Abstract
Background
The purpose of the study was to improve treatment efficacy for locally advanced rectal cancer (LARC) by shifting half of adjuvant chemotherapy preoperatively to one induction and two consolidation cycles.
Patients and methods
Between October 2011 and April 2013, 66 patients with LARC were treated with one induction chemotherapy cycle followed by chemoradiotherapy (CRT), two consolidation cycles, surgery and three adjuvant capecitabine cycles. Radiation doses were 50.4 Gy for T2-3 and 54 Gy for T4 tumours in 1.8 Gy daily fraction. The doses of concomitant and neo/adjuvant capecitabine were 825 mg/m2/12h and 1250mg/m2/12h, respectively. The primary endpoint was pathologic complete response (pCR).
Results
Forty-three (65.1%) patients were treated according to protocol. The compliance rates for induction, consolidation, and adjuvant chemotherapy were 98.5%, 93.8% and 87.3%, respectively. CRT was completed by 65/66 patients, with G ≥ 3 non-hematologic toxicity at 13.6%. The rate of pCR (17.5%) was not increased, but N and the total-down staging rates were 77.7% and 79.3%, respectively. In a median follow-up of 55 months, we recorded one local relapse (LR) (1.6%). The 5-year disease-free survival (DFS) and overall survival (OS) rates were 64.0% (95% CI 63.89–64.11) and 69.5% (95% CI 69.39–69.61), respectively.
Conclusions
In LARC preoperative treatment intensification with capecitabine before and after radiotherapy is well tolerated, with a high compliance rate and acceptable toxicity. Though it does not improve the local effect, it achieves a high LR rate, DFS, and OS.
Key words: rectal cancer, neoadjuvant chemotherapy, preoperative chemoradiotherapy, pathologic complete response, total neoadjuvant therapy
Introduction
Over the past 15 years, there have been unprecedented advances in the multimodality treatment of locally advanced rectal cancer (LARC). The shift of chemoradiotherapy (CRT) from a postoperative to a preoperative setting enabled tumour downsizing and downstaging and, consequently, increased the likelihood of microscopic complete clearance of the primary tumour (R0 resection). With a highly precise surgical technique and a total mesorectal excision, combined modality treatment resulted in an excellent local control and, as such, represents the standard of care for these patients. Still, the prognosis remains largely unsatisfactory due to a high rate of distant relapse, which is the most common cause of death.1
The results of two meta-analyses suggest that the pathological stage of the disease and/or the rate of tumour reduction (pathohistological tumour regression - TRG) after pre-operative treatment are predictive factors for disease-free survival. A particularly low risk of recurrence of the disease has a subgroup of patients with a complete pathohistological response (pCR).2,3 With standard 5-FU based CRT, pCR is reported to range between 9 and 20%.3,4 In an attempt to increase the pCR rate, many trials integrated oxaliplatin and/or molecular targeted agents into fluoropyrimidine-based preoperative CRT protocols. They achieved a high rate of pCR, but it was accompanied by higher toxicity and had no impact on survival.5, 6
In the search for improving the rate of pCR and the control of micrometastatic disease without causing greater toxicity, the intensification of standard chemotherapy (ChT) treatment in the neoadjuvant setting, namely by integrating induction chemotherapy before CRT and consolidation ChT before the operation, represents a rational approach.
In this study, we sought to determine whether the intensification of ChT in the neoadjuvant setting was associated with an improved outcome of the disease. The primary goal was to establish the proportion of complete pathohistological response to the treatment. Secondary objectives were to evaluate the pathological downstaging rate, histopathological R0 resection rate, sphincter preservation rate, perioperative surgical complication rate, local control (LC), disease-free survival (DFS), overall survival (OS), late toxicity, and the quality of life.
In this paper, we summarize the results of the phase II trial altogether and provide 5-year follow-up data.
Patients and methods
Patients
The inclusion criteria comprised a histologically proven adenocarcinoma of the rectum, a clinical TNM stage II or III based on magnetic resonance imaging (MRI) of the pelvis, and an operable disease or disease likely to become operable after neoadjuvant therapy.7 The extent of disease was determined according to the International Union Against Cancer (UICC) classification.7
Patients had to be ≥ 18 years old with a performance status 0–2 according to the World Health Organisation (WHO) scale, and had to have adequate cardiac, bone marrow, liver and renal function. All patients signed written informed consents before commencing treatment. The trial was approved by the National Medical Ethics Committee of the Republic of Slovenia (No. 163/06/11) and was registered in the ClinicalTrials.gov database (NCT01489332).
Pre-treatment evaluation
Before entering the study, the patients underwent a complete history and physical examination, full blood count, serum biochemistry profiles with liver and renal function tests, carcinoembryonic antigen (CEA), chest X-rays, ultrasonography (USG) or computed tomography (CT) of the abdomen, and colonoscopy with biopsy. Each patient underwent a magnetic resonance imaging (MRI) of the pelvis for local staging.
Study protocol
Intervention ChT included one cycle of oral capecitabine before and two cycles after CRT at a dose of 1250 mg/m2/12 hours, 14 consecutive days. The patients were irradiated with 15-MV linear accelerator photon beams, using the four-field 3-dimensional conformal technique. The total dose to the small pelvis was 45.0 Gy in 1.8 Gy daily fraction, followed by a boost to the primary tumour (1.8 Gy daily) to 5.4 Gy for T2–T3, and 9.0 Gy for T4 tumours. Oral capecitabine was administered concomitantly with radiotherapy at a dose of 825 mg/m2 twice daily from the first to the last day of radiotherapy (including weekends).
Surgery was performed 2 weeks after the completion of ChT. Adjuvant ChT began 4–6 weeks after resection and comprised three cycles of oral capecitabine 1250mg/m2/12, 14 consecutive days.
Follow-up
During therapy, acute toxicity was monitored on a three-week basis for ChT and on a weekly basis for CRT. A clinical examination and complete blood count were performed. Toxic side effects were assessed according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) (version 4.0).8
All patients were followed up every 3 months in the first 2 years after surgery, and then every 6 months for 5 years. A clinical examination was performed and the serum scanned for CEA at each follow-up. An abdominal ultrasound was performed every 6 months, chest radiograph every 12 months, and colonoscopy annually. The terminal time for the evaluation of outcomes was 5 years. The follow-up rate was 100%.
Statistics
The sample size calculation was based on a hypothesis that the experimental treatment regime will increase the pCR rate by 14%, from our 9%9 to 23%. To confirm the hypothesis that the pCR rate was greater than 23% with 80% power and to reject the hypothesis that the pCR rate was lower or equal to 9% with 5% significance, a sample size of 50 was required.11,12 In case that 10% proved not to be evaluable, we planned to include 60 patients.
Statistical calculations were performed using the SPSS statistical software package, version 18 (SPSS Inc., Chicago, IL, USA). Statistical analyses were performed using the Chi-square test with Fisher’s exact test. The survival rates were obtained using the Kaplan-Meier method and the significance of the difference in survival rate was determined by means of the log-rank test. P-values < 0.05 were considered statistically significant.
All time intervals were calculated from the date of operation or date of CRT completion (for nonoperated patients). The end dates for time calculations were the dates of the last follow-up or death for overall survival (OS); and the dates of detected local/distant relapse, last follow-up, or death for disease-free survival (DFS). In non-operated patients, the DFS time was 0 months.
Results
Patients
Between October 2011 and April 2013, 66 patients with locally advanced rectal cancer were treated in the trial. Table 1 lists the pre-treatment patient and tumour characteristics. The median age was 60 years (range 37–81) and 42 (63.6%) patients were men. The WHO performance status was graded as 0 in 54 (81.8%) patients, as 1 in 11 (16.7%) patients, and as 2 in 1 (1.5%) patient. Fifty-seven (86.4%) patients had nodal involvement. In 31 patients (47%), the primary tumour was sited ≤ 5 cm from the anal verge, 33 (50%) tumours were located at 5–10 cm, and 2 (3%) were above 10 cm from anal verge. The flow of the patients through the trial is shown in Figure 1.
Table 1.
Pre-treatment patients and tumour characteristics (N = 66)
| Median age (years) | 60 (37-81) |
| Gender | |
| Male | 42 (63.6%) |
| Female | 24 (36.4%) |
| WHO performance status7 | |
| 0 | 54 (81.8%) |
| 1 | 11 (16.7%) |
| 2 | 1 (1.5 %) |
| Stage8 | |
| T2 | 9 (13.6%) |
| T3 | 50 (75.8%) |
| T4 | 7 (10.6%) |
| N0 | 9 (13.6%) |
| N1 | 34 (51.5%) |
| N2 | 23 (34.9%) |
| Tumour differentiation (grade) | |
| Well (G1) | 8 (12.1%) |
| Moderate (G2) | 35 (53.0%) |
| Poorly (G3) | 4 (6.0%) |
| Unknown or not stated (GX) | 19 (28.9%) |
| MRF distance | |
| MRF+ | 20 |
| MRF- | 44 |
| Median tumour distance from the anal verge (cm) | 6 (0-12) |
MRF distance = distance between tumour and mesorectal fascia
Figure 1.
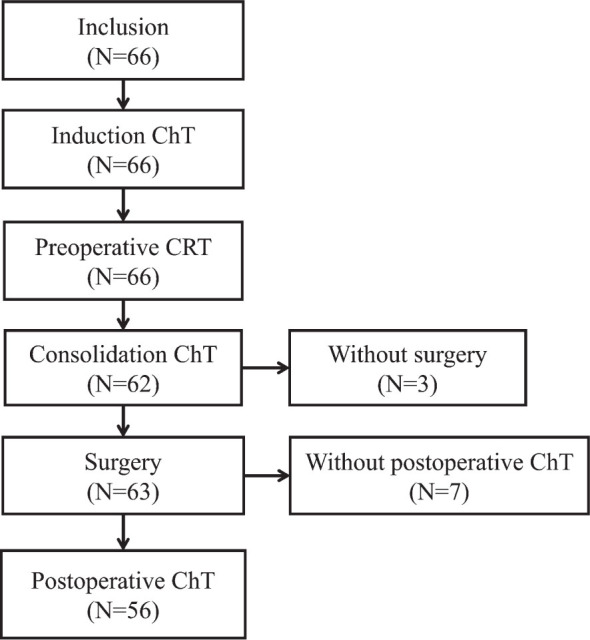
Distribution of patients through the trial.
Neoadjuvant treatment
The toxic side effects of preoperative treatment are listed in Table 2. The induction cycle of capecitabine was well tolerated with no G ≥ 2 toxicity. Sixty-five (98.5%) patients completed radiotherapy according to the treatment protocol. In one patient, radiotherapy was discontinued after the TD 45 Gy due to prolonged G4 thrombocytopenia and septic shock caused by Pseudomonas aeruginosa. The radiotherapy treatment was completed in the median time of 39 days (range 37–53 days). The median time of radiotherapy interruption was 2 days (range 0–15 days). The median duration of ChT was 39 days (6–45 days) and the median time of ChT interruption was 0 days (0–47 days). Nine (13.8%) patients received less than 90% of the planned capecitabine dose due to G ≥ 2 (3/66; 4.5%) thrombocytopenia, G3 neutropenia and G3 diarrhoea (2/66; 3%), infection (2/66; 3%), chest pain (1/66; 1.5%) and due to protocol violation by the patient (1/66; 1.5%).
Table 2.
Toxicity8 of preoperative treatment (N = 66)
| Toxicity grade (N) | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Haematological | |||||
| Anaemia | 23 | 7 | 1 | 0 | 0 |
| Neutropenia | 4 | 7 | 2 | 0 | 0 |
| Thrombocytopenia | 6 | 1 | 1 | 1 | 0 |
| Non-haematological | |||||
| Fatigue | 5 | 0 | 0 | / | / |
| Nausea/vomiting | 2 | 2 | 0 | / | / |
| Hand-foot syndrome | 11 | 5 | 1 | ||
| Radiodermatitis | 7 | 18 | 5 | ||
| Diarrhoea | 11 | 3 | 2 | ||
| Urinary infection | 6 | 0 | 0 | 0 | 0 |
| Systemic infection | 0 | 1 | 0 | 1 | 0 |
| Proctitis | 6 | 2 | 0 | ||
| Thromboembolism | 2 | 2 | 0 | 0 | 1 |
| Chest pain | 1 | ||||
After the completion of CRT, one patient died due to pulmonary thromboembolism.
Consolidation chemotherapy
After CRT, ChT was administered to 93.8% patients according to protocol. The consolidation treatment was omitted due to patient refusal in one, prolonged neutropenia in the second, and thrombocytopenia in the third patient. It was discontinued after the first cycle for a patient with L1 fracture.
Surgery and perioperative toxicity
Surgery was performed in 63/66 (95.4%) patients in median 8 weeks (range 6.6–11.3) after CRT completion. One patient refused the operation, one was unfit for surgery due to low performance status (PS), and one patient died after CRT before operation. A low anterior resection was performed in 46 patients (73%) and abdominoperineal resection in 17 (27%), with hysterectomy +/− ovariectomy in three patients. During surgery, solitary hepatic metastases were discovered in two patients and a synchronous metastasectomy was performed. In all patients but one a radical resection was achieved (98.4%).
Within 30 days of the operation we recorded thirty adverse events in 24 patients. In 10 out of 63 (15.8%) patients, toxicity was graded as G ≥ 3 with postoperative wound complications, including a local infection with delayed healing (N = 7), anastomotic leakage (N = 2), intraabdominal infection (N = 4), urinary infection (N = 1), and pneumonia (N = 2). Three patients were re-operated because of acute abdomen, intraabdominal, and anastomotic bleeding. In one patient, a revision of the necrotic epidermal bound lope under general anaesthesia was performed. There was no perioperative mortality.
Adjuvant chemotherapy
Fifty-five of the 63 operated patients (87.3%) received adjuvant capecitabine treatment, two of them with oxaliplatin on account of pathological upstaging. All three cycles of the recommended dose were able to receive 94.3% of patients. No G ≥ 3 toxicity was observed. Two patients were treated with chemotherapy and targeted agents after synchronous liver metastasectomy.
Tumour response
A complete pathological response (pCR) was observed in 11/63 (17.5%) patients. In 2 patients, liver metastases were found during the operation. The tumour, nodal and overall downstaging rates were 55.5%, 77.7%, and 79.3%, respectively. An increase in T- and/or N-stage (upstaging) was recorded in 6 patients (9.5%). The pathologic TNM stages, as assessed in histopathological examination of the resected specimens in relation to preoperative TNM status, are listed in Table 4. In 5 patients, the local pathological stage was higher than the clinical.
Table 4.
Distribution of clinical and pathological stages
| pT0 | pT1 | pT2 | pT3 | pT4 | pN0 | pN1 | pN2 | ||
| cT1 | - | - | - | - | - | cN0 | 7 | - | - |
| cT2 | 2 | - | 3 | 4 | - | cN1 | 28 | 4 | 1 |
| cT3 | 8 | 6 | 16 | 18 | 1 | cN2 | 17 | 4 | 2 |
| cT4 | 1 | - | 1 | 1 | 2 |
c = clinical; p = pathological
Table 3.
| Toxicity grade (N) | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Haematological | ||||||
| Anaemia | 23 | 1 | ||||
| Neutropenia | 1 | |||||
| Non-haematological | ||||||
| Fatigue | 2 | |||||
| Nausea/vomiting | 2 | 1 | ||||
| Hand-foot syndrome | 3 | 3 | ||||
| Diarrhoea | 1 | |||||
| Urinary infection | 2 | |||||
| Thromboembolic event | 1 | |||||
According to the Dworak criteria, the tumour regression grades (TRG) were TRG 4, TRG 3, TRG 2, TRG 1, and TRG 0 in 11, 7, 32, 12, and 1 patients, respectively [12].
The sphincter preservation rate for the low rectal tumours ≤ 5 cm from the anal verge was 45% (14/31).
There was no association between pCR and the stage of the disease, tumour grade, radiotherapy or ChT interruption, the total dose of radiation therapy, and time to operation from CRT completion on the Fisher’s exact test.
Survival and late toxicity
The median follow-up time was 55 months (range 2–71 months). One local relapse was recorded (1/63; 1.6%) among the operated patients. The 5-year local control rate was 92.7% (95% CI 85.9–99.6). Distant metastases were noted in 14 (14/66; 21.2%) patients (three patients with liver metastases, seven with lung metastases, three with both liver and lung, and one with both lung metastases and peritoneal carcinomatosis).
As of August 2017, 20 of the 66 (20/66; 30.3%) patients had died due to: treatment complications (N = 2), disease progression (N = 14), secondary cancers (i.e. gastric cancer (N = 1), prostate cancer (N = 1), new rectal cancer (N = 1)), and non-disease or treatment-related cause (suicide (N = 1)).
The 5-year DFS rate was 64.0% (95 % CI 63.89–64.11). The median survival has not yet been reached. The 5-year OS rate was 69.5% (95% CI 69.39–69.61).
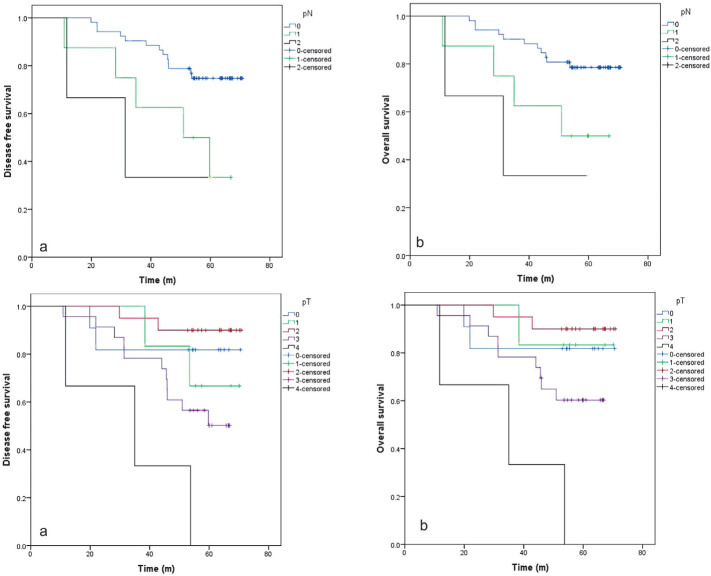
Patients with pN+ or T3–4 had a significantly worse OS and DFS (Figure 2).
Figure 2.
Prognostic significance of pathologic nodal stage (pN) and pathologic tumour stage (pT) in 5-year disease free survival and overall survival in rectal cancer after preoperative chemoradiotherapy and surgery. Time (m = months).
Forty-three (65.1 %) of all patients in the study received all treatment according to protocol and they had significantly better OS (79.1% vs. 52.2%) and DFS (74.4% vs. 47.8%) compared to patients with less treatment with p < 0.05 (Table 5).
Table 5.
Univariate analysis of overall survival (OS) and disease free survival (DFS) according to patient, disease, and treatment characteristics (N = 66)
| Parameter | OS | DFS |
|---|---|---|
| Gender | ns | ns |
| Age | ns | ns |
| WHO PSa | ns | ns |
| Tumour location in the rectum | ns | ns |
| cTumour stage | ns | ns |
| cNodal stage | ns | ns |
| Type of surgery: APR vs. LAR | ns | ns |
| pT1–2 vs. pT3–4 | 0.005 | 0.002 |
| pT0 vs. pT4 | 0.009 | 0.009 |
| pN0 vs. pN+ | 0.009 | 0.005 |
| TRG 3–4 vs. 0–2 | ns | ns |
| pCR | ns | ns |
| All treatment vs. less treatment | 0.016 | 0.018 |
APR = abdominoperineal resection; LAR = low anterior resection; ns = not specific (p > 0.05); p = pathologic; PS = performance status, PCR = pathologic complete response; TRG = tumour regression grade
Late toxicity data was available for 57 patients. The recorded rate for G ≥ 3 toxicity was: 3.5% faecal incontinence, 1.8% stoma prolapse, 3.5% rectal anastomotic leak, 1.8% rectal stenosis, 1,8% diarrhoea, 1.8% perineal abscess, 3.5% ileus, 1.8% myocutaneous flap defect and 3.5% urinary tract obstruction. One patient died due to colon perforation.
Discussion
Neoadjuvant treatment intensification with one-cycle induction capecitabine, standard CRT, and two-cycle consolidation capecitabine did not significantly improve the rate of pCR in LARC compared to standard CRT with capecitabine.
Additional three cycles of capecitabine prior to operation were not enough to achieve a better local effect in preoperative treatment, which is in agreement with the studies that tried to optimize the treatment of LARC with total neoadjuvant (NA) therapy. In a Spanish GCR-3 phase II trial, patients were randomized to 4 cycles of neoadjuvant CAPOX (capecitabine and oxaliplatin), followed by CRT and operation; or to second arm with CRT, operation, and 4 cycles of adjuvant (A) CAPOX.13 The treatment was intensified with an up-front ChT in the first arm and with the addition of oxaliplatin to concomitant capecitabine in both arms. They reported pCR rates of 14% and 13% in the first and second arms, respectively. The second randomized trial with standard CRT with or without NA ChT with 5-FU/OX was closed prematurely due to the similar rates of pCR in both arms.14 In a pooled analysis of phase II EXPERT (NA ChT CAPOX - CRT and A CAPOX) and EXPERT C (addition of cetuximab) trials, the pooled pCR rate for 269 patients was 19%.15,16,17 With only three additional cycles to CRT preoperatively with capecitabine only, we report a pCR rate of 17.5%. The negative results of our primary endpoint and the rate of pCR similar to other studies suggests that NA capecitabine does not have an additional local effect in standard CRT, nor does the second concomitant chemotherapy agent in more intensified regimes - a conclusion that is supported by a recent meta-analysis.18
Despite the questionable local effect of systemic neoadjuvant capecitabine, we have recorded an improvement in N downstaging rate from 52% with classical treatment12 to 77% with experimental treatment and, consequently, improved the total downstaging rates from 50% to 79%, respectively. With potential to impact micrometastatic disease early with an up-front ChT, the regional effect can potentially contribute to an improved treatment outcome with NA ChT, since the pathologic N stage is an independent prognostic factor for the incidence of distant metastasis.19 This observed effect addresses an additional question on the appropriateness of pCR as a primary endpoint for phase II LARC treatment optimization trials.
The expected effect of adjuvant ChT on the treatment outcome in rectal cancer is compromised by the late introduction of ChT after local treatment with CRT and surgery20 and the treatment compliance, since less than half of the patients receive the planed dose.21 The main findings of trials with total neoadjuvant ChT were the improved tolerance and completion rates of ChT.13,14,17,22,23 Our results are in concordance with the reported compliance of 85–95%13,17,23, since all the patients received an induction cycle and 93.8% received both consolidation cycles. CRT was not compromised, with 98.5% patients having completed radiotherapy according to the treatment protocol. We recorded an excellent 87% adherence to adjuvant ChT, compared to only 57% in the GCR 3 trial, probably due to the toxicity of oxaliplatin addition to the regime.13
The acute toxicity of our protocol treatment was acceptable. No G3/4 events were recorded during induction and adjuvant ChT. However, after CRT one patient died (G5 toxicity) because of pulmonary thromboembolism. The most frequent G3/4 toxicities during CRT were diarrhoea, radiodermatitis, and hematologic side effects. An intensified preoperative treatment regime did not affect the surgery complication rate, as is the case with standard treatment.24 All patients but one achieved R0 resection and we report an excellent 5-year local recurrence rate of 1.6%.
Compared to our historical cohort, we achieved a similar 5y LC (92% vs. 87%), slightly improved 5y OS (69.5% vs. 61%), and improved 5y DFS of 64% compared to 52% in standard treatment.12 The results are similar to the 5y OS 67%–77% and DFS 63%–64% results16,25,26 from total neoadjuvant therapy trials. Similarly to other studies, we found the pathologic T and N stages to be significant prognostic factors for OS and DFS.21,23 The OS and DFS were also significantly better in patients who received all planed therapy, compared to the patients with less treatment, signalling the importance of treatment compliance for disease outcome.
The main limitations of our study are the singlearm non-randomized design with pCR as a primary endpoint and the small sample size. The systemic treatment consisted of capecitabine, which was standard at the time but is suboptimal today.16,25,26 Larger randomized trials are needed to evaluate the efficacy of intensified neoadjuvant treatment.
To our knowledge, this study is, beside the Italian investigation of Zampino et al., the only neoadjuvant intensification study that does not influence the timing of CRT and surgery.27 We took advantage of the waiting time in local therapy for an additional administration of three cycles of capecitabine preoperatively, without the additional intensification with a second systemic agent. Our regime was well tolerated with excellent compliance, and although we couldn’t achieve a significantly higher rate of pCR, we report high 5y DFS and OS that are within range of the published results from more intensified regimes. We believe that our regime can be used to treat patients with LARC who are not suited for combination chemotherapy, namely because of good results, excellent compliance and the additional advantage of shorter treatment time.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L. et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200–7. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 2.Martin ST, Heneghan HM, Winter DC.. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg. 2012;99:918–28. doi: 10.1002/bjs.8702. [DOI] [PubMed] [Google Scholar]
- 3.Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu A. et al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol. 2012;19:2822–32. doi: 10.1245/s10434-011-2209-y. [DOI] [PubMed] [Google Scholar]
- 4.Velenik V, Anderluh F, Oblak I, Strojan P, Zakotnik B.. Capecitabine as a radiosensitizing agent in neoadjuvant treatment of locally advanced resectable rectal cancer: prospective phase II trial. Croat Med J. 2006;47:693–700. [PMC free article] [PubMed] [Google Scholar]
- 5.Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL. et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–65. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 6.Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G. et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 Randomized Phase III Trial. J Clin Oncol. 2011;29:2773–80. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC.. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-09854. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Common terminology criteria for adverse events v4.0 (CTCAE). 2009;2009:0–71. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Publish. Available at. [Google Scholar]
- 9.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET. et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–56. [PubMed] [Google Scholar]
- 10.Fleming TR.. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–51. [PubMed] [Google Scholar]
- 11.A’Hern RP.. Sample size tables for exact single-stage phase II designs. Stat Med. 2001;20:859–66. doi: 10.1002/sim.721. [DOI] [PubMed] [Google Scholar]
- 12.Velenik V, Oblak I, Anderluh F.. Long-term results from a randomized phase II trial of neoadjuvant combined-modality therapy for locally advanced rectal cancer. Radiat Oncol. 2010;5:88. doi: 10.1186/1748-717X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B. et al. Phase II. randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–65. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 14.Marechal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P. et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30. doi: 10.1093/annonc/mdr473. [DOI] [PubMed] [Google Scholar]
- 15.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A. et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–7. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 16.Sclafani F, Peckitt C, Cunningham D, Tait D, Giralt J, Glimelius B. et al. Short- and long-term quality of life and bowel function in patients with MRI-defined, high-risk, locally advanced rectal cancer treated with an intensified neoadjuvant strategy in the randomized phase 2 EXPERT-C Trial. Int J Radiat Oncol. 2015;93:303–12. doi: 10.1016/j.ijrobp.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 17.Sclafani F, Brown G, Cunningham D, Wotherspoon A, Tait D, Peckitt C. et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27:1557–65. doi: 10.1093/annonc/mdw215. [DOI] [PubMed] [Google Scholar]
- 18.Teo MTW, McParland L, Appelt AL. Sebag-Montefiore D. Phase 2 neoadjuvant treatment intensification trials in rectal cancer: a systematic review. Int J Radiat Oncol. 2018;100:146–58. doi: 10.1016/j.ijrobp.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C. et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32:1554–62. doi: 10.1200/JCO.2013.54.3769. [DOI] [PubMed] [Google Scholar]
- 20.Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM.. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer. JAMA. 2011;305 doi: 10.1001/jama.2011.749. 2335. [DOI] [PubMed] [Google Scholar]
- 21.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L. et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 22.Perez K, Safran H, Sikov W, Vrees M, Klipfel A, Shah N. et al. Complete neoadjuvant treatment for rectal cancer: the Brown University Oncology Group CONTRE Study. Am J Clin Oncol. 2017;40:283–7. doi: 10.1097/COC.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 23.Nogue M, Salud A, Vicente P, Arrivi A, Roca JM, Losa F. et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS Study. Oncologist. 2011;16:614–20. doi: 10.1634/theoncologist.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Connell MJ, Colangelo LH, Beart RW, Petrelli NJ, Allegra CJ, Sharif S. et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927–34. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schou J V, Larsen FO, Rasch L, Linnemann D, Langhoff J, Høgdall E. et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–33. doi: 10.1093/annonc/mds056. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C. et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–8. doi: 10.1093/annonc/mdv223. [DOI] [PubMed] [Google Scholar]
- 27.Zampino MG, Magni E, Leonardi MC, Petazzi E, Santoro L, Luca F. et al. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:421–7. doi: 10.1016/j.ijrobp.2008.11.002. [DOI] [PubMed] [Google Scholar]