patients with breast cancer have been reported [22]. These devices might motivate people to remain active and facilitate reaching a personal or recommended goal because of the feedback received in real time (eg, steps) [23-25]. In addition, activity trackers are often linked to a mobile app with a personal interface that provides a summary of the physical activity (ie, light, moderate, and vigorous intensity), sedentary time, and the number of steps accumulated per day, week, and month.
Reportedly, regular physical activity and fatigue can affect blood biomarker levels, including inflammatory markers [26] and oxidative stress [27]. Evidence from randomized controlled trials (RCTs) has indicated an effect of physical activity in patients with cancer on the levels of circulating growth factors and cytokines (eg, interleukin 6 and tumor necrosis factor alpha) [28,29] and suggests that physical activity might markedly alter the frequency and functional competence of immune cell subsets of the innate immune system (eg, neutrophils, monocytes, and natural killer cells) [30,31]. Concerning the oxidative stress, an excessive accumulation of oxidative stress in cells has been shown to induce marked cellular and molecular damages and likely plays an important role in carcinogenesis, tumor promotion, and breast cancer recurrence and metastasis [32-34]. Moreover, plasma antioxidant defenses seem to be lower in women with breast cancer [34]. On the contrary, physical activity programs have shown to decrease the oxidative stress in patients with chronic diseases other than cancer, in particular through the improvement of enzymatic antioxidant defenses [27,35].
The Advanced stage Breast cancer and Lifestyle Exercise (ABLE) Trial was designed to address the gaps in the current literature. The primary aim is to determine the feasibility of an unsupervised and personalized physical activity intervention in patients with metastatic breast cancer. The secondary aims are to investigate (1) how the physical activity intervention changes the total global physical activity, sedentary time, and physical fitness; (2) how the physical activity intervention changes patient-reported outcomes, including the quality of life and fatigue; (3) how the physical activity intervention changes patients’ anthropometric measurements and body composition; (4) the barriers and facilitators of the adherence to a physical activity program; and (5) whether the physical activity intervention affects the oxidative stress and inflammation as biomarkers of the tumor progression.
Methods
Study Design
The ABLE Trial is a single-arm trial that is being conducted in the Léon Bérard Comprehensive Cancer Center (Lyon, France). The study protocol was approved by the French ethics committee (Comité de protection des personnes Sud-Est IV), and the study database was reported to the National Commission for Data Protection and Liberties (CNIL; reference number: 1994192). The study is registered on ClinicalTrials.gov (NCT number: NCT03148886).
Study Population
The inclusion criteria for this study are as follows: (1) female; (2) aged 18-78 years; (3) newly diagnosed with primary or secondary metastatic breast cancer histologically confirmed (ie, within the last 3 months) and treated in a cancer center by chemotherapy or radiotherapy or hormonal therapy or targeted therapy; (4) Eastern Cooperative Oncology Group Performance status <2; (5) able to speak and understand French and able to complete questionnaires and follow instructions in French; and (6) valid health insurance affiliation. For patients willing to participate in the study, confirmation from their treating oncologist of no contraindications to physical activity is required.
The exclusion criteria for this study are as follows: untreated brain metastases; pregnancy; and contraindications to physical activity (eg, uncontrolled hypertension, cardiac disease). All patients must sign an informed consent form.
Recruitment
Women are screened weekly during the center’s multidisciplinary metastatic breast cancer board meetings, as seen in Figure 1. After checking the inclusion and exclusion criteria, the study is directly proposed by an oncologist or radiotherapist to patients treated by chemotherapy or radiotherapy. As patients treated by hormonal therapy do not come to the center, they receive an information letter and study brochure through post and are contacted by telephone 1 week later to know whether they intend to participate. For all enrolled patients, a physician provides a certificate indicating no contraindications to physical activity, and an appointment is subsequently planned to sign an informed consent and proceed to the baseline evaluation (day one, D1).
Figure 1.
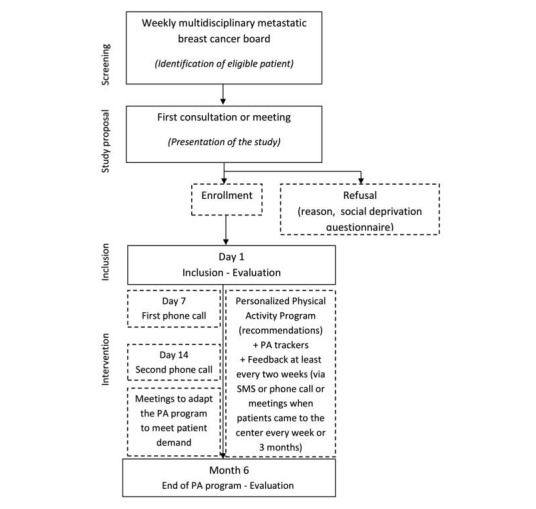
Participant flow chart for the Advanced stage Breast cancer Lifestyle and Exercise study, Lyon, France (PA: Physical activity).
Intervention
Adapted Physical Activity Program
The evaluated intervention is a home-based, personalized physical activity program. The frequency, duration, and intensity of physical activity sessions are modulated depending on patients’ capacities.
Patients are encouraged to accumulate at least 150 minutes per week of moderate-to-vigorous intensity physical activity to maintain and improve health benefits. In addition, patients are asked to walk at least 30 minutes per day, increase their activities in their daily routine, and reduce their sedentary time. Several individual strategies are established with patients to attain their objectives (eg, using stairs whenever possible and walking for groceries or shopping). Moreover, meetings at the center with physical activity instructors are proposed depending on patients’ needs. Patients receive feedback at least monthly from their instructor. For patients undergoing chemotherapy, meetings take place during their day care visits at the cancer center. For patients with hormonal therapy treatment, meetings occur by phone or during a consultation with a physician in the center.
Activity Trackers
Activity trackers (Nokia Go wristband, Nokia France, Issy-les-Moulineaux, France) are given to all study participants. Patients are instructed to wear the device every day for the duration of the 6-month intervention. In addition, patients receive real-time feedback on their number of steps per day. Recommendations are to achieve 5000-10,000 steps a day depending on patients’ ability and comorbidities. For a patient who reaches 10,000 steps per day, we will advise her to maintain her goal. For a patient who is not able to achieve 5000 steps a day, we will gradually increase her objective. The goal is adjusted regularly throughout the 6-month intervention period. The instructor has access to the data pertaining to the number of steps per day and can modify the daily steps goal directly in the app or by a phone call to the patients during meetings.
Data are collected by regular transfer to the wearable activity tracker mobile phone app (Nokia Health Mate) available on a mobile phone or Tablet PC. Patients can use the mobile phone app to follow their number of steps per day represented by a graph. After receiving the wearable activity tracker, patients are called on days 7 and 14 to ensure the proper use of the device and answer any questions. Personalized objectives might be redefined to increase their daily physical activity. For patients without a mobile phone, data are collected during the 6-month intervention when visiting the hospital for a consultation every week or 2 weeks. Instructors might use the activity tracker interface to monitor change over time and to adapt to physical activity recommendations. At the end of the 6-month intervention, patients can keep the activity tracker to continue their efforts.
Data Collection
Table 1 provides the complete data collection schedule in this study.
Table 1.
The data collection schedule for the Advanced stage Breast cancer Lifestyle and Exercise Trial.
| Assessments | Time | |||
|
|
|
Patients’ recruitment |
Day 1: Baseline |
Month 6: End of the study |
| Clinical data (patient record) | ||||
|
|
Date of birth |
|
✓ |
|
|
|
Age at diagnosis |
|
✓ |
|
|
|
Employment status |
|
✓ | ✓ |
|
|
Hormonal receptor status |
|
✓ |
|
|
|
Personal history of breast cancer |
|
✓ |
|
|
|
Metastasis localization |
|
✓ | ✓ |
|
|
Current treatment |
|
✓ | ✓ |
|
|
Tumor histology |
|
✓ |
|
|
|
Disease progression (Response Evaluation Criteria In Solid Tumors) |
|
✓ | ✓ |
| Demographic data |
|
✓ | ✓ | |
| Anthropometricsa |
|
✓ | ✓ | |
| Physical fitness | ||||
|
|
6-minute Walk Test with oxygen consumption |
|
✓ | ✓ |
|
|
Upper limb strength: handgrip |
|
✓ | ✓ |
|
|
Maximum isometric strength of quadriceps extension |
|
✓ | ✓ |
|
|
International Physical Activity Questionnaire (long form) |
|
✓ | ✓ |
|
|
Sedentary behavior: Marshall Questionnaire |
|
✓ | ✓ |
|
|
Steps per day: Activity trackerb |
|
|
✓ |
| Psychological questionnaires | ||||
|
|
Quality of life: Cancer Quality Of Life Questionnaire, breast cancer module |
|
✓ | ✓ |
|
|
Fatigue: Piper Fatigue Scale |
|
✓ | ✓ |
|
|
Physical activity preferences, facilitators, and barriers | ✓ | ✓ | ✓ |
|
|
Incentive effect of activity tracker |
|
|
✓ |
|
|
Social deprivation: EPICES (Evaluation of Deprivation and Inequalities in Health Examination Centres) score | ✓ | ✓ | ✓ |
| Other | ||||
|
|
Reason for refusal | ✓ |
|
|
aHeight, weight, waist-to-hip circumference, and body mass index.
bDuring 6 months.
All assessments are recorded at the baseline and the end of the 6-month physical activity program (M6) by the instructor, as seen in Figure 1.
Demographic and Clinical Data
Demographic and clinical data, including date of birth, age at diagnosis, living situation, employment status, hormonal status, tumor histology, personal history of breast cancer, sites of metastases, and current treatment, are collected at the baseline. The Response Evaluation Criteria In Solid Tumors (RECIST) is used to assess the tumor progression between the diagnosis and the end of the physical activity program [36]. All clinical data are extracted from patients’ electronic medical records.
Body Composition and Anthropometrics
The standing height (cm), body weight (kg), waist (cm), and hip (cm) circumferences are measured using standardized procedures. The waist circumference is measured midway between the last floating rib and the iliac crest. The hip circumference is measured at the tip of the pubis. The body mass index is calculated as the body weight in kilograms divided by the square of the height in meters.
Physical Activity Fitness and Sedentary Behavior
Cardiorespiratory fitness is measured by evaluating the peak oxygen consumption during the 6-minute walk test (6MWT) [37,38]. Patients are asked to perform the maximum walk shuttle distance on 30-meter-long flat corridors in 6 minutes. During this test, the oxygen consumption, carbon dioxide production, heart rate, and oxygen arterial saturation are continuously recorded using a portable respiratory gas analyzer (MetaMax 3b; Cortex Biophysik, Leipzig, Germany). In addition, the perception of the difficulty during 6MWT is evaluated at the end of the test using the Borg Rating of Perceived Exertion questionnaire [39].
Then, prehensile and grip strength is measured using a hand dynamometry (Jamar Plus Digital Hand Dynamometer; Patterson Medical, Huthwaite, United Kingdom), which is a validated index of the elbow extension strength [40]. Moreover, patients are asked to squeeze the handgrip as strongly as possible for 5 seconds to obtain the maximal force. Of note, two measures are performed on each hand, and the best performance is registered.
The maximum isometric strength of quadriceps extension is measured using a back-leg dynamometer (DFS II Series Digital; Force Gauges Chatillon, Largo, FL, USA). Patients are asked to sit on a chair with the knee articulation at 90°, arms crossed on the chest, and the dynamometer attached to the ankle. At the signal of the instructor, patients must try to extend the leg as strongly as possible in 3 seconds. Only the dominant leg is tested twice, and the best performance is obtained.
The International Physical Activity Questionnaire (IPAQ) [41] is used to measure the self-reported physical activity. IPAQ (long form) is a validated self-administered physical activity questionnaire with 31 items and covers 4 activity domains, work-related physical activity, transportation physical activity, domestic physical activity, and recreational physical activity [41]. IPAQ gives specific scores in the metabolic equivalent of task (MET)-minutes/week for walking, moderate-intensity, and vigorous-intensity activity within each of the work, transportation, domestic chores and gardening (yard), and leisure-time domains. Questions are coded and converted in MET per minute and per week according to the Compendium of Physical Activities [42] by multiplying the number of METs by the duration and frequency of the activity. Then, the total physical activity score for each intensity is obtained by adding the score for this intensity in each domain and by adding the number of MET-minutes per week at each intensity.
Next, the global score of physical activity is divided into 3 categories commonly used by physical activity guidelines (<600 MET-minutes/week is equivalent to low physical activity; between 600 and 3000 MET-minutes/week is equivalent to moderate physical activity; and >3000 corresponds to high physical activity [43]). Furthermore, the sedentary time in minutes per week is obtained by adding the weekday sitting time in minutes for 5 weekdays, and the weekend sitting time in minutes for 2 days.
Patient-Reported Outcomes
The quality of life is measured with the European Organization for Research and Treatment of Cancer Quality Of Life Questionnaire (QLQ-C30) and its specific module for breast cancer (BR-23) [44]. QLQ-C30 is a 30-item self-administered questionnaire that evaluates 5 functioning domains (physical, role, emotional, cognitive, and social), a global quality of life domain, 3 symptom domains (pain, fatigue, and nausea), and 6 single items (dyspnea, insomnia, anorexia, diarrhea, constipation, and financial impact). Each item is associated with a score ranging from 0 to 100. For the functioning and global scales, a higher score corresponds to a better functioning level. The specific module for breast cancer (BR-23) gathers data about perceived body image, sexual functioning, sex enjoyment, arm symptoms, breast symptoms, and systemic therapy side effects.
Fatigue is assessed with the revised Piper Fatigue Scale [45,46], which is a 22-item self-reported questionnaire with 4 subscales, behavioral and severity, affective, sensory, and cognitive and mood. All these items together produce a score for total fatigue defining categories as follows: no fatigue (score=0), mild fatigue (score 1-3), moderate fatigue (score 4-6), and severe fatigue (score 7-10).
The social deprivation is assessed using the EPICES (Evaluation of Deprivation and Inequalities in Health Examination Centres) score [47,48]. The score is computed by adding each question coefficient to intercept whenever the answer is “yes.” The score ranges from 0 to 100 with the threshold for deprivation at 30, and higher scores indicate greater deprivation levels.
Determinants of Physical Activity
Preferences, facilitators, and barriers are evaluated with the translated version of a specific questionnaire developed by Vallance et al [49]; this questionnaire includes questions regarding the interest and willingness of patients with metastatic breast cancer to participate in a physical activity program designed for patients with metastatic breast cancer. In addition, it includes questions on their interest and preferences for physical activity counseling and specific aspects of these programs, including how, where, and when they would be interested in these physical activity programs as well as the type of intervention that would be most amenable for them.
Biological Data
A 7-mL blood sample is collected at the baseline (D1) and at the end of the study after 6 months (M6). The sample is centrifuged within half an hour after drawing and kept at 4°C before and during centrifugation. The plasma is distributed into cryotube aliquots of 1 mL and buffy coat in a single 1-mL cryotube; these cryotubes are frozen and stored at −80°C at the center and used for the analyses of oxidative stress and antioxidant biomarkers. Superoxide dismutase, catalase, glutathione peroxidase enzymatic activities, and markers of DNA oxidation (8-hydroxy-2'-deoxyguanosine), prostaglandins oxidation (8-Iso Prostaglandin F2α) are measured in the plasma [50]. Furthermore, levels of circulating growth factors, cytokines, neutrophils, monocytes, and natural killer cells are assessed as previously described [51].
Statistical Analysis
Sample Size
The sample size was defined empirically to explore the feasibility of the program according to the enrollment potential in the study center. Because the main objective of the ABLE Trial was to assess the feasibility of a physical activity intervention in women with metastatic breast cancer, without major regard for the efficiency, no formal calculation was performed. Given that 200 patients are treated annually for metastatic breast cancer in the Center Léon Bérard and that 60% of patients are expected to survive for at least 6 months, with a projected acceptance rate of 50%, the pilot study will aim to recruit 60 patients within 9 months.
Statistical Methods
Patients’ characteristics will be described at D1 and M6 using the mean and SD for quantitative data, frequency, and percentage for qualitative data.
The primary outcome to assess the feasibility of the study will be the proportion of patients achieving the physical activity recommendations corresponding to 150 minutes per week of moderate physical activity evaluated by the IPAQ score during the last week of the study. In addition, we will estimate the proportion of patients who agreed to participate in the ABLE Trial among eligible patients. The reasons for refusal will be documented and described as well.
The secondary outcomes to assess will be the evolution of physical activity, anthropometric, physical fitness, psychological, and biological variables between the initiation (D1) and the completion (M6) of the physical activity intervention using Wilcoxon signed-rank tests. In addition, the level of physical activity according to biological, psychological, and clinical outcomes adjusted on potential confounders, including age, treatment, and number of visits, will be explored using multiple linear regressions. Moreover, the level of oxidative stress will be correlated to the RECIST criteria. Data on barriers and facilitators of the adherence to a physical activity program from D1 to M6 will be compared by Mac Nemar tests. All statistical analyses will be performed using SAS software (version 9.4. SAS Institute Inc, Cary, NC, USA).
Results
The recruitment and enrollment in this single-arm feasibility trial started in October 2016. A follow-up was completed in January 2018. Data analyses began in February 2018 and will be completed in October 2018. All results are expected to be available by the end of 2018.
Discussion
Principal Findings
Given the beneficial effects of physical activity in localized breast cancer, the ABLE Trial is the first European study to propose a physical activity intervention for patients with metastatic cancer that will obtain preliminary data on biological, functional, psychological, and clinical outcomes and identify the determinants of physical activity. In addition, the use of wearable activity trackers in the ABLE Trial strengthens its novelty. Although wearable activity trackers are comparable to pedometers in some aspects, they are more effective for behavioral modification [52] because the Health Mate app and the wearable activity tracker provide more detailed data on the physical activity performed over time. These types of devices are emerging in the health care field and are being shown to help motivate people to increase their physical activity level and facilitate them to reach a personal or recommended goal because of the feedback received in real time (eg, steps) [23-25]. International recommendations for patients with cancer are to practice 30 minutes of moderate physical activity per day at least 5 times a week [53]. The number of steps per day is more easily comprehensible for individuals to achieve these physical activity recommendations. For adults with health impairment, 5000-7000 steps per day might be a more appropriate target than the target of 10,000 steps per day recommended for healthy people [54]. Indeed, walking is feasible at no charge and practiced by the majority of people. In the ABLE Trial, the recommended number of steps is individualized to each patient because research has suggested that a small goal is more effective and easier to achieve than a higher activity goal. In addition, it is important to increase these physical activity targets gradually [24].
The links between the level of physical activity and the biological mechanisms involved in the tumor progression have never been studied in patients with metastatic breast cancer. A study of the oxidative stress in this population might help identify potential new biomarkers associated with physical activity and tumor progression.
Of note, some limitations are acknowledged in the ABLE Trial. First, the sample size is limited but is sufficient to test the feasibility of the intervention. Second, the device used in the study has not been validated but uses the same algorithm as Nokia Pulse that has been validated by comparing this tracker with the OptoGait system for laboratory and ActivPAL for free-living conditions [20]. Finally, the test-retest reliability for Nokia Pulse was excellent with an intraclass correlation coefficient >0.90.
The results of this trial will provide quantitative and qualitative outcomes that will help design a future multicenter RCT on a physical training intervention in patients with metastatic breast cancer. Given the paucity of data in this population of patients and the potential for measurable health benefits to them, in many domains, this trial will provide new data that will be relevant in assessing the feasibility and acceptability of a larger-scale trial for this previously underinvestigated population.
Ethics and Dissemination
The study protocol was approved by the French ethics committee (Comité de protection des personnes Sud-Est IV), and the study database was declared to the National Commission for Data Protection and Liberties (CNIL; reference number: 1994192).
Acknowledgments
The authors would like to thank the French League against Cancer, especially the League of Landes, the Cancéropôle Lyon Auvergne Rhône-Alpes, the associations Odyssea and Activ’RA for their financial support and Nokia for providing wearable activity trackers for patients. Furthermore, the authors would like to acknowledge the contribution and support of Nicole Falette for her help on the methodological design.
Abbreviations
- 6MWT
6-minute walk test
- ABLE
Advanced stage Breast cancer Lifestyle and Exercise
- D1
day one
- IPAQ
International Physical Activity Questionnaire
- M6
end of 6 months
- MET
metabolic equivalent of task
- RCT
randomized controlled trial
- SMS
Short Message Service
Footnotes
Conflicts of Interest: None declared.
References
- 1.INCa http://www.e-cancer.fr/ressources/cancers_en_france/ [2018-07-23]. Les cancers en France - Edition 2017 http://www.e-cancer.fr/ressources/cancers_en_france/
- 2.David A. Référentiel locorégional et métastatique des Cancers Cancers du sein ‐ Juin 2010. 2010. [2018-07-23]. http://www.oncolr.org/upload/Espace_patients/Referentiels_regionaux/ONCO_LR_-_Referentiel_locoregional_et_metastatique_des_Cancers_du_seinn_-_Juin_2010.pdf .
- 3.Beslija S, Bonneterre J, Burstein HJ, Cocquyt V, Gnant M, Heinemann V, Jassem J, Köstler WJ, Krainer M, Menard S, Petit T, Petruzelka L, Possinger K, Schmid P, Stadtmauer E, Stockler M, Van BS, Vogel C, Wilcken N, Wiltschke C, Zielinski CC, Zwierzina H, Central European Cooperative Oncology Group (CECOG) Third consensus on medical treatment of metastatic breast cancer. Ann Oncol. 2009 Nov;20(11):1771–85. doi: 10.1093/annonc/mdp261.mdp261 [DOI] [PubMed] [Google Scholar]
- 4.Andre F, Slimane K, Bachelot T, Dunant A, Namer M, Barrelier A, Kabbaj O, Spano JP, Marsiglia H, Rouzier R, Delaloge S, Spielmann M. Breast cancer with synchronous metastases: trends in survival during a 14-year period. J Clin Oncol. 2004 Aug 15;22(16):3302–8. doi: 10.1200/JCO.2004.08.095.22/16/3302 [DOI] [PubMed] [Google Scholar]
- 5.Krohe M, Hao Y, Lamoureux RE, Galipeau N, Globe D, Foley C, Mazar I, Solomon J, Shields AL. Patient-Reported Outcomes in Metastatic Breast Cancer: A Review of Industry-Sponsored Clinical Trials. Breast Cancer (Auckl) 2016 Jul;10:93–102. doi: 10.4137/BCBCR.S39385. http://europepmc.org/abstract/MED/27441001 .bcbcr-10-2016-093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen MJ, Redd WH, Winkel G, Badr H. Associations among pain, pain attitudes, and pain behaviors in patients with metastatic breast cancer. J Behav Med. 2014 Aug;37(4):595–606. doi: 10.1007/s10865-013-9529-2. http://europepmc.org/abstract/MED/23943140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimeo F. Effects of exercise on cancer-related fatigue. Cancer. 2001 Sep 15;92(6 Suppl):1689–93. doi: 10.1002/1097-0142(20010915)92:6+<1689::aid-cncr1498>3.0.co;2-h.10.1002/1097-0142(20010915)92:6+<1689::AID-CNCR1498>3.0.CO;2-H [DOI] [PubMed] [Google Scholar]
- 8.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010 Jun;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 9.Velthuis MJ, Agasi-Idenburg SC, Aufdemkampe G, Wittink HM. The effect of physical exercise on cancer-related fatigue during cancer treatment: a meta-analysis of randomised controlled trials. Clin Oncol (R Coll Radiol) 2010 Apr;22(3):208–21. doi: 10.1016/j.clon.2009.12.005.S0936-6555(09)00411-7 [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Huang M, Cheng ASK, Zhou Y, So WKW. Meta-analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer. 2014 Feb 26;21(3):262–274. doi: 10.1007/s12282-014-0521-7. [DOI] [PubMed] [Google Scholar]
- 11.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: A systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015 May;54(5):635–54. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 12.Bluethmann SM, Vernon SW, Gabriel KP, Murphy CC, Bartholomew LK. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat. 2015 Jan;149(2):331–42. doi: 10.1007/s10549-014-3255-5. http://europepmc.org/abstract/MED/25555831 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oldervoll LM, Loge JH, Lydersen S, Paltiel H, Asp MB, Nygaard UV, Oredalen E, Frantzen TL, Lesteberg I, Amundsen L, Hjermstad MJ, Haugen DF, Paulsen �, Kaasa S. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011 Sep;16(11):1649–57. doi: 10.1634/theoncologist.2011-0133. http://theoncologist.alphamedpress.org/cgi/pmidlookup?view=long&pmid=21948693 .theoncologist.2011-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Headley JA, Ownby KK, John LD. The effect of seated exercise on fatigue and quality of life in women with advanced breast cancer. Oncol Nurs Forum. 2004 Sep;31(5):977–83. doi: 10.1188/04.ONF.977-983. [DOI] [PubMed] [Google Scholar]
- 15.Ligibel JA, Giobbie-Hurder A, Shockro L, Campbell N, Partridge AH, Tolaney SM, Lin NU, Winer EP. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer. 2016 Apr 15;122(8):1169–77. doi: 10.1002/cncr.29899. doi: 10.1002/cncr.29899. [DOI] [PubMed] [Google Scholar]
- 16.Cormie P, Galvão DA, Spry N, Joseph D, Taaffe DR, Newton RU. Functional benefits are sustained after a program of supervised resistance exercise in cancer patients with bone metastases: longitudinal results of a pilot study. Support Care Cancer. 2014 Jun;22(6):1537–48. doi: 10.1007/s00520-013-2103-1. [DOI] [PubMed] [Google Scholar]
- 17.Scott JM, Iyengar NM, Nilsen TS, Michalski M, Thomas SM, Herndon J, Sasso J, Yu A, Chandarlapaty S, Dang CT, Comen EA, Dickler MN, Peppercorn JM, Jones LW. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: A randomized controlled trial. Cancer. 2018 Jun 15;124(12):2552–2560. doi: 10.1002/cncr.31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Hornsby WE, Coan AD, Herndon JE, Douglas PS, Haykowsky M. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012 Jul 10;30(20):2530–7. doi: 10.1200/JCO.2011.39.9014. http://europepmc.org/abstract/MED/22614980 .JCO.2011.39.9014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright SP, Hall BTS, Collier SR, Sandberg K. How consumer physical activity monitors could transform human physiology research. Am J Physiol Regul Integr Comp Physiol. 2017 Dec 01;312(3):R358–R367. doi: 10.1152/ajpregu.00349.2016. http://www.physiology.org/doi/abs/10.1152/ajpregu.00349.2016?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .ajpregu.00349.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kooiman TJ, Dontje ML, Sprenger SR, Krijnen WP, van der Schans CP, de Groot M. Reliability and validity of ten consumer activity trackers. BMC Sports Sci Med Rehabil. 2015 Oct;7:24. doi: 10.1186/s13102-015-0018-5. http://bmcsportsscimedrehabil.biomedcentral.com/articles/10.1186/s13102-015-0018-5 .18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storm FA, Heller BW, Mazzà C. Step detection and activity recognition accuracy of seven physical activity monitors. PLoS One. 2015 Mar;10(3):e0118723. doi: 10.1371/journal.pone.0118723. http://dx.plos.org/10.1371/journal.pone.0118723 .PONE-D-14-23091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uhm KE, Yoo JS, Chung SH, Lee JD, Lee I, Kim JI, Lee SK, Nam SJ, Park YH, Lee JY, Hwang JH. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2017 Dec;161(3):443–452. doi: 10.1007/s10549-016-4065-8.10.1007/s10549-016-4065-8 [DOI] [PubMed] [Google Scholar]
- 23.Mercer K, Giangregorio L, Schneider E, Chilana P, Li M, Grindrod K. Acceptance of Commercially Available Wearable Activity Trackers Among Adults Aged Over 50 and With Chronic Illness: A Mixed-Methods Evaluation. JMIR Mhealth Uhealth. 2016 Jan 27;4(1):e7. doi: 10.2196/mhealth.4225. http://mhealth.jmir.org/2016/1/e7/ v4i1e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014 Aug;16(8):e192. doi: 10.2196/jmir.3469. http://www.jmir.org/2014/8/e192/ v16i8e192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan AN, Lachman ME. Behavior Change with Fitness Technology in Sedentary Adults: A Review of the Evidence for Increasing Physical Activity. Front Public Health. 2016;4:289. doi: 10.3389/fpubh.2016.00289. doi: 10.3389/fpubh.2016.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006 Nov;42:105–17. doi: 10.1042/bse0420105.bse0420105 [DOI] [PubMed] [Google Scholar]
- 27.Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res. 2005 Aug 01;67(2):187–97. doi: 10.1016/j.cardiores.2005.04.032.S0008-6363(05)00222-1 [DOI] [PubMed] [Google Scholar]
- 28.Kang D, Lee J, Suh S, Ligibel J, Courneya KS, Jeon JY. Effects of Exercise on Insulin, IGF Axis, Adipocytokines, and Inflammatory Markers in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Cancer Epidemiol Biomarkers Prev. 2017 Mar;26(3):355–365. doi: 10.1158/1055-9965.EPI-16-0602. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=27742668 .1055-9965.EPI-16-0602 [DOI] [PubMed] [Google Scholar]
- 29.Meneses-Echávez JF, Jiménez EG, Río-Valle JS, Correa-Bautista JE, Izquierdo M, Ramírez-Vélez R. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2016 Dec 25;16(1):682. doi: 10.1186/s12885-016-2733-z. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2733-z .10.1186/s12885-016-2733-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koelwyn G, Wennerberg E, Demaria S, Jones Lee W. Exercise in Regulation of Inflammation-Immune Axis Function in Cancer Initiation and Progression. Oncology (Williston Park) 2015 Dec;29(12):908–20, 922. http://www.cancernetwork.com/oncology-journal/exercise-regulation-inflammation-immune-axis-function-cancer-initiation-and-progression .214800 [PMC free article] [PubMed] [Google Scholar]
- 31.Idorn M, Thor SP. Exercise and cancer: from “healthy” to “therapeutic”? Cancer Immunol Immunother. 2017 May;66(5):667–671. doi: 10.1007/s00262-017-1985-z. http://europepmc.org/abstract/MED/28324125 .10.1007/s00262-017-1985-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003 Apr;3(4):276–85. doi: 10.1038/nrc1046.nrc1046 [DOI] [PubMed] [Google Scholar]
- 33.Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3(5):323–7. doi: 10.1186/bcr315. http://europepmc.org/abstract/MED/11597322 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinha R, Singh R, Mehrotra S, Singh R K. Implications of free radicals and antioxidant levels in carcinoma of the breast: a never-ending battle for survival. Indian J Cancer. 2009;46(2):146–50. doi: 10.4103/0019-509x.49153. http://www.indianjcancer.com/article.asp?issn=0019-509X;year=2009;volume=46;issue=2;spage=146;epage=150;aulast=Sinha . [DOI] [PubMed] [Google Scholar]
- 35.Debevec T, Millet GP, Pialoux V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front Physiol. 2017 Feb;8:84. doi: 10.3389/fphys.2017.00084. doi: 10.3389/fphys.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009 Jan;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026.S0959-8049(08)00873-3 [DOI] [PubMed] [Google Scholar]
- 37.Galiano-Castillo N, Arroyo-Morales M, Ariza-Garcia A, Sánchez-Salado C, Fernández-Lao C, Cantarero-Villanueva I, Martín-Martín L. The Six-Minute Walk Test as a Measure of Health in Breast Cancer Patients. J Aging Phys Act. 2016 Dec;24(4):508–515. doi: 10.1123/japa.2015-0056.2015-0056 [DOI] [PubMed] [Google Scholar]
- 38.Schmidt K, Vogt L, Thiel C, Jäger E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013 Jul;34(7):631–6. doi: 10.1055/s-0032-1323746. [DOI] [PubMed] [Google Scholar]
- 39.Borg G, Hassmén P, Lagerström M. Perceived exertion related to heart rate and blood lactate during arm and leg exercise. Eur J Appl Physiol Occup Physiol. 1987;56(6):679–85. doi: 10.1007/BF00424810. [DOI] [PubMed] [Google Scholar]
- 40.Savva C, Giakas G, Efstathiou M, Karagiannis C. Test-retest reliability of handgrip strength measurement using a hydraulic hand dynamometer in patients with cervical radiculopathy. J Manipulative Physiol Ther. 2014 Mar;37(3):206–10. doi: 10.1016/j.jmpt.2014.02.001.S0161-4754(14)00035-9 [DOI] [PubMed] [Google Scholar]
- 41.Crinière Lise, Lhommet C, Caille A, Giraudeau Bruno, Lecomte Pierre, Couet Charles, Oppert Jean-Michel, Jacobi David. Reproducibility and validity of the French version of the long international physical activity questionnaire in patients with type 2 diabetes. J Phys Act Health. 2011 Aug;8(6):858–65. doi: 10.1123/jpah.8.6.858. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011 Aug;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 43.World HO. Pacific Physical Activity Guidelines for Adults. 2008. [2018-07-23]. http://www.who.int/dietphysicalactivity/publications/pacific_pa_guidelines.pdf .
- 44.Hjermstad M, Fossa S, Bjordal K, Kaasa S. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire. J Clin Oncol. 1995 May;13(5):1249–54. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 45.Piper B, Dibble S, Dodd M. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum ? 1998;25:84. [PubMed] [Google Scholar]
- 46.Gledhill J, Rodary C, Mahé Cedric, Laizet Céline. [French validation of the revised Piper Fatigue Scale] Rech Soins Infirm. 2002 Mar;(68):50–65. [PubMed] [Google Scholar]
- 47.Sass C, Moulin J, Guéguen R. Le score EPICES?: un score individuel de précarité. Construction et évaluation du score dans une population de 197 389 personnes. 2006 [Google Scholar]
- 48.Labbe E, Blanquet M, Gerbaud L, Poirier G, Sass C, Vendittelli F, Moulin J. A new reliable index to measure individual deprivation: the EPICES score. Eur J Public Health. 2015 Aug;25(4):604–9. doi: 10.1093/eurpub/cku231.cku231 [DOI] [PubMed] [Google Scholar]
- 49.Vallance J, Lavallee C, Culos-Reed N, Trudeau M. Rural and small town breast cancer survivors' preferences for physical activity. Int J Behav Med. 2013 Dec;20(4):522–8. doi: 10.1007/s12529-012-9264-z. [DOI] [PubMed] [Google Scholar]
- 50.Friedenreich CM, Pialoux V, Wang Q, Shaw E, Brenner DR, Waltz X, Conroy SM, Johnson R, Woolcott CG, Poulin MJ, Courneya KS. Effects of exercise on markers of oxidative stress: an Ancillary analysis of the Alberta Physical Activity and Breast Cancer Prevention Trial. BMJ Open Sport Exerc Med. 2016 Oct;2(1):e000171. doi: 10.1136/bmjsem-2016-000171. http://europepmc.org/abstract/MED/27900199 .bmjsem-2016-000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faёs C, Martin C, Chirico EN, Féasson L, Oyonno-Enguelle S, Dubouchaud H, Francina A, Thiriet P, Pialoux V, Messonnier L. Effect of α-thalassaemia on exercise-induced oxidative stress in sickle cell trait. Acta Physiol (Oxf) 2012 Aug;205(4):541–50. doi: 10.1111/j.1748-1716.2012.02434.x. [DOI] [PubMed] [Google Scholar]
- 52.Cadmus-Bertram LA, Marcus BH, Patterson RE, Parker BA, Morey BL. Randomized Trial of a Fitbit-Based Physical Activity Intervention for Women. Am J Prev Med. 2015 Sep;49(3):414–8. doi: 10.1016/j.amepre.2015.01.020. http://europepmc.org/abstract/MED/26071863 .S0749-3797(15)00044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.INCa Bénéfices de l?activité physique pendant et après cancer. Des connaissances scientifiques aux repères pratiques. 2017 [Google Scholar]
- 54.Tudor-Locke C, Bassett D. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001.3411 [DOI] [PubMed] [Google Scholar]


