Abstract
Toxoplasma gondii is an apicomplexan zoonotic protozoan parasite that infects most species of warm-blooded animals, including humans. The heavy incidence and severe or lethal damage caused by T. gondii infection clearly indicate a need for the development of an effective vaccine. T. gondii GRA8 is a member of the dense granules protein family and is used as a marker of acute infection. In the present study, we evaluated the protective immunity induced by DNA vaccination based on a recombinant eukaryotic plasmid, pDsRed2-GRA8, against acute toxoplasmosis in mice. BALB/c mice were intramuscularly immunized with the pDsRed2-GRA8 plasmid and then challenged by infection with the highly virulent GFP-RH strain of T. gondii. The specific immune responses and protective efficacy against T. gondii of this vaccine were analyzed by measuring cytokine and serum antibody titers, splenocyte proliferation assays, and the survival times of mice after challenge. Our results showed that mice immunized with pDsRed2-GRA8 demonstrated specific humoral and cellular responses, induced higher IgG antibody titers with predominant IgG2a production; increased levels of IL-10, IL-12 (p70), IFN-γ, TNF-α, and splenocyte proliferation; and prolonged survival times compared to those of control mice. The present study showed that DNA immunization with pDsRed2-GRA8 induced humoral and cellular immune responses, and all immunized mice showed greater Th1-type immune responses and longer survival times than those of control mice. These results indicated that T. gondii GRA8 DNA immunization induces a partial protective effect against acute toxoplasmosis.
Keywords: Toxoplasma gondii, DNA vaccine, GRA8, immune response
INTRODUCTION
Toxoplasma gondii is a ubiquitous intracellular protozoan parasite that can invade and replicate in all nucleated cells. It is prevalent in humans and animals worldwide, and one-third of the world’s population is reportedly infected with T. gondii [1,2]. Although 80–90% of individuals with primary infection are asymptomatic, the most affected are immunocompromised individuals (HIV/AIDS, cancer patients, organ and tissue transplant recipients, and congenitally infected children), and in these patients, T. gondii can lead to encephalitis, ophthalmopathy, or even death [3–5]. T. gondii infection in domestic animals can cause substantial economic losses due to abortion and neonatal loss in livestock and represents a source of transmission to humans [6–8].
To date, no safe and effective drug is available to eliminate T. gondii tissue cysts [9]. Thus, the development of a T. gondii vaccine is considered to be the most effective way to control toxoplasmosis [10]. Unfortunately, after decades of effort, only one vaccine (Toxovax®) has been licensed for use to prevent abortion in sheep and goats, and no vaccine suitable for use in humans exists [11]. In these circumstances, there is an urgent need to develop new, effective vaccines to reduce the high incidence of toxoplasmosis by preventing and treating this disease in humans and animals [12].
DNA vaccines have become a major focus of research, because they promote the specific expression of an encoded vaccine antigen by host cells and can deliver multivalent vaccines to a host in a single dose. In particular, DNA vaccines can also elicit potent, long-lasting humoral and cell-mediated immunity [13]. T. gondii has evolved unique secretory organelles to help establish infection in its hosts. Among these secretory organelles, the dense granules (GRA), micronemes (MIC), and rhoptries (ROP) are the best characterized, and these organelles play critical roles in adhesion, motility, host invasion, intracellular replication, manipulation of host signaling and innate immune pathways, and formation of the parasitophorous vacuole (PV) [14,15]. The vaccine candidate antigens involved in protective immunity against T. gondii include membrane-associated surface antigens (SAGs) and secreted GRAs, MICs and ROPs [16–24]. However, none of these antigens could completely inhibit tissue cyst formation [25].
Dense granules is an important secretory organelle in the T. gondii cytoplasm, and GRA proteins are potent antigens that trigger strong T and B cell responses upon infection [20,26]. Previous studies have demonstrated that GRA8 is a marker of acute infection, and IgG, IgM or IgA detection with GRA8 by enzyme-linked immunosorbent assay (ELISA) is useful in diagnosing T. gondii infection and discriminating acute from chronic infection [27–29]. However, no study of the protective efficacy of a DNA vaccine expressing T. gondii GRA8 to induce resistance to acute infection has been published. To evaluate the protective immune responses induced by T. gondii GRA8, we constructed a GRA8-expressing eukaryotic plasmid, pDsRed2-GRA8, and evaluated its immunogenicity and protective efficacy after vaccination of BALB/c mice through challenge with the highly virulent T. gondii GFP-RH strain.
MATERIALS AND METHODS
Animals
Specific-pathogen-free (SPF) female BALB/c mice of 6 to 8 weeks old were purchased from Guangdong Medical Laboratory Animal Center, Guangzhou, Guangdong Province, China. All mice were handled in strict accordance with the Good Animal Practice Requirements of the Animal Ethics Procedures and Guidelines of the People’s Republic of China. This study was approved by the Committee on the Ethics of Animal Experiments of Guangdong Medical University (Permit Number: SYXK [Guangdong] 2015-0147).
Toxoplasma gondii strain
The tachyzoites of the T. gondii GFP-RH strain were maintained in human retinal pigment epithelial cells (ARPE-19; ATCC, Rockville, Maryland, USA). Briefly, T. gondii GFP-RH were infected into ARPE-19 cells (parasite/cell ratio, 5: 1) and incubated at 37˚C with 5% CO2 for 2 to 3 days. Following host cell rupture, lysed tachyzoites and host cellular debris were centrifuged at 900×g for 10 min using Percoll (Sigma Chemical Co., St. Louis, Missouri, USA) to pellet the parasites. The final pellet was suspended in cold phosphate buffered saline (PBS), and the suspension was passed through a 5.0-μm-poresize filter (Millipore, Bedford, Massachusetts, USA). Purified tachyzoites were used in all experiments.
Preparation of soluble tachyzoite antigen
The soluble tachyzoite antigens (STAg) were prepared from T. gondii tachyzoites as described previously [26]. Briefly, purified tachyzoites were centrifuged at 5,000×g for 3 min and disrupted by 3 cycles of freezing at −20˚C and thawing at 4˚C. Finally, the lysate was sonicated on ice at 60 W/s and centrifuged for 40 min at 100,000×g. The supernatants containing STAg were pooled and sterile filtered (Millipore), and the protein concentration was determined via the Bradford method using bovine serum albumin (BSA) as the standard. STAg was stored in aliquots at −80˚C until used.
Construction and preparation of DNA vaccine plasmid
The total RNA of the RH strain tachyzoites was prepared by using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, California, USA) according to the manufacturer’s instructions. The coding sequence of GRA8 was obtained by RT-PCR amplification from total RNA with specific primers (forward primer: 5′-GGC CTC GAG ATG GCT TTA CCA TTG CG-3′ and reverse primer: 5′-CCG GAA TTC GCG TCG TTA CGG TGA ATC-3′, containing the XhoI and EcoRI restriction sites, respectively, which are underlined) and then inserted into the pGEM-T Easy vector (Promega Corporation, Madison, Wisconsin, USA). The GRA8 fragments were cleaved from pGEM-GRA8 by using the restriction endonucleases XhoI and EcoRI and then were subcloned into the corresponding sites of the pDsRed2-N1 vector (Invitrogen Life Technologies) to construct pDsRed2-N1-GRA8 (pDsRed2-GRA8). The recombinant plasmid was propagated in Escherichia coli DH5α and confirmed by restriction analysis and PCR sequencing (Sangon Biotech, Shanghai, China).
Large-scale plasmid DNA was prepared using the Endotox in-Free Mega kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany); concentrations were determined by spectrophotometer, and the DNA was diluted with sterile endotoxin-free PBS to a final concentration of 1 mg/ml and stored at −20˚C until use.
Expression of pDsRed2-GRA8 in vitro
The recombinant plasmids pDsRed2-GRA8 and pDsRed2-N1 were separately transfected into ARPE-19 cells grown on glass coverslips in 12-well plates by using Lipofectamine LTX and Plus reagents (Invitrogen Life Technologies) according to the manufacturer’s instructions. Forty-eight hours after transfection, cells were fixed with 4% paraformaldehyde and per-meabilized with 0.1% Triton X-100 in PBS (PBST) for 10 min. The coverslips were mounted on microscope slides using a mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, California, USA), and the cells were imaged using fluorescence microscopy.
DNA immunization and experimental design
A total of 80 female BALB/c mice were randomly divided into 4 groups (20 mice per group). For the experimental groups, mice were inoculated with an injection of 50 μg pDsRed2-GRA8 into the tibialis anterior muscles of both hind legs (100 μg/per mouse) and given booster immunizations 2 and 4 weeks later. Mice injected with empty pDsRed2-N1vector or sterile endotoxin-free PBS served as negative control groups, and the blank control group received nothing.
Serum samples collected from the tail vein from 3 mice in each group at weeks 0, 2, 4, and 6 were centrifuged at 3,000×g for 10 min and stored at −20˚C for later ELISA detection.
Spleens from 3 mice in each group at week 6 were collected under aseptic conditions and used for in vitro splenocyte proliferation and cytokine assays (IL-4, IL-10, IL-12, IFN-γ, and TNF-α).
Two weeks after the last immunization, 15 mice per group were challenged intraperitoneally with 1×103 tachyzoites of the highly virulent T. gondii GFP-RH strain, and the survival time of the mice was monitored daily (Fig. 1).
Fig. 1.
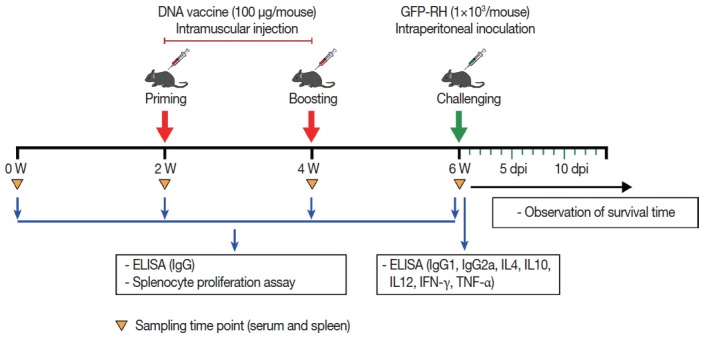
Schematic representation of DNA vaccine immunization and T. gondii challenge schedule. To monitor immune responses, serum and spleen samples were collected at the indicated time point and subsequently examined by ELISA and splenocyte proliferation assay.
Determination of serum antibodies
T. gondii-specific serum IgG, IgG1, and IgG2a antibody levels were determined by ELISA, as described previously, with some modifications [26]. Briefly, the 96-well plates were coated with 100 μl of STAg (10 μg/ml) in 50 mM carbonate buffer (pH 9.6) at 4˚C overnight. Then, the plates were blocked with PBS containing 1% BSA for 2 hr at room temperature and incubated with the diluted sera. Subsequently, the plates were incubated with horseradish peroxidase (HRP)-conjugated anti-mouse IgG, IgG1, or IgG2a, and the bound antibody was reacted with 200 μl of substrate solution in the dark for 30 min. Finally, the reaction was stopped with 3N HCl, and the absorbance was measured with a spectrophotometer at 490 nm. All samples were evaluated in triplicate.
Splenocyte proliferation assay
Spleens from immunized mice were collected under aseptic conditions in RPMI 1640 medium (Invitrogen Life Technologies). Erythrocytes were removed by erythrocyte lysis buffer (Sigma), and the remaining cells were washed and suspended in RPMI 1640 medium supplemented with 10% FBS, 1% antibiotic-antimycotic (Gibco-BRL, Life Technologies Ltd, Paisley, UK), HEPES (10 mM), L-glutamine (2 mM), sodium pyruvate (1 mM), and β-mercaptoethanol (50 μM). Splenocytes were stimulated with STAg (10 μg/ml) or medium alone as positive and negative controls, respectively, and then incubated at 37˚C in 5% CO2. Splenocyte proliferation was measured using a chemiluminescent bromodeoxyuridine (BrdU) ELISA kit according to the manufacturer’s instructions (Roche Molecular Biochemicals, Indianapolis, Indiana, USA).
Cytokine assays
Splenocytes from each of 3 mice per group were harvested as described above. Single-cell suspensions of splenocytes were stimulated with STAg or medium alone (negative control) in flat-bottomed 96-well microtiter plates as described for the lymphocyte proliferation assay. Culture supernatants were harvested at 24 hr for the determination of IL-4, 72 hr for IL-10, 96 hr for IL-12 (p70) and IFN-γ, 4 and 8 hr for TNF-α, using commercial ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, Minnesota, USA). Cytokine concentrations were determined by reference to standard curves constructed with known amounts of mouse recombinant IL-4, IL-10, IL-12 (p70), IFN-γ or TNF-α. The sensitivity limits for the assays were 2 pg/ml for IL-4, 1.97 pg/ml for IL-10, 5.0 pg/ml for IL-12 (p70), 2 pg/ml for IFN-γ, and 1.88 pg/ml for TNF-α.
Statistical analysis
Results are presented as the means±standard deviation (SD) of 3 independent experiments. The statistical evaluation of the differences in survival rates was checked by the Kaplan-Meier test, and then a log-rank test was performed. Statistical differences in parasite burdens, antibody titers, in vitro splenocyte proliferation, and cytokine levels were determined by one-way ANOVA with Dunnett’s multiple comparisons test. P-values lower than 0.05 were considered statistically significant.
RESULTS
Expressed recombinant T. gondii GRA8
The T. gondii GRA8 gene was amplified by PCR (814 bp) from the cDNA of T. gondii strain GFP-RH (Fig. 2A). Subsequently, the PCR products were digested with the appropriate restriction enzymes and subcloned into the XhoI and EcoRI sites of the pDsRed2-N1 expression vector to construct a recombinant plasmid, which was named pDsRed2-GRA8 (Fig. 2B). To identify the expression of the recombinant plasmid in vitro, the presence of GRA8 mRNA in transfected ARPE-19 cells was analyzed using RT-PCR. As shown in Fig. 2C, no expression of GRA8 was detected in pDsRed2-N1 transfected cells, whereas the expression of GRA8 was detected in pDsRed2-GRA8 transfected cells. In addition, we also confirmed the expression of pDsRed2-GRA8 by immunofluorescence assay. DsRed2 fluorescence was observed in the cytoplasm of ARPE-19 cells transfected with the pDsRed2-N1 vector or pDsRed2-GRA8 (Fig. 2D). These results indicated that the T. gondii GRA8 was expressed successfully in ARPE-19 cells.
Fig. 2.
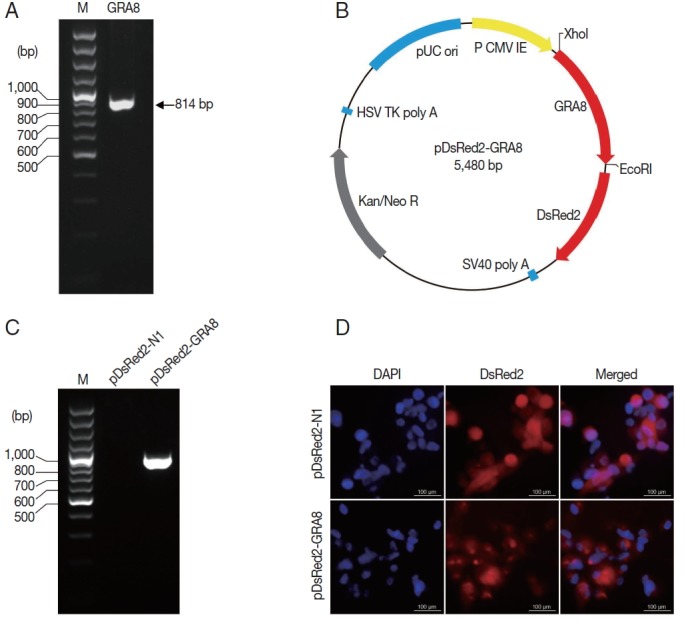
T. gondii GRA8 was expressed successfully in ARPE-19 cells. (A) The coding sequence of the T. gondii GRA8 gene was amplified by PCR (814 bp) from the cDNA of T. gondii strain GFP-RH. (B) The PCR products were digested with the appropriate restriction enzymes and cloned into the XhoI and EcoRI sites of the pDsRed2-N1 expression vector, which contains the DsRed open reading frame. The constructed recombinant plasmid was named pDsRed2-GRA8. (C) ARPE-19 cells transfected with pDsRed2-GRA8 showed specific expression of GRA8 mRNA. (D) pDsRed2-GRA8 and the pDsRed2-N1 empty vector both expressed the DsRed2 protein, which localized to the cytoplasm of the transfected cells.
Humoral immune responses induced by DNA immunization
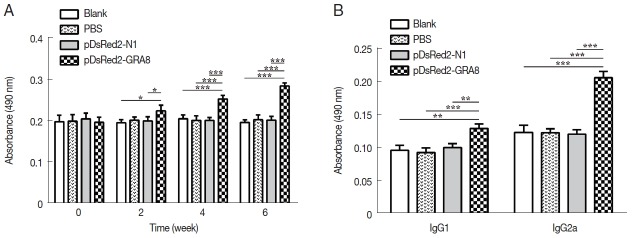
Serum samples from immunized and control mice were used to evaluate the levels of specific antibodies against T. gondii. As shown in Fig. 3A, immunization induced a significant IgG response in all immunized mice from 2 weeks after immunization, with higher antibody levels in pDsRed2-GRA8 immunized mice than in the blank, PBS, or pDsRed2-N1 control groups (2 weeks: F(3,8)=5.082, P=0.0294; 4 weeks: F(3,8)=21.21, P=0.0004; 6 weeks: F(3,8)=66.82, P <0.0001). In contrast, the levels of IgG antibodies in the mice of the 3 control groups did not statistically increase with successive immunizations.
Fig. 3.
pDsRed2-GRA8 DNA vaccine immunization induced a humoral response in mice. (A) BALB/c mice immunized with pDsRed2-GRA8 showed higher levels of IgG antibody than pDsRed2-N1, PBS and blank controls at 4 and 6 weeks. (B) T. gondii-specific IgG1 and IgG2 antibody levels were significantly increased in the sera of mice at 2 weeks after the last immunization. The results are expressed as the means of OD490±SD (n=3). *P<0.05, **P<0.01, ***P<0.001 versus the pDsRed2-N1, PBS and blank control groups.
To characterize whether a Th1 and/or Th2 response was induced in the immunized mice, the distribution of subclasses of IgG (IgG1 and IgG2a) was analyzed individually in the sera of mice from all groups at 2 weeks after the last immunization. As depicted in Fig. 3B, the levels of IgG1 and IgG2a in the immunized groups were significantly higher than those in the 3 control groups (IgG1: F(3,8)=17.05, P =0.0008; IgG2a: F(3,8)= 72.47, P<0.0001). The ratios of IgG2a/IgG1 in mice immunized with pDsRed2-GRA8 (1.60±0.11) were significantly different from those in the control groups (blank, 1.29±0.19; PBS, 1.28±0.15; pDsRed2-N1, 1.19±0.81) (F(3,8)=4.908, P=0.032). These results suggested that a specific humoral response with a mixed Th1/Th2 profile was elicited by these DNA immunizations, with a predominant Th1-type immune response in mice immunized with pDsRed2-GRA8.
Splenocyte proliferation
To assess proliferative immune responses to STAg, the splenocytes from the immunized and control groups (3 mice per group) were analyzed by a BrdU ELISA kit. As shown in Fig. 4, in vitro splenocyte proliferation in mice immunized with pDsRed2-GRA8 was significantly higher than that in the 3 control groups (2 weeks: F(3,8)=7.595, P =0.01; 4 weeks: F(3,8)=32.32, P <0.0001; 6 weeks: F(3,8)=51.46, P <0.0001) and gradually increased over time starting 2 weeks after immunization. In contrast, the in vitro splenocyte proliferation among the mice in the 3 control groups did not show significant differences.
Fig. 4.
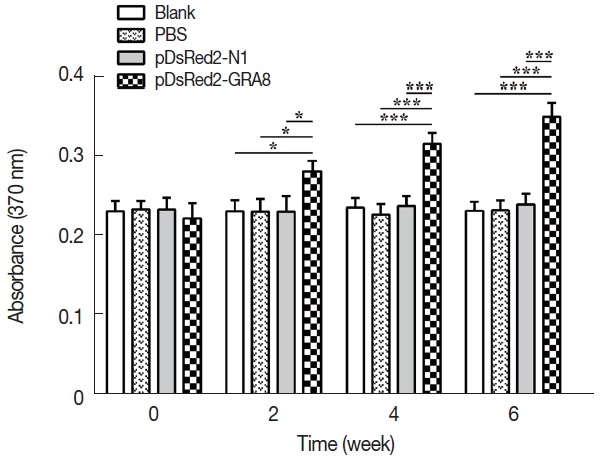
In vitro splenocyte proliferation was significantly higher in pDsRed2-GRA8 immunized mice. Spleens were aseptically harvested from 3 mice per group 2 weeks after the last immunization and stimulated with the soluble tachyzoite antigen of T. gondii (STAg). Splenocyte proliferation was measured using a chemiluminescent BrdU ELISA kit, and absorbance was evaluated by an ELISA reader at 370 nm with a 492 nm reference. A significantly higher level of splenocyte proliferative response was induced by DNA immunization with pDsRed2-GRA8 than in the 3 control groups. The experiment was repeated 3 times with similar results. *P<0.05, ***P<0.001 versus the pDsRed2-N1, PBS and blank control groups.
Cytokine production
To determine whether pDsRed2-GRA8 immunization augments the Th1 or Th2 cytokine response, STAg-treated culture supernatants of splenocytes were obtained from immunized mice 2 weeks after the final immunization, and the levels of IL-4, IL-10, IL-12 (p70), IFN-γ and TNF-α were quantified by ELISA. As shown in Table 1, the levels of IL-10, IL-12 (p70), IFN-γ, and TNF-α in spleen cell cultures from mice immunized with pDsRed2-GRA8 were significantly increased compared to those in the 3 control groups (IL-10: F(3,8)=38.72, P <0.0001; IL-12: F(3,8)=224.4, P <0.0001; IFN-γ: F(3,8)=219.8, P <0.0001; TNF-α: F(3,8)=88.22, P <0.0001). However, the level of IL-4 in spleen cell cultures from the pDsRed2-GRA8 immunized group was not significantly higher than those in the 3 control groups.
Table 1.
Cytokine production of splenocytes stimulated by soluble tachyzoite antigens of T. gondii (STAg) evaluated by ELISA
| Groups (n=3) | Cytokine productiona (pg/ml) | ||||
|---|---|---|---|---|---|
| IL-4 | IL-10 | IL-12 (p70) | IFN-γ | TNF-α | |
| Blank | 13.0±2.3 | ND | ND | 34.4±1.9 | 19.5±3.2 |
| PBS | 12.2±3.5 | ND | ND | 33.6±2.6 | 20.7±2.2 |
| pDsRed2-N1 | 14.6±1.4 | ND | ND | 33.1±3.5 | 22.1±3.6 |
| pDsRed2-GRA8 | 15.8±2.7 | 27.5±5.4*** | 32.5±4.1*** | 105.2±6.8*** | 65.4±6.3*** |
Values for IL-4, IL-10, IL-12 (p70), IFN-γ, and TNF-α are at 24, 72, 96, 96, and 48 hr, respectively. ND=non-detectable. Data represent the means±SD from 3 mice per group. Three independent experiments were performed, and the data from 1 representative experiment are shown.
P<0.001 compared with the blank, PBS and pDsRed2-N1 empty vector groups.
Protection of immunized mice
To evaluate the protective immunity induced by immunization with pDsRed2-GRA8, 15 mice from each group were challenged intraperitoneally with 1×103 tachyzoites of the virulent T. gondii GFP-RH strain at 2 weeks after the last immunization. The survival times of the mice were observed. The average survival time of the mice immunized with pDsRed2-GRA8 (9.00 ± 1.77 days) was significantly longer than that of the 3 control groups (blank, 5.73±1.16 days, χ2=20.43, df=1, P <0.0001; PBS, 6.27±1.03 days, χ2=17.34, df=1, P <0.0001; pDsRed2-N1, 6.33±0.82 days, χ2=17.31, df=1, P <0.0001). However, the difference in the average survival time of mice among the 3 control groups was not statistically significant, and all died within 8 days after challenge (Fig. 5).
Fig. 5.
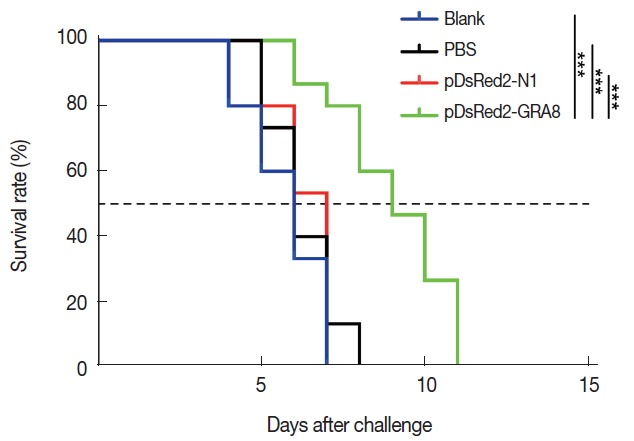
Immunization with pDsRed2-GRA8 significantly increased the survival times of mice in acute toxoplasmosis. Survival rates of mice immunized with pDsRed2-GRA8, pDsRed2-N1, PBS, and blank control mice followed by challenge with 1×103 tachyzoites of the T. gondii strain GFP-RH at 2 weeks after the last immunization. The differences between the pDsRed2-GRA8 vaccinated group and each of the 3 control groups were significant. ***P<0.001 versus the pDsRed2-N1, PBS and blank control groups.
DISCUSSION
In recent years, owing to the easy production and low cost of DNA vaccines and the fact that DNA vaccines can elicit long-lasting humoral and cellular immune responses, the development of effective DNA vaccines has emerged as an active research field in the fight against toxoplasmosis [22,30,31]. Numerous studies have evaluated the protective efficacy of different antigens, such as SAG4, GRA15, MIC13, ROP18, NTPase II, TrxLp, and ENO2 [18,24,32–35]. Most of them showed partial protection against toxoplasmosis. Herein, we evaluated the protective immunity induced by DNA vaccination with a plasmid encoding GRA8 proteins against acute toxoplasmosis in a mouse model. Our results demonstrated that immunization with pDsRed2-GRA8 can trigger different levels of humoral and cellular responses, with a slightly longer survival time than the blank, PBS or pDsRed2-N1 controls, suggesting that T. gondii GRA8 DNA immunization induces a partial protective effect against acute toxoplasmosis.
When T. gondii invasion occurs for the first time in vivo, the parasite can be captured and processed by antigen-presenting cells and then presented to T lymphocytes, which further build adaptive immunity. When T. gondii invades subsequently, special anti-T. gondii IgG antibodies adhere to the surfaces of the parasites and limit their spread by preventing attachment to host cell receptors, resulting in their elimination by macrophages [36]. Therefore, humoral response, by promoting macrophages to kill intracellular parasites, has been considered to be of great importance in immunity against T. gondii infection. In the present study, we evaluated humoral response intensity on the basis of specific anti-T. gondii IgG levels. The immunization of mice with pDsRed2-GRA8 could induce significantly higher levels of IgG antibodies than those in the controls (P <0.05), which would contribute to strong protective efficacy against T. gondii infection. These results were in agreement with those of previous studies that vaccinated mice with plasmids encoding GRA7, GRA17, GRA23, ROM5, and ROP38 [10,26,37,38].
High levels of IgG1 and IgG2a were also detected in the serum of mice in the pDsRed2-GRA8 immunized group compared to those in the control groups. This result was consistent with those of previous studies, showing that DNA immunization could elicit a mixed Th1/Th2 immune response, with a more significant IgG2a response [10,39–41]. The higher ratio of IgG2a/IgG1 in mice immunized with pDsRed2-GRA8 shows that a predominantly Th1-type immune response was elicited.
A Th1-type immune response associated with high levels of IFN-γ is essential for protection against T. gondii infection [42]. By restimulation of splenocytes in vitro with STAg, significant increases in IFN-γ, IL-12 (p70) and TNF-α production were induced after DNA immunization with pDsRed2-GRA8. These results were similar to previous data showing that immunization with a plasmid encoding the GRA7 gene enhanced the production of IFN-γ in vitro and GRA7-specific IgG2a antibody [26]. These results suggested that immunization with a pDsRed2-GRA8 DNA vaccine could elicit the Th1-type immune response that contributes to effective protection against acute T. gondii infection. In addition, the Th2-type cytokines (IL-4 and IL-10) have been shown to play important roles during the early phase of acute T. gondii infection [43]. In our study, increased levels of IL-10 were observed in only the mice immunized with pDsRed2-GRA8. As a regulatory cytokine, IL-10 is important for protection against the potential immunopathological mechanism caused by a vigorous Th1 response characterized by high levels of IFN-γ production [44]. Therefore, the high level of IL-10 contributed to the longer survival time of mice immunized with pDsRed2-GRA8. However, the levels of IL-4 in all immunized groups were similar to those in all control groups. These results may further explain the inability of these DNA vaccines to completely protect mice against acute T. gondii infection.
Cellular immunity plays an important role in the control of T. gondii infection. In this study, a significantly higher level of splenocyte proliferative response was induced by DNA immunization with pDsRed2-GRA8. This result was similar to those of previous studies reporting that DNA vaccination with GRA7 and GRA16 could increase splenocyte proliferation compared with that in controls [25,26]. These results suggest that a cellular immune response was elicited in the immunized mice. Immunization with pDsRed2-GRA8 induced partial immune protection against acute toxoplasmosis, and all immunized mice had prolonged survival times compared to those of all controls. These results indicate that pDsRed2-GRA8 represents a potential candidate for the further development of effective vaccines against acute toxoplasmosis. Nevertheless, further studies are needed to assess the induction of stronger immune protection by multiantigenic DNA vaccines combining GRA8 with other proteins to combat acute and chronic toxoplasmosis. The present study showed that DNA immunization with pDsRed2-GRA8 induced humoral and cellular immune responses, and all immunized mice showed greater Th1-type immune responses and longer survival times than those of control mice. However, the changes of immune factors by pDsRed2-GRA8 immunization just had a little effect on the survival of acute toxoplasmosis mice. These results indicated that T. gondii GRA8 DNA immunization induces a partial protective effect against acute toxoplasmosis.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81771612, 81701575) and the Natural Science Foundation of Shandong Province of China (ZR2015HQ033). We thank our anonymous reviewers for their critical comments and helpful suggestions on this manuscript.
Footnotes
CONFLICT OF INTREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Dubey JP. The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- 2.Dubey JP, Rajendran C, Ferreira LR, Martins J, Kwok OC, Hill DE, Villena I, Zhou H, Su C, Jones JL. High prevalence and genotypes of Toxoplasma gondii isolated from goats, from a retail meat store, destined for human consumption in the USA. Int J Parasitol. 2011;41:827–833. doi: 10.1016/j.ijpara.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Nissapatorn V, Lee C, Quek KF, Leong CL, Mahmud R, Abdullah KA. Toxoplasmosis in HIV/AIDS patients: a current situation. Jpn J Infect Dis. 2004;57:160–165. [PubMed] [Google Scholar]
- 4.Thomas F, Lafferty KD, Brodeur J, Elguero E, Gauthier-Clerc M, Missé D. Incidence of adult brain cancers is higher in countries where the protozoan parasite Toxoplasma gondii is common. Biol Lett. 2012;8:101–103. doi: 10.1098/rsbl.2011.0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebech M, Andersen O, Christensen NC, Hertel J, Nielsen HE, Peitersen B, Rechnitzer C, Larsen SO, Nørgaard-Pedersen B, Petersen E. Feasibility of neonatal screening for toxoplasma infection in the absence of prenatal treatment. Lancet. 1999;353:1834–1837. doi: 10.1016/s0140-6736(98)11281-3. [DOI] [PubMed] [Google Scholar]
- 6.Dubey JP, Hill DE, Jones JL, Hightower AW, Kirkland E, Roberts JM, Marcet PL, Lehmann T, Vianna MC, Miska K, Sreekumar C, Kwok OC, Shen SK, Gamble HR. Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol. 2005;91:1082–1093. [Google Scholar]
- 7.Fajardo HV, D’ávila S, Bastos RR, Cyrino CD, de Lima Detoni M, Garcia JL, das Neves LB, Nicolau JL, Amendoeira MR. Seroprevalence and risk factors of toxoplasmosis in cattle from extensive and semi-intensive rearing systems at Zona da Mata, Minas Gerais state, Southern Brazil. Parasit Vectors. 2013;6:191. doi: 10.1186/1756-3305-6-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey JP. Toxoplasmosis in pigs—the last 20 years. Vet Parasitol. 2009;164:89–103. doi: 10.1016/j.vetpar.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Abugri DA, Witola WH, Jaynes JM. In vitro antagonistic and indifferent activity of combination of 3-deoxyanthocyanidins against Toxoplasma gondii . Parasitol Res. 2017;116:3387–3400. doi: 10.1007/s00436-017-5661-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhu WN, Wang JL, Chen K, Yue DM, Zhang XX, Huang SY, Zhu XQ. Evaluation of protective immunity induced by DNA vaccination with genes encoding Toxoplasma gondii GRA17 and GRA23 against acute toxoplasmosis in mice. Exp Parasitol. 2017;179:20–27. doi: 10.1016/j.exppara.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Buxton D, Innes EA. A commercial vaccine for ovine toxoplasmosis. Parasitology. 1995;110(suppl):11–16. doi: 10.1017/s003118200000144x. [DOI] [PubMed] [Google Scholar]
- 12.Cao A, Liu Y, Wang J, Li X, Wang S, Zhao Q, Cong H, He S, Zhou H. Toxoplasma gondii: vaccination with a DNA vaccine encoding T-and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice. Vaccine. 2015;33:6757–6762. doi: 10.1016/j.vaccine.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 13.Beláková J, Horynová M, Krupka M, Weigl E, Raska M. DNA vaccines: are they still just a powerful tool for the future? Arch Immunol Ther Exp (Warsz) 2007;55:387–398. doi: 10.1007/s00005-007-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriquez FL, Woods S, Cong H, McLeod R, Roberts CW. Immunogenetics of Toxoplasma gondii informs vaccine design. Trends in Parasitol. 2010;26:550–555. doi: 10.1016/j.pt.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Hiszczyńska-Sawicka E, Holec-Gasior L, Kur J. DNA vaccines and recombinant antigens in prevention of Toxoplasma gondii infections--current status of the studies. Wiad Parazytol. 2009;55:125–139. (in Polish) [PubMed] [Google Scholar]
- 16.Mendes ÉA, Fonseca FG, Casério BM, Colina JP, Gazzinelli RT, Caetano BC. Recombinant vaccines against T. gondii: comparison between homologous and heterologous vaccination protocols using two viral vectors expressing SAG1. PLoS One. 2013;8:e63201. doi: 10.1371/journal.pone.0063201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cong H, Zhang M, Xin Q, Wang Z, Li Y, Zhao Q, Zhou H, He S. Compound DNA vaccine encoding SAG1/SAG3 with A2/B subunit of cholera toxin as a genetic adjuvant protects BALB/c mice against Toxoplasma gondii . Parasit Vectors. 2013;6:63. doi: 10.1186/1756-3305-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J, Wang L. SAG4 DNA and peptide vaccination provides partial protection against T. gondii infection in BALB/c mice. Front Microbiol. 2017;8:1733. doi: 10.3389/fmicb.2017.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiszczyńska-Sawicka E, Olędzka G, Holec-Gąsior L, Li H, Xu JB, Sedcole R, Kur J, Bickerstaffe R, Stankiewicz M. Evaluation of immune responses in sheep induced by DNA immunization with genes encoding GRA1, GRA4, GRA6 and GRA7 antigens of Toxoplasma gondii . Vet Parasitol. 2011;177:281–289. doi: 10.1016/j.vetpar.2010.11.047. [DOI] [PubMed] [Google Scholar]
- 20.Ahmadpour E, Sarvi S, Hashemi Soteh MB, Sharif M, Rahimi MT, Valadan R, Tehrani M, Khalilian A, Montazeri M, Daryani A. Evaluation of the immune response in BALB/c mice induced by a novel DNA vaccine expressing GRA14 against Toxoplasma gondii . Parasite Immunol. 2017;39:e12419. doi: 10.1111/pim.12419. [DOI] [PubMed] [Google Scholar]
- 21.Yuan ZG, Ren D, Zhou DH, Zhang XX, Petersen E, Li XZ, Zhou Y, Yang GL, Zhu XQ. Evaluation of protective effect of pVAX-TgMIC13 plasmid against acute and chronic Toxoplasma gondii infection in a murine model. Vaccine. 2013;31:3135–3139. doi: 10.1016/j.vaccine.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 22.Liu Q, Wang F, Wang G, Zhao Q, Min J, Wang S, Cong H, Li Y, He S, Zhou H. Toxoplasma gondii: immune response and protective efficacy induced by ROP16/GRA7 multicomponent DNA vaccine with a genetic adjuvant B7-2. Hum Vaccin Immunother. 2014;10:184–191. doi: 10.4161/hv.26703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HL, Wang YJ, Pei YJ, Bai JZ, Yin LT, Guo R, Yin GR. DNA vaccination with a gene encoding Toxoplasma gondii rhoptry protein 17 induces partial protective immunity against lethal challenge in mice. Parasite. 2016;23:4. doi: 10.1051/parasite/2016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nabi H, Rashid I, Ahmad N, Durrani A, Akbar H, Islam S, Bajwa AA, Shehzad W, Ashraf K, Imran N. Induction of specific humoral immune response in mice immunized with ROP18 nanospheres from Toxoplasma gondii . Parasitol Res. 2017;116:359–370. doi: 10.1007/s00436-016-5298-5. [DOI] [PubMed] [Google Scholar]
- 25.Hu LY, Zhang NZ, Zhang FK, Wang M, Gao Q, Wang JL, Zhu XQ. Resistance to Chronic Toxoplasma gondii infection induced by a DNA vaccine expressing GRA16. Biomed Res Int. 2017;2017:1295038. doi: 10.1155/2017/1295038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan JH, Chu JQ, Ismail HA, Zhou W, Jo EK, Cha GH, Lee YH. Induction of protective immune responses by a multiantigenic DNA vaccine encoding GRA7 and ROP1 of Toxoplasma gondii . Clin Vaccine Immunol. 2012;19:666–674. doi: 10.1128/CVI.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babaie J, Zare M, Sadeghiani G, Lorgard-Dezfuli M, Aghighi Z, Golkar M. Bacterial production of dense granule antigen GRA8 of Toxoplasma gondii . Iran Biomed J. 2009;13:145–151. [PubMed] [Google Scholar]
- 28.Babaie J, Miri M, Sadeghiani G, Zare M, Khalili G, Golkar M. Expression and Single-step Purification of GRA8 Antigen of Toxoplasma gondii in Escherichia coli. Avicenna J Med Biotechnol. 2011;3:67–77. [PMC free article] [PubMed] [Google Scholar]
- 29.Costa JG, Duré AB. Immunochemical evaluation of two Toxoplasma gondii GRA8 sequences to detect acute toxoplasmosis infection. Microb Pathog. 2016;100:229–236. doi: 10.1016/j.micpath.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Verma R, Khanna P. Development of Toxoplasma gondii vaccine: a global challenge. Hum Vaccin Immunother. 2013;9:291–293. doi: 10.4161/hv.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ching XT, Fong MY, Lau YL. Evaluation of the protective effect of deoxyribonucleic acid vaccines encoding granule antigen 2 and 5 against acute Toxoplasmosis in BALB/c Mice. Am J Trop Med Hyg. 2017;96:1441–1447. doi: 10.4269/ajtmh.16-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Li ZY, Petersen E, Huang SY, Zhou DH, Zhu XQ. DNA vaccination with genes encoding Toxoplasma gondii antigens ROP5 and GRA15 induces protective immunity against toxoplasmosis in Kunming mice. Expert Rev Vaccines. 2015;14:617–624. doi: 10.1586/14760584.2015.1011133. [DOI] [PubMed] [Google Scholar]
- 33.Yuan ZG, Zhang XX, Lin RQ, Petersen E, He S, Yu M, He XH, Zhou DH, He Y, Li HX, Liao M, Zhu XQ. Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine. 2011;29:6614–6619. doi: 10.1016/j.vaccine.2011.06.110. [DOI] [PubMed] [Google Scholar]
- 34.Zheng L, Hu Y, Hua Q, Luo F, Xie G, Li X, Lin J, Wan Y, Ren S, Pan C, Tan F. Protective immune response in mice induced by a suicidal DNA vaccine encoding NTPase-II gene of Toxoplasma gondii . Acta Trop. 2017;166:336–342. doi: 10.1016/j.actatropica.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Yang XY, Zhang NZ, Zhang DL, Zhu XQ. Evaluation of protective immune responses induced by recombinant TrxLp and ENO2 proteins against Toxoplasma gondii infection in BALB/c mice. Biomed Res Int. 2016;2016:3571962. doi: 10.1155/2016/3571962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen K, Wang JL, Huang SY, Yang WB, Zhu WN, Zhu XQ. Immune responses and protection after DNA vaccination against Toxoplasma gondii calcium-dependent protein kinase 2 (TgCDPK2) Parasite. 2017;24:41. doi: 10.1051/parasite/2017045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang NZ, Xu Y, Wang M, Petersen E, Chen J, Huang SY, Zhu XQ. Protective efficacy of two novel DNA vaccines expressing Toxoplasma gondii rhomboid 4 and rhomboid 5 proteins against acute and chronic toxoplasmosis in mice. Expert Rev Vaccines. 2015;14:1289–1297. doi: 10.1586/14760584.2015.1061938. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y, Zhang NZ, Tan QD, Chen J, Lu J, Xu QM, Zhu XQ. Evaluation of immuno-efficacy of a novel DNA vaccine encoding Toxoplasma gondii rhoptry protein 38 (TgROP38) against chronic toxoplasmosis in a murine model. BMC Infect Dis. 2014;14:525. doi: 10.1186/1471-2334-14-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han Y, Zhou A, Lu G, Zhao G, Sha W, Wang L, Guo J, Zhou J, Zhou H, Cong H, He S. DNA vaccines encoding Toxoplasma gondii cathepsin C 1 induce protection against Toxoplasmosis in mice. Korean J Parasitol. 2017;55:505–512. doi: 10.3347/kjp.2017.55.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SH, Kim AR, Lee DH, Rubino I, Choi HJ, Quan FS. Protection induced by virus-like particles containing Toxoplasma gondii microneme protein 8 against highly virulent RH strain of Toxoplasma gondii infection. PLoS One. 2017;12:e0175644. doi: 10.1371/journal.pone.0175644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han Y, Zhou A, Lu G, Zhao G, Wang L, Guo J, Song P, Zhou J, Zhou H, Cong H, He S. Protection via a ROM4 DNA vaccine and peptide against Toxoplasma gondii in BALB/c mice. BMC Infect Dis. 2017;17:59. doi: 10.1186/s12879-016-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu CH, Fan YT, Dias A, Esper L, Corn RA, Bafica A, Machado FS, Aliberti J. Cutting edge: dendritic cells are essential for in vivo IL-12 production and development of resistance against Toxoplasma gondii infection in mice. J Immunol. 2006;177:31–35. doi: 10.4049/jimmunol.177.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Gazzinelli RT, Wysocka M, Hayashi S, Denkers EY, Hieny S, Caspar P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii . J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 44.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]