Abstract
Research on the human parental brain implicated brain networks involved in simulation, mentalization and emotion processing and indicated that stimuli of own parent–child interaction elicit greater integration among networks supporting attachment. Here, we examined children’s neural activation while viewing own parent–child interactions and asked whether similar networks activate when children are exposed to attachment stimuli. Sixty-five 11-year-old children underwent magnetoencephalography (MEG) while observing own vs unfamiliar mother-child interaction. Own mother–child interactions elicited a greater neural response across distributed brain areas including alpha suppression in posterior regions, theta enhancement in the fusiform gyrus and beta- and gamma-band oscillations across a wide cluster in the right temporal cortex, comprising the superior temporal sulcus/superior temporal gyrus and insula. Theta and gamma activations were associated with the degree of mother–child social synchrony in the home ecology. Findings from this exploratory study are the first to show activations in children that are similar to previous findings in parents and comparable associations between social synchrony and gamma oscillations in temporal regions. Results indicate that attachment stimuli elicit a strong neural response in children that spreads across a wide range of oscillations, underscoring the considerable neural resources allocated to this fundamental, survival-related cue.
Keywords: MEG, social synchrony, attachment, affiliative brain, mother–child, relationship
Introduction
Humans, like any altricial mammal born with immature brain, depend on the mother's attuned caregiving for the development of neural systems that enable participation in social life. To support the care of offspring, mammalian mothers activate a distinct subcortical neural network that underpins motherhood (Barrett and Fleming, 2011; Numan and Young, 2016), which in humans evolved to include multiple cortical regions implicated in empathy, theory of mind, embodied simulation and emotion regulation (Swain et al., 2014; Feldman, 2015). This global caregiving network provides the neural basis of attachment and enables humans to form close relationships, resonate with social partners and inhibit momentary needs toward long-term affiliative goals (Feldman, 2017). Infants' attachment-related neural systems develop in the context of the parent's caregiving networks and parenting behavior via processes of bio-behavioral synchrony; the coordination of biological and behavioral signals during social contact wherein the parent's mature systems externally regulate the infant's environment-dependent systems and tune them to social life (Feldman, 2015, 2017; Kundakovic and Champagne, 2015). Imaging studies exposing parents to their own infant cues or to own parent–infant interaction videos, as compared to unfamiliar infants, defined the specific areas comprising the parent's ‘affiliative brain’ and showed their links with sensitive and synchronous parenting (Feldman, 2015; Kim, 2016). Yet, little research applied this well-studied paradigm to children, exposing children to own mother–child interaction vs unfamiliar interaction. Animal studies indicate that mothers and offspring activate the same brain areas in affiliative contexts and the infant's social brain matures via the experience of well-adapted parenting behavior (Champagne and Meaney, 2001; Francis et al., 2001; Numan and Young, 2016). It is thus of interest to test whether human children show similar activations as their parents in response to attachment cues, thus charting the cross-generational transmission of human attachment.
In the current study, we targeted the neural basis of attachment in 11-year-old children using MEG. Our focus was on neural oscillations and we hypothesized that children, similar to adults, would show greater neural activations when exposed to own mother–child interaction compared to unfamiliar interaction in key nodes of the affiliative brain and that this response would be associated with the degree of mother–child social synchrony. Social synchrony is a key feature of the mother–child relationship beginning in the third month of life that carries a long-term impact on children's social, emotional, neuroendocrine and mental health outcomes across childhood and adolescence (for review, Feldman, 2012, 2016). Understanding how mother–child synchrony is related to children's affiliative brain may shed further light on the mechanisms by which human parenting reorganizes children's brain toward participation in meaningful social relationships.
Oscillations are a pervasive feature of neuronal activity that afford a unique perspective on complex brain functioning (Sedley et al., 2016). The maturation of neural oscillations indexes structural development and can provide novel insights on social brain development beyond anatomy, particularly on network interactions underlying key socio-cognitive functions (Donner and Siegel, 2011). Different oscillatory rhythms are thought to capture distinct neural functions. Theta rhythms play a role in the formatting and retrieval of episodic and spatial memory (Buzsáki, 2002) and are frequently examined in spontaneous brain activity tasks. Alpha oscillations are the predominant rhythms in humans during rest and are suppressed during the processing of novel information (Klimesch, 1999; Başar, 2012); hence, alpha is thought to index top-down attention processes (Klimesch, 1999) with greater suppression indexing greater allocation of processing resources to the cue (Haegens et al., 2014). Beta rhythms participate in active information processing and monitor precision (Donner and Siegel, 2011), while gamma defines a late-maturing rhythm (Uhlhaas et al., 2010) associated with higher-order cognition, visceromotor resonance and consciousness (Buzsáki and Wang, 2012; Schulz et al., 2015). Complex cognitive functions involve an orchestrated response of multiple rhythms across widely distributed brain areas (Segalowitz et al., 2010; Vertes and Bullmore, 2015; Levy et al., 2018). It is thus likely that attachment, a fundamental process serving a core survival function, activates a range of low- and high-frequency rhythms across multiple brain areas which integrate online to support the formation of human affiliative bonds.
Studies assessing oscillatory response to attachment-related cues in adults focused on beta and gamma rhythms in temporal areas as the neural markers of attachment. Kinreich et al. (2017) examined brain-to-brain synchrony during naturalistic social interactions, comparing long-term romantic couples and male–female stranger pairs and found higher inter-brain synchronization in gamma rhythms localized to temporal–parietal regions among couples. Brain-to-brain synchrony was anchored in moments of social synchrony; when partners coordinated their gaze and positive affective expression neural synchrony was higher, highlighting the link between social synchrony and brain synchrony in temporal regions. Infant faces elicited higher beta oscillations as compared to adult faces in the medial orbitofrontal cortex of non-parents (Kringelbach et al., 2008), and the authors interpreted the findings as pointing to the existence of an intrinsic neural system for attachment in humans. Mothers showed higher beta- and gamma-band activations to infant crying and laughing in temporal-parietal regions (Hernández-González et al., 2016), suggesting the involvement of beta- and gamma-band rhythms in the brain basis of attachment. Overall, these studies provide evidence for the involvement of multiple oscillatory rhythms across distributed areas in the processing of attachment.
Very little research focused on the neurobiology of attachment from the child's end. Arias and Pena (2016) found that infants exhibited higher beta- and gamma-band activity to infant-directed speech compared to singing and suggested that episodes of personal, species-typical behavior elicit beta and gamma oscillations in areas of the affiliative brain. A study assessing brain-to-brain synchronization between infants and mothers while viewing a video of mother singing showed that maternal singing had a direct causal effect on the infant's oscillatory patterns in the theta and alpha bands. Moreover, during a real-time interaction, infant signals strongly impacted the adult's oscillatory response, underscoring the effect of synchronous, mutually regulating interactions on the neural basis of attachment in caregiving adults (Leong et al., 2017). Only one MEG study, to our knowledge, exposed mothers and their 9-year-old children to own vs unfamiliar interactions and showed brain-to-brain synchronization of gamma oscillations between mother and child during the viewing of own interaction in the right superior temporal sulcus (rSTS), a hub integrating mirror and mentalizing properties. Mother–child neural synchrony was anchored in moments of gaze and affect synchrony, further attesting to the close links between behavioral coordination and gamma activity in temporal regions in the context of attachment (Levy et al., 2017).
In the current exploratory study, we attempted to look at the orchestration of oscillatory patterns as they coalesce to form the neural basis of attachment in 11-year-old children. We utilized the well-validated ecological paradigm used to assess the brain basis of attachment in parents, in which own parent–child interaction is videotaped in the home ecology and is used as imaging stimuli in comparison with matched unfamiliar interaction (Atzil et al., 2011, 2012; Abraham et al., 2014, 2017). To further support our findings, we also examined links between the degree of social synchrony during mother–child interaction observed in the home environment and the difference between brain activations to own vs unfamiliar stimuli. As this was an exploratory study, we examined differences in activity across a wide range of oscillations and in all brain areas, expecting primarily to find differences in activations to own vs unfamiliar mother-child interactions across multiple rhythms. Yet, there were several regions found in previous research to be involved in the brain basis of attachment in parents which guided our search for comparable activations in children. In particular, the right posterior STS was found to be sensitive to synchronous own mother–child interaction (Atzil et al., 2011; Levy et al., 2017) and romantic partner interaction (Kinreich et al., 2017) and studies in parents found higher activations to own compared with unfamiliar interactions in this region (Kim et al., 2010; Abraham et al., 2014). There were three additional regions of interest that were consistently found to show greater activations to own vs unfamiliar interaction in functional magnetic resonance imaging (fMRI) studies in adults (Feldman, 2015; Kim, 2016), the orbitofrontal cortex, the right insula and the right fusiform gyrus (rFG) (Noriuchi et al., 2008; Kim et al., 2010; Atzil et al., 2012; Abraham et al., 2014), and we thus examined whether these areas would show increased activations to attachment cues in children as well. Moreover, we expected that attachment, being a complex survival-related function, would elicit a multi-rhythmic response integrating the entire range from low- to high-frequency rhythms, including theta, alpha, beta and gamma across multiple nodes in the affiliative brain. Finally, consistent with the associations found between parent–child social synchrony and activations in the parent's affiliative brain (Atzil et al., 2011; Abraham et al., 2014; Abraham et al., 2016), we expected correlations between the degree of mother–child synchrony and activations in the child's affiliative brain. In line with electroencephalography/MEG studies showing links between behavioral and neural synchrony of mother and child as expressed in gamma rhythms in temporal areas (Kinreich et al., 2017; Levy et al., 2017), we expected social synchrony to show a parallel pattern and link with greater gamma oscillations in temporal areas, particularly the STS/STG.
Method and materials
Participants
Sixty-five children participated in the study, recruited from a longitudinal follow-up to participate in an MEG study at 11 years. All children were of typical development, healthy and lived in a two-parent family. Families were of middle-class socio-economic status; mothers’ mean education was 14.24 (s.d. = 2.54; range = 12–19), and fathers’ mean education was 13.42 (s.d. = 3.24; range = 12–19), with 34.15% of the children being firstborn. A home visit was conducted at 6 years in which parent–child interactions were videotaped. Mean child age at the home visit was 6.20 (s.d. = 1.33) years, and 61.97% were boys. At 11 years (mean age 11.25; s.d. = 1.13; range = 10.6–12.3 years) children underwent MEG study (60% boys) with own home videos used as stimuli. The study was approved by the institutional ethics review board, procedures were explained to the accompanying parent before sessions and all families signed informed consent. Families received a small gift for participation. Eight subjects were excluded from the final analysis because of exaggerated muscle artifact, movement or inability to complete session, yielding a final sample of 57 children with full MEG data.
Measures
Mother–child interaction
Ten minutes of mother–child free-play interaction were filmed at 6 years. In line with previous fMRI and MEG studies (Atzil et al., 2012; Abraham et al., 2014; Levy et al., 2017), 2-min segments from the own mother–child and of unfamiliar mother–child interactions with the least unrelated background noise were chosen for viewing in the MEG session. Order of movies was counterbalanced and a 2 s fixation point separated the movies. Movies were presented on a 17 inch screen located 60 cm in front of children using e-Prime software (Psychology Software Tools, Inc.).
Behavioral coding
Interactions were coded using a well-validated micro-coding of synchrony (Feldman and Eidelman, 2004, 2007; Granat et al., 2017) using a computerized system (Noldus, Wageningen, the Netherlands). The system enables 0.01 s precision, the closest to the temporal resolution of brain activity. Two non-verbal categories were coded for parent and child separately: affect—including positive, neutral, negative-angry and negative-withdrawn; and physical proximity—including approach, stable and withdraw. Inter-rater reliability, conducted for 15% of the interactions, averaged 98%, κ = 0.94. Social synchrony was calculated as the conditional probability indexing the total number of positive affect episode in mother and child when partners are in a non-withdrawn position, consistent with previous studies (Kinreich et al., 2017; Levy et al., 2017). Social synchrony scores varied from 0 to 14 with a mean score of 7.63, and an s.d. of 2.72. For the unfamiliar mother–child interaction, we chose an interaction between a healthy mother and a healthy child, both screened using a full psychiatric diagnosis for any psychiatric disorder, whose interaction contained high levels of social synchrony (z = 2.87, i.e 2.87 s.d. from the mean). This was selected to eliminate the effect of synchrony in light of research on the maternal brain indicating that interaction marked by high levels of interactive synchrony elicited response in the maternal brain when contrasted with interaction containing minimal synchrony (Atzil et al., 2011).
Data acquisition and analysis
MEG was recorded with a 248-channel magnetometer array (4-D Neuroimaging) in a magnetically shielded room with sampling rate of 1017 Hz and online 1–400 Hz bandpass filter in supine position. Reference coils located short distance away from the head were used to record environmental noise. Five coils were attached to the participant's scalp for recording of the head position. External noise (power-line, mechanical vibrations) and heartbeat artifacts were removed from the data as previously described (Tal and Abeles, 2013). Signal pre-processing was carried out using MATLAB and the FieldTrip toolbox (Oostenveld et al., 2011). Data were segmented into 1 s epochs with an overlap of 0.5 s (Levy et al., 2017). Segments containing muscle artifacts and power jumps were discarded by visual inspection, resulting in a total mean of 158.21 viable segments (s.d. = 27.86) per subject, with a mean of 156.01 segments in own (s.d. = 28.65) and 162.88 segments in other condition (s.d. = 24.95). There was no condition effect for the number of trials. The remaining trials were bandpass filtered in the 1–80 Hz range, and independent component analysis (ICA) was used to remove eye movement and blinks and other remaining artifacts from the data.
Data were source localized with Synthetic Aperture Magnetometry (SAM) beamformer (Robinson and Vrba, 1999) with a spatial resolution of 0.5 cm in four traditional frequency bands: theta (4–7), alpha (8–12), beta (13–29) and gamma (30–40 Hz) (Wang, 2010). Covariance estimates were calculated using the full raw data (without ICA correction) throughout the whole paradigm and were uniform for both conditions. SAM estimates were noise-normalized via division by the mean square of the active weights. Following, the spatial filter was applied on each trial of the segmented data and then averaged across trials. Finally, the data were presented as functional maps on a template MRI (Colin27) that was modified to fit each subject’s digitized head shape using SPM8 (Wellcome Department of Imaging Neuroscience, University College London, London, UK). These templates were then used to transform the beamformer functional images into a common Talairach space in order to allow group comparisons.
Statistical analysis
Source-level statistics were calculated using the Analysis of Functional NeuroImages (AFNI) toolbox (http://afni.nimh.nih.gov/afni). To estimate whether activation in the four oscillatory bands differed according to condition (own/other) a paired t-test was done using AFNI’s 3dttest++. To control for multiple comparisons we applied non-parametric permutation approach (Nichols and Holmes, 2002) in which the t-test was repeated 2000 times, with condition randomly assigned within subject at each permutation, calculating the maximal cluster size. The critical cluster size corresponded to the 100th maximal cluster size (5%) in the distribution and was 49 voxels for theta, 996 voxels for alpha, 561 voxels for beta and 881 voxels for gamma. Next, for each subject, the average for each cluster in each condition was used for computing Pearson correlations.
Results
Oscillatory neural response to social interactions
As a first step, we measured neural response in the various oscillatory rhythms to social interactions as compared to rest, to ascertain the direction of children's brain response to social interactions in these bands. Differences in theta (4–7 Hz) were localized to frontal, occipital and temporal areas, including the orbitofrontal gyrus; superior, middle and inferior temporal gyrus; the fusiform gyrus (FG); and visual areas, and consisted of higher power during interaction viewings compared to rest. Differences in alpha (8–12 Hz) were localized mainly to occipital–parietal areas, including primary and secondary visual areas, and the temporal–parietal junction and consisted of lower alpha power during interaction viewings compared to rest, indicating alpha suppression. Differences in beta (13–29 Hz) were localized mainly to occipital and parietal and precuneus right posterior temporal gyrus, insula and visual processing areas and consisted of higher power during interaction viewings than in rest. Differences in gamma (30–40 Hz) were localized to frontal, occipital and temporal areas, including the prefrontal gyrus; orbitofrontal gyrus; superior, middle and inferior temporal gyrus; insula; precuneus; sensorimotor areas; and visual areas, and consisted of higher activation during interaction viewings than in rest. These data, presented in Table 1, suggest that viewing social interactions elicits increased neural activity in the theta, beta and gamma oscillatory bands and a desynchronization in alpha band oscillations.
Table 1.
Coordinates and sizes of significant clusters in mother–child interactions compared to rest
| Side | X | Y | Z | Cluster size | P | |
|---|---|---|---|---|---|---|
| Theta: Occipital gyrus, posterior temporal gyruses, fusiform-gyrus and orbitofrontal gyrus | B | 60.5 | −9.5 | −21.5 | 2382 | <0.001 |
| Alpha:Occipital gyrus, precuneus, posterior temporal gyruses and sensorimotor areas | B | 22.5 | 92.5 | 2.5 | 6185 | <0.001 |
| Beta:Occipital gyrus, precuneus, posterior temporal gyruses and insula | B | 17.5 | 87.5 | −2.5 | 3216 | <0.001 |
| Beta:Occipital gyrus, sensorimotor areas, frontal temporal gyruses, insula, orbitofrontal gyrus and prefrontal cortex | B | −37.5 | 2.5 | −32.5 | 4673 | <0.001 |
Coordinates reflect the voxel of peak activity.
Oscillatory neural response to own vs unfamiliar mother–child interactions
Source-level analysis of own vs unfamiliar mother–child interaction revealed one significant cluster for each frequency band. Exact coordinates and sizes of clusters appear in Table 2. Differences between own and unfamiliar mother–child interactions in theta (4–7 Hz) rhythms were localized to the rFG with higher activations to own interaction (Figure 1). In the alpha band (8–12 Hz) lower activations to own interaction were localized to bilateral occipital areas. Higher activations in the beta (13–29 Hz) (Figure 2) and gamma (30–40 Hz) (Figure 3) bands were both localized to a wide cluster in the right temporal cortex, including the STS/STG and insula, consistent with findings for adults.
Table 2.
Coordinates and sizes of significant clusters in own mother–child interaction compared to an unfamiliar mother–child interaction
| Side | X | Y | Z | Cluster size | P | |
|---|---|---|---|---|---|---|
| Theta: FG | R | −12.5 | 32.5 | −52.5 | 94 | <0.01 |
| Alpha: Occipital gyrus | B | −12.5 | 62.5 | 67.5 | 1278 | <0.001 |
| Beta: STS insula | R | −52.5 | 7.5 | −17.5 | 604 | <0.001 |
| Gamma: Temporal gyrus Insula |
R | −62.5 | 12.5 | 2.5 | 944 | <0.001 |
Coordinates reflect the voxel of peak activity.
Fig. 1.
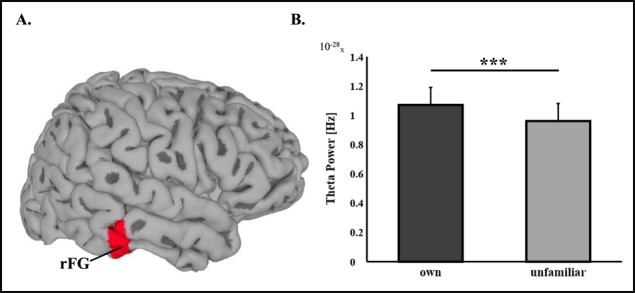
Theta oscillations source-level statistics, *** P < 0.001. (A) Regions of significant activations in the theta frequency band (4–7 Hz) frequency band. (B) Difference between theta power during viewing own mother–child interaction video compared to unfamiliar mother–child interaction video. rFG is located in a more internal part of the brain than can be visualized here.
Fig. 2.
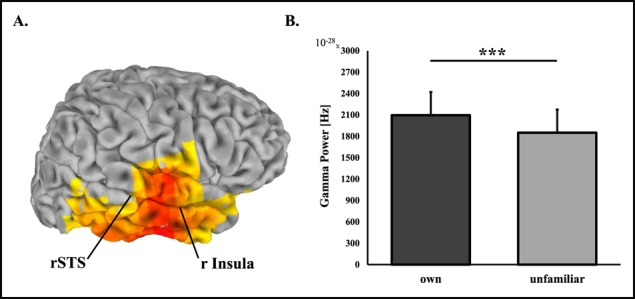
Beta oscillations source-level statistics, *** P < 0.001. (A) Regions of significant activations in the beta frequency band (13–29 Hz) frequency band. (B) Difference between beta power during viewing own mother–child interaction video compared to unfamiliar mother–child interaction video. The insula, approximately pointed to here, is located in a more internal part of the brain than can be visualized here.
Fig. 3.
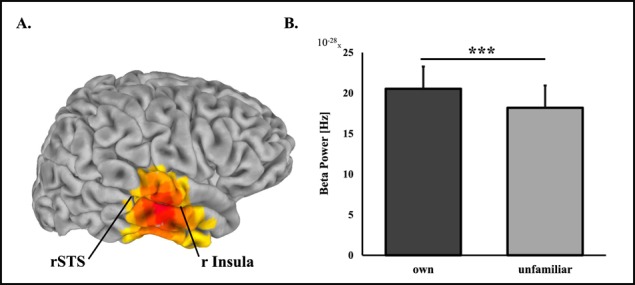
Gamma oscillations source-level statistics, *** P < 0.001. (A) Regions of significant activations in the gamma frequency band (30–40 Hz) frequency band. (B) Difference between gamma power during viewing own mother–child interaction video compared to unfamiliar mother–child interaction video. The insula, approximately pointed to here, is located in a more internal part of the brain than can be visualized here.
Associations of neural patterns with social synchrony
To examine the correlation between Social Synchrony and neural activity across the oscillatory bands, we conducted Pearson correlations between Social Synchrony and the mean power of activation to own mother–child interaction in each frequency band. Results are presented in Table 3 and indicate that the degree of Social Synchrony the child experienced with the mother was significantly related to the oscillatory power to own mother–child interaction in the theta and gamma frequency bands.
Table 3.
Correlation matrix between social synchrony and oscillatory activations to own mother–child interaction
| 2 | 3 | 4 | 5 | |
|---|---|---|---|---|
| Social synchrony | 0.35** | 0.09 | 0.08 | 0.29* |
| Theta | 0.14 | 0.31* | 0.12 | |
| Alpha | 0.06 | −0.13 | ||
| Beta | 0.34** | |||
| Gamma |
*P < 0.05, **P < 0.01
Discussion
Results of this exploratory study, the first to test children's oscillatory brain response to attachment cues, demonstrate that human children show a widely distributed response across multiple areas, which have previously been found to activate in adults' brain in response to attachment stimuli. Children's brain response integrated a large spectrum of frequencies from low-frequency theta to high-frequency gamma. A growing literature has described the global ‘human caregiving network’, the key areas in the adult brain activated in response to stimuli of the attachment target, whether children, romantic partners or close friends (Swain, 2014; Feldman, 2015, 2017; Kim, 2016). Our findings show comparability between adults and children and indicate that children activate the same areas when exposed to stimuli of the parent–child relationship. Consistent with findings in adults (Atzil et al., 2011; Abraham et al., 2014), we show that social synchrony, the online mutual adaptation of mother and child to each other's signals, linked with greater activations in a wide network in the right temporal and insular cortices, including the STS/STG, the FG and insula, areas implicated in multiple social functions, empathic resonance, mentalization and action understanding (Noriuchi et al., 2008; Strathearn et al., 2009; Atzil et al., 2011; Kim et al., 2011; Laurent and Ablow, 2012; Abraham et al., 2014). These findings accord with research in animal models (Champagne and Meaney, 2001; Francis et al., 2001; Numan and Young, 2016) which found activations in parallel areas in mother and offspring as mediated by well-adapted maternal behavior. Importantly, in animal models, these parallel activations were found to shape the neural basis of attachment (Francis et al., 2002; Numan and Young, 2016), highlighting the importance of our findings in humans. Using MEG, we tapped, for the first time, not only the brain areas which activate in children to attachment-related stimuli but also their oscillatory patterns. Neural oscillations across various frequency bands provide important insights into the brain basis of key socio-cognitive processes (Donner and Siegel, 2011; Sedley et al., 2016), and thus, our findings may shed light on how multiple oscillatory frequencies coalesce to support neural activations in attachment-related contexts in human children.
Attachment stimuli elicited greater response in the child's affiliative brain across four oscillatory bands, including the suppression of posterior alpha oscillations and the enhancement of right temporal theta, beta and gamma rhythms in regions implicated in visual-sensory processing, affect salience, embodiment, mentalization and interoceptive representations of one's bodily signals (Klimesch, 1999; Başar et al., 2001; Buzsáki, 2002; Uhlhaas et al., 2010). A multi-oscillatory pattern of activation, which combines alpha suppression with beta- and gamma-band enhancement, has been shown in a study on the development of empathy to index a mature brain response such that across the transition from childhood to adulthood the range of frequencies involved in the neural empathic response to pain increased from alpha oscillation in childhood, to alpha and beta band activity in adolescence and to an integration of alpha-, beta- and gamma-band activations across multiple brain areas (Levy et al., 2018). Moreover, gamma-band activation has been associated with brain maturation processes that range from childhood through adolescence to adulthood (Uhlhaas et al., 2010) and the transition from childhood to early adolescence is characterized by an increase in gamma oscillatory power (Uhlhaas et al., 2009). This change has been associated with other neuro-maturation processes that lead to a more specific and integrated neural response (Vertes and Bullmore, 2015). In adults, own-child stimuli has been shown to elicit a more integrated neural response as indexed by greater coherence in networks implicated in simulation and mentalization and greater synchronization among distinct brain areas (Atzil et al., 2013; Abraham et al., 2017). Processes that enable greater neural efficiency, such as pruning and myelination, which occur during the first years of life (Vertes and Bullmore, 2015), depend on the availability of well-adapted caregiving (Insel and Young, 2001; Feldman, 2016). While our results are insufficient to support the conclusion that neural maturation in late childhood/early adolescence is supported by the mother–child attachment, we did find higher theta- and gamma-band activations to interactions marked by greater synchrony. As patterns of mother–child interaction are individually stable from infancy to adolescence (Kochanska and Murray, 2000; Feldman, 2010) and mother–child synchrony longitudinally predicts a host of positive child outcomes, including emotion regulation, empathy, mental health and stress management (Feldman, 2007; Feldman, 2012), the increased neural activations in children experiencing greater synchrony may contribute over time to maturation of the child's brain. Yet this hypothesis is preliminary and requires much further research in longitudinal and experimental designs.
We found suppression of alpha power during the processing of own vs unfamiliar interaction. Alpha oscillations are the prominent rhythm in the awake, conscious brain and are suppressed during the processing of relevant information pending the degree of potential relevance the information carries (Başar, 2012). Alpha rhythms function as a mechanism of neural efficiency via the inhibition of stimuli that interfere with the task at hand (Klimesch et al., 2007), thus defining a top-down inhibitory process that regulates the focus of ongoing neural processing, specifically to novel and salient cues (Knyazev, 2007). As such, lower alpha power in posterior regions in response to own interaction may be an index of greater visual processing, supporting the evolutionary-based proposition that attachment stimuli carry increase saliency, particularly in primates (Dunbar, 2014; Rilling, 2014). Yet, as not only the attachment context of the own video could enhance saliency but also the child's self-recognition, future studies must expand these findings to examine their strength when controlling for more confounders.
Theta oscillations, a rhythm mostly implicated in memory and emotion regulation processes (Başar et al., 2001; Buzsáki, 2002; Knyazev, 2007), showed greater power in response to own interaction in the FG. The FG is a key structure subserving face and body recognition (McGugin et al., 2016) and has shown to be specifically active when processing personally familiar faces (Weibert et al., 2016), even when these are compared to famous faces (Ida Gobbini et al., 2004), thus highlighting the importance of the FG for attachment. Moreover, information that carries a special relevance to the individual activates the FG; for instance, car experts had stronger FG activations to cars and bird experts to birds (Gauthier et al., 2000), and thus, the FG appears to respond strongly to emotionally or motivationally salient images (Schultz, 2005). Taking into account the findings that show the involvement of theta rhythms in the processing of salient emotional cues and memory retrieval, it appears that the stronger theta activations to own interaction in FG may index the greater motivation and saliency attributed to the attachment context. Notably, studies of the maternal brain have similarly shown FG activations to infant stimuli, particularly to infant cry sounds (Kim et al., 2010; Laurent and Ablow, 2013), and this underscores the comparability in FG engagement between mother and child to mother–child interaction. Yet, since the FG may be sensitive to the viewing of familiar faces not only in the attachment-related cues, future research is required to disentangle the FG response to attachment from its response to self-recognition.
Beta- and gamma-band activations to own mother–child interactions were localized to a right temporal cluster including the STS and insula, areas of the mentalizing and empathy networks. These regions activate during emotion understanding and social decision-making and participate in the embodiment of others’ emotions (Gallese et al., 2004; Paulus et al., 2005; Domes et al., 2010). The STS receives projections from primary auditory and visual areas and integrates bottom-up processes of social perception and biological motion with top-down processes of mentalization (Mar, 2011). The insula participates in visceromotor processes and similarly combines higher-order socio-cognitive processes with lower-level sensory perception (Gallese et al., 2004). Previous studies on brain-to-brain synchrony within attachment relationships, including romantic couples (Kinreich et al., 2017) and mother–child dyads (Levy et al., 2017), found higher gamma activity in similar regions. Moreover, the amount of synchrony and positive affect within the dyadic interaction predicted the degree of brain coordination in these regions (Kinreich et al., 2017; Levy et al., 2017), highlighting the sensitivity of gamma rhythms in the rSTS to attachment cues. Beta rhythms are implicated in active information processing and monitor precision (Donner and Siegel, 2011) while gamma is associated with higher-order cognition, visceromotor resonance and consciousness (Buzsáki and Wang, 2012; Schulz et al., 2015). Thus, higher beta- and gamma-band oscillations in these brain areas may indicate the online mental processing of the self-mother interaction. Interestingly, in contrast to adults' response to attachment stimuli (Feldman, 2015; Hernández-González et al., 2016), children did not exhibit frontal activations to own interactions. Possibly the orbitofrontal cortex (OFC), observed in adults' brain response to infant stimuli (Kringelbach et al., 2008; Feldman, 2015), underpins emotion regulation capacities, a role that may be unique to parents as they modulate child behaviors to accommodate long-term parenting goals.
Predictions from patterns of dyadic synchrony were specific to attachment cues and we found that the oscillatory power to own interaction in the theta and gamma bands correlated with the degree of social synchrony in the mother–child interaction videos. Theta oscillations are thought to originate in limbic areas and are sensitive to emotional saliency (Başar et al., 2001; Knyazev, 2007). This may make theta rhythms specifically sensitive to attachment cues, as mother–child interaction patterns depend on ongoing detection and adjustment to novelty in the partner's behavior (Feldman, 2012; Feldman, 2015). This is consistent with a recent study which found that adults and infants showed brain-to-brain coordination in theta rhythms that were anchored in moments of direct gaze to the infant's face (Leong et al., 2017). Parent social gaze was found to trigger higher oxytocin release in both parents and their 4-month-old infants (Feldman, Gordon, & Zagoory-Sharon, 2010), indicating that parental gaze can activate the child's affiliative biology. Gamma is considered as a bottom-up mechanism linked with the intensity of perceived stimuli, and long-range gamma phase synchronization supports the large-scale integration of neural information (Uhlhaas et al., 2010; Buzsáki and Wang, 2012). Prior research found that gamma activity in the STS supports mother–child brain-to-brain coupling when viewing own interactions as anchored in episodes of gaze and affect synchrony (Levy et al., 2017). Furthermore, when mothers viewed interactions that are more synchronous, they showed greater activations in the STS and insula compared to interactions that contained lower levels of social synchrony (Atzil et al.,2014). Overall, these findings suggest that temporal theta- and gamma-rhythms may chart mechanisms that are specifically sensitive to social synchrony. Future research is needed to assess whether such increased activations to synchronized cues may over time shape the neurobiological substrate of attachment.
Our main study limitation involves the choice to present attachment stimuli collected in the natural home ecology, which, by nature, cannot be fully controlled. Studies in social and affective neuroscience must always oscillate between ecological validity and experimental control. Since the early studies of attachment by (Lorenz, 1935; Bowlby, 1958) it has been suggested that attachment processes must be researched and understood within the specific needs and constrains of the ecological niche, wherein the affiliative brain evolves in relation to specific contextual conditions and where patterns of caregiving are most clearly expressed. While we believe that to study attachment one must begin with research in the natural ecology, insights drawn from such research must be complemented by studies that exert greater empirical control. Moreover, as our study was inspired by research on the parent’s neural response to own vs unfamiliar interaction, we did not apply sufficient control to the effects of self-recognition and future studies are required to examine children's neural response to attachment cues under more controlled settings. We used a video collected in the home environment several years prior the study and memory processes could have also played a role in the neural activations. Additionally, as our social synchrony measurement was computed from the same interaction which the children viewed, we cannot determine whether it was a predictor of oscillatory power to own interaction or whether it elicited the greater power and thus, no causal relationships can be inferred and this question remains for future research. Our study was exploratory, and thus, much further research is required to understand the development of children's affiliative brain across developmental epochs, cultural contexts and various risk conditions in order to understand the neurobiological mechanisms supporting the cross-generational transmission of attachment from human parents to their children.
Funding
Study was supported by the National Association for Research on Schizophrenia and Affective Disorders independent investigator award to R.F., the Irving B. Harris Foundation and the Simms/Mann Foundation.
Financial Disclosure
Drs. Pratt, Goldstein, and Feldman have no conflict of interest to disclose.
References
- Abraham E., Hendler T., Shapira-Lichter I., et al. (2014). Father’s brain is sensitive to childcare experiences. Proceedings of the National Academy of Sciences of the United States of America, 111, 9792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E., Hendler T., Zagoory-Sharon O., et al. (2016). Network integrity of the parental brain in infancy supports the development of children’s social competencies. Social Cognitive and Affective Neuroscience, 11, 1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E., Raz G., Zagoory-Sharon O., et al. (2017). Empathy networks in the parental brain and their long-term effects on children’s stress reactivity and behavior adaptation. Neuropsychologia. [DOI] [PubMed] [Google Scholar]
- Arias D., Pena M. (2016). Mother–infant face-to-face interaction: the communicative value of infant-directed talking and singing. Psychopathology, 49, 217–27. [DOI] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2011). Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 36, 2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2013). The brain basis of social synchrony. Social cognitive and affective neuroscience, nst105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Feldman R. (2014). The brain basis of social synchrony. Social, Cognitive, and Affective Neuroscience, 9(8), 1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S., Hendler T., Zagoory-Sharon O., et al. (2012). Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 798–811. [DOI] [PubMed] [Google Scholar]
- Barrett J., Fleming A.S. (2011). Annual research review: all mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry, 52, 368–97. [DOI] [PubMed] [Google Scholar]
- Başar E. (2012). A review of alpha activity in integrative brain function: fundamental physiology, sensory coding, cognition and pathology. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 86, 1–24. [DOI] [PubMed] [Google Scholar]
- Başar E., Başar-Eroglu C., Karakaş S., et al. (2001). Gamma, alpha, delta, and theta oscillations govern cognitive processes. International Journal of Psychophysiology, 39, 241–8. [DOI] [PubMed] [Google Scholar]
- Bowlby J. (1958). The nature of the child's tie to his mother. International Journal of Psychoanalysis, 39, 350–73. [PubMed] [Google Scholar]
- Buzsáki G. (2002). Theta oscillations in the hippocampus. Neuron, 33, 325–40. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Wang X.-J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35, 203–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F., Meaney M.J. (2001). Like mother , like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Progress in Brain Research, 133, 287–302. [DOI] [PubMed] [Google Scholar]
- Domes G., Lischke A., Berger C., et al. (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology, 35, 83–93. [DOI] [PubMed] [Google Scholar]
- Donner T.H., Siegel M. (2011). A framework for local cortical oscillation patterns. Trends in Cognitive Sciences, 15, 191–9. [DOI] [PubMed] [Google Scholar]
- Dunbar R.I.M. (2014). The social brain: psychological underpinnings and implications for the structure of organizations. Current Directions in Psychological Science, 23, 109–14. [Google Scholar]
- Feldman R. (2007). Parent-infant synchrony and the construction of shared timing; Physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48, 329–54. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2010). The relational basis of adolescent adjustment: trajectories of mother--child interactive behaviors from infancy to adolescence shape adolescents’ adaptation. Attachment and Human Development, 12, 173–92. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2012). Biobehavioral synchrony: A model for integrating biological and microsocial behavioral processes in the study of parenting. Parenting; Science and Practice, 12, 154–64. [Google Scholar]
- Feldman R. (2015). The adaptive human parental brain: implications for children’s social growth. Trends in Neurosciences, 38, 387–99. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2016). The neurobiology of mammalian parenting and the biosocial context of human caregiving. Hormones and Behavior, 77, 3–17. [DOI] [PubMed] [Google Scholar]
- Feldman R. (2017). The neurobiology of human attachments. Trends in Cognitive Sciences, 21, 80–99. [DOI] [PubMed] [Google Scholar]
- Feldman R., Eidelman A.I. (2004). Parent-infant synchrony and the social-emotional development of triplets. Developmental Psychology, 40, 1133–47. [DOI] [PubMed] [Google Scholar]
- Feldman R., Eidelman A.I. (2007). Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Developmental Psychobiology. 49, 290–302. [DOI] [PubMed] [Google Scholar]
- Feldman R., Gordon I., Zagoory-Sharon O. (2010). The cross-generation transmission of Oxytocin in humans. Hormones and Behavior, 58, 696–76. [DOI] [PubMed] [Google Scholar]
- Francis D.D., Champagne F.C., Meaney M.J. (2001). Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. Journal of Neuroendocrinology, 12, 1145–8. [DOI] [PubMed] [Google Scholar]
- Francis D.D., Young L.J., Meaney M.J., et al. (2002). Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. Journal of neuroendocrinology, 14, 349–53. [DOI] [PubMed] [Google Scholar]
- Gallese V., Keysers C., Rizzolatti G. (2004). A unifying view of the basis of social cognition. Trends in Cognitive Sciences, 8, 396–403. [DOI] [PubMed] [Google Scholar]
- Gauthier I., Skudlarski P., Gore J.C., et al. (2000). Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience, 3, 191–7. [DOI] [PubMed] [Google Scholar]
- Granat A., Gadassi R., Gilboa-Schechtman E., et al. (2017). Maternal depression and anxiety, social synchrony, and infant regulation of negative and positive emotions. Emotion (Washington, D.C.), 17, 11–27. [DOI] [PubMed] [Google Scholar]
- Haegens S., Cousijn H., Wallis G., et al. (2014). Inter- and intra-individual variability in alpha peak frequency. NeuroImage, 92, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-González M., Hidalgo-Aguirre R.M., Guevara M.A., et al. (2016). Observing videos of a baby crying or smiling induces similar, but not identical, electroencephalographic responses in biological and adoptive mothers. Infant Behavior and Development, 42, 1–10. [DOI] [PubMed] [Google Scholar]
- Ida Gobbini M., Leibenluft E., Santiago N., et al. (2004). Social and emotional attachment in the neural representation of faces. NeuroImage, 22, 1628–35. [DOI] [PubMed] [Google Scholar]
- Insel T.R., Young L.J. (2001). The neurobiology of attachment. Nature Reviews. Neuroscience, 2, 129–36. [DOI] [PubMed] [Google Scholar]
- Kim P. (2016). Human maternal brain plasticity: adaptation to parenting. New Directions for Child and Adolescent Development, 2016, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Feldman R., Mayes L.C., et al. (2011). Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 52, 907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P., Leckman J.F., Mayes L.C., et al. (2010). Perceived quality of maternal care in childhood and structure and function of mothers’ brain. Developmental Science, 13, 662–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinreich S., Djalovski A., Kraus L., et al. (2017). Brain-to-brain synchrony during naturalistic social interactions. Scientific Reports, 7, 17060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews, 29, 169–95. [DOI] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. (2007). EEG alpha oscillations: the inhibition-timing hypothesis. Brain Research Reviews, 53, 63–88. [DOI] [PubMed] [Google Scholar]
- Knyazev G.G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience and Biobehavioral Reviews, 31, 377–95. [DOI] [PubMed] [Google Scholar]
- Kochanska G., Murray K.T. (2000). Mother–child mutually responsive orientation and conscience development: from toddler to early school age. Child Development, 71, 417–31. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Lehtonen A., Squire S., et al. (2008). A specific and rapid neural signature for parental instinct. PLoS ONE, 3, e1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M., Champagne F.A. (2015). Early-life experience, epigenetics, and the developing brain. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 40, 141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H.K., Ablow J.C. (2012). A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience, 7, 125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent H.K., Ablow J.C. (2013). A face a mother could love: depression-related maternal neural responses to infant emotion faces. Social Neuroscience, 8, 228–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong V., Byrne E., Clackson K., et al. (2017). Speaker gaze increases information coupling between infant and adult brains. Proceedings of the National Academy of Sciences, 114, 201702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Goldstein A., Feldman R. (2017). Perception of social synchrony induces mother-child gamma coupling in the social brain. Social Cognitive and Affective Neuroscience. 12, 1036–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J., Goldstein A., Pratt M., et al. (2018). Maturation of pain empathy from child to adult shifts from single to multiple neural rhythms to support interoceptive representations. Scientific Reports, 8, 1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K. (1935). Der Kumpan in der Umwelt des Vogels. J. für Ornithol, 83, 137–213. [Google Scholar]
- Mar R. a (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62, 103–34. [DOI] [PubMed] [Google Scholar]
- McGugin R.W., Van Gulick A.E., Gauthier I. (2016). Cortical thickness in fusiform face area predicts face and object recognition performance. Journal of Cognitive Neuroscience, 28, 282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping, 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M., Kikuchi Y., Senoo A. (2008). The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biological Psychiatry, 63, 415–23. [DOI] [PubMed] [Google Scholar]
- Numan M., Young L.J. (2016). Neural mechanisms of mother–infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior, 77, 98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., et al. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus M.P., Feinstein J.S., Leland D., et al. (2005). Superior temporal gyrus and insula provide response and outcome-dependent information during assessment and action selection in a decision-making situation. NeuroImage, 25, 607–15. [DOI] [PubMed] [Google Scholar]
- Rilling J.K. (2014). Comparative primate neuroimaging: insights into human brain evolution. Trends in Cognitive Sciences, 18, 46–55. [DOI] [PubMed] [Google Scholar]
- Robinson S.E., Vrba J. (1999). Recent advances in biomagnetism. In: Yoshimoto T., Kotani M., Kuriki S., et al., editors. Functional Neuroimaging by Synthetic Aperture Magnetometry (SAM), Sendai, Japan: Tohoku University Press. [Google Scholar]
- Schultz R.T. (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience, 23, 125–41. [DOI] [PubMed] [Google Scholar]
- Schulz E., May E.S., Postorino M., et al. (2015). Prefrontal gamma oscillations encode tonic pain in humans. Cerebral Cortex, 25, 4407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedley W., Gander P.E., Kumar S., et al. (2016). Neural signatures of perceptual inference. eLife, 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalowitz S.J., Santesso D.L., Jetha M.K. (2010). Electrophysiological changes during adolescence: a review. Brain and Cognition, 72, 86–100. [DOI] [PubMed] [Google Scholar]
- Strathearn L., Fonagy P., Amico J., et al. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 34, 2655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain J.E., Dayton C.J., Kim P., Tolman R.M., Volling B.L. (2014). Progress on the paternal brain: theory, animal models, human brain research, and mental health implications. Infant Mental Health Journal, 5, 394–408. [DOI] [PubMed] [Google Scholar]
- Swain J.E., Kim P., Spicer J., et al. (2014). Approaching the biology of human parental attachment: brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Research, 1580, 78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal I., Abeles M. (2013). Cleaning MEG artifacts using external cues. Journal of Neuroscience Methods, 217, 31–8. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Rodriguez E., et al. (2010). Neural synchrony and the development of cortical networks. Trends in Cognitive Sciences, 14, 72–80. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Roux F., Singer W., et al. (2009). The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proceedings of the National Academy of Sciences, 106, 9866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes P.E., Bullmore E.T. (2015). Annual research review: growth connectomics—the organization and reorganization of brain networks during normal and abnormal development. Journal of Child Psychology and Psychiatry and Allied Disciplines, 56, 299–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.-J. (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiological Reviews, 90, 1195–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibert K., Harris R.J., Mitchell A., et al. (2016). An image-invariant neural response to familiar faces in the human medial temporal lobe. Cortex, 84, 34–42. [DOI] [PubMed] [Google Scholar]


