Abstract
Sibling relationships have been linked to adolescent externalizing behaviors, but the neurobiological factors that underlie this association have not been identified. This study investigated sibling closeness and birth order as a predictor of adolescent externalizing behavior via differences in neural processes during safe decision-making. A total of 77 adolescents (range = 12–15 years, Mage = 13.45 years, 40 females) completed a computerized driving task during a functional MRI scan. Results showed that adolescents’ perceptions of sibling closeness were associated with greater neural activation in the anterior insula, ventral striatum and left ventrolateral prefrontal cortex when making safe decisions, suggesting that the quality of sibling relationships modulates adolescent neurocognition even without being present. Furthermore, moderated mediation analyses revealed that higher sibling closeness was associated with lower externalizing behavior via left anterior insula activation during safe decision-making, but only for adolescents without older siblings (i.e. eldest children) compared to adolescents who had multiple older siblings. Importantly, these findings persisted above and beyond parental and peer closeness and sibling characteristics (i.e. sex, relatedness, birth order), highlighting the significant influence of sibling relationships on adolescent externalizing behavior through the brain.
Keywords: sibling relations, decision making, externalizing behavior, adolescence, fMRI
Adolescents engage in higher rates of risky decision-making compared to children and adults (Defoe et al., 2015), which is often associated with the onset of, and increases in, externalizing behaviors, such as substance use and risky sexual behavior (Steinberg, 2008). Less widely studied is the process of safe decision-making, a regulation process underlying risk avoidance, which has been associated with low engagement in problematic behaviors across adolescence (Wulfert et al., 2002). Given that safe decision-making predicts lower levels of externalizing behavior, understanding which factors can promote this process is critical. As adolescents navigate novel environments and new developmental goals, their risky decision-making increases, particularly in socially salient contexts (Crone and Dahl, 2012; Blakemore and Mills, 2014). Indeed, social relationships heavily contribute to adolescents’ decisions to abstain from risk-taking, compared to children and adults. For example, experimental studies examining the association between relationship quality and task-based decision-making have found that positive parent–adolescent relationships (Qu et al., 2015) and supportive peer relationships (Telzer et al., 2015) are associated with adolescent choices to make safe decisions, as opposed to risky decisions, in the laboratory.
While much prior research has focused almost exclusively on peer and parent relationships, siblings play a key role in adolescent decision-making and externalizing problems. For example, sibling hostility and deviancy are linked to higher rates of adolescent externalizing behavior (Slomkowski et al., 2001; Rende et al., 2005), whereas positive sibling relationships serve as a buffer for adolescent externalizing behavior (e.g. Conger et al., 1994). Importantly, sibling influence on adolescent decision-making is oftentimes greater than that of parents and peers, underscoring the key role of siblings in adolescents externalizing behavior (e.g. Stormshak et al., 2004; Defoe et al., 2013). Although developmental social neuroscience has investigated the role of parent and peer relationships on adolescent decisions to take risks or play it safe (Telzer et al., 2017), no prior study has examined sibling relationships. Because sibling relationships are an important and understudied factor in adolescent decision-making, the aim of this study is to investigate whether sibling closeness is associated with differences in neural processes during safe decision-making, and in turn less externalizing behavior, above and beyond relationships with parents and peers.
Sibling relationships as a salient influence on adolescent externalizing behavior
The majority of children in the United States live with at least one sibling (81%; Kreider, 2008). Positive adolescent sibling relationships, characterized by closeness and support, have been linked to a variety of psychosocial adjustment outcomes during adolescence and emerging adulthood (Milevsky and Levitt, 2005; Melby et al., 2008; Alfaro and Umaña-Taylor, 2010; Hollifield and Conger, 2015; Rogers et al., 2017), including lower externalizing behavior such as substance use, risky sexual behaviors and deviant acts (Conger et al., 1994; Slomkowski et al., 2001; Yeh and Lempers, 2004; Buist et al., 2014), above and beyond the effects of parent and peer relationships (Stormshak et al., 2004; Defoe et al., 2013; Whiteman et al., 2013; Samek et al., 2015). Sibling relationships distinguish themselves from parents and peers because they are our longest lasting relationships (Cicirelli, 1995), and thus, provide a stable and safe environment for unique learning experiences (Furman and Buhrmester, 1985; Feinberg et al., 2012). The context of close sibling relationships can provide adolescents with a space to disclose and feel validated, empowered and supported (Campione-barr et al., 2015; Kramer et al., 2019), which engenders adolescent autonomy and competence (Dailey, 2009; Hollifield and Conger, 2015) to make more optimal decisions.
In addition to the relationship quality between siblings, the number of older siblings in a collective sibling culture also plays a role in adolescent attitudes and behavior (Hurtado-Ortiz and Gauvain, 2007). Specifically, older siblings typically exert more influence over their younger siblings than vice versa during adolescence (Buhrmester and Furman, 1990; for a review, McHale et al., 2012). A large body of research has shown that older siblings’ engagement in risky behavior (e.g. substance use, risky sexual behavior, deviant acts) predicts similar levels of risk-taking behavior in their younger sibling (Rende et al., 2005; Craine et al., 2009; Whiteman et al., 2013, 2014). Although seemingly positive for youth adjustment, high perceived sibling closeness may actually increase susceptibility to risk taking when the older sibling is also risky. Indeed, a warm and supportive relationship with a delinquent older sibling represents the highest risk for a younger sibling’s delinquency, even after controlling for younger siblings’ initial levels of delinquency, whereas older siblings who perceive high cohesion and support may abstain from risk taking in order to be a positive role model for their younger sibling (Slomkowski et al., 2001). Thus, sibling cohesion may differentially predict externalizing behavior depending on whether the individual is the eldest or has older siblings themselves. As such, older sibling quantity and sibling relationship quality are intertwined in determining adolescent decision-making, and thus, must be investigated concurrently to better understand whether positive sibling relationships can promote safe decision-making, and in turn, less engagement in externalizing behavior.
Neurobiology of social relationships on safe decision-making
Recent advances in developmental neuroscience have identified key neurobiological processes linking social relationships to adolescent decision-making (see Schriber and Guyer, 2016). Although no research to date has examined the unique role of siblings, evidence from neuroimaging research on parents and peers provides a compelling framework for examining siblings. The exaggerated focus on social cues during adolescence has been explained as a neurobiological phenomenon—that is, adolescents may be neurobiologically susceptible to social influence (Schriber and Guyer, 2016; Telzer et al., 2017). The adolescent brain is proposed to act as a pathway that explains, in part, the association between social contexts and adolescent decision-making (Telzer et al., 2017). Specifically, the quality of social relationships is associated with adolescent neural activity when making cautious decisions to avoid risks. Of interest, the anterior insula is a critical region in the association between family discord and adolescent decision-making to avoid risks (i.e. safe decision-making; Guassi Moreira and Telzer, 2017). In addition, mother presence is associated with heightened activity in the ventral striatum (VS; Guassi Moreira and Telzer, 2018) and ventrolateral prefrontal cortex (vlPFC) during safe decision-making (Telzer et al., 2015). The VS is a dopaminergic region involved in reward processing (Fareri et al., 2008; Van Leijenhorst et al., 2010; Telzer, 2016), the vlPFC is a region implicated in inhibitory control and social flexibility (Luna et al., 2010; Nelson and Guyer, 2011), and the insula serves as a neural hub integrating affective and cognitive responses during decision-making (for a review, Smith et al., 2014). Together, this growing body of research suggests that high quality relationships contribute to less risky behavior via modulation of neural processes involved in safe decision-making.
Current study
In the current study, we examined whether high sibling closeness relates to lower adolescent engagement in externalizing behavior via neural activation during safe decision-making, and whether this was modulated by birth order. We investigated this aim using a community sample to better understand how sibling relations and safe decision-making contribute to normative levels of externalizing behavior to ultimately inform how problematic levels may develop in at-risk adolescents. Similar to previous findings examining parents’ impact on adolescent risk-taking (e.g. Guassi Moreira and Telzer, 2017), we hypothesized that sibling closeness would be associated with modulated neural responses in the anterior insula, VS and vlPFC during safe decision-making, and this neural activation would, in turn, predict less self-reported externalizing problems. Given that sibling relationships contribute to adolescent externalizing behavior above and beyond the effects of parent and peer relationships (Stormshak et al., 2004; Defoe et al., 2013), we examined the unique role of sibling closeness controlling for parent and peer closeness. Moreover, given that birth order (i.e. being the eldest sibling or having multiple older siblings) plays a key role in social influence susceptibility, particularly in the domain of externalizing behavior (Whiteman et al., 2014), we tested whether these associations would be moderated by the number of older siblings.
Methods
Participants
Participants were from a larger project investigating parent and peer influences on adolescent decision-making from a neurodevelopmental perspective, which included 89 adolescents, 12 of whom were excluded from the current manuscript; seven had incomplete data such that there were too few trials to model or the task was not fully completed (explained below), three did not have any siblings and two had excessive head movement (>2.0 mm inter-slice movement on ≥10% of slices). The current study included 77 participants (Mage = 13.45 years, range = 12.05–15.92; 40 females) and their primary caregiver (63 mothers), who were recruited through community flyers, listservs and in-person handouts at a school open house. Adolescent participants were screened for and free from MRI contraindications, learning disabilities and neurological-altering medications. Screening occurred on the following three occasions: first, research staff asked parents using a systemized script over the phone prior to scheduling; second, research staff asked parents using the biomedical center check list and third, the scan technician reviewed the checklist with the parent. Full biological, half biological, step, adopted and foster siblings were included in the sample, with the majority of adolescents having full biological siblings (n = 64; Table 1). The majority of adolescents had both sisters and brothers (49%), whereas 31% had only sisters and 20% had only brothers. The age distributions of siblings were evenly distributed with 31% of adolescents having both older and younger siblings, 38% with only younger siblings and 31% with only older siblings. Demographic information on adolescent ethnicity, parental education and parental marital status are displayed in Table 2. All participants provided written assent with parental consent in accordance with the Institutional Review Board.
Table 1.
Descriptive and frequency statistics of sibling characteristics (n = 77)
| Descriptives | Frequencies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Min | Max | Mean | SD | 0 | 1 | 2 | 3 | 4 | 5+ | |
| Sibling characteristics | |||||||||||
| Total | 1 | 9 | 2.57 | 1.80 | 0 | 27 | 21 | 12 | 3 | 14 | |
| Younger | 0 | 8 | 1.32 | 1.46 | 24 | 28 | 14 | 3 | 6 | 2 | |
| Older | 0 | 5 | 1.22 | 1.34 | 29 | 25 | 8 | 9 | 4 | 2 | |
| Brothers | 0 | 4 | 1.22 | 1.14 | 24 | 27 | 15 | 7 | 4 | 0 | |
| Sisters | 0 | 6 | 1.35 | 1.18 | 15 | 37 | 15 | 7 | 0 | 3 | |
| Full biological | 0 | 5 | 1.52 | 1.12 | 13 | 30 | 20 | 10 | 3 | 1 | |
| Half biological | 0 | 5 | 0.70 | 1.22 | 52 | 9 | 8 | 4 | 3 | 1 | |
| Step | 0 | 4 | 0.31 | 0.82 | 65 | 4 | 5 | 2 | 1 | 0 | |
| Adopted | 0 | 1 | 0.03 | 0.16 | 75 | 2 | 0 | 0 | 0 | 0 | |
| Foster | 0 | 1 | 0.01 | 0.11 | 76 | 1 | 0 | 0 | 0 | 0 | |
Table 2.
Demographics of adolescent ethnicity, parental education and parental marital status
| Variables | N |
|---|---|
| Adolescent ethnicity | |
| Latino/Hispanic | 2 |
| African American/Black | 15 |
| Asian/Pacific Islander | 5 |
| Caucasian/White | 41 |
| American or Native American | 1 |
| Multiethnic | 13 |
| Parental education | |
| High school | 2 |
| Vocational or trade school | 2 |
| Some college | 18 |
| College | 32 |
| Some medical, law or graduate school | 4 |
| Medical, law or graduate school | 19 |
| Parental marital status | |
| Single | 12 |
| Married | 60 |
| Separated, cohabitating or other | 5 |
Risk-taking task
The Yellow Light Game (YLG) is a driving simulation adapted from the Stoplight task (Steinberg et al., 2008; Chein et al., 2011), which is used to examine both performance and the neural correlates of risky decision-making (Op de Macks et al., 2018). During the YLG, participants complete a virtual driving course, in which they are instructed to choose to either stop or go at each intersection with the goal of completing the course as quickly as possible (see Figure 1). A go decision is the fastest option and results in no delay if successful. However, if there is a car crossing the intersection, a go decision results in a crash, which causes a 5 s delay. A successful go is paired with a positive chiming sound and a visual cue (a blue tilde; see Figure 1), whereas a crash is paired with a honking car sound, crash noise and visual cue (broken windshield). A stop decision causes a 2.5 s delay and is paired with either an approaching car which honks or a blue tilde if no car is in the intersection. Participants were explicitly informed about the associated time delays, or lack thereof, of the decisions and outcomes. Thus, go decisions are risky, whereas stop decisions are safe. In the current study, we focus on stop decisions because they represent safe decision-making, which has been shown to underlie associations between family relationships and insula activation (Guassi Moreira and Telzer, 2018). Of the seven participants who were excluded due to incomplete data, five were specifically excluded because their performance on both rounds of the YLG contained less than four stop decisions, which was too few to appropriately model. Participants were trained on the YLG by playing two full rounds before the scan. To discourage participants from not responding, they received a warning (red X) and error noise as well as a 5 s delay if they did not decide fast enough to stop or go. During the actual scan task, the no-response trials resulted in a 1 s delay, although this was not communicated explicitly to participants.
Fig. 1.
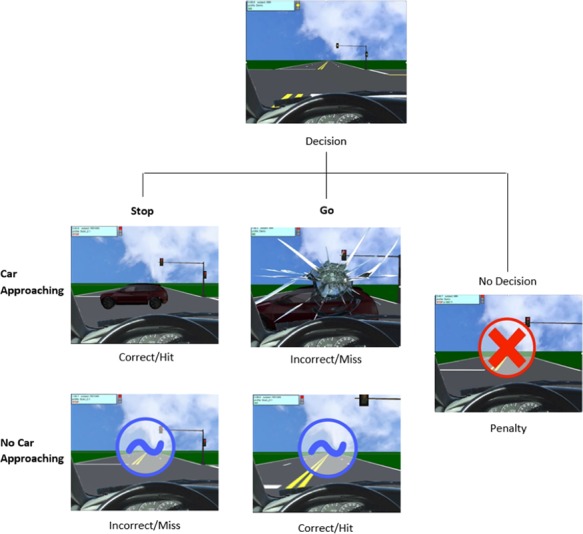
YLG paradigm.
Participants then completed two runs of the task during the fMRI scan. Each run of the task included 20 intersections, resulting in 40 total intersections. The probability of a car passing through the intersection was kept constant at 50%, and the perceived distance of the yellow light varied between 200 and 250 feet. Participants were not made explicitly aware about the probability of crashing. The two practice runs and two scan runs of the YLG were different from one another in the onset of yellow and red lights, as well as the intersections in which cars approached the intersection. All participants completed the same two practice runs and two scanner runs of the YLG. If a participant had four or more no decision trials in one YLG run, data from that run were not included in the fMRI analyses. These runs were excluded to ensure that the remaining data reflected participants who understood the instructions and displayed a minimal level of competence in their performance. Of the seven participants who were excluded due to incomplete data, two were excluded because their performance on both rounds of the YLG contained four or more (i.e. >20% of trials) no decision trials, which reflected low understanding or attention to the task.
Self-report measures
Closeness. Adolescents completed seven items each for parents and peers from the Inventory of Parent and Peer Attachment (IPPA; Armsden and Greenberg, 1987), and seven items for siblings from a new subscale on Sibling Attachment (items based identically on the IPPA), to measure how much adolescents feel they can trust, communicate with and are supported by their siblings, peers and parents. Using a 5-point Likert scale (1 = almost never to 5 = almost always), adolescents answered seven items each about their siblings, parents and peers in the past month [e.g. ‘I trusted my sibling(s) (parents/peers)’ and ‘I could count on my sibling(s) (parents/peers) when I needed to talk’]. These items were averaged for each subscale (Sibling Attachment, Parent Attachment and Peer Attachment) with higher scores indicating greater closeness (α = 0.89–0.92). While the sibling subscale is newly developed for this study, we have used the seven-item version of the IPPA successfully in prior research linking family and peer relationships to neural processing during risk taking (e.g. Qu et al., 2015; Telzer et al., 2015). The full list of items is displayed in Appendix A.
Externalizing Behavior. Adolescents completed the strengths and difficulties questionnaire (Goodman, 1997), which includes five subscales and 25 items, 10 of which are specific to externalizing behavior. Thus, the possible score range was 0–20, with 0–8 indicative of normative behavior from early- to mid-adolescence, and scores above 9 indicating problematic levels of externalizing behavior (Goodman et al., 1998). Adolescents indicated the extent to which each behavior was true (0 = not true, 1 = somewhat true and 2 = certainly true) regarding their recklessness, anger, concentration and lack of inhibition. Examples include ‘I get very angry and often lose my temper’, and ‘I am easily distracted, I find it difficult to concentrate’. The conduct problems and hyperactivity subscales were each summed to form a composite score of externalizing problems (Goodman, 1997), each of which had satisfactory internal reliability (conduct problems, α = 0.64; hyperactivity, α = 0.65). Higher average scores between these subscales represent greater frequency of externalizing behaviors.
Sibling(s) characteristics. The primary caregiver provided the age, sex and relatedness of their children. This information was recoded to create several variables to capture sibling characteristics. The number of older siblings was coded as 0 = no older siblings, 1 = one older sibling and 2 = two or more older siblings and used as a moderator in analyses given the salience of older siblings on adolescent risky decision-making (e.g. Slomkowski et al., 2001). Other variables were included as covariates based on previous literature on sibling relations were as follows: the proportion of sisters compared to brothers (total sisters divided by total siblings) and the proportion of full biological siblings compared to half biological, step, adopted and fostered siblings (total biological siblings divided by total siblings). Given that the sibling closeness scale captured adolescent perceptions of their sibling collective, that is, all of their sibling relationships, the proportion of sisters, and biological siblings, were used to index the variation of sex and biological relatedness within the sibling group for each family.
fMRI data acquisition
Brain images were collected using a research dedicated 3 Tesla Siemens Trio MRI scanner. The YLG was presented on a computer screen and projected through a mirror. A high-resolution structural T2*-weighted echo-planar imaging (EPI) volume (TR = 2000 ms; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; 38 slices; slice thickness = 3 mm; voxel size 2.5 × 2.5 x 3 mm3) was acquired coplanar with a T2*-weighted structural matched-bandwidth (MBW), high-resolution, anatomical scan (TR = 4000 ms; TE = 64 ms; matrix = 192 × 192; FOV = 230 mm; 38 slices; slice thickness = 3 mm). In addition, a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1900 ms; TE = 2.32 ms; matrix = 256 × 256; FOV = 230 mm; sagittal plane; slice thickness = 0.9 mm; 192 slices) was acquired. The orientation for the EPI and MBW scans was oblique axial to maximize brain coverage and to reduce noise.
fMRI data preprocessing and analysis
fMRI data was preprocessed and analyzed using FSL FMRIBs Software Library (FSL v6.0; https://fsl.fmrib.ox.ac.uk/fsl/). Preprocessing for each participant’s images included resampling the functional images to 2 × 2 × 2 mm, spatial realignment to correct for head motion using MCFLIRT (only participants whose motion was less than 2 mm for inter-slice movement on 10% or fewer slices were included), spatial smoothing using a 6 mm FWHM Gaussian kernel to increase the signal-to-noise ratio in the functional images, high-pass temporal filtering of 128 s to eliminate low-frequency drift across the time series and skull stripping for all images using BET. Co-registration of the MBW and EPI images was conducted with the high-resolution T1* MPRAGE images utilizing FLIRT so that we could normalize them into standard stereotactic space as defined by the Montreal Neurological Institute and the International Consortium for Brain Mapping. Finally, individual-level independent component analysis (ICA) was applied using MELODIC, in conjunction with an automated component classifier (Tohka et al., 2008; Neyman–Pearson threshold = 0.03), to remove artifact signals such as motion and physiological noise.
The YLG was modeled as an event-related design using Statistical Parametric Mapping (SPM8; Welcome Department of Cognitive Neurology, Institute of Neurology, London, UK) software package. Using a two-level procedure, we first conducted a fixed effects fMRI data analysis at the subject level. First, linear contrasts were created for each planned condition for each participant including two decision regressors: go decisions and stop decisions; four outcome regressors: successful go outcome, unsuccessful go outcome (i.e. crash), safe stop outcome (i.e. with approaching car) and unnecessary stop outcome (i.e. without approaching car) and no decision trials, which were modeled in a separate junk regressor. The onset of the go and stop decision was modeled when the traffic light turned yellow, and the duration of the go and stop decision trials was modeled as the reaction time from which the yellow light appeared (onset time) until the participant made a response. The onsets of the go outcome trials were modeled when the blue tilde occurred for a successful go or when a crash occurred. For stop outcome trials, the onset began when the car stopped. The duration of outcomes after deciding to go lasted 1 s and 2.5 s following stop decisions. The individual subject contrasts were used to create linear contrast images for the contrasts of interest. In the current study, our contrast of interest was stop decisions (i.e. making a safe choice) relative to baseline. The baseline, which was not explicitly modeled, was the driving time between stoplights.
Random-effects, group-level analyses were conducted using GLMFlex, which corrects for variance–covariance inequality and partitions error terms, as well as removes outliers of spontaneous activation changes in the brains, to analyze all voxels containing data (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). Whole-brain analyses were computed at the group level to assess neural activation when adolescents made safe decisions (i.e. stop trials). We included sibling closeness in whole brain regression analyses, controlling for parental closeness, peer closeness and sibling characteristics simultaneously. These models were computed again to include the interaction between sibling closeness and number of older siblings to investigate whether having older siblings moderated the association between sibling closeness and neural activation during safe decision-making.
In order to correct for multiple comparisons, Monte Carlo simulations were run using the group-level brain mask for stop decisions. The simulations were run through 3dClustSim within the AFNI software package (Ward, 2000; updated April 2016), as well as the acf option to estimate the intrinsic smoothness. The simulation yielded a minimum cluster size of 61 contiguous voxels using a P < 0.005 voxel-wise threshold, corresponding to P < 0.05, Family Wise Error corrected. All reported results are available on NeuroVault (Gorgolewski et al., 2015; see /collections/NCTQNTAP/).
Results
Descriptives
Descriptive and frequency statistics of sibling characteristics and closeness are displayed in Table 1. An analysis of variance with the number of older siblings (0 = no older siblings, 1 = one older sibling and 2 = two or more older siblings) predicting sibling closeness demonstrated no significant differences, F (76) = 1.056, P = 0.35. Correlations between variables of closeness, stop decisions in the YLG and self-reported externalizing behavior are presented in Table 3. Closeness with siblings was positively correlated with closeness with parents and peers and closeness with parents was positively correlated with closeness with peers. Perceptions of closeness with siblings, peers and parents were not significantly associated with stop decisions during the YLG. Higher levels of sibling, parental and peer closeness were associated with lower levels of externalizing behavior. Importantly, adolescents who made more safe decisions during the YLG reported lower levels of externalizing behavior, suggesting the task is ecologically valid. Finally, t-tests indicated that there were no gender differences in any of the variables of interest.
Table 3.
Descriptive statistics and bivariate correlations between variables of closeness, stop decisions in the YLG and self-reported externalizing behavior
| Measure | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Sibling closeness | — | 0.44* | 0.24* | 0.21 | −0.42* |
| 2. Parent closeness | — | 0.24* | 0.02 | −0.30* | |
| 3. Peer closeness | — | 0.05 | −0.44* | ||
| 4. Mean stop decisions in YLG | — | −0.25* | |||
| 5. Self-reported externalizing | — | ||||
| Min | 7 | 11 | 8 | 3.5 | 0 |
| Max | 34 | 35 | 35 | 16.5 | 15 |
| M | 19.87 | 27.00 | 25.90 | 9.05 | 5.03 |
| SD | 6.97 | 6.25 | 6.24 | 2.92 | 2.75 |
Note: M = Mean; SD = standard deviation.
*P < 0.05. All correlations tested at this level. Two-tailed significance.
fMRI results
Our first set of fMRI analyses examined the neural correlates of sibling closeness during stop decisions using whole brain regression analyses controlling for parental closeness, peer closeness and sibling characteristics. We found a positive correlation between sibling closeness and activity in the right precentral gyrus (see Figure 2), such that sibling closeness was associated with higher levels of activation in this region during stop decisions (Table 4). Sibling closeness was not associated with lower levels of activation in any region (see Appendix B for analyses examining parental and peer closeness).
Fig. 2.
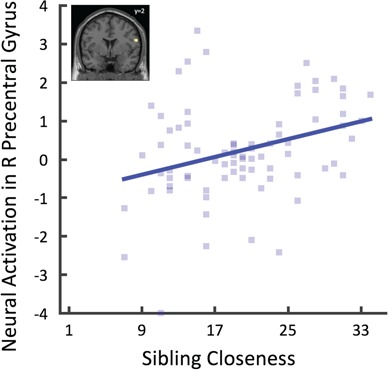
Neural correlates of sibling closeness.
Table 4.
Neural regions that associated with sibling closeness during stop trials in the YLG
| Predictor | Anatomical region | + / − | x | y | z | t | k |
|---|---|---|---|---|---|---|---|
| Sibling closeness | R Precentral Gyrus | + | 62 | 2 | 24 | 2.87 | 128 |
| Sibling closeness X older sibling quantity | R Anterior Insula | ⎼ | 46 | 8 | 14 | 3.37 | 139 |
| L Anterior Insula | ⎼ | ⎼46 | 4 | 14 | 3.72 | 232 | |
| R Ventral Striatum | ⎼ | 16 | 10 | ⎼18 | 2.87 | 264a | |
| L Ventral Striatum | ⎼ | ⎼8 | 6 | ⎼2 | 3.39 | a | |
| R Caudate | ⎼ | 14 | 24 | 6 | 3.34 | a | |
| L Caudate | ⎼ | ⎼6 | 10 | 2 | 3.13 | a | |
| R Parahippocampal Gyrus | ⎼ | 16 | ⎼40 | ⎼18 | 3.48 | 150 | |
| L IFG | ⎼ | ⎼40 | 30 | 22 | 3.37 | 490b | |
| L vlPFC | ⎼ | ⎼22 | 60 | 0 | 3.93 | b |
Note: L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y and z refer to MNI coordinates. All regions are significant at P < 0.005. Regions that share the same superscript are part of the same cluster.
Next, we conducted our primary analysis of interest by adding the interaction between sibling closeness and the number of older siblings an adolescent had onto neural activation during stop decisions. This analysis revealed that the association between sibling closeness and activation in the bilateral anterior insula, bilateral VS and left ventrolateral PFC (vlPFC) is moderated by the number of older siblings (Table 4). For descriptive purposes, we extracted parameter estimates of signal intensity from these regions and plotted the interactions following the guidelines of Aiken and West (1991) and Dawson (2014). As shown in Figure 3, we plotted the slopes for adolescents with zero older siblings (i.e. the oldest child in the family) and those with two or more older siblings. Simple slope analyses corroborated that the slope is significant for adolescents with no older siblings (left insula: t = 2.77, P = 0.007; right insula: t = 2.94, P = 0.004; bilateral VS: t = 3.18, P = 0.002; left dlPFC: t = 2.21, P = 0.03), such that greater sibling closeness was related to more activation in these regions. In contrast, adolescents who had several older siblings showed a significant negative association between sibling closeness and brain activation in these regions (left insula: t = –2.43, P = 0.018; bilateral VS: t = –2.75, P = 0.008; left dlPFC: t = –2.94, P = 0.004), with the right insula at a trend level (t = –1.87, P = 0.066). These results suggest that the association between perceptions of sibling closeness and neural activity in the anterior insula, VS and vlPFC significantly differs depending on whether an adolescent is the eldest child in the family compared to having multiple older siblings.
Fig. 3.
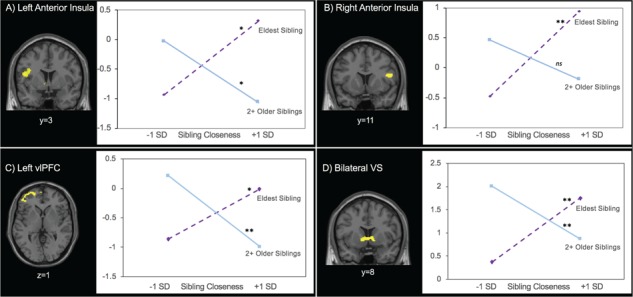
Having multiple older siblings moderates the association between sibling closeness and activation in the left and right anterior insula, left and right VS and left vlPFC during safe decision-making. Simple slopes analysis was used to examine whether the association between sibling closeness and neural activity was significant for eldest siblings and adolescents with two or more older siblings. *P < 0.05, **P < 0.01.
Next, we computed a moderated mediation model to examine whether the interaction between sibling closeness and number of older siblings was associated with externalizing behavior via heightened neural activation during stop decisions. Four separate models were computed for the left and right anterior insula, VS and vlPFC using the PROCESS macro in SPSS. Parent and peer closeness, and sibling characteristics, were added as covariates in this model. The results showed that the left anterior insula was a significant mediator, such that the interaction between sibling closeness and older sibling quantity was related to lower adolescent externalizing via activation in the left anterior insula during safe decision-making [ß = 0.062, SE = 0.027, 95% CI (0.0194, 0.1293); Figure 4]. Specifically, adolescents with no older siblings (i.e. they were the eldest sibling) showed an indirect effect of high sibling closeness associating with less externalizing via insula activation [–0.055, SE = 0.030, 95% CI (–0.1279, –0.0135)], whereas adolescents with two or more older siblings showed an indirect effect of high sibling closeness associating with more externalizing via insula activation [–0.047, SE = 0.027, 95% CI (0.0085, 0.1182)]. The right insula, VS and vlPFC were not significant mediators. These results suggest that the association between perceptions of sibling closeness and externalizing behavior is mediated by the left anterior insula during stop decisions, and significantly differs depending on whether an adolescent is the eldest child in the family compared to having multiple older siblings.
Fig. 4.
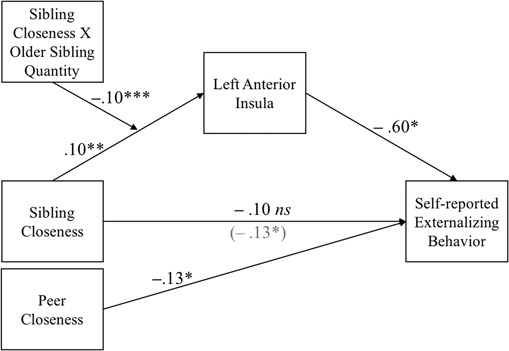
Moderated mediation model examining the association between sibling closeness and self-reported externalizing behavior. Activation in the left anterior insula during stop decisions was modeled as a mediator between sibling closeness and externalizing behavior. The number of older siblings an adolescent had was examined as a moderator between sibling closeness and activation in the left anterior insula. Coefficients are unstandardized. The covariates of parent and peer closeness, and percentage of siblings who were sisters and fully biological, were regressed onto the mediator and outcome variables. Only significant covariate pathways are displayed. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
The field of social neuroscience has witnessed a surge of research focusing on the neural mechanisms that underlie how social relationships may influence adolescent decision-making and externalizing behavior (Schriber and Guyer, 2016; Telzer et al., 2017). However, the theoretical and empirical emphasis of this research has been focused solely on parent and peer relationships, and has not addressed relationships with siblings. This is an important limitation, given that siblings are one of the most ubiquitous and enduring relationships in individuals’ lives (Kramer et al., 2019) and often influence adolescents’ behavior above and beyond that of peers or parents (Defoe et al., 2013; Whiteman et al., 2014; Rogers et al., 2017). Given the important role of sibling relationships on adolescent externalizing behavior (Defoe et al., 2013; Slomkowski et al., 2001; Whiteman et al., 2014), we examined the role of sibling closeness on the neurobiology of adolescent decision-making. Our findings underscore the salience of sibling relationships on adolescent neural processing during safe decision-making. In addition, moderated mediation analyses highlight the role of the anterior insula in the association between sibling relationships and externalizing behavior, and how this relationship is modulated by having older siblings. Because the sample represented both normative and problematic levels of externalizing behavior, the way in which adolescents perceive closeness with their siblings and the extent to which the anterior insula is recruited during safe decision-making explains meaningful differences in whether adolescents engage in problematic behavior. Importantly, these findings persisted above and beyond parental and peer closeness, highlighting the significant influence of sibling relationships on adolescent externalizing behavior through the brain.
Our findings show that close sibling relationships were associated with adolescents’ neural processing while making safe decisions. Although sibling closeness did not associate with behavior during the task, sibling closeness was associated with meaningful neurobiological processes that underlie adolescent decisions to avoid risk. Furthermore, neural processes during safe decisions were related to externalizing behaviors, whereas behavior on the task was not. These findings suggest that adolescents who perceive close sibling bonds differentially recruit neural regions to make safe decisions which is ultimately related to real-life decision-making and associated externalizing behavior.
Importantly, we found that the association between insula activation during safe decision-making and sibling closeness was moderated by the quantity of older siblings an adolescent had. Thus, perceptions of sibling closeness differentially predicted activation in these brain regions during safe decision-making depending on whether adolescents were the eldest child in the family or had older siblings themselves. Furthermore, insula activation while making safe decisions served as a pathway through which sibling closeness and older sibling quantity were associated with adolescent experiences of externalizing behavior. This finding is consistent with previous research that has identified neural mechanisms through which social relationship quality is associated with adolescent risky behavior to inform our understanding of various factors that contribute to the development of adolescent decision-making (Qu et al., 2015; McCormick et al., 2016). Specifically, eldest children exhibited a negative association between sibling closeness and externalizing behavior via insula activation during safe decision-making, whereas adolescents with older siblings showed a positive association between sibling closeness and externalizing via insula activation. Given that the anterior insula has been linked to making decisions under conditions of uncertainty (Singer et al., 2009; Van Leijenhorst et al., 2010) and the integration of affective and cognitive processes during decision-making (Smith et al., 2014), adolescents may make safer decisions when they have younger siblings they care about, and thus, experience less externalizing problems than adolescents with older siblings. This finding reflects research that proposes that oftentimes, older siblings fill the role as caregivers, models and advice-givers for their younger siblings (Tucker et al., 1997; Whiteman et al., 2007). Thus, being an older sibling and feeling close to one’s younger siblings may increase the level of affective sensitivity adolescents show when making safe decisions.
In contrast, adolescents who have several older siblings reported higher externalizing behavior through activation in the anterior insula as their perceived closeness with their siblings increased. This finding may suggest that adolescents who have several older siblings tend to experience safe decisions as less rewarding compared to older siblings (Sulloway, 1996). Furthermore, this finding is consistent with research that suggests that younger siblings who are close with their older siblings oftentimes exhibit higher levels of risk-taking than adolescents who do not perceive close sibling ties (Slomkowski et al., 2001; Slomkowski et al., 2005). Still, this literature also suggests that the closer adolescent siblings are to one another, the more likely they are to emulate the level of problematic behaviors as exemplified by their older siblings. As such, we propose that adolescents who are close with older siblings who exhibit high levels of externalizing problems would show an amplified association between suppressed anterior insula activity and self-reported externalizing behaviors. Our findings emphasize the importance of recruiting adolescent sibling dyads to tease apart these associations in future studies, and highlight the importance of considering birth order when investigating the effect of sibling relationships on the neurobiology of adolescent decision-making, particularly since sibling closeness appears to operate as a risk or protective factor depending on whether an adolescent has older siblings or is the eldest child in the family.
Of note, perceiving close sibling relationships indirectly predicted externalizing behavior via safe decision-making at the level of the brain above and beyond parent and peer relations, underscoring sibling closeness as a unique influence on adolescent externalizing behavior through an endogenous process in the brain (Fuligni and Telzer, 2013). Sibling closeness uniquely predicted adolescent recruitment of the insula, VS and vlPFC during safe decision-making and self-reported externalizing behavior that parental and peer closeness did not, suggesting that sibling relationships are an idiosyncratic predictor of whether adolescents decide to play it safe, and ultimately, whether they decide to avoid engaging in externalizing behavior. Furthermore, examining parental closeness and peer closeness separately showed that neither relationship was associated with recruitment of the insula during safe decision-making (Appendix B). It is no surprise that closeness between siblings uniquely predicts adolescent decision-making (Kramer et al., 2019), as sibling relationships not only reinforce family values and can offer familial support like parent–child relationships (e.g. Updegraff et al., 2005), but also provide a space to navigate relevant developmental issues and goals like peer relationships (e.g. Craine et al., 2009; Campione-barr et al., 2015). Thus, siblings can act as a salient resource for adolescents to acquire advice, validation and support to make decisions in their day-to-day lives (Tucker et al., 2001), for better or for worse and depending on birth order.
The limitations of this study are important to address for future investigations on social influences on the neurobiology of adolescent decision-making. First, the measure of sibling closeness was not specific to one sibling, but global to include all sibling relationships for each adolescent. In light of this, it is important to interpret the findings as adolescent perceptions of their sibling culture as a whole, rather than individual sibling relationships. Future work would benefit from focusing on one sibling dyad, particularly siblings closest in age to better understand sibling influence on risk-taking (e.g. Slomkowski et al., 2001) and how neural processes play a role in this association. Additionally, obtaining the externalizing behavior of sibling participants would be helpful in discerning whether this factor modulates the association between sibling closeness, adolescent decision-making and adolescent externalizing behavior. Second, although the pattern of results shown in this study suggest that close sibling ties predict the neural correlates of safe decision-making, future research in this area should create research designs (e.g. experimental; Telzer et al., 2015) and conduct longitudinal analyses (e.g. cross lagged panel analyses; Selig and Little, 2012) that can appropriately tease out causal association between sibling relations and neural activity. Furthermore, because using a moderated mediation model assumes causality from one construct to another, and our research design was cross-sectional, it is paramount for future work to utilize a longitudinal design to more appropriately test the brain as a mediator between sibling closeness and externalizing behavior (e.g. Maxwell et al., 2011). Last, although stop decisions were the condition of interest because they represent safe decision-making, they were contrasted with the baseline. Thus, our findings could be related to decision-making more generally, providing an opportunity for future work to examine how sibling closeness relates to different types of decision-making. These future directions can inform our understanding of the role of sibling relationships in the development of risk-taking behavior during adolescence.
In conclusion, our findings provide support for sibling relationships as a salient factor in the neurobiology of adolescent decision-making. We found that close sibling ties predict real-life externalizing behavior during adolescence via neural mechanisms associated with salience detection, and coordinating the processes of cognitive control and reward valuation. Furthermore, having several older siblings modifies the association between sibling closeness and the neural underpinnings of safe decision-making. Findings from this study contribute toward our understanding of how important social relationships influence adolescent externalizing behavior, and which neural processes may be integral for engendering social information from these relationships to inform adolescent decisions to take, or abstain from, risks. Furthermore, these findings inform our understanding of the social antecedents and processes associated with externalizing behavior in a community sample, and provide a comparison for future work to investigate these processes in adolescents at-risk for severe externalizing problems.
Acknowledgements
We greatly appreciate the assistance of the Biomedical Imaging Center at the University of Illinois as well as Elizabeth Lozano, Kathy Do, Michael Perino, Heather Ross, Lynda Lin, Paul Sharp and Tae-Ho Lee for assistance in collecting data. We thank Dr Jennifer Pfeifer and the Developmental Social Neuroscience Lab at the University of Oregon for creating the Yellow Light Game and providing support on the task.
Conflict of interest. None declared.
Funding
This work was supported by grants from the National Institutes of Health (R01DA039923) and National Science Foundation (SES 1459719).
A.
Sibling attachment subscale of the IPPA
Indicate the extent to which the following statements relate to you and your sibling(s) :
Table A1.
| Almost never or never | Seldom | Sometimes | Often | Almost always or always | |
|---|---|---|---|---|---|
| My siblings respect my feelings. | ○ | ○ | ○ | ○ | ○ |
| My siblings encourage me to talk about my difficulties. | ○ | ○ | ○ | ○ | ○ |
| My siblings understand me. | ○ | ○ | ○ | ○ | ○ |
| When I am angry about something, my siblings try to be understanding. | ○ | ○ | ○ | ○ | ○ |
| I trust my siblings. | ○ | ○ | ○ | ○ | ○ |
| I can count on my siblings when I need to get something off my chest. | ○ | ○ | ○ | ○ | ○ |
| If my siblings know something is bothering me, they ask me about it. | ○ | ○ | ○ | ○ | ○ |
Note: The sibling attachment subscale was adapted based on the IPPA (Armsden and Greenberg, 1987) to measure how much adolescents feel they can trust, communicate with and are supported by their sibling(s).
B.
Neural regions that positively associated with parental closeness and peer closeness during stop trials in the YLG
Table B1.
| Predictor | Anatomical region | x | y | z | t | k |
|---|---|---|---|---|---|---|
| Parental closeness | ||||||
| Precuneus | ⎼10 | ⎼70 | 42 | 3.83 | 117 | |
| L Cerebelum (IV-V) | ⎼16 | ⎼46 | ⎼22 | 3.80 | 76 | |
| L SupraMarginal Gyrus | ⎼64 | ⎼40 | 38 | 3.66 | 6 | |
| Peer closeness | ||||||
| Thalmus | 6 | ⎼16 | 0 | 4.23 | 172 | |
| L Medial Temporal Pole | ⎼26 | 8 | ⎼34 | 3.77 | 72 | |
| L Putamen | ⎼32 | ⎼12 | 6 | 3.30 | 67 |
Note: Analyses controlled for sibling closeness, and percentage of related siblings and percentage of sisters in sibling collective. In addition, the parental closeness analysis controlled for peer closeness and the peer closeness analysis controlled for parental closeness. L and R refer to left and right hemispheres; k refers to the number of voxels in each significant cluster; t refers to peak activation level in each cluster; x, y and z refer to MNI coordinates. All regions are significant at P < 0.005. Regions that share the same superscript are part of the same cluster.
References
- Aiken L.S., West S.G. (1991). Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage. [Google Scholar]
- Alfaro E.C., Umaña-Taylor A.J. (2010). Latino adolescents’ academic motivation: the role of siblings. Hispanic Journal of Behavioral Sciences, 32, 549–70 10.1177/0739986310383165. [DOI] [Google Scholar]
- Armsden G.C., Greenberg M.T. (1987). The inventory of parent and peer attachment: individual differences and their relationship to psychological well-being in adolescence. Journal of Youth and Adolescence, 16, 427–54. 10.1007/BF02202939. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Buhrmester D., Furman W. (1990). Perceptions of sibling relationships during middle childhood and adolescence. Child Development, 61, 1387–98. Retrieved fromhttp://www.ncbi.nlm.nih.gov/pubmed/2245732. [DOI] [PubMed] [Google Scholar]
- Buist K.L., Paalman C.H., Branje S.J.T., et al. (2014). Longitudinal effects of sibling relationship quality on adolescent problem behavior: a cross-ethnic comparison. Cultural Diversity & Ethnic Minority Psychology, 20(2), 266–75. 10.1037/a0033675. [DOI] [PubMed] [Google Scholar]
- Campione-barr N., Lindell A.K., Giron S.E., Killoren S.E.,Basset K., Campione-barr N. et al. (2015). Domain differentiated disclosure to mothers and siblings and associations with sibling relationship quality and youth emotional adjustment. Developmental Psychology, 51, 1278–91. [DOI] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14, F1–10. 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli V.G. (1995). Sibling Relationships Across the Life Span, New York: Plenum Press. [Google Scholar]
- Conger K.J., Conger R.D., Elder J.H. Jr. (1994). Sibling relations during hard times. In: Conger R.D., Elder G.H. Jr., editors. Families in Troubled Times: Adapting to Change in Rural America, Hawthorne, NY: Aldine. [Google Scholar]
- Craine J.L., Tanaka T.A., Nishina A., Conger K.J. (2009). Understanding adolescent delinquency: the role of older siblings’ delinquency and popularity with peers. Merrill-Palmer Quarterly (Wayne State University Press), 55(4), 436–53. 10.1353/mpq.0.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–50. 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dailey R.M. (2009). Confirmation from family members: parent and sibling contributions to adolescent psychosocial adjustment. Western Journal of Communication, 73, 273–99. 10.1080/10570310903082032. [DOI] [Google Scholar]
- Dawson J.F. (2014). Moderation in management research: what, why, when, and how. Journal of Business and Psychology, 29, 1–19. 10.1007/s10869-013-9308-7. [DOI] [Google Scholar]
- Defoe I.N., Dubas J.S., Figner B., Aken M.A.G. (2015). A meta-analysis on age differences in risky decision making: adolescents versus children and adults. Psychological Bulletin, 141, 48–84. 10.1037/a0038088 [DOI] [PubMed] [Google Scholar]
- Defoe I.N., Keijsers L., Hawk S.T.,Branje S., Dubas J.S., Buist K., Meeus W. et al. (2013). Siblings versus parents and friends: longitudinal linkages to adolescent externalizing problems. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54, 881–9. 10.1111/jcpp.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Martin L.N., Delgado M.R. (2008). Reward-related processing in the human brain: developmental considerations. Development and Psychopathology, 20, 1191–211. 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- Feinberg M.E., Solmeyer A.R., McHale S.M. (2012). The third rail of family systems: sibling relationships, mental and behavioral health, and preventive intervention in childhood and adolescence. Clinical Child and Family Psychology Review, 15, 43–57. 10.1007/s10567-011-0104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni A.J., Telzer E.H. (2013). Another way family can get in the head and under the skin: the neurobiology of helping the family. Child Development Perspectives, 7, 138–42. 10.1111/cdep.12029. [DOI] [Google Scholar]
- Furman W., Buhrmester D. (1985). Children’s perceptions of the personal relationships in their social networks. Developmental Psychology, 21(6), 1016–24. 10.1037//0012-1649.21.6.1016. [DOI] [Google Scholar]
- Goodman R. (1997). The strengths and difficulties questionnaire: a research note. Journal of Child Psychology and Psychiatry, 38, 581–6. 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Goodman R., Meltzer H., Bailey V. (1998). The strengths and difficulties questionnaire: a pilot study on the validity of the self-report version. International Review of Psychiatry (Abingdon, England), 15, 173–7. 10.1080/0954026021000046137. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K. J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S.S., Maumet C., Sochat V.V., Nichols T.E., Poldrack R.A., Poline J., Yarkoni T., Margulies D.S. (2015). NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers Neuroinformatics. 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J., Telzer E.H. (2017). Family conflict is associated with longitudinal changes in insular-striatal functional connectivity during adolescent risk taking under maternal influence. Developmental Science. 10.1111/desc.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Telzer E.H. (2018). Mother still knows best: maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science, 21, 1–11. 10.1111/desc.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollifield C.R., Conger K.J. (2015). The role of siblings and psychological needs in predicting life satisfaction during emerging adulthood. Emerging Adulthood, 3, 143–53. 10.1177/2167696814561544. [DOI] [Google Scholar]
- Hurtado-Ortiz M.T., Gauvain M. (2007). Postsecondary education among Mexican American youth: contributions of parents, siblings, acculturation, and generational status. Hispanic Journal of Behavioral Sciences, 29, 181–91. 10.1177/0739986307299584. [DOI] [Google Scholar]
- Kahn L.E., Peake S.J., Dishion T.J., Stormshak E.A., Pfeifer J.H. (2014). Learning to play it safe (or not): stable and evolving neural responses during adolescent risky decision-making. Journal of Cognitive Neuroscience, 27, 13–25. 10.1162/jocn_a_00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer L., Conger K.J., Rogers C.R., Ravindran N. (2019). Siblings. In: Fiese B.H., editor. APA Handbook of Contemporary Family Psychology,Vol. 1, Washington, D.C.: American Psychological Association, 1–18. [Google Scholar]
- Kreider R.M. (2008). Living arrangements of children: 2004. Current Population Reports. Washington, D.C.: U.S. Census Bureau. [Google Scholar]
- van Leijenhorst L., Zanolie K., van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. (2010). What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex, 20, 61–9. 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. (2010). What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition, 72(1), 101–13. 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick E.M., Qu Y., Telzer E.H. (2016). Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. NeuroImage, 124, 989–96. 10.1016/j.neuroimage.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale S.M., Updegraff K.A., Whiteman S.D. (2012). Sibling relationships and influences in childhood and adolescence. Journal of Marriage and Family, 74, 913–30. 10.1111/j.1741-3737.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby J.N., Conger R.D., Fang S., Wickrama K.A.S., Conger K.J. (2008). Adolescent family experiences and educational attainment during early adulthood. Developmental Psychology, 44, 1519–36. 10.1037/a0013352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milevsky A., Levitt M.J. (2005). Sibling support in early adolescence: buffering and compensation across relationships. European Journal of Developmental Psychology, 2, 299–320. 10.1080/17405620544000048. [DOI] [Google Scholar]
- Nelson E.E., Guyer A.E. (2011). The development of the ventral prefrontal cortex and social flexibility. Developmental Cognitive Neuroscience, 1(3), 233–45. 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks Z.A., Flannery J.E., Peake S.J., Flournoy J.C., Mobasser A., Alberti S.L., Fisher P.A., Pfeifer J.H. (2018). Novel insights from the Yellow Light Game: Safe and risky decisions differentially impact adolescent outcome-related brain function. NeuroImage, 181, 568–581. 10.1016/j.neuroimage.2018.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomery E.A., Gibbons F.X., Gerrard M., Cleveland M.J., Brody G.H., Wills T.A. (2005). Families and risk: prospective analyses of familial and social influences on adolescent substance use. Journal of Family Psychology, 19, 560–70. 10.1037/0893-3200.19.4.560. [DOI] [PubMed] [Google Scholar]
- Qu Y., Fuligni A.J., Galvan A., Telzer E.H. (2015). Buffering effect of positive parent–child relationships on adolescent risk taking: a longitudinal neuroimaging investigation. Developmental Cognitive Neuroscience, 15, 26–34. 10.1016/j.dcn.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rende R., Slomkowski C., Lloyd-Richardson E., Niaura R. (2005). Sibling effects on substance use in adolescence: social contagion and genetic relatedness. Journal of Family Psychology, 19, 611–8. 10.1037/0893-3200.19.4.611. [DOI] [PubMed] [Google Scholar]
- Rogers C.R., Guyer A.E., Nishina A., Conger K.J. (2017). Developmental change in sibling support and school commitment across adolescence. Journal of Research in Personality, 1–17. [DOI] [PubMed] [Google Scholar]
- Samek D.R., Rueter M.A., Keyes M.A., Mcgue M., Iacono W.G. (2015). Parent involvement, sibling companionship, and adolescent substance use: a longitudinal, genetically informed design. Journal of Family Psychology, 29, 614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriber R.A., Guyer A.E. (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. 10.1016/j.dcn.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selig J.P., Little T.D. (2012). Chapter 16: autoregressive and cross-lagged panel analysis for longitudinal data. In: Laursen B., Little T.D., Card N.A., editors. Handbook of Developmental Research Methods, New York: Guilford Press. [Google Scholar]
- Singer T., Critchley H.D., Preuschoff K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences, 13(8), 334–40. 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Slomkowski C., Rende R., Conger K.J., Simons R.L., Conger R.D. (2001). Sisters, brothers, and delinquency: evaluating social influence during early and middle adolescence. Child Development, 72, 271–83. [DOI] [PubMed] [Google Scholar]
- Slomkowski C., Rende R., Novak S., Lloyd-Richardson E., Niaura R. (2005). Sibling effects on smoking in adolescence: evidence for social influence from a genetically informative design. Addiction, 100, 430–8. 10.1111/j.1360-0443.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Chein J. (2014). The role of the anterior insula in adolescent decision making. Developmental Neuroscience, 36, 196–209. 10.1159/0003589188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28, 78–106. 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Albert D., Cauffman E., Banich M., Graham S., Woolard J. (2008). Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: evidence for a dual systems model. Developmental Psychology, 44, 1764–78. 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Stormshak E.A., Comeau C.A., Shepard S.A. (2004). The relative contribution of sibling deviance and peer deviance in the prediction of substance use across middle childhood. Journal of Abnormal Child Psychology, 32, 635–49. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15648530. [DOI] [PubMed] [Google Scholar]
- Sulloway F.J. (1996). Born to rebel: Birth order, family dynamics, and creative lives. New York, NY, US: Pantheon Books. [Google Scholar]
- Telzer E.H. (2016). Dopaminergic reward sensitivity can promote adolescent health: a new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galvan A. (2013). Meaningful family relationships: neurocognitive buffers of adolescent risk taking. Journal of Cognitive Neuroscience, 25, 374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Miernicki M.E., Galván A. (2015). The quality of adolescents’ peer relationships modulates neural sensitivity to risk taking. Social Cognitive and Affective Neuroscience, 10, 389–98. 10.1093/scan/nsu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E., Ichien N.T., Qu Y. (2015). Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience, 10, 1383–91. 10.1093/scan/nsv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Rogers C.R., Hoorn J. (2017). Neural correlates of social influence on risk taking and substance use in adolescents. Addictions Report, 4, 333–41. Retrieved fromhttp://dsnlab.web.unc.edu/files/2017/08/Telzer-E.H.-Rogers-C.R.-Van-Hoorn-J.-2017-Addition-Reports.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga W., Poldrack R.A. (2008). Automatic independent component labeling for artifact removal in fMRI. NeuroImage, 39, 1227–1245. 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker C., Barber B., Eccles J. (1997). Advice about life plans and personal problems in late adolescent sibling relationships. Journal of Youth and Adolescence, 26, 63–76. Retrieved fromhttp://link.springer.com/article/10.1023/A:1024540228946. [Google Scholar]
- Tucker C.J., McHale S.M., Crouter A.C. (2001). Conditions of sibling support in adolescence. Journal of Family Psychology, 15, 254–71. 10.1037//o893-32oo. [DOI] [PubMed] [Google Scholar]
- Updegraff K.A., McHale S.M., Whiteman S.D., Thayer S.M., Delgado M.Y. (2005). Adolescent sibling relationships in Mexican American families: exploring the role of familism. Journal of Family Psychology, 19, 512–22. 10.1037/0893-3200.19.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman S.D., Jensen A.C., Maggs J.L. (2013). Similarities in adolescent siblings’ substance use: testing competing pathways of influence. Journal of Studies on Alcohol and Drugs, 74, 104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman S.D., McHale S.M., Crouter A.C. (2007). Competing processes of sibling influence: observational learning and sibling deidentification. Social Development, 16, 642–61. 10.1111/j.1467-9507.2007.00409.x. [DOI] [Google Scholar]
- Whiteman S.D., Zeiders K.H., Killoren S.E., Rodriguez S.A., Updegraff K.A. (2014). Sibling influence on mexican-origin adolescents’ deviant and sexual risk behaviors: the role of sibling modeling. The Journal of Adolescent Health, 54, 587–92. 10.1016/j.jadohealth.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfert E., Block J.A., Ana E.S., Rodriguez M.L., Colsman M. (2002). Delay of gratification: impulsive choices and problem behaviors in early and late adolescence. Journal of Personality, 70, 533–52. [DOI] [PubMed] [Google Scholar]
- Yeh H.-C., Lempers J.D. (2004). Perceived sibling relationships and adolescent development. Journal of Youth and Adolescence, 33, 133–47. 10.1023/B:JOYO.0000013425.86424.0f. [DOI] [Google Scholar]


