Abstract
This meta-analysis (k = 48, N = 2196) examined the effect of transcranial direct current brain stimulation (tDCS) applied to the prefrontal cortex on a variety of social behaviors, including aggression, overeating, impulsivity, bias, honesty, and risk-taking. tDCS showed an overall significant effect on reducing undesirable behaviors, with an average effect size of d = −0.20. tDCS was most effective at reducing risk-taking behavior, bias, and overeating. tDCS did not affect aggression, impulsivity, or dishonesty. We examined moderators such as brain region of interest, online vs offline stimulation, within- vs between-subjects designs, dose, and duration, but none showed significant interactions. We also tested for potential publication bias using two different tools, which indicated signs of publication bias in the literature. After correcting for potential publication bias, the effect of tDCS was still significant, but the size was reduced (d = −0.10). These findings suggest the presence of tDCS studies with null findings outside of the published literature. Taken together, these results suggest that although tDCS can reduce undesirable behaviors, researchers should consider the types of behaviors they measure and use strategies to ensure sufficient power to detect a possible effect of tDCS on social behavior.
Keywords: transcranial direct current stimulation, meta-analysis, social psychology, brain stimulation, prefrontal cortex, social behavior
Introduction
Since the genesis of the field, social psychologists have shown an interest in understanding the neural underpinnings of social behavior. Functional magnetic resonance imaging (fMRI) seems an ideal method for studying how the brain affects social behavior because it offers a means of showing how interaction with social stimuli—interaction partners, sounds and images—affects brain function. But no method is perfect. fMRI has limitations, including expense and difficulty establishing causality. Although fMRI can help social psychologists peek into the brain, no neuroimaging magnet can manipulate neural activity.
Transcranial direct current brain stimulation (tDCS) offers a relatively new tool to understand the relationship between brain and behavior. Applying electricity to a targeted brain region can excite that area of the brain, modulating how people think and feel. Moreover, compared with fMRI, this method is inexpensive and safe (see Filmer et al., 2014 for review).
Despite the increase of social psychologists harnessing this tool in the past 10 years, no existing meta-analysis examines the effects of tDCS on multiple realms of social behavior. Hence, social psychologists may have difficulty understanding whether tDCS reliably affects all social behaviors, some social behaviors or none. This meta-analysis represents the first paper synthesizing the effect of tDCS on multiple areas of social behavior. We begin by briefly summarizing how tDCS works, followed by details regarding how we conducted our meta-analysis.
How tDCS works
tDCS works by applying a tiny electrical current to a targeted area of the brain. It can make neurons more likely to fire or less likely to fire depending on which polarity is used (Bruoni et al., 2012; Filmer et al., 2014). With anodal stimulation, the electrical current partially depolarizes these neurons, which brings them closer to an action potential. With cathodal stimulation, the electrical current partially hyperpolarizes these neurons, which means the energy needed for an action potential is reduced (Bruoni et al., 2012; Filmer et al., 2014). Although the currents used in tDCS are exceptionally small (Nistche, 2000; Fregni, 2005; Brunoni et al., 2012), they can modulate different emotions and behaviors including aggression, motivation and decision-making (Fregni et al., 2006; Brunoni et al., 2012).
During tDCS brain stimulation, two electrodes are applied to the brain (Nitsche et al., 2000; Filmer et al., 2014). These electrodes are quite large. For example, 5 cm by 7 cm is a common electrode size (Figure 1). The anode electrode is where the electricity goes into the brain. The cathode electrode is where the electricity comes out of the brain.
Fig. 1.

Visual representation of tDCS.
Table 1.
The overall effect of tDCS and the effect of tDCS on different dependent variables
| DV | k | N | d | Q | P |
|---|---|---|---|---|---|
| Aggression | 6 | 339 | −0.18 | 7.09 | 0.19 |
| Overeating | 7 | 326 | −0.29 (−0.15c) | 7.29 | 0.03* |
| Impulsivity | 9 | 667 | −0.04 | 11.97 | 0.70 |
| Bias | 7 | 447 | −0.25 | 6.88 | 0.02* |
| Honesty | 4 | 322 | −0.06 | 0.22 | 0.57 |
| Risk-taking | 13 | 676 | −0.36 (−0.35c) | 34.79 | 0.01* |
| Overall | 46 | 2196 | −0.20 (−0.10c) | 78.17 | < 0.001*** |
Note: Some of the numbers under d have numbers in parentheses next to them with a subscript of c. These numbers are corrected effect sizes from using the trim and fill method. * = P < 0.05, ** = P < 0.01, *** = P < 0.001; s.d.weighted = weighted standard deviation.
Either electrode can be applied to a targeted region to stimulate the brain; anode electrodes generally cause excitation in the neurons of the target area, while cathode electrodes are primarily thought to cause inhibition in the neurons of the target area (Bruoni et al., 2012; Filmer et al., 2014). However, in cognitive domains, cathodal stimulation sometimes causes excitation instead of inhibition (Jacobson et al., 2012). For this reason, this meta-analysis only includes studies using anodal stimulation.
The stimulating electrode is applied to the targeted region of the cortex. The reference electrode is typically placed in the contralateral subpraorbital area, just above the eyebrow, because of excess sinus cavity located there (Bruoni et al., 2012; Filmer et al., 2014). The current at the stimulating electrode is most concentrated in that region (Fregni et al., 2005; Keeser et al., 2011), but the targeted region is not the only stimulated area. Current often travels across the path between the two electrodes, expanding the affected region beyond the targeted area (Wagner et al., 2007; Bikson et al., 2010; Stagg and Nitsche, 2011). This meta-analysis consists of papers where the stimulating electrode was on the prefrontal cortex.
Role of PFC
The prefrontal cortex affects various social behaviors (Grafman, 1995; Anderson, 1999). Lesions to the prefrontal cortex in animals change social behavior (Kolb, 1974; Nonneman et al., 1974; De Bruin, 1983). More recently, fMRI work has shown that the activity in the prefrontal cortex changes during social tasks such as aggression (Eisenberger, 2003; Lotze et al., 2007; Chester et al., 2013), risk-taking (Kuhnen and Knutson, 2005; Rao, 2008; Chein et al., 2011), dishonesty (Ganis et al., 2003; Langleben, 2005; Abe et al., 2007) and implicit bias (Forie et al., 2014), among others. Thus, the prefrontal cortex is a key to social behavior.
The balance model of the brain lays a theoretical basis for how parts of the prefrontal cortex control different types of social behaviors such as aggression, impulsivity and more (Heatherton and Wagner, 2011). According to this theory, one role of the prefrontal cortex is to control urges for rewards that arise from more central parts of the brain including the nucleus accumbens (NAcc). The prefrontal cortex works in a circuit with reward centers deeper in the brain. When the prefrontal cortex is more active, we are better at controlling maladaptive social impulses that may arise from the NAcc and other associated reward-sensitive regions of the brain. However, when the prefrontal cortex is less active, the balance between the two parts of the brain can become off-kilter in favor of the reward areas (Heatherton and Wagner, 2011).
Because of the importance of the prefrontal cortex to social behavior, we narrowed the scope of this meta-analysis to just that region. Different sub-regions of the prefrontal cortex have different specializations (Hare et al., 2014; Davis, 2017; Vanuk et al., 2017). However, it is important to remember that wherever the current begins in the prefrontal cortex, it still travels across multiple regions in the prefrontal cortex. While we examined distinct brain regions of the prefrontal cortex as moderators in this meta-analysis, overall, due to the nature of how the stimulation travels, all of the studies included in this meta-analysis generally stimulate the prefrontal cortex.
Variability of parameters across studies
tDCS is growing in popularity among social psychologists. Three tDCS and social psychology articles were published in 2005; 22 tDCS and social psychology articles were published in 2010 and 73 tDCS and social psychology articles were published in 2015 (Boggio et al., 2016). Despite this increased interest, there is no standard operating procedure for how to conduct a tDCS study. We have included several parameters that researchers often vary as part of our statistical models. These parameters include brain region within the prefrontal cortex, dose, duration, whether stimulation was online or offline and whether the design was within or between subjects.
Materials and Methods
Eligibility criteria
Inclusion criteria and coding
We used three search engines for this meta-analysis: PsycINFO, PubMed, and Dissertations and Thesis Abstracts Global Proquest. These searches were conducted on July of 2017. Figure 2 shows a schematic flowchart of inclusion criteria (for more information, see Supplementary Materials). After undergoing extensive training by the first author, two coders extracted relevant information from the articles. Inter-rater reliability was 94%.
Fig. 2.
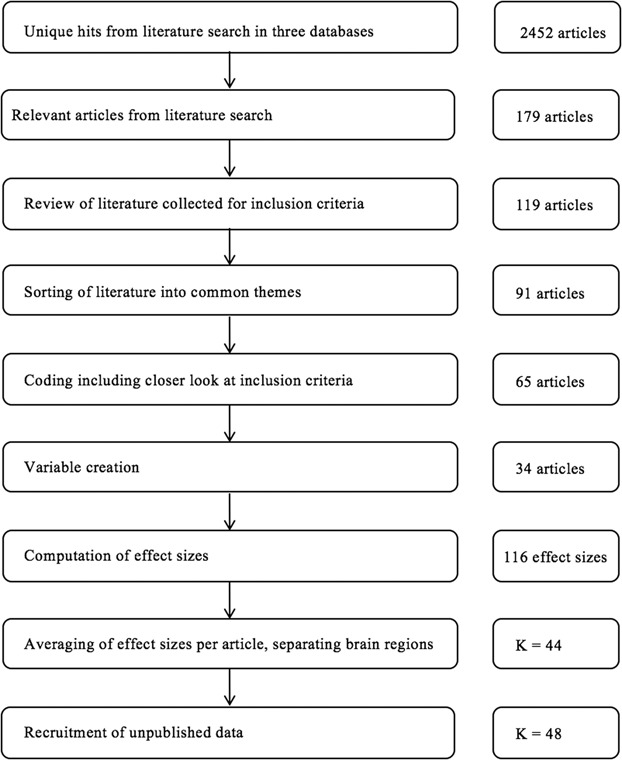
Flow chart of inclusion process for articles in analysis.
There were 116 relevant effects of tDCS on a dependent variable across 65 articles. Next, effect sizes from the same article were averaged (Lipsey and Wilson, 2001). There was one exception to this averaging. If the researchers tested more than one area of the prefrontal cortex, each region tested was treated as an independent study. For example, authors often test the effect of left vs right hemisphere stimulation to the same area of interest of the prefrontal cortex to test hypotheses related to asymmetry. Ten studies did this and were treated as two separate studies, which resulted in 75 effect sizes across 65 articles.
Determination of sub-areas of interest
Next, the coders sorted the 65 studies according to their dependent variables. Thirty-one articles were removed after this phase because there were not adequate studies (k < 4) to assess a reliable estimate of tDCS on that outcome. The variables they identified were aggression, overeating, impulsivity, bias, cooperation, rejection, honesty and risk-taking. The coders agreed on how to categorize 48 out of 65 of these articles, resulting in an inter-rater reliability of 74%. Coding discrepancies were resolved through discussion.
Once we narrowed the literature to 34 articles, we identified studies that examined two different regions of the prefrontal cortex and examined them separately. After this step, our total number of experiments included in the study totaled 44 (k = 44, N = 1664). Next, we contacted authors for unpublished data. There are four unpublished studies in this meta-analysis, resulting in a final number of k = 48, N = 2196.
The average sample size in this meta-analysis is N = 60, standard deviation (s.d.) = 50. Therefore, there was a large range of sample sizes, with some studies having low power. The smallest study included had 14 participants, and the largest had 224 participants.
Planned analyses
Overall effect sizes were computed using random effects models from the Lipsey & Wilson macro from their book Practical Meta-Analysis (2001). Moderation was tested with analog analysis of variances (ANOVAs) for the categorical moderators and with regressions on continuous moderators. Publication bias was assessed with both the trim and fill method (Duval and Tweedie, 2000; Duval, 2005) and the PET–PEESE method (Stanley, 2008; Stanley and Doucouliagos, 2014).
Results
Study characteristics
Results of individual studies
Figure 3 shows a forest plot of all of the studies included in this meta-analysis. The squares represent the effect sizes of each individual study included in the meta-analysis. The lines show 95% confidence intervals for each study included in the meta-analysis. The 95% confidence intervals for each study are also written on the side of the plot. This is a visual representation of the size of effects and variability in each study included. At the bottom, the overall effect size is shown visually. The supplemental materials of this paper include a table of all studies included.
Fig. 3.
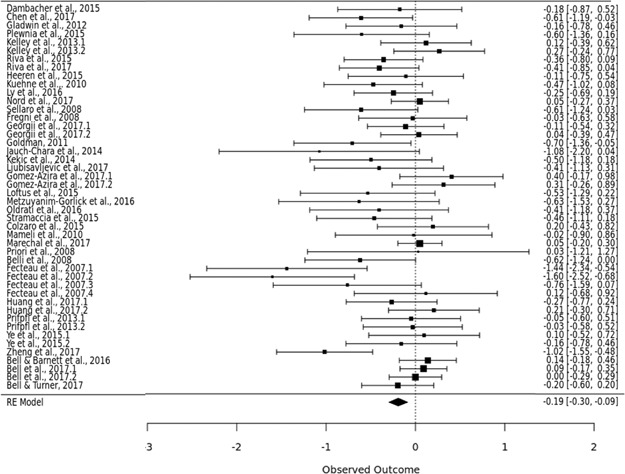
Forest plot showing effect sizes across studies.
Effect sizes and moderators in the overall model
The overall effect of tDCS
Overall, across domains, tDCS reduced maladaptive problems by a small but significant amount, d = −0.20, s.d.weighted = 0.45, P < 0.001. There was a significant heterogeneity in this model, Q(45) = 78.17, P = 0.002. This amount of heterogeneity is considered small (Higgins et al., 2003; Kovalchik, 2013).
Moderators
Analog ANOVAs were conducted on the three categorical moderators: (i) different brain regions, (ii) online/offline stimulation and (iii) experimental design. No significant difference was found among the five brain regions that were included in this meta-analysis, Q = 46.17, P = 0.42.
Online stimulation means the dependent variable was tested while the brain stimulator was still on, and offline stimulation means the dependent variable was tested 0–30 min after the brain stimulator was taken off. An analog ANOVA showed no significant difference between the two types of stimulation, Q = 52.22, P = 0.21.
The third analog ANOVA compared studies that used within-subjects designs and studies that used between-subjects designs. An analog ANOVA showed no significant difference between the two types of designs, Q = 48.22, P = 0.34.
Regressions were used to examine continuous moderators. The two moderators that were examined with regressions were dose of tDCS in milliamps and duration of tDCS in seconds. There was no significant effect of tDCS dose on behavior, B = 0.08, Q(45) = 48.29, P = 0.58. There was also no significant effect of tDCS duration on behavior, B = 0, Q(45) = 48.29, P = 0.39. Supplemental materials provide thorough tables about all five moderators.
The effect of tDCS in specific realms of social behavior
Aggression
tDCS did not reduce aggressive behavior by a significant amount, d = −0.18, s.d.weighted = 0.30, P = 0.19. There was not significant publication bias in this literature, t(4) = −0.17, P = 0.87.
Overeating
tDCS significantly reduced maladaptive overeating by a small amount, d = −0.29, s.d.weighted = 0.33, P = 0.03. There was significant publication bias in this literature, t(5) = −3.58, P = 0.02. Using the trim and fill method, the effect size of overeating was adjusted from d = −0.29 to d = −0.15.
Impulsivity
tDCS did not reduce impulsivity by a significant amount, d = −0.04, s.d.weighted = 0.27, P = 0.70. There was not significant publication bias in this literature, t(7) = −1.75, P = 0.12.
Bias
tDCS significantly reduced bias by a small amount, d = −0.25, s.d.weighted = 0.25, P = 0.02 (Supplementary Table S2). There was not significant publication bias in this literature, t(5) = −2.34, P = 0.07.
Honesty
tDCS did not reduce dishonest behavior by a significant amount, d = −0.06, s.d.weighted = 0.05, P = 0.57. There was not significant publication bias in this literature, t(2) = 0.22, P = 0.84.
Risk-taking
tDCS significantly reduced risk-taking by a small to medium amount, d = −0.36, s.d.weighted = 0.47, P = 0.01. There was significant publication bias in this literature, t(11) = −2.24, P = 0.046. Using the trim and fill method, the effect size of risk-taking was adjusted from d = −0.36 to d = −0.35.
The supplemental materials section of this paper provides tables about the effect of tDCS on the above realms of behavior.
Publication bias in the overall model
Trim and fill
Trim and fill uses weighted standard error to estimate how many studies may be missing from the meta-analysis because they were never published (Duval and Tweedie, 2000; Duval, 2005). It tests for publication bias and corrects for publication bias by providing a new estimate. The trim and fill statistical test we used was a non-parametric weighed regression with multiplicative dispersion. The predictor was standard error. The test for funnel asymmetry was significant, indicating probable publication bias, t(44) = −4.21, P < 0.001 (Figure 4).
Fig. 4.
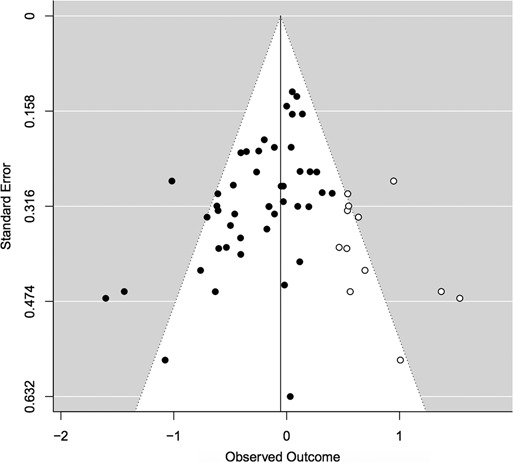
Funnel plot examining publication bias in the overall model. Model: weighted regression with multiplicative dispersion, predictor: standard error. Test for funnel asymmetry: t(44) = −4.21, P < 0.001, signifying likely publication bias.
The trim and fill test corrected the effect size of tDCS on overall social behavior. The effect size computed from the random effects model above was d = −0.20. The adjusted effect size from the trim and fill method was d = −0.10.
PET and PEESE
In PET–PEESE, standard errors or squared standard errors are used to predict effect sized with weighted least squares. This can be plotted as a scatterplot. The line of best fit on the scatterplot would be flat if there is no publication bias. A significant slope indicates publication bias (Stanley, 2008).
Both the PET and the PEESE tests suggested publication bias in this meta-analysis. For PET, B = −2.05, P = 0.01. For PEESE, B = −2.63, P = 0.002. The supplemental materials provide a visual representation of this bias.
Discussion
Summary of findings
This meta-analysis found that there was a reliable overall effect of tDCS on reducing maladaptive social behavior. tDCS did not affect all social behaviors equally. Although tDCS did not significantly reduce aggressive behavior, impulsivity or honesty, it did reduce maladaptive overeating and bias (see Table 1). The largest effect of tDCS was on reducing risk-taking behavior, showing a small-to-medium effect size. Two methods for detecting potential publication bias suggested the presence of unpublished null findings that exist outside of the published literature. Therefore, we urge researchers and clinicians to exercise caution in drawing conclusions from these results because of the potential for publication bias to influence the strength and reliability of tDCS on social behaviors.
tDCS affected risk-taking but not impulsivity, even though these constructs share conceptual similarity. At first glance, this provokes the question of whether one domain measured accuracy more often while the other domain measured reaction time more often as the outcome variable. However, all of the tasks used in this meta-analysis for impulsivity and risk-taking were accuracy measures. This difference may be occurring because the risk-taking tasks are producing more robust effects than impulsivity tasks, but other unknown factors also may be contributing to this difference.
The broader implication of these findings is that social psychologists will benefit from developing theoretical models to identify behaviors that will be most amenable to change through the use of tDCS. Although tDCS showed the greatest effectiveness in reducing risk-taking, the size of effect indicated that numerous factors could strengthen or weaken the size of that effect. Of particular importance, our results suggest that most tDCS studies do not have adequate power to detect a valid and reliable effect on social behavior. If social psychologists wish to continue using tDCS, they will benefit the field by developing strategies to ensure they have adequate power to test their hypotheses.
Our results cast doubt on the possibility that tDCS has sufficient specificity in order to enable researchers to meaningfully test the effects of stimulation on multiple areas of the prefrontal cortex. Although electrical current is the most concentrated in the target region, it travels across the entire prefrontal cortex. Thus, researchers may consider tDCS stimulation as relatively global across larger regions such as the prefrontal cortex. A newer device called high-definition tDCS provides more focality than traditional tDCS and is useful for researchers seeking to stimulate smaller regions of the brain (Edwards et al., 2013). With regard to focality, researchers can also use current dispersion models to help them chose appropriate electrode placements, especially when choosing the location of the reference electrode (Bikson et al., 2012).
Limitations
One limitation of this meta-analysis is lack of consistency in tDCS paradigms. There is no gold standard for how to conduct tDCS. A push for standard guidelines with tDCS is needed. While conferences and conceptual papers discuss a variety of best practice ideas, the field will benefit from a standard operating procedure on the parameters with which tDCS should be conducted.
A second limitation is that we were unable to examine the potential impact of several moderators because most authors do not report them. For example, few authors reported the amount, salinity and sterility of saline used. Because saline conducts the electricity, it is an important parameter to examine because it could change the magnitude of the stimulation. Another parameter often not reported is if the electrodes were held on the head by a headband and if so, the headband’s tightness. A tighter attachment of the electrode would increase the amount of electricity getting into the brain, which may influence the strength of the predicted effect. Additionally, exclusion criteria for participants vary, including whether researchers test people who take psychotropic medicine and if so, which ones. This is important because certain medications can increase the magnitude of tDCS, including selective serotonin reuptake inhibitors (SSRIs) (Nitsche et al., 2009; Brunoni et al., 2013; Kou et al., 2016) and amphetamines (Liebetanz et al., 2002; McLaren et al., 2018).
In addition, recent research indicates that tDCS may be most effective after multiple sessions (Mancuso et al., 2016). This paper did include studies that had multiple sessions, but only included results from session 1, to make studies across this meta-analysis more comparable. However, multiple sessions could be a key factor in the efficacy of tDCS.
Finally, site of the reference electrode varies quite a bit. This is perhaps the most pressing variation across these studies, as reference electrode placement is a key factor in where the current goes (Bikson et al., 2012). Supplementary Table S1 highlights this lack of standardization. More standardization in this emerging field could help make findings more appropriate to compare.
Concluding remarks
The results of this meta-analysis are promising. While there is potential for publication bias in this literature, we found that tDCS was effective at reducing maladaptive social behaviors. In addition, tDCS affected some social behaviors more than others. These differences can help guide future research.
Social psychologists have long been fascinated with the relationship between brain function and social behavior. tDCS offers a relatively inexpensive, easy-to-use tool to learn more. Brain stimulation has only become a popular tool to investigate social behavior in the past 10 years. We may be on the frontier of exciting discoveries about how neuromodulation can influence social behavior.
Supplementary Material
Acknowledgements
We would like to acknowledge the two coders, Tori Dennie and Lesley Phillips, for their contribution to this work.
References
- Abe N., Suzuki M., Mori E., Itoh M., Fujii T. (2007). Deceiving others: distinct neural responses of the prefrontal cortex and amygdala in simple fabrication and deception with social interactions. Journal of Cognitive Neuroscience, 19(2), 287–95. [DOI] [PubMed] [Google Scholar]
- Anderson S.W., Bechara A., Damasio H., Tranel D., Damasio A.R. (1999). Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience, 2(11), 1032–7. [DOI] [PubMed] [Google Scholar]
- Bikson M., Datta A., Rahman A., Scaturro J. (2010). Electrode montages for tDCS and weak transcranial electrical stimulation: role of ‘return’ electrode’s position and size. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 121(12), 1976–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M., Rahman A., Datta A. (2012). Computational models of transcranial direct current stimulation. Clinical EEG and Neuroscience, 43(3), 176–83. [DOI] [PubMed] [Google Scholar]
- Boggio P.S., Rêgo G.G., Marques L.M., Costa T.L. (2016). Social psychology and noninvasive electrical stimulation. European Psychologist, 21, 30–40 . [Google Scholar]
- Brunoni A.R., Ferrucci R., Bortolomasi M., et al. (2013). Interactions between transcranial direct current stimulation (tDCS) and pharmacological interventions in the major depressive episode: findings from a naturalistic study. European Psychiatry, 28(6), 356–61. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Nitsche M.A., Bolognini N., et al. (2012). Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimulation, 5(3), 175–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. (2011). Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Developmental Science, 14(2), F1–F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester D.S., Eisenberger N.I., Pond R.S. Jr., Richman S.B., Bushman B.J., DeWall C.N. (2013). The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Social Cognitive and Affective Neuroscience, 9(5), 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.W., Stanley M.L., Moscovitch M., Cabeza R. (2017). Resting-state networks do not determine cognitive function networks: a commentary on Campbell and Schacter (2016). Language, Cognition and Neuroscience, 32(6), 669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruin J.P., Van Oyen H.G., Van De Poll N. (1983). Behavioural changes following lesions of the orbital prefrontal cortex in male rats. Behavioural Brain Research, 10(2), 209–32. [DOI] [PubMed] [Google Scholar]
- Duval S. (2005). The trim and fill method In: Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments, John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England.
- Duval S., Tweedie R. (2000). Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics, 56(2), 455–63. [DOI] [PubMed] [Google Scholar]
- Edwards D., Cortes M., Datta A., Minhas P., Wassermann E.M., Bikson M. (2013). Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage, 74, 266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302(5643), 290–2. [DOI] [PubMed] [Google Scholar]
- Filmer H.L., Dux P.E., Mattingley J.B. (2014). Applications of transcranial direct current stimulation for understanding brain function. Trends in Neurosciences, 37(12), 742–53. [DOI] [PubMed] [Google Scholar]
- Fourie M.M., Thomas K.G., Amodio D.M., Warton C.M., Meintjes E.M. (2014). Neural correlates of experienced moral emotion: an fMRI investigation of emotion in response to prejudice feedback. Social Neuroscience, 9(2), 203–18. [DOI] [PubMed] [Google Scholar]
- Fregni F., Boggio P.S., Lima M.C., et al. (2006). A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain, 122(1), 197–209. [DOI] [PubMed] [Google Scholar]
- Fregni F., Boggio P.S., Nitsche M., et al. (2005). Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Experimental Brain Research, 166(1), 23–30. [DOI] [PubMed] [Google Scholar]
- Ganis G., Kosslyn S.M., Stose S., Thompson W.L., Yurgelun-Todd D.A. (2003). Neural correlates of different types of deception: an fMRI investigation. Cerebral Cortex, 13(8), 830–6. [DOI] [PubMed] [Google Scholar]
- Grafman J. (1995). Similarities and distinctions among current models of prefrontal cortical functions. Annals of the New York Academy of Sciences, 769(1), 337–68. [DOI] [PubMed] [Google Scholar]
- Hare T.A., Hakimi S., Rangel A. (2014). Activity in dlPFC and its effective connectivity to vmPFC are associated with temporal discounting. Frontiers in Neuroscience, 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Wagner D.D. (2011). Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences, 15(3), 132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. (2003). Measuring inconsistency in meta-analyses. BMJ: British Medical Journal, 327(7414), 557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L., Koslowsky M., Lavidor M. (2012). tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Experimental Brain Research, 216(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Keeser D., Meindl T., Bor J., et al. (2011). Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. Journal of Neuroscience, 31(43), 15284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B., Nonneman A.J. (1974). Frontolimbic lesions and social behavior in the rat. Physiology & Behavior, 13(5), 637–43. [DOI] [PubMed] [Google Scholar]
- Kovalchik S. (2013). Tutorial on meta-analysis in R. R useR, 204. [Google Scholar]
- Kuhnen C.M., Knutson B. (2005). The neural basis of financial risk taking. Neuron, 47(5), 763–70. [DOI] [PubMed] [Google Scholar]
- Langleben D.D., Loughead J.W., Bilker W.B., et al. (2005). Telling truth from lie in individual subjects with fast event-related fMRI. Human Brain Mapping, 26(4), 262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D., Nitsche M.A., Tergau F., Paulus W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain, 125, 2238–47. [DOI] [PubMed] [Google Scholar]
- Lipsey M.W. & Wilson D.B. (2001). Practical meta-analysis, Thousand Oaks, Calif: Sage Publications. [Google Scholar]
- Lotze M., Veit R., Anders S., Birbaumer N. (2007). Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: an interactive fMRI study. Neuroimage, 34(1), 470–8. [DOI] [PubMed] [Google Scholar]
- Mancuso L.E., Ilieva I.P., Hamilton R.H., Farah M.J. (2016). Does transcranial direct current stimulation improve healthy working memory?: a meta-analytic review. Journal of Cognitive Neuroscience, 28(8), 1063–89. [DOI] [PubMed] [Google Scholar]
- McLaren M.E., Nissim N.R., Woods A.J. (2018). The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation, 11(1), 52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M.A., Boggio P.S., Fregni F., Pascual-Leone A. (2009). Treatment of depression with transcranial direct current stimulation (tDCS): a review. Experimental Neurology, 219, 14–9. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Paulus W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. The Journal of Physiology, 527(3), 633–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonneman A.J., Voigt J., Kolb B.E. (1974). Comparisons of behavioral effects of hippocampal and prefrontal cortex lesions in the rat. Journal of Comparative and Physiological Psychology, 87(2), 249. [DOI] [PubMed] [Google Scholar]
- Rao H., Korczykowski M., Pluta J., Hoang A., Detre J.A. (2008). Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART). Neuroimage, 42(2), 902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C.J., Nitsche M.A. (2011). Physiological basis of transcranial direct current stimulation. The Neuroscientist, 17(1), 37–53. [DOI] [PubMed] [Google Scholar]
- Stanley T.D. (2008). Meta-regression methods for detecting and estimating empirical effects in the presence of publication selection Oxford Bulletin of Economics and statistics, 70(1), 103–127. [Google Scholar]
- Stanley T.D., Doucouliagos H. (2014). Meta-regression approximations to reduce publication selection bias. Research Synthesis Methods, 5(1), 60–78. [DOI] [PubMed] [Google Scholar]
- Vanuk J., Shane B., Bajaj S., Millan M., Killgore W.D. (2017). 538. Blue light therapy following a mild traumatic brain injury improves mPFC-amygdala functional connectivity and mood. Biological Psychiatry, 81(10), S218. [Google Scholar]
- Wagner T., Fregni F., Fecteau S., Grodzinsky A., Zahn M., Pascual-Leone A. (2007). Transcranial direct current stimulation: a computer-based human model study. Neuroimage, 35(3), 1113–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


