Abstract
Adolescence is a developmental period associated with increased health-risk behaviors and unique sensitivity to the input from the social context, paralleled by major changes in the developing brain. Peer presence increases adolescent risk taking, associated with greater reward-related activity, while parental presence decreases risk taking, associated with decreased reward-related activity and increased cognitive control. Yet the effects specific to peers and parents are still unknown. The current functional magnetic resonance imaging (fMRI) study compared within-person peer and parent influences on risky decision-making during adolescence (ages 12–15 years; N = 56). Participants completed the Yellow Light Game (YLG), a computerized driving task, during which they could make safe or risky decisions, in the presence of a peer and their parent. Behavioral findings revealed no effects of social context on risk taking. At the neural level, a collection of affective, social and cognitive regions [ventral striatum (VS), temporo-parietal junction (TPJ), and dorsolateral prefrontal cortex (dlPFC)] was more active during decision-making with peers than parents. Additionally, functional connectivity analyses showed greater coupling between affective, social and cognitive control regions (VS-insula, VS-TPJ) during decision-making with parents than peers. These findings highlight the complex nature of social influence processes in peer and parent contexts, and contribute to our understanding of the opportunities and vulnerabilities associated with adolescent social sensitivity.
Keywords: adolescence, risk taking, brain development, social influence, peers, parents
Adolescence is characterized by heightened social-affective sensitivity and substantial increases in risk-taking behaviors (Crone and Dahl, 2012; Blakemore and Mills, 2014; Patton et al., 2016; Van Duijvenvoorde et al.,2016). As such, adolescent decision-making is a topic of common interest among parents and policy makers, as well as developmental neuroscientists, who aim to leverage an understanding of brain development to inform adolescent health (Dahl et al.,2018; Fuligni et al.,2018). Changes in the developing brain reorient adolescents towards social cues, making them especially sensitive to their social context, such as in the presence of parents or peers (Blakemore and Mills, 2014; Nelson et al.,2016; Scriber and Guyer, 2017). Although previous neuroimaging research has examined how the presence of peers (Chein et al., 2011) or parents (Telzer et al., 2015) impacts adolescent risk taking, no study to date has directly compared these social influences on risk taking within the same group of adolescents, a significant limitation given that prior approaches cannot disentangle the effects specific to each source of social influence. The current fMRI study aimed to fill this gap in the literature by comparing within-person peer and parent influences on risky decision-making.
The adolescent social world is characterized by increased salience of peer relations, importance of fitting, in and attunement to social evaluation (Somerville, 2013; Lam et al., 2014; Blakemore, 2018). In the presence of peers, adolescents display greater risk taking in several health-risk domains, including risky driving, gambling and smoking (Gardner and Steinberg, 2005; Loke et al., 2013; Van Hoorn et al., 2016). The increase in risk-taking behaviors with peers is paralleled by heightened activation in reward-related regions, including the ventral striatum (VS) and orbitofrontal cortex (OFC), in adolescents but not (young) adults (Chein et al., 2011). This suggests that risk taking in the presence of peers may be more rewarding and salient during adolescence, an effect that seems specific to risk-taking, as peer presence does not disrupt neural activity during response inhibition (Smith et al., 2018). Together, this work highlights that adolescents’ social-affective sensitivities may place them in a position of vulnerability for increased health-risk behaviors in the presence of their peers.
Although adolescents’ social reorientation involves moving towards their peers and becoming independent from parents (Nelson et al., 2005; Nelson et al., 2016), the family still plays a large role in shaping adolescents’ behaviors and attitudes (Tsai et al., 2013; Telzer et al., 2018; Van Ryzin et al., 2012). Indeed, the presence of mothers reduces adolescents’ risky behavior relative to being alone, and this is modulated by greater activation in the ventrolateral prefrontal cortex (vlPFC) during safe decisions, and decreased VS activation following risky decisions (Telzer et al., 2015). Buffering of risk-taking behaviors is specific to mothers, as unfamiliar adults relative to mothers do not seem to have this protective effect (Guassi Moreira and Telzer, 2018; but see Silva et al., 2016 for a decrease in risk taking in a group of one young adult and peers relative to a group of solely peers). Collectively, this work suggests that parental presence may serve as a buffer to dissuade adolescents from engaging in risky behaviors (Telzer et al., 2015), such that social-affective sensitivities may also constitute an opportunity for positive influences on development (Scriber and Guyer, 2017; Dahl et al., 2018; Telzer et al., 2018).
Understanding the different roles of peers and parents in adolescent risk taking is crucial to gain a better grasp on the opportunities and vulnerabilities associated with adolescent sensitivity to social contexts. The presence of peers and parents has the potential to impact risky behavior through their modulation of affective, cognitive control and social-cognitive processes in the brain. Indeed, the regions associated with these processes are intimately involved in risk-taking, particularly in a social context. For instance, reward-related regions such as the VS, OFC and ventromedial prefrontal cortex (vmPFC) are consistently activated across risk-taking and social influence tasks (Chein et al. 2011; Telzer et al., 2015; Welborn et al., 2015; Telzer et al., 2018), underscoring how social contexts can modulate the rewarding and salient nature of risk taking. Cognitive control-related regions, such as the vlPFC and dlPFC, are also implicated in the context of risk taking, serving as a neural brake to decrease risky choices, whereas the insula serves as a neural hub that integrates reward-related and cognitive processes, especially in the context of risky decision-making (Blakemore, 2008; Lamm and Singer, 2010; Smith et al., 2014a, 2014b). Social influence on attitudes and behaviors also instantiates mentalizing about other people’s social norms, values and expectations, which is facilitated by social-cognitive regions such as the TPJ and dorsomedial prefrontal cortex (dmPFC) (Somerville et al., 2013; Blakemore and Mills, 2014; Welborn et al., 2015; Van Hoorn et al., 2016).
These neural regions implicated in social influence are part of complex and dynamically interacting neural circuits (Casey, 2015), and functional coupling between affective, cognitive and social regions affects adolescent risk-taking behavior. Indeed, functional coupling between the VS and prefrontal cortex has been linked to a decrease in risky behavior (Qu et al., 2015), and can be modulated by the social context, such as parental presence (Telzer et al., 2015; Guassi Moreira and Telzer, 2017). There is also initial evidence for the involvement of functional connectivity between VS and TPJ after social exclusion by peers in a risky context (Peake et al., 2013). As such, we take a complementary approach, examining the neural correlates of parent and peer presence on affective, cognitive control and social brain regions, as well as functional coupling of these regions, to gain traction on adolescent risk-taking in social contexts.
Methods
Participants
The final sample for analyses included 56 early adolescents (Mage(s.d.) = 13.2(0.69) years, range 12.08–14.82 years, 26 females). An additional five participants were excluded due to excessive motion (n = 2; >2.0 mm inter-slice movement on ≥10% of slices) or technical difficulties in acquiring data for parent or peer runs (n = 3). Moreover, n = 13 additional participants were excluded because they did not have enough behavioral data (i.e. stop or go decisions) to model at the neural level, which is detailed in the task section below. The age range was based on previous work (Gardner and Steinberg, 2005) and recent evidence suggesting that across development, early adolescents (ages 12–14) are most susceptible to social influence, for better or for worse (Knoll et al., 2015; Van Hoorn et al., 2016). Participants were recruited via local schools, community flyers and Listservs. They were from diverse ethnic backgrounds, including White (n = 31, 55%), African American (n = 10, 18%), Asian (n = 4, 7%), mixed race (n = 9, 16%) and other (n = 2, 4%).
We screened participants to ensure they were free from neurological disorders, taking psychotropic medication, or any MRI contraindications. Based on parent report, a small subset of our sample had a lifetime history of a psychological disorder: attention deficit (hyperactivity) disorder (AD(H)D) n = 4 (7 %); anxiety n = 1 (2%); ADHD and anxiety n = 1 (2%); depression and anxiety n = 1 (2%)1. If participants were taking AD(H)D medication, they were asked to refrain from taking their medication for a 24 h period before their scan. Participants were accompanied to the scan by their primary caregiver, which included mostly biological mothers (73%), fathers (16%), or other legal guardians, such as adoptive parents or grandparent (11%). All participants and their legal guardians provided written consent and assent, and the Institutional Review Board of the University of Illinois at Champaign–Urbana approved all procedures.
Manipulation of social context: peers and parents
During a behavioral session, a week or more prior to the scan session, each participant’s photo was taken, and they completed a short bio indicating their grade, their favorite subject in school and what they liked to do for fun. When they arrived to the scan, they were shown a picture of an age-, race- and gender-matched peer and received the peer’s bio, who was ostensibly also completing the study and undergoing their own brain scan. The participant was told that the peer was currently completing a brain scan, and would be playing the same game [a computerized driving game; see task description YLG below] as the participant. After participants practiced two rounds of the YLG themselves, the researcher communicated via cell phone to the scan tech, who indicated the peer was ready to begin the task in the scanner. The participant was then given a notecard to read to the peer through the cell phone: ‘Hi, this is [PARTICIPANT’S NAME], and I’ll be watching you play this round’. The participant then saw a ‘live feed’ of the peer supposedly playing the YLG in the scanner, which was in fact a recording of a game set to be a representative teen’s behavior [choosing to go 50% of the time, which was based on a similar peer manipulation (Kahn et al., 2015) and the ‘average’ peer in prior work (Peake et al., 2013)].
During their scan, participants completed the YLG. In the Peer Presence condition, the participant was told that the same peer would be watching them play now; they essentially ‘switched roles’ with the peer. The experimenter then played an audio recording of the same script from this supposed peer, telling the participant they were watching them play this round. In the Parent Presence condition, the participant was told their parent would be entering the scan room to watch them play. The parent spoke through the intercom using the identical script as the peer, a method we have employed in prior studies to examine social influence on risk taking (Telzer et al., 2015; Guassi Moreira and Telzer, 2017; Guassi Moreira and Telzer, 2018). The social context conditions were counterbalanced across participants.
Risk-taking paradigm
The YLG (Op de Macks et al., 2018) is an adaption of the widely used Stoplight Task (Gardner and Steinberg, 2005; Chein et al., 2011;) that examines risk-taking at the behavioral and neural level. In the YLG, participants were asked to drive a virtual car from the driver’s point of view along a straight track, during which they encountered several intersections with yellow lights (Figure 1A). They were instructed that the goal of the game was to get through all of the intersections in the shortest amount of time. At each intersection, participants had to indicate by button press whether they wanted to accelerate and go through the yellow light (go decision) or brake before arriving at the intersection (stop decision). Deciding to accelerate through the intersection—a go decision—constitutes a risky decision, and could result either in a successful go associated with no delay (i.e. if there was no other car passing through the intersection), or a delay of 5 s in the event of a crash (i.e. if there was another car passing through the intersection). A successful go was shown on the screen with a blue tilde and a positive chiming sound (Figure 1B), whereas a crash was shown as a cracked car window, honking car and crash sound (Figure 1C). At the behavioral level, we assessed risk taking as the percentage of go decisions out of the total decisions made (i.e. stop and go were exact opposites).
Fig. 1.
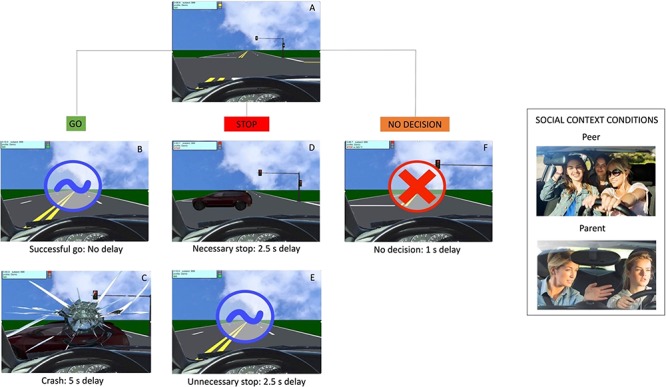
Illustration of the YLG and social context conditions.
Braking before the intersection—a stop decision—resulted in a 2.5 s delay and constitutes a safe decision because participants avoided a potential crash. After a stop decision, participants either saw an approaching car and heard a honking noise (i.e. necessary stop, because going would have resulted in a crash; Figure 1D), or an empty intersection (i.e. unnecessary stop, because going would have been successful; Figure 1E). At the behavioral level, safe decisions were defined as the percentage of stop decisions out of the total decisions made. Finally, if participants did not make a decision, this resulted in a 1 s delay, a red X on the screen and an error noise (Figure 1F).
Participants played the YLG in the MRI scanner while alone, in presence of a peer and in the presence of a parent. In the current paper, we focused on the Peer presence and Parental presence conditions, because comparisons with an alone condition have been described in previous work (peer > alone, Chein et al., 2011; parent > alone, Telzer et al., 2015), and the alone condition is part of a separate manuscript focused on individual differences (Rogers et al., 2018). Participants completed two runs for each social context condition, which were counterbalanced across participants. Within each run, participants encountered 20 intersections, totaling 40 trials for each social context condition. The onset of the yellow light was 1500 ms after the previous trial which corresponded to a varying distance on the track (200–250 ft) to avoid predictability. While the runs were varied in terms of probability of crashes for each intersection, the probability of crashing was kept at 50% for each run, and all participants completed the exact same runs in the scanner for consistency. Before entering the scanner, participants were trained on how to properly play the task by completing two practice runs in order to account for learning effects (Kahn et al., 2015). The practice runs were based on the same parameters as the scanner task, but unbeknownst to the participants, were slightly different in terms of the no-decisions. To dissuade participants from not responding, no-decisions were paired with a larger 5 s delay in the practice runs (the shorter 1 s delay in the scan runs was not explicitly told to participants).
We used stringent quality control criteria in order to model high-quality fMRI data with a sufficient number of trials. Participants were excluded from analyses if they had (a) more than four no-decision trials within one run and/or (b) fewer than four go or stop decisions within one run. More than four no-decisions were considered problematic because this suggested that the participant was disengaged from the task, generating both invalid behavioral and neural data. This cutoff was determined based on the total number of trials in a run (i.e. 20), such that participants were included only if they had least 75% of the trials with decisions in order to properly fit the fMRI model. Moreover, participants were excluded when they made fewer than four go or stop decisions in a peer or parent run, in order to effectively model risky and safe decisions for parent and peer conditions. Based on these criteria, our final sample excluded n = 3 adolescents for having more than four no-decision trials in a run, n = 7 for having fewer than four go or stop decisions in a peer or parent run and n = 3 for having both exclusion criteria. Of note, we also ran our analyses with a slightly less stringent threshold (i.e. including those with fewer than four go/stop decisions), using a strategy of reweighting the data where possible. Although results with this sample yielded nearly identical results, it also added more noise, such that the intrinsic smoothness of the residual file was significantly higher. Thus, to present the cleanest data, we used the conservative exclusion criteria presented above.
fMRI data acquisition
Data were collected with a 3-T Siemens Trio MRI scanner, using a 32-channel head coil. The task was presented on a computer screen, which participants could see through a mirror attached to the head coil. We obtained the functional data using T2*-weighted echoplanar images (EPI; slice thickness = 3 mm; 38 slices; TR = 2 sec; TE = 25 ms; matrix = 92 × 92; FOV = 230 mm; voxel size 2.5 × 2.5 × 3 mm3). In order to provide an anatomical reference, structural scans were also acquired, including a T2*-weighted, matched-bandwidth (MBW; TR = 4 s; TE = 64 ms; FOV = 230; matrix = 192 × 192; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (TR = 1.9 s; TE = 2.32 ms; FOV = 230; matrix = 256 × 256; sagittal acquisition plane; slice thickness = 0.9 mm; 192 slices). MBW and EPI scans were collected with an oblique axial orientation to prevent signal drop-out in orbital and temporal regions, thereby maximizing coverage of the brain.
fMRI data preprocessing and analysis
Standard preprocessing was conducted using the FSL FMRIBs Software Library (FSL v6.0, Oxford, UK; https://fsl.fmrib.ox.ac.uk/fsl/). We corrected for head motion using MCFLIRT (Jenkinson, 2002). Data were skull-stripped with BET (Smith, 2002), spatially smoothed with a 6 mm full width half maximum (FWHM) Gaussian kernel, and a high-pass temporal filtering with a 128 s cutoff was applied to remove low-frequency drift across time (Gaussian-weighted least squares straight line fitting; sigma = 64.0 s). Image co-registration was done using a three-step registration procedure (EPI to T2 to T1), and each functional image was resampled to 2 × 2 × 2 mm and warped to the standard Montreal Neurological Institute 2 mm brain using FLIRT (Jenkinson, 2001; 2002). Moreover, to remove artifact signals such as motion and physiological noise, we applied an independent component analysis (ICA) denoising procedure using MELODIC (Beckmann, 2004), combined with an automated signal classification toolbox (an average of 4.02 components or 11.33% were removed; classifier NP-threshold = 0.3; for more details see Tohka et al., 2008).
After preprocessing, statistical analyses were conducted on the individual subjects’ data using the general linear model in the Statistical Parametric Mapping software package (SPM8; Welcome Department of Cognitive Neurology, London, UK). Each trial was convolved with the canonical hemodynamic response function. The YLG was modeled as an event-related design. In the fixed-effect model, we included two decision regressors (go and stop decisions); four outcome regressors [successful go (i.e. no car approaching), crash, necessary stop (i.e. car approaching), and unnecessary stop (i.e. no car approaching)]; as well as no-decisions, which were modeled in a separate regressor together with volumes that contained excessive motion (>2.0 mm framewise displacement; participants included had motion on < 10% of total slices). These regressors were estimated separately for each social context condition: Alone, Peer presence, and Parental presence. The time when participants were driving on the course between the intersections was not explicitly modeled and therefore served as an implicit baseline, which included the driving time across all runs, and controlled for basic visual characteristics.
The decision phase (go and stop decisions) was modeled with the onset of the yellow light and duration of their decision (i.e. when participants made a button press to either go or stop). The outcome phase for go decisions was modeled from either the onset of blue tilde (successful go) or onset of the crash (unsuccessful go), each with a 2.5 s duration, in order to make the outcome equitable across conditions. The outcome phase for stop decisions was modeled from the onset of the stopped car and had a duration of 2.5 s for both necessary and unnecessary stops. Note that we included the four possible outcomes in our model, but do not report on the outcome phase in the current paper, because we were specifically interested in the effects of parent and peer presence on decision-making (i.e. stop and go decisions).
The resulting contrast images, computed at the individual level, were submitted to random-effects group analyses. In the current study, our contrasts of interest were go and stop decisions, under peer vs parental presence (i.e. go decisions peer > parent, stop decisions peer > parent, as well as the reverse contrasts). At the group level, we conducted analyses on our contrasts of interest using GLMFlex, which removes outliers and sudden activation changes in the brain, partitions error terms, analyzes all voxels containing data and corrects for variance-covariance inequality (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). In addition, we conducted psychophysiological interaction (PPI) analyses. Given the a priori hypotheses about the involvement of the VS (Chein et al., 2011; Telzer et al., 2015) in social context effects on decision-making, and the link between VS and cognitive control (Qu et al., 2015), insula (Guassi Moreira and Telzer, 2017) and social brain (Peake et al., 2013) regions, the bilateral VS was specified as our seed region of interest (ROI). We structurally defined the VS using the WFUpickatlas (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003).
The generalized PPI toolbox in SPM involves a three-step approach to conduct PPI analyses (gPPI; McLaren et al., 2012). First, deconvolved time-series were extracted from the VS ROI for each participant to create the physiological variables; then each trial type was convolved with the canonical hemodynamic response function (HRF) to create the psychological regressor and finally the psychological regressors were multiplied with the physiological variable to create the PPI interaction terms. As such, the PPI interaction terms constitute regions that covary with the VS during the contrasts of interest. At the individual level, we included the deconvolved blood oxygen level dependent (BOLD) signal as a regressor, together with the psychological and PPI interaction terms to create our gPPI model. Then, at the group level, we conducted random-effects, whole-brain analyses using GLMFlex to examine differences in functional connectivity across the Peer and Parent conditions, for stop and go decision separately.
We corrected all analyses for multiple comparisons using Monte Carlo simulations through 3DClustSim (updated version November 2016) in the software package AFNI (Ward, 2000), and accounted for the smoothness of the data with the acf function within the 3dFWHMx command. For the main effects of decision, the simulation resulted in a voxel-wise threshold of P < 0.005 and minimum cluster size of 150 (stop) and 159 (go) voxels for the whole brain, which corresponds to P < 0.05, family-wise error (FWE) cluster-corrected. Given our a priori hypotheses of activation in the VS, an anatomically small structure which typically does not survive stringent correction, we applied a small volume correction for the VS, with a voxel-wise threshold of P < 0.005, and minimum cluster size of k = 20 (Giuliani and Pfeifer, 2015; Guassi Moreira and Telzer, 2018). For the PPI analyses, the simulation resulted in a voxel-wise threshold of P < 0.005 and minimum cluster size of 115 (stop) and 116 (go) voxels for the whole-brain. All reported results are available on NeuroVault (Gorgolewski et al., 2015; see /collections/XPPOFEMU/).
Results
Behavioral analyses
To test the effects of parental and peer presence on risk-taking behavior, we compared the percentage of go decisions in the parent, peer and alone conditions. A one-way analysis of variance (ANOVA) indicated a significant effect of condition F(2,110 = 5.312, P = 0.006). To probe this effect, we conducted a series of paired-samples t-tests. We found no significant differences in the percentage of go decisions between parent and peer [t(55) = −0.437, P = 0.663; M(SD)peer = 47.66(12.04); M(SD)parent = 48.23(11.09)]. Replicating our prior work (Telzer et al., 2015), adolescents were less risky in the presence of their parent compared to alone [t(55) = 2.529, P = .014; M(SD)alone = 52.254(13.69)]. However, inconsistent with prior work (Chein et al., 2011) adolescents were less risky in the presence of their peer compared to alone (t(55) = 2.774, P = 0.008). Note that percentage of stop decisions is the exact opposite of go decisions and so are not reported. Next, we compared reaction times (s) for go and stop decisions between parent, peer and alone conditions. Results indicated no significant differences between the conditions (go: F(2,110) = 0.937, P = 0.395; stop: F(2,110) = 2.057, P = 0.133).
fMRI analyses
Differences in neural activation during peer presence compared to parent presence. We first examined the main effects for the peer > parent contrasts. For go decisions, we observed greater activity in the VS (Figure 2A), as well as regions involved in social cognition (precuneus, fusiform, left TPJ; for the latter, see Figure 2A), motor (supplementary motor area, postcentral gyrus) as well as visual processing (occipital cortex), and cerebellum when peers were present compared to when parents were present (see Table 1 for all regions). For descriptive purposes, we extracted parameter estimates of signal intensity from the VS and TPJ clusters, separately for peer go > baseline and parent go > baseline and plotted the activation (Figure 2A).
Fig. 2.
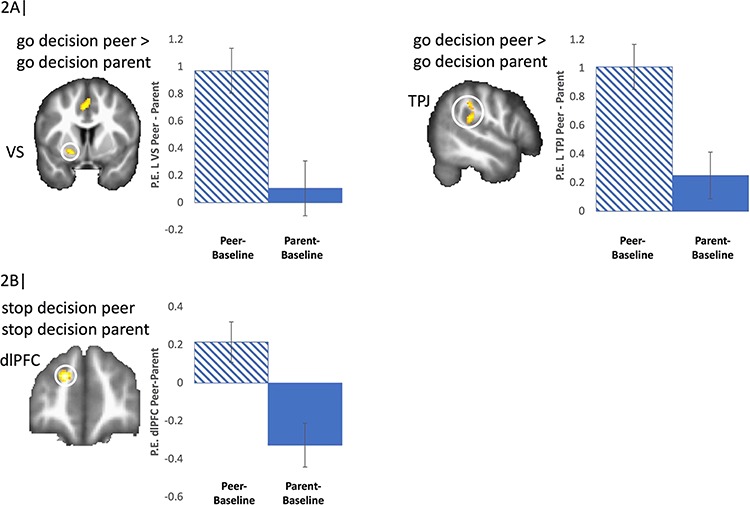
(A) VS and TPJ activity when adolescents took risks (go-decision) in presence of peers compared to their parents. (B) dlPFC activity when adolescents made safe decisions (stop-decision) in presence of peers compared to their parents. For descriptive purposes, parameter estimates of intensity were extracted from Peer presence > baseline and Parent presence > baseline. Error bars represent standard error of the mean (SEM).
Table 1.
Brain regions that showed positive activity for the main effects of peer presence > parental presence, separately for go and stop decisions at P < 0.005, FWE-cluster corrected
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Region | Volumea | t-value | x | y | z |
| Go decision peer > parent | |||||
| L VS | 87b | 3.554 | −14 | 22 | −8 |
| L precuneus | 1081 | 3.837 | 2 | −74 | 44 |
| L cerebellum | 176 | 3.823 | −30 | −72 | −20 |
| L TPJ | 164 | 3.811 | −50 | −34 | 46 |
| L STS | c | 3.289 | −54 | −34 | 24 |
| SMA | 168 | 3.778 | 2 | 12 | 64 |
| Stop decision peer > parent | |||||
| L dorsolateral PFC | 228 | 4.288 | −18 | 48 | 26 |
Abbreviations: L = left, R = right, MNI = Montreal Neurological Institute. aVolume of activation in mm3. T-value is at local maximum. Analyses for negative relationships (i.e. parent > peer) showed no significant clusters of activation. bSmall-volume correction P < 0.005, 20 voxels. cPart of left TPJ cluster.
When making stop-decisions in the presence of peers compared to parents, adolescents displayed greater activity in the dlPFC. For descriptive purposes, we extracted parameter estimates of signal intensity from the dlPFC cluster, separately for stop peer > baseline and stop parent > baseline and plotted the activation (Figure 2B). No regions were more active in the parent > peer contrasts for either go or stop decisions.
Differences in neural connectivity during parent presence compared to peer presence. Next, we conducted PPI analyses using the VS as the seed region, comparing neural coupling separately for go and stop decisions in the presence of parents compared to peers. For go decisions, we found a significant interaction between the VS and insula in the presence of their parent compared to their peer. To further examine this effect, we extracted parameter estimates of signal intensity from the insula cluster for each condition separately relative to baseline and plotted these effects.
As shown in Figure 3A, adolescents displayed greater connectivity between the VS and insula when making go decisions in the presence of their parent compared to their peer. In addition, we found greater coupling between the VS and visual regions in the presence of parents vs peers (Table 2).
Fig. 3.
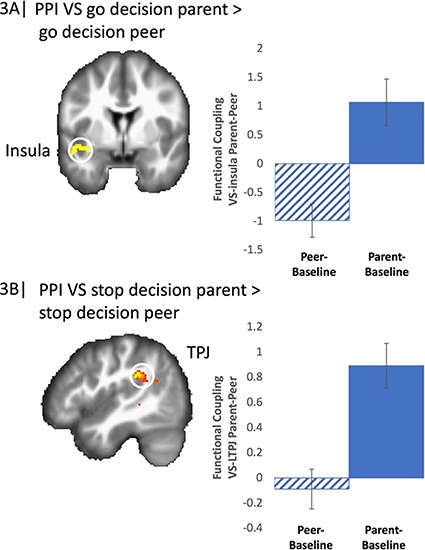
(A) VS-insula connectivity when adolescents took risks (go-decision) in presence of parents compared to their peers. (B) VS-TPJ connectivity when adolescents engaged in safe decision-making (stop-decision) in presence of parents compared to their peers. For descriptive purposes, parameter estimates of intensity were extracted from each condition compared to baseline. Error bars represent the SEM.
Table 2.
Brain regions that showed positive activity for the PPI analysis with VS as seed region and effects of parental presence > peer presence, separately for go and stop decisions at P < 0.005, FWE-cluster corrected
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Region | Volumea | t-value | x | y | z |
| PPI VS go decision parent > peer | |||||
| L insula | 153 | 3.583 | −50 | 10 | −20 |
| Occipital lobe | 478 | 3.932 | −16 | −82 | 4 |
| PPI VS stop decision parent > peer | |||||
| L STS | 255 | 5.841 | −54 | −36 | 2 |
| L TPJ | 164 | 4.552 | −46 | −40 | 30 |
| L precuneus | 320 | 3.860 | −8 | −64 | 48 |
| L precentral gyrus | 160 | 3.883 | −30 | −28 | 64 |
| R TPJ/STS | 361 | 3.878 | 70 | −26 | 4 |
Abbreviations: MNI=Montreal Neurological Institute, L = left, R = right. T-value is at local maximum. aVolume of activation in mm3. Analyses for negative relationships (i.e. peer > parent) showed no significant clusters of activation.
For safe decisions (i.e. when adolescents chose to stop), the PPI results yielded a significant interaction between the VS and bilateral TPJ in the presence of their parent compared to their peer. To further examine this effect, we extracted parameter estimates of functional connectivity from the left TPJ cluster for each condition separately relative to baseline and plotted these effects. As shown in Figure 3B, adolescents displayed greater connectivity between the VS and TPJ when making safe decisions in the presence of their parent compared to their peer. In addition, we found greater coupling between the VS and bilateral STS as well as precuneus in the presence of parents vs peers (Table 2). No regions were functionally connected to the VS in the peer > parent contrasts for either go or stop decisions.
Discussion
This study aimed to gain traction on the different roles of two key social influences on risk taking during adolescence: peers and parents. A sample of 12–15-year-old adolescents completed the YLG, a computerized risky driving task, while their performance was observed by their peers and parents, which for the first time allowed us to directly compare effects of peer and parental presence on risk taking within the same group of adolescents. Unexpectedly, behavioral findings revealed no effects of social context on risk taking. At the neural level, during risky decisions, adolescents displayed greater activity in the VS and TPJ with a peer present compared to their parent, as well as greater activity in the dlPFC during safe decisions when peers relative to parents were present. Analyses of functional coupling showed greater connectivity during parental presence compared to peer presence for both risky and safe decisions—greater VS-insula coupling during risky decisions and greater VS-TPJ coupling during safe decisions. Together, these findings suggest that peer presence elicits greater activity of an interplay of affective, social and cognitive control regions, while parental presence elicits greater functional coupling between affective, social and cognitive control regions.
Many decisions in adolescents’ everyday lives take place in a social context, either with social others intimately involved or with their perspectives in mind. Empirical work shows the robust effects of social context on adolescent decision-making (Van Hoorn et al., 2017; Blakemore, 2018). Yet, it is currently less well understood how different social sources may uniquely impact behavior and the underlying neural substrates which guide that behavior. Status and acceptance in the peer group become crucially important during adolescence, which may be one factor involved in the adolescents’ pursuit of riskier behavior in the presence of peers (Prinstein and Wang, 2005; Somerville, 2013), especially with the popular notion (i.e. perceived social norm) that fellow teenagers show high levels of risk-taking (Van Hoorn et al., 2016; Powers et al., 2018). Alternatively, it has been suggested that adolescents conform to their peers to avoid social risks and maintain their acceptance in the peer group (Blakemore, 2018), an effect corroborated by experimental fMRI research (Peake et al., 2013; Falk et al., 2014; Telzer et al., 2018). Across these explanations, processes of social learning and conformity to perceived social norms from the social context play a key role in the effects of peers (i.e. perceived endorsement of risk- taking, leading to greater risks), as well as parents (i.e. perceived endorsement of safe behavior, leading to fewer risks) (Telzer et al., 2018).
Despite the wealth of studies showing separate peer and parent effects on risk-taking behaviors, the current study yielded no such effects at the behavioral level. While one could argue that a computerized driving task may be more appropriate for older adolescent samples (i.e. legal driving age > 16 in the USA and > 18 in Europe), previous work has successfully found social influence effects using the Stoplight task in similar age ranges (Gardner and Steinberg, 2005: ages 13–16 years; Telzer et al., 2015: ages 14 years). Moreover, the age range appears appropriate in light of recent work suggesting greater sensitivity to peers in early adolescence (Knoll et al., 2015; Van Hoorn et al., 2016), a time when parental scaffolding is also more prominent than in mid- or late adolescence (Scriber and Guyer, 2017). The social context manipulations employed in the current study were based off of previous work (‘average peer’ video from Peake et al., 2013; parent protocol from Telzer et al., 2015, Guassi Moreira and Telzer, 2018), and entailed an active observation of performance (Somervilleet al., in press). While we replicated behavioral effects comparing risk taking when alone compared to a parent present (Telzer et al., 2015), the effects for the peer condition are inconsistent with prior research (Chein et al., 2011). We speculate that the lower level of risk taking in the presence of the peer may be related to the video manipulation we used. When adolescents supposedly watched their peer play, they saw them taking about 50% risk. This video manipulation may have set the norm for task behavior, which may have resulted in a social conformity effect (i.e. incorporating the norm set by the peer into the adolescents’ own behavior). Because we did not also have an observation of parent behavior, this social conformity may have only occurred for the peer condition. The absence of significant behavioral differences between parent and peers has interesting implications for future research, suggesting that peer and parent effects may be dependent on the modes through which social influence occur. Showing a video of a peer’s behavior may have different consequences for risk-taking behavior than having peers just present. In conjunction with our neural findings, which do show differences between the two social contexts, we speculate that the same behavior is a result of different processes, which, when taking place in the real world, may affect decision-making in the ways we expect—greater risk taking with peers and more safe decisions with parents.
At the neural level, risky decisions in the presence of peers relative to parents elicited greater activity in the VS, a region previously associated with processing of reward motivation and salience (Delgado, 2007; Telzer, 2016; Schreuders et al., 2018). Previous work shows that when adolescents take risks, the VS response is amplified in the presence of peers relative to alone as well as following social rejection, suggesting an important role in perceived social status and expected value of risk behaviors in a peer context (Chein et al., 2011; Peake et al., 2013; Telzer et al., 2018). Our findings provide additional evidence for the hypothesis that risk-taking in the presence of peers is potentially related to greater reward motivation, an effect not found in the presence of parents.
In addition to the VS, we found increased activation in the fusiform, TPJ and precuneus, regions implicated in detection and processing of social cues (Nelson et al., 2005; Blakemore, 2008), with greater recruitment of these regions in the presence of peers relative to parents. The fusiform is part of the social-detection node (Nelson et al., 2005) and has a role in processing salient affective components of the social environment, particularly in appetitive social contexts (Perino et al., 2016). The TPJ is a key component of the so-called social brain, generally involved in perspective-taking and (social) attention processes (Mitchell et al., 2006; Van den Bos et al., 2011), and has been linked to greater risk-taking after social exclusion (Peake et al., 2013). Interestingly, in a neutral setting (i.e. art work attitudes), parent and peer influences rely on the same neural bases, including social brain regions such as the TPJ (Welborn et al., 2015), while our findings highlight that an interplay of affective and social brain regions are more active when taking risks with a peer present relative to a parent. This suggests that risky decisions may be processed as more socially salient events with the psychological presence of a peer (Shah, 2003), and perhaps this increased allocation of neural resources is related to a greater need for social reward and connection in the peer domain, relative to parents who are more likely to endorse (i.e. provide social reward for) safe behaviors.
During safe decisions, adolescents recruited the dlPFC to a greater extent in the presence of a peer than their parent. The dlPFC is a neural region commonly found in the context of safe decision-making (Peake et al., 2013), and is often associated with general cognitive control, as well as more specific suppression of affective responses (Aron et al., 2004; Ridderinkhof et al., 2004). The presence of peers may make a risky decision more attractive and salient, and as such, adolescents likely need to recruit more cognitive control resources to make a safe decision in front of their peers than in front of parents, in order to compute the same safe behavior.
Functional coupling between the VS and prefrontal and social regions also affects adolescent risk-taking (Guassi Moreira and Telzer, 2017; Peake et al., 2013; Qu et al., 2015). Adolescents displayed increased VS-insula coupling when they made a risky decision in the parent condition relative to the peer condition. The insula is generally seen as neural hub integrating affective and cognitive cues, and helps guide attention during goal-directed behavior (Menon and Uddin, 2010; Touroutoglou et al., 2012; Smith et al., 2014a, 2014b). Hence, it is not surprising that the insula is consistently implicated in adolescent decision-making in social contexts (Van Hoorn et al., 2017). Increased functional connectivity between the VS and insula has been linked to attenuated risk-taking (Van Duijvenvoorde et al., 2014), as well as increased risk taking in high-conflict relationships with parents (Guassi Moreira and Telzer, 2017). Linking this finding to the main effect, which showed a relatively low VS response when adolescents took risks while their parent was observing, we speculate when the insula comes online in conjunction with the VS, this potentially downregulates VS activation with parental presence. The insula may function as a relay center that indicates the level of activity of the VS to prefrontal cognitive control regions (Guassi Moreira and Telzer, 2017). Note that analyses of functional connectivity assess correlations, so future work is needed to detect the direction of this relationship, for example using techniques such as Group Iterative Multiple Model Estimation (GIMME) (Gates and Molenaar, 2012).
Finally, parent relative to peer presence elicited heightened VS-TPJ coupling during safe decisions, suggesting that within adolescents, those who engage the VS to a greater extent also engage the TPJ more. These results highlight the importance of examining crosstalk between regions implicated in reward and social cognition when examining social influence. Interestingly, Peake et al. (2013) report exploratory analyses of increased TPJ-VS coupling during risky decisions after social exclusion that did not reach the threshold of significance. Our findings build on this work, and suggest that adolescents engage in mentalizing to make safe decisions, and that this may elicit a reward response in the context of parental presence. As such, functional coupling between the VS and social regions such as the TPJ may result in adaptive or maladaptive outcomes depending on the context (Pfeifer et al., 2011; Telzer et al., 2015).
While the current study provides important insights into two salient sources of influence, the influence of each source may change with development (Scriber and Guyer, 2017). We used a relatively large sample of early adolescents, but did not include other age groups to test whether these effects are unique to adolescence. Potentially, as compared to adolescence, parental influences may be more potent than peer influences in childhood, and perhaps both sources decrease in saliency when individuals enter adulthood (Knoll et al., 2015). Future research should focus on gaining more traction on why and how questions of social influences and determining the boundaries between which social influences occur (Somerville et al., in press), for example comparing active (i.e. through feedback) and passive (i.e. observing performance) influences across different contexts and behaviors. In daily life, adolescents may also have to deal with the simultaneous (and potentially conflicting) influences from parents and peers, which no study today has addressed. Moreover, relationship quality with peers and parents, including aspects of conflict and support likely modulates these effects (Guassi Moreira and Telzer, 2018). Studying relationship quality will be an important direction for future research, and should include the comparison of an actual friend and parent, in order to account for potential effects of similarity and closeness.
In conclusion, we employed a sample of early adolescents and, for the first time, disentangled within-person neural mechanisms that differentiate risky and safe decision-making in peer and parent contexts. An interplay of affective, social and cognitive regions (VS, TPJ, dlPFC) was more activated during decision-making with peers. On the other hand, findings showed more crosstalk between affective and social regions (VS-insula, VS-TPJ) during decision-making with parents. Our results highlight the salient nature of peers during adolescence, and at the same time show the complex nature of social influence processes in peer and parent contexts, emphasizing the utility of combining analytic techniques assessing mean levels of activity and neural coupling. These findings contribute to an increased understanding of the opportunities and vulnerabilities associated with adolescent sensitivity to potent social contexts, and ultimately may provide ways to mitigate the increase in risk-taking and help adolescents navigate this phase of life safely and successfully.
Funding
This work was supported by grants from the National Institutes of Health (R01DA039923) and the National Science Foundation (SES1459719).
Acknowledgements
We greatly appreciate the assistance of the Biomedical Imaging Center at the University of Illinois as well as Elizabeth Lozano, Kathy Do, Michael Perino, Heather Ross, Lynda Lin, Paul Sharp and Tae-Ho Lee for assistance in collecting data. We thank Dr Jennifer Pfeifer and the Developmental Social Neuroscience Laboratory at the University of Oregon for creating the YLG and providing support on the task.
Footnotes
All analyses were also run excluding these participants, and results were nearly identical. Hence, we report the results including these n = 7 participants.
References
- Aron A.R., Robbins T.W., Poldrack R.A. (2004). Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences, 8, 170–7. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging, 23, 137–52. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9, 266–77. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. (2018). Avoiding social risk in adolescence. Current Directions in Psychological Science, 1–7. [Google Scholar]
- Blakemore S.J., Mills K.L. (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Van den Bos W., Dijk E., Westenberg M., Rombouts S.A., Crone E.A. (2011). Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychological Science, 22, 60–70. [DOI] [PubMed] [Google Scholar]
- Casey B.J. (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annual Review of Psychology, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14, F1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636. [DOI] [PubMed] [Google Scholar]
- Dahl R.E., Allen N.B., Wilbrecht L., Suleiman A.B. (2018). Importance of investing in adolescence from a developmental science perspective. Nature, 554, 441. [DOI] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Van Duijvenvoorde A.C., Op de Macks Z.A., Overgaauw S., Moor B.G., Dahl R.E., Crone E.A. (2014). A cross-sectional and longitudinal analysis of reward-related brain activation: effects of age, pubertal stage, and reward sensitivity. Brain and Cognition, 89, 3–14. [DOI] [PubMed] [Google Scholar]
- Van Duijvenvoorde A.C., Peters S., Braams B.R., Crone E.A. (2016). What motivates adolescents? Neural responses to rewards and their influence on adolescents’ risk taking, learning, and cognitive control. Neuroscience & Biobehavioral Reviews, 70, 135–47. [DOI] [PubMed] [Google Scholar]
- Falk E.B., Cascio C.N., O’Donnell M.B., et al. (2014). Neural responses to exclusion predict susceptibility to social influence. Journal of Adolescent Health, 54, S22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni A.J., Dapretto M., Galván A. (2018). Broadening the impact of developmental neuroscience on the study of adolescence. Journal of Research on Adolescence, 28, 150–3. [DOI] [PubMed] [Google Scholar]
- Gardner M., Steinberg L. (2005). Peer influence on risk taking, risk preference, and risky decision-making in adolescence and adulthood: an experimental study. Developmental Psychology, 41, 625–35. [DOI] [PubMed] [Google Scholar]
- Gates K.M., Molenaar P.C.M. (2012). Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. NeuroImage, 63, 310–9. [DOI] [PubMed] [Google Scholar]
- Giuliani N.R., Pfeifer J.H. (2015). Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage, 108, 173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., et al. (2015). NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Frontiers in Neuroinformatics, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Telzer E.H. (2017). Family conflict is associated with longitudinal changes in insular-striatal functional connectivity during adolescent risk taking under maternal influence. Developmental Science, e12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guassi Moreira J.F., Telzer E.H. (2018). Mother still knows best: maternal influence uniquely modulates adolescent reward sensitivity during risk taking. Developmental Science, 21, e12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoorn J., Crone E.A., Leijenhorst L. (2017). Hanging out with the right crowd: Peer influence on risk-taking behavior in adolescence. Journal of Research on Adolescence, 27, 189–200. [DOI] [PubMed] [Google Scholar]
- Van Hoorn J., Van Dijk E., Güroğlu B., Crone E.A. (2016). Neural correlates of prosocial peer influence on public goods game donations during adolescence. Social Cognitive and Affective Neuroscience, 11, 923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–41. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–56. [DOI] [PubMed] [Google Scholar]
- Kahn L.E., Peake S.J., Dishion T.J., Stormshak E.A., Pfeifer J.H. (2015). Learning to play it safe (or not): Stable and evolving neural responses during adolescent risky decision-making. Journal of Cognitive Neuroscience, 27, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll L.J., Magis-Weinberg L., Speekenbrink M., Blakemore S.J. (2015). Social influence on risk perception during adolescence. Psychological Science, 26, 583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam C.B., McHale S.M., Crouter A.C. (2014). Time with peers from middle childhood to late adolescence: developmental course and adjustment correlates. Child Development, 85, 1677–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Singer T. (2010). The role of anterior insular cortex in social emotions. Brain Structure and Function, 214, 579–91. [DOI] [PubMed] [Google Scholar]
- Logue S., Chein J., Gould T., Holliday E., Steinberg L. (2014). Adolescent mice, unlike adults, consume more alcohol in the presence of peers than alone. Developmental Science, 17, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke A.Y., Mak Y.W. (2013). Family process and peer influences on substance use by adolescents. International Journal of Environmental Research and Public Health, 10, 3868–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61, 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214, 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. (2006). Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron, 50, 655–63. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Jarcho J.M., Guyer A.E. (2016). Social re-orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, 17, 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. (2005). The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–74. [DOI] [PubMed] [Google Scholar]
- Op de Macks Z.A., Flannery J.E., Peake S.J., Flournoy J.C., Mobasser A., Alberti S.L., Pfeifer J.H. (2018). Novel insights from the Yellow Light Game: Safe and risky decisions differentially impact adolescent outcome-related brain function. Neuroimage. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton G.C., Sawyer S.M., Santelli J.S., et al. (2016). Our future: a Lancet commission on adolescent health and wellbeing. The Lancet, 387, 2423–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake S.J., Dishion T.J., Stormshak E.A., Moore W.E., Pfeifer J.H. (2013). Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. NeuroImage, 82, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino M.T., Miernicki M.E., Telzer E.H. (2016). Letting the good times roll: adolescence as a period of reduced inhibition to appetitive social cues. Social Cognitive and Affective Neuroscience, 11, 1762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J. H. (2015). Yellow Light Game. https://dsn.uoregon.edu/research/yellow-light-game/
- Pfeifer J.H., Masten C.L., Moore W.E. III, et al. (2011). Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69, 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers K.E., Yaffe G., Hartley C.A., Davidow J.Y., Kober H., Somerville L.H. (2018). Consequences for peers differentially bias computations about risk from adolescence to adulthood. Journal of Experimental Psychology: General, advanced online publication. [DOI] [PubMed] [Google Scholar]
- Prinstein M.J., Wang S.S. (2005). False consensus and adolescent peer contagion: Examining discrepancies between perceptions and actual reported levels of friends’ deviant and health risk behaviors. Journal of Abnormal Child Psychology, 33, 293–306. [DOI] [PubMed] [Google Scholar]
- Qu Y., Galvan A., Fuligini A.J., Lieberman M.D., Telzer E.H. (2015). Longitudinal changes in prefrontal cortex activation underlie declines in adolescent risk taking. Journal of Neuroscience, 35 (32), 11308–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Van Den Wildenberg W.P., Segalowitz S.J., Carter C.S. (2004). Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition, 56, 129–40. [DOI] [PubMed] [Google Scholar]
- Rogers C.R., McCormick E.M., van Hoorn J., Ivory S.L., Telzer E.H. (2018). Neural Correlates of Sibling Closeness and Association with Externalizing Behavior in Adolescence. Social Cognitive and Affective Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ryzin M.J., Fosco G.M., Dishion T.J. (2012). Family and peer predictors of substance use from early adolescence to early adulthood: an 11-year prospective analysis. Addictive Behaviors, 37, 1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuders E., Braams B.R., Blankenstein N.E., Peper J.S., Güroğlu B., Crone E.A. (2018). Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Development, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriber R.A., Guyer A.E. (2017). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J. (2003). The motivational looking glass: how significant others implicitly affect goal appraisals. Journal of Personality and Social Psychology, 85, 424. [DOI] [PubMed] [Google Scholar]
- Smith S.M. (2002). Fast robust automated brain extraction. Human brain mapping, 17, 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.R., Chein J., Steinberg L. (2014a). Peers increase adolescent risk taking even when the probabilities of negative outcomes are known. Developmental Psychology, 50, 1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K., Chein J., Steinberg L. (2016). Adolescents in peer groups make more prudent decisions when a slightly older adult is present. Psychological Science, 27(3), 322–30. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Rosenbaum G.M., Botdorf M.A., Steinberg L., Chein J.M. (2018). Peers influence adolescent reward processing, but not response inhibition. Cognitive, Affective, & Behavioral Neuroscience, 1–12. [DOI] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Chein J. (2014b). The role of the anterior insula in adolescent decision making. Developmental Neuroscience, 36, 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Strang N., Chein J. (2015). Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Developmental cognitive Neuroscience, 11, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H. (2013). The teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22, 121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Haddara N., Sasse S.F., Skwara A.C., Moran J.M., Figner B. (in press). Dissecting ‘peer presence’ and ‘decisions’ to deepen understanding of peer influence on adolescent risky choice. Child Development. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 8, 1554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga A.W., Poldrack R.A. (2008). Automatic independent component labeling for artifact removal in fMRI. NeuroImage, 39, 1227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touroutoglou A., Hollenbeck M., Dickerson B.C., Barrett L.F. (2012). Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. NeuroImage, 60, 1947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H. (2016). Dopaminergic reward sensitivity can promote adolescent health: a new perspective on the mechanism of ventral striatum activation. Developmental Cognitive Neuroscience, 17, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Hoorn J., Rogers C.R., Do K.T. (2018). Social influence on positive youth development: A developmental neuroscience perspective. Advances in Child Development and Behavior, 54, 215–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Ichien N.T., Qu Y. (2015). Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Social Cognitive and Affective Neuroscience, 10, 1383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Miernicki M.E., Rudolph K.D. (2018). Chronic peer victimization heightens neural sensitivity to risk taking. Development and Psychopathology, 30(1), 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai K.M., Telzer E.H., Fuligni A.J. (2013). Continuity and discontinuity in perceptions of family relationships from adolescence to young adulthood. Child Development, 84, 471–84. [DOI] [PubMed] [Google Scholar]
- Ward B.D. (2000). Simultaneous inference for fMRI data [WWW]. Available: https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf [27 February 2018, last accessed date].
- Weigard A., Chein J., Albert D., Smith A., Steinberg L. (2014). Effects of anonymous peer observation on adolescents’ preference for immediate rewards. Developmental Science, 17, 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welborn B.L., Lieberman M.D., Goldenberg D., Fuligni A.J., Galván A., Telzer E.H. (2015). Neural mechanisms of social influence in adolescence. Social Cognitive and Affective Neuroscience, 11, 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


