Abstract
Purpose of the Study
Interest in cognitive training for healthy older adults to reduce cognitive decline has grown considerably over the past few decades. Given the shift toward a more diverse society, the purpose of this review is to examine the extent of race/ethnic minority participation in cognitive training studies and characteristics of studies that included race/ethnic minority participants.
Design and Methods
This review considered peer-reviewed studies reporting cognitive training studies for cognitively healthy, community-dwelling older adults (age 55+) in the United States published in English before December 31, 2015. A total of 31 articles published between 1986 and 2015 meeting inclusion criteria were identified and included in the review.
Results
A total of 6,432 participants were recruited across all of the studies, and ranged in age from 55 to 99 years. Across all studies examined, 39% reported racial/ethnic background information. Only 3 of these studies included a substantial number of minorities (26.7% in the ACTIVE study; 28.4% in the SeniorWISE study; 22.7% in the TEAM study). Race/ethnic minority older adults were disproportionately underrepresented in cognitive training studies.
Implications
Further research should aim to enroll participants representative of various race/ethnic minority populations. Strategies for recruitment and retention of ethnic minority participants in cognitive training research are discussed, which could lead to the development of more culturally appropriate and perhaps more effective cognitive interventions.
Keywords: Cognition, Diversity and ethnicity, Intervention, Preventive medicine/care/services
The U.S. population will undergo a demographic shift in the coming decades. By 2050, the number of U.S. adults aged 65 and older is projected to reach 88.5 million, which is more than double the 40.2 million older adults captured in the 2010 U.S. Census (Vincent & Velkoff, 2010). Age is the greatest risk factor for cognitive decline (Hebert, Weuve, Scherr, & Evans, 2013), which is the clinical hallmark of Alzheimer’s disease and related dementias (ADRD). The Alzheimer’s Association estimated that there will be a 40% increase of persons with Alzheimer’s disease, from 5.3 million in 2015 to 7.1 million in 2025 (Alzheimer’s Association, 2015). Older individuals will be disproportionately affected, and this fast-approaching shift highlights the need for prevention and early intervention.
The older population in the United States is not only growing at a fast pace but is also increasingly ethnically and culturally diverse. Minority older adults are expected to comprise 42% of the total U.S. older adult population in 2050 compared to 20% in 2010, and the most notable projected increase will be a sixfold increase in the older Hispanic American population (Vincent & Velkoff, 2010). Older African Americans made up 9% of the total U.S. older population in 2014 and by 2060, that figure is projected to grow to 12% (Administration for Community Living, 2015). Following a similar upward trend, Asian Americans 65 years and older made up 4% of the total U.S. older population in 2014, and the percentage is projected to increase to 9% by 2060 (Administration for Community Living, 2014).
Although findings are not consistent, most studies have shown that certain groups of ethnic minority older adults are at greater risk of developing cognitive impairments or ADRD when compared to non-Hispanic Whites (Glymour & Manly, 2008; Yeo, 2006), including African Americans (Dilworth-Anderson et al., 2008; Gurland et al., 1999; Hendrie et al., 2001; Potter et al., 2009; Tang et al., 2001) and Hispanic/Latino Americans (Clark et al., 2005; Gurland et al., 1999; Haan et al., 2003). Much less is known about Asian Americans. To our knowledge, only two Asian American subgroups, Japanese Americans from Seattle, WA and Honolulu, HI (Graves et al., 1996; White et al., 1996) and Chinese Americans in the Greater Chicago area (Dong, Wong, & Simon, 2014), have been systematically included in epidemiologic aging studies.
Taken as a whole, these factors have led to an increased focus on methods for reducing cognitive decline as people get older. Several recent reports have enumerated risk factors that may be modified to reduce risk of cognitive decline among older persons (Baumgart et al., 2015; Glymour & Manly, 2008; Hughes & Ganguli, 2009; IOM [Institute of Medicine], 2015; Whalley, Dick, & McNeill, 2006).
In this context, cognitive training has gained considerable interest in the past decade in the research community, as evidenced by the rapid growth of the number of publications that contain terms such as “cognitive training,” “cognitive remediation,” or “cognitive rehabilitation” over the last 2 decades (Walton, Mowszowski, Lewis, & Naismith, 2014). This increased interest is also evidenced by a number of recently published reviews of cognitive training studies in healthy adults (defined most commonly as having no known cognitive impairments; Baumgart et al., 2015; Coyle, Traynor, & Solowij, 2015; Gross et al., 2012; Hertzog, Kramer, Wilson, & Lindenberger, 2008; Kelly et al., 2014; Kueider, Parisi, Gross, & Rebok, 2012; Lampit, Hallock, & Valenzuela, 2014; Martin, Clare, Altgassen, Cameron, & Zehnder, 2011; Moreau & Conway, 2014; Owen et al., 2010; Papp, Walsh, & Snyder, 2009; Rebok, Carlson, & Langbaum, 2007; Saczynski et al., 2012; Valenzuela & Sachdev, 2009; Williams, Plassman, Burke, Holsinger, & Benjamin, 2010).
Cognitive training refers to standardized, systematic training on cognitive tasks designed to improve cognitive functions (Coyle et al., 2015; Lampit et al., 2014). Most cognitive training has been carried out in small group settings by trained facilitators over a set number of sessions (Rebok et al., 2007; Rebok, Parisi, Gross, & Spira, 2010). An example is the ACTIVE (Advanced Cognitive Training for Independent and Vital Elderly) trial, the largest randomized controlled trial (N = 2,832 across six sites) to date that examined the efficacy and durability of cognitive training on cognitive abilities and everyday functioning among community-dwelling older adults who were free of cognitive impairment at baseline (Ball et al., 2002; Rebok et al., 2014; Willis et al., 2006).
Given that the cognitive training field has grown substantially over the years, the demographic shift toward a more diverse elderly population described above, and the fact that some ethnic minority populations bear a greater risk of developing ADRD (Dilworth-Anderson et al., 2008; Manly & Mayeux, 2004; Yeo, 2006), surprisingly little is known about the use and effectiveness of cognitive training among U.S. community-dwelling ethnic minority populations. This review summarizes past cognitive training research to address gaps in the current literature with regard to: (a) the extent to which cognitive training studies have included ethnic minority participants and changes in their inclusion over time and (b) characteristics of studies that included ethnic minority participants. Implications for future research, including methods to encourage more participation of older ethnic minority adults in cognitive training studies, are discussed.
Methods
Selection Criteria
To be eligible for inclusion in this review, studies were required to be randomized controlled trials of cognitive training interventions performed in the United States and reported results in peer-reviewed journals prior to December 31, 2015. Participants needed to be community-dwelling older adults with no known existing cognitive impairment and to be at least 55 years of age at the time of the training. Considering the variations in the race/ethnic makeup and demographic structures across countries, we excluded international studies to focus the discussion on trends in minority participation in the specific context of the pending demographic changes in the United States. Previously published reviews of cognitive training studies have applied different age cutoffs to their inclusion/exclusion criteria, for example, an age cutoff of 60 in Lampit and coworkers (2014), and an age cutoff of 55 in Kueider and coworkers (2012). Considering the recent trend toward broadening the age of eligibility as shown in several cognitive training studies (Anderson, White-Schwoch, Parbery-Clark, & Kraus, 2013; Chapman et al., 2015; McDaniel et al., 2014), we established the age cutoff at 55 for this review to capture a wider range of cognitive training studies that have been developed and implemented targeting older adults. Studies that did not screen for cognitive impairment were excluded because the design of cognitive training may differ substantially when targeted toward persons with cognitive impairment. All modalities of cognitive training (e.g., individual vs. group, in-person vs. computerized) were included. To ensure that each cognitive training study was only included once, we only included the primary outcome paper from each study in this review. Studies that included cognitive training conducted in other languages were considered but were required to be published in English due to resource constraints.
Databases that were searched included PubMed, PsychInfo, and ClinicalTrials.gov to identify past and ongoing randomized controlled trials conducted in the United States. To avoid risk of bias, two independent reviewers screened titles and abstracts and excluded articles not meeting selection criteria. The reviewers then conducted a full-text review after excluding ineligible abstracts and removing duplicate articles. Disagreements were discussed and resolved by an expert in cognitive training (G. W. Rebok). Efforts were made to contact lead authors in cases where key information was missing from the publications. The final list of included studies was approved by G. W. Rebok.
Search Strategy
Combinations of the following search terms were used: (a) cognitive intervention, cognitive training, brain training, memory training, mnemonic training, cognitive retraining, cognitive retraining, cognitive support, memory retraining, memory support, memory stimulation, memory strategy; (b) healthy elderly, elderly, older adults, old adults, cognitive ag(e)ing, normal, cognitively healthy, cognition, prevention, delay, cognitive decline, cognitive reserve, cognitive performance, cognitive function, cognitive decline, cognitive abilities. Filters were used to search for publications with age groups identified as 55 years and older and for clinical trials in PubMed. The search also included examining references from published review articles, book chapters, and Google Scholar to identify any missed articles. Cognitive training programs were divided into two categories: (a) single, domain-specific programs that targeted only memory, reasoning, processing speed, attention, or visuospatial skills and (b) multidomain programs that trained a combination of cognitive tasks and/or were conducted with other interventions such as physical activity.
Results
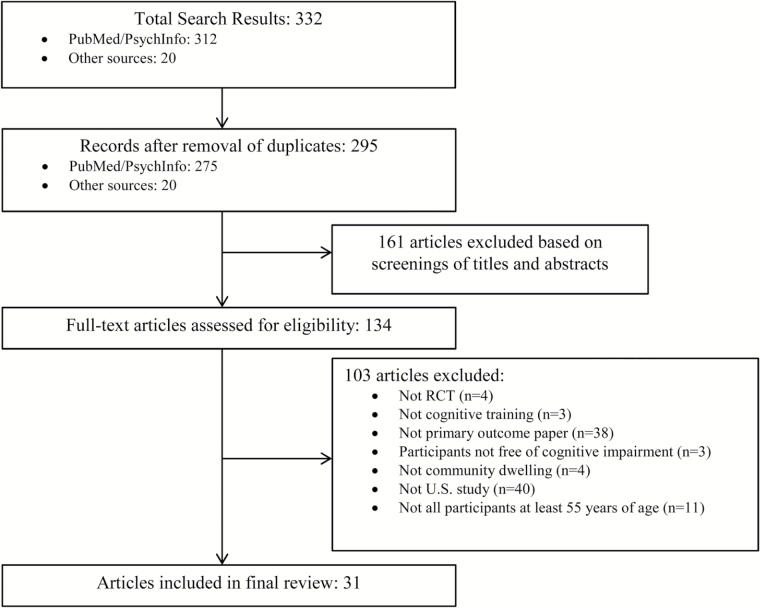
A total of 332 records were identified through the electronic database search and from other sources (222 from PubMed, 90 from PsychInfo, 20 from references in book chapters and review papers). In total, 295 duplicates were identified and removed. After review of titles and abstracts, 161 additional papers were excluded; 134 articles remained and underwent full-text review by two independent reviewers. After consulting with study authors and an expert (G. W. Rebok), 103 articles were excluded for the following reasons: (a) not meeting the randomization criteria (n = 4), (b) not cognitive training (n = 3), (c) not a primary outcome paper (n = 38), (d) study participants had some form of cognitive impairment (n = 3), (e) some or all of the participants were not living independently in the community (n = 4), (f) study was not conducted in the United States (n = 40), and (g) some or all of the participants were younger than 55 years at the time of the study (n = 11). Figure 1 illustrates the search and screening process. A total of 31 papers were included in the final review.
Figure 1.
Summary of study identification and selection.
Race/Ethnic Minority Participation in Included Studies and Changes in Participation Over Time
A total of 6,432 participants were recruited across all 31 studies examined in this review, and participant age ranged from 55 to 99 years. The included studies were published between 1986 and 2015. Figure 2 displays the number of cognitive training studies meeting review inclusion criteria over this interval. Only 1–2 cognitive training studies meeting inclusion criteria were published in each of the following 5-year periods: 1986–1990, 1991–1995, 1996–2000, and 2001–2005. There was a steady increase in the number of cognitive training studies published beginning in 2005. Twenty-five (i.e., 81%) of the cognitive training studies meeting inclusion criteria were published between 2005 and 2015.
Figure 2.
Number of cognitive training studies meeting review inclusion criteria for cognitively health, community-dwelling older adults from 1986 to 2015 (N = 31).
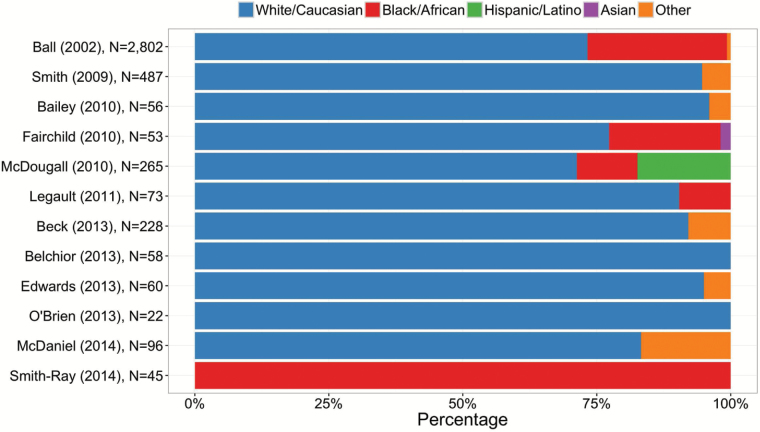
Only 12 of the 31 (39%) studies provided data on the participants’ race and/or ethnicity. No included studies published prior to year 2000 provided information on the ethnic/racial characteristics of their sample. Among studies that reported the ethnic/racial breakdown (n = 12), study participants were predominantly White (around 81%). African Americans participated in 5 of the 12 studies; all Hispanic/Latino participants were from a single study, the SeniorWISE study (McDougall et al., 2010b). Only one Asian American participant was explicitly reported to have participated in cognitive training research since the first cognitive training study meeting inclusion criteria was published in 1986. It is possible, however, that some Hispanic/Latino and Asian American participants may have been included under the “Other” category (Figure 3).
Figure 3.
Participation by racial/ethnic groups in cognitive training studies meeting review inclusion criteria from 1986 to 2015 (%; n = 12).
Characteristics of Included Studies
Participant demographics and study characteristics are displayed in Table 1. Eighteen studies targeted a single cognitive domain including memory (n = 11), speed of processing (n = 4), reasoning (n = 2), and attention (n = 1). Thirteen studies involved multidomain training and in some of these studies, other forms of training that have been shown to improve cognitive health (e.g., physical activity) were included in the intervention.
Table 1.
Characteristics of Cognitive Training Studies in Healthy, Community-Dwelling Older Adults in the United States From 1986 to 2015 (N = 31)
| Participant demographics | Study characteristics | |||||
|---|---|---|---|---|---|---|
| Author (year) | N (TC, CC) | Race/ethnicity | Age range (mean) | Type of training | Duration/total hours | Outcomes of interest |
| Anderson et al. (2013) | 67 | NR | NR (63) | Auditory-based cognitive training vs. general education stimulation program | 8 weeks | Memory, speed of processing, speech-in-noise perception |
| Bailey, Dunlosky, & Hertzog (2010) | 56 (29, 27) | C: 54 | 60–89 (NR) | Metacognitive training at home vs. control | 2 weeks/2.5+ h | Paired associates |
| O: 2 | ||||||
| Ball et al. (2002) | 2,802 | C: 2,054 | 65–94 (74) | Memory training or reasoning training or speed training vs. control | Ten 60–75-min sessions over 5–6 weeks | Cognitive abilities (memory, reasoning, speed of processing) and daily functions (everyday problem-solving, everyday speed, activities of daily living, driving habits) |
| Memory training: 711 | B: 729 | |||||
| Reasoning training: 705 | O: 19 | |||||
| Speed training: 712 | ||||||
| Control: 704 | ||||||
| Beck et al. (2013) | 228 (116, 112) | C: 210 | NR (59) | Cognitive intervention vs. control, weight-loss intervention | 4 months/32 h | Memory, attention, visuospatial, language |
| O: 18 | ||||||
| Belchior et al. (2013) | 58 | C: 58 | 65–91 (75) | Target action game vs. placebo control arcade game vs. clinically validated UFOV training program vs. no-contact control group | 2–3 weeks/9 h | Speed, divided attention, selective attention |
| Target action game: 14 | ||||||
| Placebo control arcade game: 15 | ||||||
| UFOV: 16 | ||||||
| Control: 13 | ||||||
| Berry et al. (2010) | 32 | NR | NR (72) | Perceptual discrimination training vs. control | 10 h | Working memory |
| Bozoki, Radovanovic, Winn, Heeter, & Anthony (2013) | 60 (32, 28) | NR | NR (69) | Computer game-based cognitive training “My Better Mind” vs. control | 6 weeks | The CogState battery |
| Brooks, Friedman, Pearman, Gray, & Yesavage (1999) | 268 (224, 44) | NR | 55–88 (69) | Mnemonic training vs. control | 2 weeks | Name recall, word recall |
| Chapman et al. (2015) | 37 | NR | 56–71 (63) | Cognitive training vs. wait-list control | 12 weeks | Neural changes in the brain |
| Edwards, Ruva, O’Brien, Haley, & Lister (2013) | 60 (27, 33) | C: 57 | 59–95 (74) | InSight cognitive training vs. control | 2–3 times/week for 10–12 weeks | UFOV |
| O: 3 | ||||||
| Fairchild & Scogin (2010) | 53 | C: 41 | 57–99 (72) | Memory enhancement vs. minimal social support | 6 weeks | Face-name recall, delayed recall |
| B: 11 | ||||||
| A: 1 | ||||||
| Hill, Sheikh, & Yesavage (1987) | 76 (59, 17) | NR | NR (68) | Mnemonic training vs. control | 8-h group training (2 h/ day, twice a week for 2 weeks) | Name–face recall |
| Hill, Allen, & McWhorter (1991) | 71 | NR | 60–83 (70) | Narrative story training vs. method of loci training; travel training (placebo) | 1 h | Free recall |
| Lachman, Weaver, Bandura, Elliott, & Lewkowicz (1992) | 105 | NR | NR (69) | Cognitive restructuring vs. memory skills training vs. combined cognitive restructuring and memory skills training vs. practice on memory tasks vs. no-contact control group | Varies by training type | Memory |
| Legault et al. (2011) | 73 | C: 66 | 70–85 (78) | Physical activity training vs. cognitive training vs. combined training vs. health education control | 4 months | Immediate recall, delayed recall, working memory, attention, cognitive function |
| B: 7 | ||||||
| Lustig & Flegal (2008) | 32 | NR | TC: NR (75) CC: NR (76) |
Memory training with strategy instruction vs. memory training with strategy choice | 3 weeks | Memory |
| Integrated sentences: 16 | ||||||
| Strategy choice: 16 | ||||||
| Mahncke et al. (2006) | 182 | NR | 60–87 (71) | Experimental computer-based training vs. active computer-based control vs. no-contact control | 60 min per day, 5 days per week for 8–10 weeks | Auditory cognition: list learning, story memory, digit span forward, delayed free list recall, relayed list recognition, delayed free story recall |
| Experimental training: 62 | ||||||
| Matched active control: 61 | ||||||
| No-contact control: 59 | ||||||
| Margrett & Willis (2006) | 98 | NR | 61–89 (71) | In-home individual inductive reasoning training vs. collaborative training vs. no-treatment control | Reasoning | |
| In-home individual inductive reasoning training: 30 | ||||||
| Collaborative training: 34 | ||||||
| No-treatment control: 34 | ||||||
| McDaniel et al. (2014) | 96 | C: 80 | NR (65) | Cognitive training vs. exercise training vs. combined training vs. control | 6 months | Laboratory tasks that simulate everyday activities: cooking breakfast, virtual week, and memory for health information |
| Cognitive training: 23 | O: 16 | |||||
| Exercise training: 24 | ||||||
| Combined: 24 | ||||||
| Control: 25 | ||||||
| McDougall et al. (2010b) | 265 (135, 130) | C: 189 | 65–94 (NR) | Memory training vs. health promotion training | 6 months | Verbal memory, visual memory, memory complaints, memory self- efficacy, cognitive function, activities of daily living |
| B: 30 | ||||||
| H: 46 | ||||||
| Mozolic, Long, Morgan, Rawley-Payne, & Laurienti (2011) | 62 (30, 32) | NR | NR (69) | Modality-specific attention training vs. educational lecture control | 8 weeks/8 h | Immediate recall, delayed recall, selective attention, processing speed, attention, working memory |
| O’Brien et al. (2013) | 22 (11, 11) | C: 22 | NR (72) | Behavioral speed of processing training vs. no-contact control | 10 weeks | Attention |
| Richmond, Morrison, Chein, & Olson (2011) | 40 (21, 19) | NR | 60–80 (66) | Working memory training vs. trivia learning regime | 4–5 weeks/12.5 h | Working memory reading span, working memory digit span, attention, general intelligence, verbal learning |
| Scogin, Fairchild, Yon, Welsh, & Presnell (2014) | 53 | NR | NR (68) | Cognitive bibliotherapy plus memory training vs. cognitive bibliotherapy alone vs. wait-list control | 8 weeks | Memory, depression |
| Shatil (2013) | 122 | NR | 65–93 (77) | Cognitive training, physical activity training, combined cognitive and physical activity vs. control | Forty-eight 40-min sessions (3 times a week for 16 weeks)—total of 32 h | Hand–eye coordination, global visual memory, speed of information processing, visual scanning, naming |
| Cognitive training: 33 | ||||||
| Physical activity training: 31 | ||||||
| Combined cognitive and physical activity training: 29 | ||||||
| Book club control: 29 | ||||||
| Smith et al. (2009) | 487 (242, 245) | C: 461 | TC: NR (76) | Brain plasticity-based computerized cognitive training vs. general cognitive stimulation program | 8 weeks/40 h | Overall memory, immediate recall, delayed recall, reasoning, working memory, processing speed, cognitive function, subjective cognitive function |
| O: 26 | CC: NR (75) | |||||
| Smith-Ray et al. (2014) | 45 (23, 22) | B: 45 | NR (73) | Computer-based cognitive training vs. control | 10 weeks/20 h | Balance, gait speed under visuospatial dual-task condition |
| Stine-Morrow, Parisi, Morrow, & Park (2008) | 150 (87, 63) | NR | 59–93 (73) | Senior Odyssey program vs. control | 20 weeks | Processing speed, working memory, inductive reasoning, visual-spatial processing, divergent thinking |
| Stine-Morrow et al. (2014) | 461 | NR | 60–94 (NR) | Inductive reasoning training vs. competitive program in creative problem-solving vs. wait-list control | 16 weeks | Reasoning, problem- solving, processing speed, visuospatial processing, verbal episodic memory |
| Strenziok et al. (2014) | 42 | NR | Brain fitness: NR (70) Rise of Nations: NR (69) Space Fortress: NR (69) |
Brain fitness vs. Rise of Nations vs. Space Fortress | 6 weeks | Everyday problem-solving, reasoning |
| Willis & Schaie (1986) | 229 | NR. Most were Caucasian | 64–95 (73) | Inductive reasoning training vs. spatial orientation training | 2 weeks/5 h | Spatial orientation, inductive reasoning |
Note: A = Asian American; B = Black/African American; H = Hispanic/Latino American; O = other race/ethnicity; W = non-Hispanic White/Caucasian. CC = control condition; NR = not reported; TC = training condition; UFOV = useful field of view.
Of the 12 studies that reported race/ethnic background information, 8 studies targeted specific cognitive domains including memory (n = 4) and speed of processing (n = 4) and 4 studies involved multidomain training. No differential training effects among racial/ethnic groups were explicitly reported except in the SeniorWISE study (McDougall et al., 2010b). Investigating race and ethnicity as a covariate, McDougall and coworkers (2010a) reported that African American and Hispanic participants had greater training-related improvements on some cognitive measures such as visual memory but not on other memory measures compared to White participants. Only one study in this review specifically targeted cognitive training as a tool for fall prevention to a specific racial/ethnic minority group, in this case, African Americans (Smith-Ray, Makowski-Woidan, & Hughes, 2014). This study showed that a cognitive training program could be efficacious in preventing falls in a group of older African Americans.
Discussion
Cognitive training programs for older adults have proliferated in recent years (Walton et al., 2014). However, to our knowledge, this is the first review dedicated to examining ethnic/minority participation in cognitive training research in the United States. This review reports results from 30 years of cognitive training studies for community-dwelling, cognitively normal older adults in the United States. Of the cognitive training studies reviewed, only 39% reported the race/ethnic makeup of their study participants. In fact, in the 15 years since the first study identified in this review was published (i.e., 1986–2000), none of the studies reported participant race/ethnic information. Since these data first became available in the early 2000s, 10 of the 12 studies that reported race/ethnic data enrolled non-White/Caucasian participants, but the participants were predominantly White/Caucasian. However, with the exception of three studies (Ball et al., 2002; Fairchild & Scogin, 2010; McDougall et al., 2010b), the percentage of non-White participants in the remaining studies that reported the race/ethnic breakdown (n = 9) was minimal, ranging from 0% to 17%. According to our review, the ACTIVE trial (Ball et al., 2002), the SeniorWISE study (McDougall et al., 2010b), and the Training to Enhance Adult Memory (TEAM) (Fairchild & Scogin, 2010) were the only studies that systematically recruited (or over-recruited) from diverse populations—African Americans (26%) in ACTIVE, African Americans (11.5%) and Hispanic Americans (16.9%) in SeniorWISE, and African Americans (20.8%) and one Asian American (1.9%) in TEAM, out of all participants in these respective studies. It is assumed that some race/ethnic data may have been grouped under the catch-all “other” category, which prevented us from calculating exact participation rates of persons from specific ethnic minority groups over time.
Cognitive training studies have encountered challenges similar to those faced by researchers in other areas of aging in terms of recruitment and retention of race/ethnic minority populations. One example of the low participation rate among ethnic minority older adults was a study that examined neuropathologic data collected by 29 Alzheimer’s Disease Centers across the United States, in which only 5.1% of autopsies came from minorities (Beekly et al., 2004). Findings in this review are consistent with others in aging research in that ethnic/minority Americans are understudied, and there is much room for improvement in the inclusion of diverse populations (Curry & Jackson, 2003; Hughes, Varma, Pettigrew, & Albert, 2015; Manly & Mungas, 2015; Schneider, 2005).
Reducing cognitive decline has a significant public health impact because maintenance of cognitive abilities is essential for preserving independence and influential in predicting long-term care needs. Comparing the ethnic minority participation rate found in this review with the current and projected populations of each race/ethnic group (Ortman, Velkoff, & Hogan, 2014), it is clear that race/ethnic minority older adults have been disproportionately underrepresented in cognitive training studies. This is concerning given that in coming decades, we will see a narrowing of the gap between the proportion of White/Caucasian and non-White, ethnic minority Americans in the entire U.S. population.
Further complicating the changing demographic landscape is the already well-documented health disparities among older minority Americans. Studies have found that ethnic minority older adults not only encounter more barriers to accessing quality health care, but also experience disparities in research, including cognitive aging research (Chin, Negash, & Hamilton, 2011; Glymour & Manly, 2008; Sloan & Wang, 2005; Zuckerman et al., 2008). As an example, it was found that female and non-White individuals were underrepresented in Phase III ADRD clinical trials due to the typically strict inclusion/exclusion criteria used (Schneider, Olin, Lyness, & Chui, 1997). The higher prevalence of certain health problems (e.g., diabetes, certain cancers, cardiovascular events), potential race-related differences in pathobiology and responses to interventions, and the need to increase generalizability of research results are additional reasons for the need to increase ethnic minority participation (Moreno-John et al., 2004). We cannot begin to answer questions such as who will benefit most from cognitive training studies, which factor or combination of factors lead(s) to differential training effects, and other questions critical to eliminating health disparities in older Americans until we have sufficient numbers of participants in intervention studies to adequately represent the existing and future demographic makeup of the United States.
Although it is not the intention of this paper to discuss in great detail the various strategies identified from previous studies, it is important to highlight some of the “best-practices” and how they have been applied to cognitive training studies (mostly from the SeniorWISE study) to foster a platform for generating ideas toward a more inclusive cognitive aging research environment.
One of the strategies is developing relationships with key community members or agencies that are respected in their community and would be helpful in recruiting potential research participants. In SeniorWISE, cultural liaisons and gatekeepers in the community helped build program credibility and facilitated social engagement to increase the research team’s presence, which forged a stronger sense of trust among ethnic/minority participants and increased interest in participating in the program (McDougall, Simpson, & Friend, 2015).
Commitment to ongoing communications with research participants, including providing feedback regarding study results to the broader community, is also important. For example, the Minority Aging Research Study in Chicago maintains frequent contact with participants including quarterly phone calls, newsletters, sending participants special occasion cards for birthdays and holidays, and actively disseminating research updates and educational presentations on healthy aging related topics (Barnes, Shah, Aggarwal, Bennett, & Schneider, 2012). Similarly, in SeniorWISE, there were ongoing interactions with the community (e.g., birthday cards, monthly newsletters) (Austin-Wells, McDougall, & Becker, 2006). The newsletter provided study updates and contained columns that addressed various topics of interest to the participants. By being more visible and open, these strategies reportedly helped with study retention (McDougall et al., 2015). Clearly, this requires significant resources. Unfortunately, many past studies likely did not anticipate the level of resource and budget required, and this is something that future studies can learn from and include in the budget in the study-planning phase.
The issue of cultural competence cannot be emphasized enough with regard to cognitive training research, especially when there is a need to address culture-specific beliefs and concerns. Recruitment, assessment, and intervention materials (e.g., study pamphlets, outreach presentation, media ads, questionnaires) should be developed with cultural sensitivity (e.g., translated materials) and written at a level appropriate to the health literacy level in the target race/ethnic community. Efforts to hire bi-cultural/bilingual staff are highly recommended because they are sensitive to cultural nuances and oftentimes become the primary broker between race/ethnic group and the research team. In addition to partnering with cultural liaisons in the community, a number of culturally appropriate strategies were employed in SeniorWISE, including: (a) Recognizing the impact of cultural stigma surrounding dementia by avoiding language that some minority participants might view as stigmatizing. For example, the memory training program was described as “successful strategies to age well.” (b) The original 50-item Memory Self-Efficacy Questionnaire was reduced to 35 items, taking out items that Hispanic and African American participants had difficulty comprehending, so that it was more user-friendly without sacrificing reliability and validity. In an earlier version of SeniorWISE with Puerto Rican elders, the Spanish version of the Mini-Mental State Examination and Geriatric Depression Scale were used, and all other measures were translated into Spanish (McDougall, 1998). (c) Adaptations were made to the performance-based Direct Assessment of Functional Status to assess participants who may have little experience with certain functions that are tested (e.g., driving, writing a check). (d) Focus groups were held in advance of the SeniorWISE study with minority elders to determine the optimal presentation format for the intervention (Austin-Wells, Zimmerman, & McDougall, 2003), and examples given in lectures for control group participants reflected life situations familiar to the participants (Austin-Wells et al., 2006; McDougall et al., 2015).
Lastly, a number of more personalized strategies have been recommended to overcome economic and time constraints (i.e., schedule/time conflicts, lack of transportation). Studies have reported that scheduling visits at the older participants’ homes, making reminder calls, providing financial or in-kind incentives, among other strategies, have shown better participant retention in minority elders (Areán & Gallagher-Thompson, 1996; Barnes et al., 2012; Gallagher-Thompson et al., 2006). For example, classes were scheduled at a time most convenient for participants immediately after recruitment in SeniorWISE, and this flexibility resulted in high retention rates (McDougall et al., 2015). Although the effectiveness of these personalized strategies is not limited to studies that enroll minority participants, they have been repeatedly advocated for as strategies associated with greater minority participation in aging research (Areán & Gallagher-Thompson, 1996; Barnes et al., 2012; Gallagher-Thompson et al., 2006; Yancey, Ortega, & Kumanyika, 2006). Building upon the knowledge base for recruitment and retention of minority participation from the broader aging research literature, future cognitive training studies will need to consider ways to deliver the intervention in these communities. We observed enhanced interactions with the community as a distinctive feature of the three studies (ACTIVE, SeniorWISE, and TEAM) that were more successful in enrolling ethnic minority participants. Successful partnerships with key community members may involve using a number of different strategies, including but not limited to holding focus groups or interviews in the early stages of the study. These are nontrivial steps that can help build trust in the community, thus facilitating enrollment of community members in research (Moreno-John et al., 2004). Furthermore, successful partnerships may improve the project teams’ abilities to gather crucial feedback that can be incorporated into designing more engaging and appealing cognitive training programs that cater to diverse participants in the community.
Population aging will be driving a rise in the prevalence of cognitive impairment in older adults in the United States. The needs for health care and services to older adults will continue to challenge public health efforts in preventing or delaying cognitive decline. One aim of the National Plan to address Alzheimer’s disease, as a result of the 2014 National Alzheimer’s Project Act, was to increase enrollment and retention of minority older adults to inform clinical practice and reduce health disparities (https://aspe.hhs.gov/national-plan-address-alzheimers-disease-2015-update). In the recently proposed “National Institute on Aging (NIA) Health Disparities Research Framework,” ethnicity and race are two of the “fundamental factors” (others are gender, age, disability status, identity) that determine priority populations for health disparities research (Hill, Pérez-Stable, Anderson, & Bernard, 2015), and the framework will be used to assess progress in health disparities research. It is important to establish a common understanding of the underlying mechanisms in cognitive training that may or may not be beneficial in race/ethnic minority older adults. More research needs to be conducted, with sufficiently sampled sizes of participants representative of various race/ethnic minority populations. For example, minority participants in the SeniorWISE study received significantly greater benefits when compared to White participants from memory training, but the authors were unable to compare their findings with other cognitive training studies, since no other cognitive training studies have examined potential moderating effects of race and ethnicity on cognitive training interventions (McDougall et al., 2010b). Cognitive training researchers will also need to be sensitive to methodological challenges related to recruitment methods. Broadly relevant to the field of cognitive aging research, but certainly applicable to cognitive training research, is the issue of recruitment bias. Manly and Mungas (2015) argued that moving away from more traditional recruitment methods may result in a very select group of race/ethnic minority older adults, while older research participants are already more likely to be from a selected group (e.g., healthier, better educated). This is a valid concern, but we want to recognize that we have much more to learn about how different recruitment and retention approaches affect intervention effectiveness. It could be that a more tailored approach to recruitment and retention would attract more diverse (and perhaps larger) samples of race/ethnic racial groups to cognitive training studies, and actually result in a reduction of recruitment bias.
Perhaps it is also time to start asking research questions differently when conducting cognitive training studies with diverse populations. Whitfield, Allaire, Belue, & Edwards (2008) thoughtfully presented their argument that simple comparisons between race/ethnic groups, and the fact that Caucasians are most often the default comparison group can lead to misconstrued conclusions about race/ethnic differences. More studies are needed to explore training effects within race/ethnic groups. For example, in the context of cognitive training research, instead of asking, “Are there race differences in the outcomes of the cognitive training?” one might ask “Does sex (or other potential modifiers or mediators, e.g., education, acculturation, socioeconomic status) change the effect of cognitive training for ethnic minority elderly?”
Cognitive training interventions hold significant potential to improve cognitive health both at the individual and population level. Given the rapid demographic shift, a better understanding of race/ethnic related factors could lead to developing more culturally appropriate and effective interventions that may improve the lives of an even greater number of older adults in the United States.
Funding
This work was supported by the National Institute on Aging/National Institute of Health Research Training in Age-Related Cognitive Disorders (T32-AG027668 to M. S. Albert, PI, supporting M. Tzuang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors wish to give our sincere thanks to Donna Hesson, MLS, BA, for her feedback in developing the search strategy. We also gratefully acknowledge the invaluable insight provided by Graham J. McDougall, Jr., PhD, RN, FAAN, FGSA as primary investigator of the SeniorWISE study. Dr. Spira has agreed to serve as a consultant to Awarables, Inc. in support of an NIH grant. All other authors report no conflicts of interest.
References
- Administration for Community Living.(2014). A statistical profile of Asian older Americans aged 65+ Retrieved from http://www.aoa.acl.gov/Aging_Statistics/minority_aging/Facts-on-API-Elderly2008-plain_format.aspx
- Administration for Community Living (2015). A statistical profile of Black older Americans aged 65+ Retrieved from http://www.aoa.acl.gov/Aging_Statistics/minority_aging/Facts-on-Black-Elderly-plain_format.aspx
- Alzheimer’s Association.(2015). 2015 Alzheimer’s disease facts and figures Retrieved from https://www.alz.org/facts/downloads/facts_figures_2015.pdf
- Anderson S. White-Schwoch T. Parbery-Clark A., & Kraus N (2013). Reversal of age-related neural timing delays with training. Proceedings of the National Academy of Sciences of the United States of America, 110, 4357–4362. doi:10.1073/pnas.1213555110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areán P. A., & Gallagher-Thompson D (1996). Issues and recommendations for the recruitment and retention of older ethnic minority adults into clinical research. Journal of Consulting and Clinical Psychology, 64, 875–880. [DOI] [PubMed] [Google Scholar]
- Austin-Wells V. McDougall G. J. Jr., & Becker H (2006). Recruiting and retaining an ethnically diverse sample of older adults in a longitudinal intervention study. Educational Gerontology, 32, 159–170. doi:10.1080/03601270500388190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin-Wells V. Zimmerman T., & McDougall G. J (2003). An optimal delivery format for presentations targeting older adults. Educational Gerontology, 29, 493–501. doi:10.1080/713844396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey H. Dunlosky J., & Hertzog C (2010). Metacognitive training at home: Does it improve older adults’ learning?Gerontology, 56, 414–420. doi:10.1159/000266030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K. Berch D. B. Helmers K. F. Jobe J. B. Leveck M. D. Marsiske M., … Willis S. L; Advanced Cognitive Training for Independent and Vital Elderly Study Group (2002). Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association, 288, 2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes L. L. Shah R. C. Aggarwal N. T. Bennett D. A., & Schneider J. A (2012). The Minority Aging Research Study: Ongoing efforts to obtain brain donation in African Americans without dementia. Current Alzheimer research, 9, 734–745. doi:10.2174/156720512801322627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M. Snyder H. M. Carrillo M. C. Fazio S. Kim H., & Johns H (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s & Dementia, 11, 718–726. doi:10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Beck C. Fausett J. K. Krukowski R. A. Cornell C. E. Prewitt T. E. Lensing S., … West D. S (2013). A randomized trial of a community-based cognitive intervention for obese senior adults. Journal of Aging and Health, 25, 97–118. doi:10.1177/0898264312467374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly D. L. Ramos E. M. van Belle G. Deitrich W. Clark A. D. Jacka M. E., … Kukull W. A; NIA-Alzheimer’s Disease Centers (2004). The National Alzheimer’s Coordinating Center (NACC) Database: An Alzheimer disease database. Alzheimer Disease and Associated Disorders, 18, 270–277. [PubMed] [Google Scholar]
- Belchior P. Marsiske M. Sisco S. M. Yam A. Bavelier D. Ball K., & Mann W. C (2013). Video game training to improve selective visual attention in older adults. Computers in Human Behavior, 29, 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A. S. Zanto T. P. Clapp W. C. Hardy J. L. Delahunt P. B. Mahncke H. W., & Gazzaley A (2010). The influence of perceptual training on working memory in older adults. PLoS ONE, 5, e11537. doi:10.1371/journal.pone.0011537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozoki A. Radovanovic M. Winn B. Heeter C., & Anthony J. C (2013). Effects of a computer-based cognitive exercise program on age-related cognitive decline. Archives of Gerontology and Geriatrics, 57, 1–7. doi:10.1016/j.archger.2013.02.009 [DOI] [PubMed] [Google Scholar]
- Brooks J. O. Friedman L. Pearman A. M. Gray C., & Yesavage J. A (1999). Mnemonic training in older adults: Effects of age, length of training, and type of cognitive pretraining. International Psychogeriatrics, 11, 75–84. doi:10.1017/S1041610299005608 [DOI] [PubMed] [Google Scholar]
- Chapman S. B. Aslan S. Spence J. S. Hart J. J. Jr. Bartz E. K. Didehbani N., … Lu H (2015). Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cerebral Cortex (New York, N.Y.: 1991), 25, 396–405. doi:10.1093/cercor/bht234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A. L. Negash S., & Hamilton R (2011). Diversity and disparity in dementia: The impact of Ethnoracial differences in Alzheimer disease. Alzheimer Disease and Associated Disorders, 25, 187–195. doi:10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. M. DeCarli C. Mungas D. Chui H. I. Higdon R. Nuñez J., … van Belle G (2005). Earlier onset of Alzheimer disease symptoms in Latino individuals compared with Anglo individuals. Archives of Neurology, 62, 774–778. doi:10.1001/archneur.62.5.774 [DOI] [PubMed] [Google Scholar]
- Coyle H. Traynor V., & Solowij N (2015). Computerized and virtual reality cognitive training for individuals at high risk of cognitive decline: Systematic review of the literature. The American Journal of Geriatric Psychiatry, 23, 335–359. doi:10.1016/j.jagp.2014.04.009 [DOI] [PubMed] [Google Scholar]
- Curry L., & Jackson J (2003). The science of including older ethnic and racial group participants in health-related research. The Gerontologist, 43, 15–17. [DOI] [PubMed] [Google Scholar]
- Dilworth-Anderson P. Hendrie H. C. Manly J. J. Khachaturian A. S., & Fazio S; Social, Behavioral and Diversity Research Workgroup of the Alzheimer’s Association (2008). Diagnosis and assessment of Alzheimer’s disease in diverse populations. Alzheimer’s & Dementia, 4, 305–309. doi:10.1016/j.jalz.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Dong X. Wong E., & Simon M. A (2014). Study design and implementation of the PINE study. Journal of Aging and Health, 26, 1085–1099. doi:10.1177/0898264314526620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. D. Ruva C. L. O’Brien J. L. Haley C. B., & Lister J. J (2013). An examination of mediators of the transfer of cognitive speed of processing training to everyday functional performance. Psychology and Aging, 28, 314–321. doi:10.1037/a0030474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild J. K., & Scogin F. R (2010). Training to Enhance Adult Memory (TEAM): An investigation of the effectiveness of a memory training program with older adults. Aging & Mental Health, 14, 364–373. doi:10.1080/13607860903311733 [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D. Rabinowitz Y. Tang P. C. Y. Tse C. Kwo E. Hsu S., … Thompson L. W (2006). Recruiting Chinese Americans for dementia caregiver intervention research: Suggestions for success. American Journal of Geriatric Psychiatry, 14, 676–683. doi:10.1097/01.JGP.0000221234.65585.f9 [DOI] [PubMed] [Google Scholar]
- Glymour M. M., & Manly J. J (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18, 223–254. doi:10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Graves A. B. Larson E. B. Edland S. D. Bowen J. D. McCormick W. C. McCurry S. M., … Uomoto J. M (1996). Prevalence of dementia and its subtypes in the Japanese American population of King County, Washington state. The KAME project. American Journal of Epidemiology, 144, 760–771. doi:10.1093/oxfordjournals.aje.a009000 [DOI] [PubMed] [Google Scholar]
- Gross A. L. Parisi J. M. Spira A. P. Kueider A. M. Ko J. Y. Saczynski J. S., … Rebok G. W (2012). Memory training interventions for older adults: A meta-analysis. Aging & Mental Health, 16, 722–734. doi:10.1080/13607863.2012.667783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland B. J. Wilder D. E. Lantigua R. Stern Y. Chen J. Killeffer E. H., & Mayeux R (1999). Rates of dementia in three ethnoracial groups. International Journal of Geriatric Psychiatry, 14, 481–493. doi:10.1002/(SICI)1099-1166(199906)14:6<481::AID-GPS959>3.3.CO;2-X [PubMed] [Google Scholar]
- Haan M. N. Mungas D. M. Gonzalez H. M. Ortiz T. A. Acharya A., & Jagust W. J (2003). Prevalence of dementia in older Latinos: The influence of type 2 diabetes mellitus, stroke and genetic factors. Journal of the American Geriatrics Society, 51, 169–177. doi:10.1046/j.1532-5415.2003.51054.x [DOI] [PubMed] [Google Scholar]
- Hebert L. E. Weuve J. Scherr P. A., & Evans D. A (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology, 80, 1778–1783. doi:10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie H. C. Ogunniyi A. Hall K. S. Baiyewu O. Unverzagt F. W. Gureje O., … Hui S. L (2001). Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. Journal of the American Medical Association, 285, 739–747. doi:10.1001/jama.285.6.739 [DOI] [PubMed] [Google Scholar]
- Hertzog C. Kramer A. F. Wilson R. S., & Lindenberger U (2008). Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced?Psychological Science in the Public Interest: A Journal of the American Psychological Society, 9, 1–65. doi:10.1111/j.1539-6053.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Hill C. V. Pérez-Stable E. Anderson N. A., & Bernard M. A (2015). The National Institute on Aging Health Disparities Research Framework. Ethnicity & Disease, 25, 245–254. doi:10.18865/ed.25.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. D. Allen C., & McWhorter P (1991). Stories as a mnemonic aid for older learners. Psychology and Aging, 6, 484–486. doi:10.1037/0882-7974.6.3.484 [DOI] [PubMed] [Google Scholar]
- Hill R. D. Sheikh J. I., & Yesavage J (1987). The effect of mnemonic training on perceived recall confidence in the elderly. Experimental Aging Research, 13, 185–188. doi:10.1080/03610738708259323 [DOI] [PubMed] [Google Scholar]
- Hughes T. B. Varma V. R. Pettigrew C., & Albert M. S (2015). African Americans and clinical research: Evidence concerning barriers and facilitators to participation and recruitment recommendations. The Gerontologist. First published November 9, 2015, doi:10.1093/geront/gnv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. F., & Ganguli M (2009). Modifiable midlife risk factors for late-life cognitive impairment and dementia. Current Psychiatry Reviews, 5, 73–92. doi:10.2174/157340009788167347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine).(2015). Cognitive aging: Progress in understanding and opportunities for action. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Kelly M. E. Loughrey D. Lawlor B. A. Robertson I. H. Walsh C., & Brennan S (2014). The impact of cognitive training and mental stimulation on cognitive and everyday functioning of healthy older adults: A systematic review and meta-analysis. Ageing Research Reviews, 15, 28–43. doi:10.1016/j.arr.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Kueider A. M. Parisi J. M. Gross A. L., & Rebok G. W (2012). Computerized cognitive training with older adults: A systematic review. PLoS ONE, 7, e40588. doi:10.1371/journal.pone.0040588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman M. E. Weaver S. L. Bandura M. Elliott E., & Lewkowicz C. J (1992). Improving memory and control beliefs through cognitive restructuring and self-generated strategies. Journal of Gerontology: Psychological Sciences, 47, 293–299. doi:10.1093/geronj/47.5.P293 [DOI] [PubMed] [Google Scholar]
- Lampit A. Hallock H., & Valenzuela M (2014). Computerized cognitive training in cognitively healthy older adults: A systematic review and meta-analysis of effect modifiers. PLoS Medicine, 11, e1001756. doi:10.1371/journal.pmed.1001756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault C. Jennings J. M. Katula J. A. Dagenbach D. Gaussoin S. A. Sink K. M.,… SHARP-P Study Group. (2011). Designing clinical trials for assessing the effects of cognitive training and physical activity interventions on cognitive outcomes: The Seniors Health and Activity Research Program Pilot (SHARP-P) study, a randomized controlled trial. BMC Geriatrics, 11, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C., & Flegal K. E (2008). Targeting latent function: Encouraging effective encoding for successful memory training and transfer. Psychology and Aging, 23, 754–764. doi:10.1037/a0014295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke H. W. Connor B. B. Appelman J. Ahsanuddin O. N. Hardy J. L. Wood R. A., … Merzenich M. M (2006). Memory enhancement in healthy older adults using a brain plasticity-based training program: A randomized, controlled study. Proceedings of the National Academy of Sciences of the United States of America, 103, 12523–12528. doi:10.1073/pnas.0605194103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly J. L., & Mayeux R (2004). Ethnic differences in dementia and Alzheimer’s disease. In Anderson N. B., Bulatao R. A., & Cohen B. (Eds.), Critical perspectives on racial and ethnic differences in health in late life (p. 95). Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Manly J. J., & Mungas D (2015). JGPS special series on race, ethnicity, life experiences, and cognitive aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 509–511. doi:10.1093/geronb/gbv030 [DOI] [PubMed] [Google Scholar]
- Margrett J. A., & Willis S. L (2006). In-home cognitive training with older married couples: Individual versus collaborative learning. Neuropsychology, Development, and Cognition, Section B: Aging, Neuropsychology and Cognition, 13, 173–195. doi:10.1080/138255890969285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Clare L. Altgassen A. M. Cameron M. H., & Zehnder F (2011). Cognition-based interventions for healthy older people and people with mild cognitive impairment. Cochrane Database Systematic Review, 1, CD006220. doi:10.1002/14651858.cd006220.pub2 [DOI] [PubMed] [Google Scholar]
- McDaniel M. A. Binder E. F. Bugg J. M. Waldum E. R. Dufault C. Meyer A., … Kudelka C (2014). Effects of cognitive training with and without aerobic exercise on cognitively demanding everyday activities. Psychology and Aging, 29, 717–730. doi:10.1037/a0037363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J. (1998). Increasing memory self-efficacy and strategy use in Hispanic elders. Clinical Gerontologist, 19, 57–76. doi:10.1300/J018v19n02_05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J. Jr. Becker H. Pituch K. Acee T. W. Vaughan P. W., & Delville C. L (2010a). Differential benefits of memory training for minority older adults in the SeniorWISE study. The Gerontologist, 50, 632–645. doi:10.1093/geront/gnq017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J. Jr. Becker H. Pituch K. Acee T. W. Vaughan P. W., & Delville C. L (2010b). The SeniorWISE study: Improving everyday memory in older adults. Archives of Psychiatric Nursing, 24, 291–306. doi:10.1016/j.apnu.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J. Jr. Simpson G., & Friend M. L (2015). Strategies for research recruitment and retention of older adults of racial and ethnic minorities. Journal of Gerontological Nursing, 41, 14–23. doi:10.3928/00989134-20150325-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau D., & Conway A. R (2014). The case for an ecological approach to cognitive training. Trends in Cognitive Sciences, 18, 334–336. doi:10.1016/j.tics.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Moreno-John G. Gachie A. Fleming C. M. Nápoles-Springer A. Mutran E., … Pérez-Stable E (2004). Ethnic minority older adults participating in clinical research: Developing trust. Journal of Aging and Health, 16, 93S–123S. doi:10.1177/0898264304268151 [DOI] [PubMed] [Google Scholar]
- Mozolic J. L. Long A. B. Morgan A. R. Rawley-Payne M., & Laurienti P. J (2011). A cognitive training intervention improves modality-specific attention in a randomized controlled trial of healthy older adults. Neurobiology of Aging, 32, 655–668. doi:10.1016/j.neurobiolaging.2009.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. L. Edwards J. D. Maxfield N. D. Peronto C. L. Williams V. A., & Lister J. J (2013). Cognitive training and selective attention in the aging brain: An electrophysiological study. Clinical Neurophysiology, 124, 2198–2208. doi:10.1016/j.clinph.2013.05.012 [DOI] [PubMed] [Google Scholar]
- Ortman J. M. Velkoff V. A., & Hogan H (2014). An aging nation: The older population in the United States. Current population reports. Washington, DC: U.S. Census Bureau. [Google Scholar]
- Owen A. M. Hampshire A. Grahn J. A. Stenton R. Dajani S. Burns A. S., … Ballard C. G (2010). Putting brain training to the test. Nature, 465, 775–778. doi:10.1038/nature09042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp K. V. Walsh S. J., & Snyder P. J (2009). Immediate and delayed effects of cognitive interventions in healthy elderly: A review of current literature and future directions. Alzheimer’s & Dementia, 5, 50–60. doi:10.1016/j.jalz.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Potter G. G. Plassman B. L. Burke J. R. Kabeto M. U. Langa K. M. Llewellyn D. J., … Steffens D. C (2009). Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimer’s & Dementia, 5, 445–453. doi:10.1016/j.jalz.2009.04.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok G. W. Ball K. Guey L. T. Jones R. N. Kim H. Y. King J. W.,… ACTIVE Study Group. (2014). Ten-year effects of the Advanced Cognitive Training for Independent and Vital Elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society, 62, 16–24. doi:10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebok G. W. Carlson M. C., & Langbaum J. B (2007). Training and maintaining memory abilities in healthy older adults: Traditional and novel approaches. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 62, 53–61. doi:10.1093/geronb/62.special_issue_1.53 [DOI] [PubMed] [Google Scholar]
- Rebok G. W. Parisi J. M. Gross A. L., & Spira A. P (2010). Assessment of cognitive training. In Lichtenberg P. A. (Ed.), Handbook of assessment in clinical gerontology (2nd ed, p. 211). New York: Academic Press. [Google Scholar]
- Richmond L. L. Morrison A. B. Chein J. M., & Olson I. R (2011). Working memory training and transfer in older adults. Psychology and Aging, 26, 813–822. doi:10.1037/a0023631 [DOI] [PubMed] [Google Scholar]
- Schneider L. S. (2005). Drug development, clinical trials, cultural heterogeneity in Alzheimer disease: The need for pro-active recruitment. Alzheimer Disease and Associated Disorders, 19, 279–283. [DOI] [PubMed] [Google Scholar]
- Schneider L. S. Olin J. T. Lyness S. A., & Chui H. C (1997). Eligibility of Alzheimer’s disease clinic patients for clinical trials. Journal of the American Geriatrics Society, 45, 923–928. [DOI] [PubMed] [Google Scholar]
- Scogin F. Fairchild J. K. Yon A. Welsh D. L., & Presnell A (2014). Cognitive bibliotherapy and memory training for older adults with depressive symptoms. Aging & Mental Health, 18, 554–560. doi:10.1080/13607863.2013.825898 [DOI] [PubMed] [Google Scholar]
- Shatil E. (2013). Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Frontiers in Aging Neuroscience, 5, 8. doi:10.3389/fnagi.2013.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan F. A., & Wang J (2005). Disparities among older adults in measures of cognitive function by race or ethnicity. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, P242–P250. [DOI] [PubMed] [Google Scholar]
- Smith G. E. Housen P. Yaffe K. Ruff R. Kennison R. F. Mahncke H. W., & Zelinski E. M (2009). A cognitive training program based on principles of brain plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. Journal of the American Geriatrics Society, 57, 594–603. doi:10.1111/j.1532-5415.2008.02167.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ray R. L. Makowski-Woidan B., & Hughes S. L (2014). A randomized trial to measure the impact of a community-based cognitive training intervention on balance and gait in cognitively intact Black older adults. Health Education & Behavior, 41(Suppl. 1), 62S–69S. doi:10.1177/1090198114537068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. Parisi J. M. Morrow D. G., & Park D. C (2008). The effects of an engaged lifestyle on cognitive vitality: A field experiment. Psychology and Aging, 23, 778–786. doi:10.1037/a0014341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow E. A. Payne B. R. Roberts B. W. Kramer A. F. Morrow D. G. Payne L., … Parisi J. M (2014). Training versus engagement as paths to cognitive enrichment with aging. Psychology and Aging, 29, 891–906. doi:10.1037/a0038244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenziok M. Parasuraman R. Clarke E. Cisler D. S. Thompson J. C., & Greenwood P. M (2014). Neurocognitive enhancement in older adults: Comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. NeuroImage, 85(Pt 3), 1027–1039. doi:10.1016/j.neuroimage.2013.07.069 [DOI] [PubMed] [Google Scholar]
- Tang M. X. Cross P. Andrews H. Jacobs D. M. Small S. Bell K., … Mayeux R (2001). Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology, 56, 49–56. [DOI] [PubMed] [Google Scholar]
- Valenzuela M., & Sachdev P (2009). Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. The American Journal of Geriatric Psychiatry, 17, 179–187. doi:10.1097/JGP.0b013e3181953b57 [DOI] [PubMed] [Google Scholar]
- Vincent G. K., & Velkoff V. A (2010). The next four decades: The older population in the United States: 2010 to 2050. Washington, DC: Administration on Aging. [Google Scholar]
- Walton C. C. Mowszowski L. Lewis S. J., & Naismith S. L (2014). Stuck in the mud: Time for change in the implementation of cognitive training research in ageing?Frontiers in Aging Neuroscience, 6, 43. doi:10.3389/fnagi.2014.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley L. J. Dick F. D., & McNeill G (2006). A life-course approach to the aetiology of late-onset dementias. The Lancet Neurology, 5, 87–96. doi:10.1016/S1474-4422(05)70286-6 [DOI] [PubMed] [Google Scholar]
- White L. Petrovitch H. Ross G. W. Masaki K. H. Abbott R. D. Teng E. L., … Curb J. D (1996). Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. Journal of the American Medical Association, 276, 955–960. [PubMed] [Google Scholar]
- Whitfield K. E. Allaire J. C. Belue R., & Edwards C. L (2008). Are comparisons the answer to understanding behavioral aspects of aging in racial and ethnic groups?The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63, P301–P308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W. Plassman B. L. Burke J. Holsinger T., & Benjamin S (2010). Preventing Alzheimer’s disease and cognitive decline. Rockville, MD: Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. [Google Scholar]
- Willis S. L., & Schaie K. W (1986). Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychology and Aging, 1, 239–247. [DOI] [PubMed] [Google Scholar]
- Willis S. L. Tennstedt S. L. Marsiske M. Ball K. Elias J. Koepke K. M., … Wright E; ACTIVE Study Group (2006). Long-term effects of cognitive training on everyday functional outcomes in older adults. Journal of the American Medical Association, 296, 2805–2814. doi:10.1001/jama.296.23.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey A. K. Ortega A. N., & Kumanyika S. K (2006). Effective recruitment and retention of minority research participants. Annual Review of Public Health, 27, 1–28. doi:10.1146/annurev.publhealth.27.021405.102113 [DOI] [PubMed] [Google Scholar]
- Yeo G. (2006). Prevalence of dementia among different ethnic populations. In Yeo G., Gallagher-Thompson D. (Eds.), Ethnicity and the dementias (2nd ed, p. 3). New York: Routledge. [Google Scholar]
- Zuckerman I. H. Ryder P. T. Simoni-Wastila L. Shaffer T. Sato M. Zhao L., & Stuart B (2008). Racial and ethnic disparities in the treatment of dementia among Medicare beneficiaries. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63, S328–S333. [DOI] [PMC free article] [PubMed] [Google Scholar]