Abstract
Background
The association of subacute thyroiditis (SAT) and papillary thyroid carcinoma is a rare finding. In this study, we aimed to investigate the prevalence of differentiated thyroid cancer in a cohort of patients followed with the diagnosis of SAT.
Patients and methods
We retrospectively screened medical records of Endocrinology and Metabolism outpatient clinic in the past 20 years for patients with SAT. Patients with nodules and suspicious ultrasonography findings who underwent fine needle aspiration biopsy (FNAB) and operated due to malignancy risk were identified.
Results
We identified 137 (100 females, 37 males) patients with reliable records to confirm the diagnosis of SAT. The mean age of female patients was 41.1 ± 9.1 (range, 20–64) and of male patients was 43.0 ± 9.3 (range, 20–65). One or more FNAB was performed in 23 of the patients (16.8%) at the beginning and/or during the follow-up period when needed. Seven patients with suspicious FNAB findings were operated, and histopathological examination of the nodules confirmed the diagnosis of papillary thyroid carcinoma in 6 patients (4.4%).
Conclusions
Our observations suggesting a relatively higher prevalence of thyroid cancer in a small series of SAT patients warrant further studies to identify the real frequency of differentiated thyroid cancer and its association with inflammatory pathogenesis of SAT. This finding is compatible with the trend of increased thyroid cancer incidence all over the world. A repeat ultrasonography after resolution of clinical and inflammatory findings, and FNAB should be recommended to all patients with suspicious nodules.
Key words: subacute thyroiditis, thyroid nodule, thyroid cancer, ultrasonography
Introduction
Subacute thyroiditis (SAT) is a self-limited, granulomatous inflammatory disorder of the thyroid gland. The diagnosis of SAT is based on the clinical findings including fever, pain and tenderness in the thyroid gland and laboratory findings of acute phase response such as elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR), elevated free T4 (fT4) and decreased thyroid stimulating hormone (TSH) concentrations in serum.1 The scintigraphy findings and/or low 24 h radioiodine uptake results are also used to confirm the diagnosis.1
Although it is not necessary for diagnosis of SAT, most of the patients undergo an ultrasound imaging of the thyroid gland, and the presence of typical thyroiditis findings support the diagnosis.2,3,4 Thyroid ultrasonography is currently the most sensitive method to detect the presence of nodules in the thyroid, which is a common and usually benign disorder. Among persons without suspected thyroid disease, the frequency of thyroid nodules detected by ultrasound is ranging between 19% to 67%.5 Ultrasonographic features of the nodules may give important clues in terms of their potential for malignancy5, 6, and about 8% to 16% of the nodules can be documented as malignant.5
There are very few studies reporting the prevalence of thyroid cancer in patients with SAT.1, 7,8,9,10 In addition to the findings compatible with thyroiditis, ultrasonographic examination of the patients with SAT may also reveal thyroid nodules incidentally. In some patients, pseudo-nodules seen in association with thyroiditis, which cannot be distinguished easily from malignant nodules with irregular margins; and a close follow-up in parallel with the resolution of inflammatory findings of SAT may be helpful in differential diagnosis.
In this study, we aimed to investigate the prevalence of differentiated thyroid cancer in a cohort of patients followed with the diagnosis of SAT.
Patients and methods
We retrospectively screened available medical records of Endocrinology and Metabolism outpatient clinic archive in the past 20 years for patients with SAT. To confirm the diagnosis of SAT from the charts, we re-evaluated their records for the clinical findings compatible with the diagnosis such as fever, painful or tender thyroid gland, laboratory findings of acute phase response such as elevated ESR (>20 mm/hour) and/or serum CRP levels (>5 mg/L), thyroid function tests such as elevated serum fT4 and decreased serum TSH, compatible thyroid scintigraphy findings and decreased 24 h radioiodine uptake, and when available histopathological evaluation of thyroidectomy material.
Figure 1.
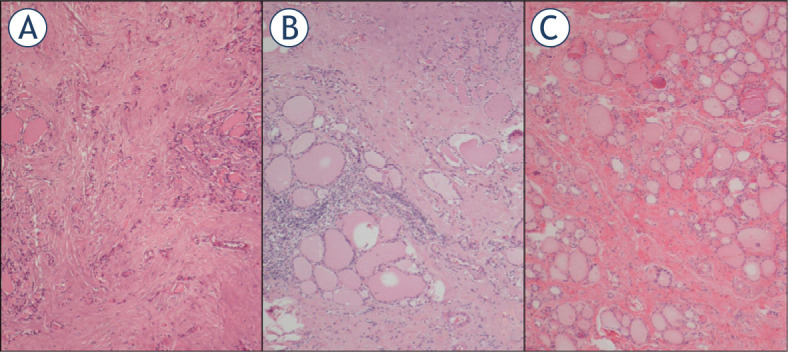
Haematoxylin and eosin stained sections of Case 4 (A), Case 5 (B) and Case 6 (C). Follicular atrophy and fibrosis, fibrosis accompanied by chronic inflammatory cells and fibrosis are seen, respectively.
Available records of ultrasonographic findings of the patients during follow-up were evaluated, and those patients who underwent fine needle aspiration biopsy (FNAB) and operated due to malignancy risk were identified.
Haematoxylin and eosin stained sections of thyroidectomy specimens of all cases diagnosed with papillary thyroid cancer, except one case who was operated in another hospital, were re-evaluated for this study by one of the authors (GY) to confirm the diagnosis of thyroid cancer according to the WHO 2017 classification of thyroid tumors; and histopathological changes of the non-tumoral thyroid tissue were also evaluated for the subacute thyroiditis associated findings.
The study adhered to the tenets of the Declaration of Helsinki and was submitted and approved by Institution Ethical Committee. All data were recorded using a standard form.
Results
We screened the records of the 9156 charts, which included 4757 patients with a thyroid disease, including 699 with Graves disease, 658 with Hashimoto thyroiditis and 2453 with papillary thyroid cancer. Among these 4757 patients with thyroid disease, we identified 137 (100 females, 37 males) patients with reliable records to confirm the diagnosis of SAT. The mean age of female patients was 41.1 ± 9.1 (range, 20–64) and of male patients was 43.0 ± 9.3 (range, 20–65).
One or more FNAB was performed in 23 of the patients with SAT (16.8%) at the beginning and/or during the follow-up period when needed according to the ultrasonography findings suspicious for thyroid malignancy. Because of cytological examination, 7 out of 23 patients with suspicious FNAB findings underwent thyroidectomy, and histopathological examination of the nodules confirmed the diagnosis of papillary thyroid carcinoma in 6 patients (4.4%).
In one of the operated 7 patients (54-year-old, female), diagnosis of SAT was done after the pathological evaluation of thyroidectomy material. She had been previously followed for hyperthyroidism at another center, and she was referred to our center for operation because of the nodules with suspicious ultrasonographic findings and FNAB findings, which were reported as suspicious for papillary carcinoma. Histopathological examination revealed colloid nodules, chronic lymphocytic thyroiditis as well as findings of subacute thyroiditis including granulomatous thyroiditis. There was no evidence of malignancy, and retrospective evaluations revealed only elevated acute phase response but no clinical findings related to SAT such as neck pain or fever.
Demographic characteristics and laboratory findings of the remaining six patients with papillary thyroid carcinoma at the time of diagnosis of SAT are given in Table 1; and their presurgical ultrasonographic findings and histopathologic features are summarized in Table 2.
Table 1.
Demographic characteristics and laboratory findings at disease onset of patients with subacute thyroiditis and papillary thyroid cancer
| Cases | Age | Sex | FT3 (pmol/L) (3.1–6.8) | FT4 (pmol/L) (12–22) | TSH (miU/L) (0.27–4.2) | CRP (mg/L) (0–5) | ESR (mm/h) (0–20) | ∗Tc 99m /RAI Uptake (%) (0.3–3 vs. 20–50) | Ultrasonography |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 42 | F | NA | 19.9 | 0.2 | NA | 55 | Low/0.2 | 3.2 cm hypoechoic nodule |
| 2 | 56 | F | 6.73 | 26.3 | 0.009 | NA | 34 | 0.06/1.35 | Diffuse HEAs, 2.2 cm hypo-isoechoic nodule |
| 3 | 56 | F | 4.5 | 21.1 | 0.68 | 125.0 | 100 | 0.59/NA focal hypoactivity | 1.8 cm focal HEA, 0.7 cm hypoechoic nodule with microcalcification |
| 4 | 51 | M | NA | 44.2 | 0.01 | NA | 91 | Low/NA | Focal HEAs, 1.6 cm isoechoic nodule |
| 5 | 52 | F | 7.01 | 24.7 | 0.02 | 15.6 | 60 | Low/NA | 2.4 cm heterogenous nodule with calcification and 1.1 cm isoechoic nodule |
| 6 | 45 | F | 13 | 45 | 0.005 | 138.8 | 132 | Low/NA | 2.2 cm hypoechoic, 1.9 cm isoechoic nodules |
CRP =C-reactive protein; ESR = erythrocyte sedimentation rate; F = female; FT3 = free triiodothyronine; FT4 = free thyroxine; HEA = hypoechogenic area; M = male; NA, not available; RAI = radioactive iodine; Tc 99m = Technetium-99m; TSH = thyroid-stimulating hormone
All of the patients had Technetium-99m scintigraphy, additionally some of them had either Technetium-99m uptake or 24-h RAI uptake.
Table 2.
Presurgical ultrasonographic findings and histopathologic features of subacute thyroiditis patients with papillary thyroid cancer
| Cases | Op.Time(mo) | Nodule size in USG (cm)∗ | Sonographic features of nodules | FNAB | Tumor subtype/Histology | Tumor size (cm) | Stage (8th TNM) | Treatment |
|---|---|---|---|---|---|---|---|---|
| 1 | 107 | 0.55 and 0.50 | Hypoechoic, indefinite margins | Suspicious for malignancy | Papillary-tall cell and classical Focal fibrosis |
0.5 and 0.05 | I | TT+RAI |
| 2 | 13 | 2.4 and 1.0 | Hypo-isoechoic, calcification | Dyskaryotic thyrocytes | Papillary-classical Chronic lymphocytic thyroiditis |
1.0 | I | TT+RAI |
| 3 | 29 | 0.7 | Hypoechoic, microcalcification | FLUS Suspicious for malignancy | Papillary-follicular variant Fibrosis, chronic lymphocytic thyroiditis |
0.6 | I | TT |
| 4 | 16 | 1.9 | Isoechoic | Suspicious for malignancy | Papillary-follicular variant Fibrosis, focal follicular atrophy |
0.4 | I | Lobectomy |
| 5 | 13 | 1.1 and 0.73 | Isoechoic, microcalcification | Suspicious for malignancy | Papillary-classical and follicular | 1.1, 0.7, 0.3, 0.2 | I | TT+ RAI |
| Fibrosis, chronic lymphocytic thyroiditis | ||||||||
| 6 | 37 | 1.7 and 0.9 | Hypoechoic and isoechoic | AUS Papillary carcinoma | Papillary-classical and follicular Fibrosis |
1.2, 0.3, 0.2 | I | TT+RAI |
AUS = atypia of undetermined significance; FLUS = follicular lesion of undetermined significance; FNAB = fine needle aspiration biopsy; Op = operation time after the diagnosis of subacute thyroiditis in months; RAI = radioactive iodine; TT = total thyroidectomy; USG = ultrasonography
In patients with more than two nodules, the sizes of the dominant ones are given.
Three of the 137 patients with SAT and papillary thyroid cancer described a positive family history for papillary thyroid cancer. Case 3 and Case 5 were first cousins, and the elderly sister of Case 3 was also diagnosed with SAT, and her father had a history of thyroid cancer diagnosed elsewhere.
Another patient (Case 1) described a positive family history for papillary thyroid cancer in her elderly brother. She was diagnosed with acromegaly and papillary cancer during the follow-up period, about 9 years after the diagnosis of SAT. She was first operated for thyroid nodule following findings of FNAB compatible with papillary thyroid cancer. She was later operated for acromegaly by endoscopic trans-sphenoidal pituitary surgery, which resulted in remission.
In four of the patients, the tumor size was ≤1cm and the remaining two patients had multifocal thyroid cancers with only one focus >1cm (the largest tumor diameter was 1.1 cm and 1.2 cm, respectively).
Non-tumoral thyroid tissue findings were summarized in Table 2. Patients underwent thyroidectomy 35.8 ± 36.2 (range, 13–107) months after SAT diagnosis (Table 2). Re-examination of all cases except Case 2, who was operated at another center, revealed findings of focal fibrosis. Case 1 and Case 5 had additional findings of chronic inflammation. In Case 4, focal follicular atrophy was also observed. No evidence of granulomatous or acute inflammation was seen in the investigated samples.
Another patient who underwent FNAB twice was still being followed-up closely, since his first biopsy was suspicious and the second biopsy was considered as benign.
Discussion
This retrospective investigation of 137 patients with confirmed diagnosis of SAT revealed 6 patients (4.4%) with papillary thyroid carcinoma. The association of SAT and thyroid carcinoma is a very rare finding, and they were usually published as case reports.1,7,8,9,10,11,12 The most comprehensive study on this subject is the work of Nishihara et al. in Japanese patients with SAT.8 In this study, 5 papillary thyroid carcinomas were detected in 1152 cases (0.4%) of SAT. Another study conducted with data of 160 SAT patients from Olmsted County, Minnesota, USA documented no thyroid cancer.1 In this study a subgroup of 94 patients were followed-up for 28 years, which revealed 11.4% cumulative malignancy rate, but none of them had thyroid cancers.1
Relatively high prevalence of papillary thyroid carcinoma in our study may have some explanations. First, the records of our Endocrinology and Metabolism Outpatient Clinic may have biases as a tertiary referral center which lead to the accumulation of refractory cases or of patients requiring advanced care. An important proportion of SAT patients could be managed at the general internal medicine outpatient clinic of our hospital, and 137 out of 9156 screened archive patients being followed at Endocrinology clinic may not represent the whole SAT patients.
In tertiary referral centers, the co-incidence of two or more rare conditions may be seen at higher rates than expected due to Berkson’s bias. Similarly, co-incidental conditions may affect the risk of other diseases. One of our patients who underwent total thyroidectomy approximately 9 years after the diagnosis of SAT also had acromegaly and a positive family history for papillary thyroid carcinoma, which are known to be associated with increased thyroid cancer risk.13,14,15
Differences in the prevalence of papillary cancer in various populations may also contribute to the conflicting results. Epidemiological surveys from different regions of Turkey revealed a thyroid cancer incidence rate of 5.5/100,000 in healthy male population and 20.7/100,000 in healthy female population.16 These rates are compatible with the rates of thyroid cancer reported from other countries including Japan (approximately 5/100,000 in males and 20/100,000 in females) and USA (overall 14.3/100,000, and 6.9/100,000 in males, 21.4/100,000 in females).17,18 Therefore, it is hard to explain the results of current study with the amount of variability of papillary cancer rates in different countries.
The widespread use of imaging methods is usually considered as an important factor for the current trend all over the world documenting an increase in the incidence of thyroid cancer.17,18,19,20,21,22 Increased use of diagnostic imaging procedures results in the identification of previously undiagnosed subclinical thyroid cancers. All our patients were operated after 2008. Therefore, closer follow-up due to another thyroid disease may result in increased diagnosis of sub-centimeter thyroid cancers, which otherwise would not be noticed.
Another explanation for the increased prevalence may be due to environmental factors such as ionizing radiation exposure, which has the strongest association with thyroid cancers. Chernobyl disaster related radioactive dispersion in 1986 affected mainly the North Eastern part of the Black Sea region of Turkey, and this exposure may have a role on the observed findings.23,24,25,26,27,28 Epidemiological surveys provided contradictory results regarding the effects of Chernobyl disaster in Turkey, which happened in an area more than 1500 km away from the shores of Black Sea shores. All but one of our 6 patients lived in cities around the Black Sea and their mean age was 25.2 ± 6.6 (range, 17–35) in 1986. We think that available data do not provide hard evidences associated with Chernobyl disaster for any type of cancer in Turkey within 30 years and it is not possible to draw a conclusive decision for ionizing radiation exposure as a possible etiological factor.
Other environmental factors like cigarette smoking, iodine excess, obesity and endocrine disrupting chemicals may also be associated with the increased thyroid cancer risk.20 Mandatory iodization of household salt in Turkey after 1999 may also be speculated as an additional environmental factor.29,30,31 However no comparative data could be found regarding the risk for thyroid cancer associated with increased iodine uptake following the changes in household salts.
On the other hand, inflammation is one of the most critical components affecting the cancer risk in patients with an inflammatory disorder. Increased rates of papillary cancer were reported in autoimmune thyroid disorders.32,33 Papillary cancer rate was found 8% in patients with Graves’ disease, (13% and 5.4% in those with and without a nodule, respectively) in a study from Turkey.34 On the other hand, studies in Hashimoto thyroiditis provided conflicting results.35,36 Within the same context, SAT may also be considered as a risk factor for the development of papillary thyroid cancer by its unique inflammatory changes within the thyroid tissue. Findings of the current study warrant further investigations to understand the dynamics of different inflammatory pathways and associated risk for thyroid cancers.
Lastly, guidelines affect the indications for FNAB in the follow-up of patients with thyroid nodules.37,38 Two of the patients with nodules <1cm at ultrasonography were evaluated before 2015, and both had a family history for thyroid cancers in first degree relatives. FNAB investigation may not have been performed according to current guidelines since papillary microcarcinomas are considered as clinically not significant.38 However, one of these patients had a 0.5 cm tall cell variant of papillary microcarcinoma, and this early intervention would be beneficial for her long-term survival.39,40
Our work has several limitations. It is a retrospective study, and it lacks some important information. Ultrasonographic examination findings were the main clues for the decision of thyroidectomy, but some of the investigations were performed by different radiologists in different hospitals before being referred to us. Since SAT is a self-limiting disorder, some of the patients lost to follow-up and had no repeated ultrasonographic examinations. However, all these limitations may only lower the possibility of diagnosed patients among this series and cannot explain the relatively higher frequency compared to the Japanese and American series of SAT patients.
Conclusions
In conclusion, our observations suggesting a relatively higher prevalence of thyroid cancer compared to healthy controls in a small series of SAT patients warrant further studies to identify the real frequency of differentiated thyroid cancer and its association with inflammatory pathogenesis of SAT. Considering the possibly increased prevalence rate of thyroid cancer in SAT patients, a repeat ultrasonography after resolution of clinical and inflammatory findings for all patients and FNAB for those with suspicious nodules should be recommended.
Disclosure
No potential conflicts of interest were disclosed.
References
- 1.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ.. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88:2100–5. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 2.Nishihara E, Amino N, Ohye H, Ota H, Ito M, Kubota S. et al. Extent of hypoechogenic area in the thyroid is related with thyroid dysfunction after subacute thyroiditis. J Endocrinol Invest. 2009;32:33–6. doi: 10.1007/BF03345675. [DOI] [PubMed] [Google Scholar]
- 3.Frates MC, Marqusee E, Benson CB, Alexander EK.. Subacute granulomatous (de Quervain) thyroiditis: grayscale and color Doppler sonographic characteristics. J Ultrasound Med. 2013;32:505–11. doi: 10.7863/jum.2013.32.3.505. [DOI] [PubMed] [Google Scholar]
- 4.Lee YJ, Kim DW.. Sonographic characteristics and interval changes of subacute thyroiditis. J Ultrasound Med. 2016;35:1653–9. doi: 10.7863/ultra.15.09049. [DOI] [PubMed] [Google Scholar]
- 5.Burman KD, Wartofsky L.. Thyroid nodules. N Engl J Med. 2015;373:2347–56. doi: 10.1056/NEJMcp1415786. [DOI] [PubMed] [Google Scholar]
- 6.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L.. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6:225–37. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam KY, Lo CY.. Papillary carcinoma with subacute thyroiditis. Endocr Pathol. 2002;13:263–5. doi: 10.1007/s12022-002-0003-x. [DOI] [PubMed] [Google Scholar]
- 8.Nishihara E, Hirokawa M, Ohye H, Ito M, Kubota S, Fukata S. et al. Papillary carcinoma obscured by complication with subacute thyroiditis: sequential ultrasonographic and histopathological findings in five cases. Thyroid. 2008;18:1221–5. doi: 10.1089/thy.2008.0096. 10.1089=thy.2008.0096. [DOI] [PubMed] [Google Scholar]
- 9.Choia YS, Kima BK, Kwon HJ, Lee JS, Heo JJ, Jung SB. et al. Subacute lymphocytic thyroiditis with coexisting papillary carcinoma diagnosed by immediately repeat fine needle aspiration: a case report. J Med Cases. 2012;3:308–11. [Google Scholar]
- 10.Valentini RB, Macedo BM, Izquierdo RF, Meyer EL.. Painless thyroiditis associated to thyroid carcinoma: role of initial ultrasonography evaluation. Arch Endocrinol Metab. 2016;60:178–82. doi: 10.1590/2359-3997000000104. [DOI] [PubMed] [Google Scholar]
- 11.Ucan B, Delibasi T, Cakal E, Arslan MS, Bozkurt NC, Demirci T. et al. Papillary thyroid cancer case masked by subacute thyroiditis. Arq Bras Endocrinol Metabol. 2014;58:851–4. doi: 10.1590/0004-2730000003222. [DOI] [PubMed] [Google Scholar]
- 12.Şenel F, Karaman H, Ertan T.. Co-occurrence of subacute granulomatous thyroiditis and papillary microcarcinoma. Kulak Burun Bogaz Ihtis Derg. 2016;26:248–50. doi: 10.5606/kbbihtisas.2016.36776. [DOI] [PubMed] [Google Scholar]
- 13.Gullu BE, Celik O, Gazioglu N, Kadioglu P.. Thyroid cancer is the most common cancer associated with acromegaly. Pituitary. 2010;13:242–8. doi: 10.1007/s11102-010-0224-9. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos MC, Nascimento GC, Nascimento AG, Carvalho VC, Lopes MH, Montenegro R. et al. Thyroid cancer in patients with acromegaly: a case-control study. Pituitary. 2013;16:109–14. doi: 10.1007/s11102-012-0383-y. [DOI] [PubMed] [Google Scholar]
- 15.Wolinski K, Stangierski A, Dyrda K, Nowicka K, Pelka M, Iqbal A. et al. Risk of malignant neoplasms in acromegaly: a case-control study. J Endocrinol Invest. 2017;40:319–22. doi: 10.1007/s40618-016-0565-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Republic of Turkey Ministry of Health. Health Statistics Yearbook 2015. 2017 Nov 15; http://ekutuphane.sagem.gov.tr/kitaplar/health_statistics_yearbook_2015.pdf cited. Available from. [Google Scholar]
- 17.Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M. et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Jpn J Clin Oncol. 2015;45:390–401. doi: 10.1093/jjco/hyv002. [DOI] [PubMed] [Google Scholar]
- 18.Davies L, Welch HG.. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–22. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 19.Morris LG, Sikora AG, Tosteson TD, Davies L.. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitahara CM, Sosa JA.. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646–53. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris LG, Tuttle RM, Davies L.. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142:709–11. doi: 10.1001/jamaoto.2016.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiltshire JJ, Drake TM, Uttley L, Balasubramanian SP.. Systematic review of trends in the incidence rates of thyroid cancer. Thyroid. 2016;26:1541–52. doi: 10.1089/thy.2016.0100. [DOI] [PubMed] [Google Scholar]
- 23.Acar H, Cakabay B, Bayrak F, Evrenkaya T.. Effects of the Chernobyl disaster on thyroid cancer incidence in Turkey after 22 years. ISRN Surg. 2011;2011 doi: 10.5402/2011/257943. 257943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocakusak A.. Did Chernobyl accident contribute to the rise of thyroid cancer in Turkey? Acta Endo (Buc) 2016;12:362–67. doi: 10.4183/aeb.2016.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emral R, Baştemir M, Güllü S, Erdoğan G.. Thyroid consequences of the Chernobyl nuclear power station accident on the Turkish population. Eur J Endocrinol. 2003;148:497–503. doi: 10.1530/eje.0.1480497. [DOI] [PubMed] [Google Scholar]
- 26.Zengi A, Karadeniz M, Erdogan M, Ozgen AG, Saygili F, Yilmaz C. et al. Does Chernobyl accident have any effect on thyroid cancers in Turkey? A retrospective review of thyroid cancers from 1982 to 2006. Endocr J. 2008;55:325–30. doi: 10.1507/endocrj.K08E-007. [DOI] [PubMed] [Google Scholar]
- 27.Ozdemir D, Dagdelen S, Kiratli P, Tuncel M, Erbas B, Erbas T.. Changing clinical characteristics of thyroid carcinoma at a single center from Turkey: before and after the Chernobyl disaster. Minerva Endocrinol. 2012;37:267–74. [PubMed] [Google Scholar]
- 28.Yildiz SY, Berkem H, Yuksel BC, Ozel H, Kendirci M, Hengirmen S.. The rising trend of papillary carcinoma in thyroidectomies: 14-years of experience in a referral center of Turkey. World J Surg Oncol. 2014;12:34. doi: 10.1186/1477-7819-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess JR.. Temporal trends for thyroid carcinoma in Australia: an increasing incidence of papillary thyroid carcinoma (1982–1997) Thyroid. 2002;12:141–9. doi: 10.1089/105072502753522374. [DOI] [PubMed] [Google Scholar]
- 30.Słowińska-Klencka D, Klencki M, Sporny S, Lewiński A.. Fine-needle aspiration biopsy of the thyroid in an area of endemic goiter: influence of restored sufficient iodine supplementation on the clinical significance of cytological results. Eur J Endocrinol. 2002;146:19–26. doi: 10.1530/eje.0.1460019. [DOI] [PubMed] [Google Scholar]
- 31.Zimmermann MB, Galetti V.. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Res. 2015;8(8) doi: 10.1186/s13044-015-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen YK, Lin CL, Chang YJ, Cheng FT, Peng CL, Sung FC. et al. Cancer risk in patients with Graves’ disease: a nationwide cohort study. Thyroid. 2013;23:879–84. doi: 10.1089/thy.2012.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YK, Lin CL, Cheng FT, Sung FC, Kao CH.. Cancer risk in patients with Hashimoto’s thyroiditis: a nationwide cohort study. Br J Cancer. 2013;109:2496–501. doi: 10.1038/bjc.2013.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tam AA, Kaya C, Kiliç FB, Ersoy R, Çakir B.. Thyroid nodules and thyroid cancer in Graves’ disease. Arq Bras Endocrinol Metabol. 2014;58:933–8. doi: 10.1590/0004-2730000003569. [DOI] [PubMed] [Google Scholar]
- 35.Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras NS, Ersoy R. et al. The association between thyroid carcinoma and Hashimoto’s thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010;20:873–8. doi: 10.1089/thy.2009.0118. [DOI] [PubMed] [Google Scholar]
- 36.Anil C, Goksel S, Gursoy A.. Hashimoto’s thyroiditis is not associated with increased risk of thyroid cancer in patients with thyroid nodules: a single-center prospective study. Thyroid. 2010;20:601–6. doi: 10.1089/thy.2009.0450. [DOI] [PubMed] [Google Scholar]
- 37.American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL. et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 38.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE. et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghossein RA, Leboeuf R, Patel KN, Rivera M, Katabi N, Carlson DL. et al. Tall cell variant of papillary thyroid carcinoma without extrathyroid extension: biologic behavior and clinical implications. Thyroid. 2007;17:655–61. doi: 10.1089/thy.2007.0061. 10.1089=thy.2007.0061. [DOI] [PubMed] [Google Scholar]
- 40.Morris LG, Shaha AR, Tuttle RM, Sikora AG, Ganly I.. Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid. 2010;20:153–8. doi: 10.1089/thy.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]


