Summary
The development of efficient synthetic strategies for the discovery of novel antitumor molecules is a major goal in current research. In this context, we report here a catalytic double cyclization process leading to bicyclic heterocycles with significant antitumor activity on different human breast cancer (BC) cell lines. The products, 6,6a-dihydrofuro[3,2-b]furan-2(5H)-ones, were obtained in one step, starting from simple substrates (4-yne-1,3-diols, CO, and O2), under the catalytic action of PdI2 in conjunction with KI. These compounds have significant antiproliferative activity in vitro on human BC cell lines, both hormone receptor positive (MCF-7) and triple negative (triple-negative breast cancer [TNBC]; MDA-MB-231 and MDAMB-468), while exhibiting practically no effects on normal MCF-10A (human mammary epithelial) and 3T3-L1 (murine fibroblasts) cells. Thus, these compounds have the potential to expand the therapeutic options against BC, and in particular, against its most aggressive forms (TNBCs). Moreover, the present synthetic approach may provide an economic benefit for their production.
Subject Areas: Drugs, Organic Synthesis, Toxicology Evaluation
Graphical Abstract

Highlights
-
•
Novel catalytic double cyclization method leading to bicyclic heterocycles in one step
-
•
Direct synthesis of antitumor agents from simple substrates (4-yne-1,3-diols and CO)
-
•
Identification of a new class of antitumor agents against breast cancer (BC) cells
-
•
Significant antitumor activity against the most aggressive triple-negative BC cells
Drugs; Organic Synthesis; Toxicology Evaluation
Introduction
Breast cancer (BC) is the leading cause of cancer death in women worldwide (Siegel et al., 2016, Siegel et al., 2017), particularly in developed countries. Genetic factors, such as mutations of tumor suppressor genes BRCA1 and BRCA2; endocrine factors (such as estrogen exposure); as well as environmental and dietary factors may be involved in the onset of this disease. Several studies of gene expression profile have been performed to characterize BCs in different molecular subtypes, which may have a prognostic value (Voduc et al., 2010). An important classification can be made on the basis of specific receptor expression. Indeed, BCs are divided into hormone receptor (HR)-negative (HR−) and HR-positive (HR+) tumors. The latter group includes estrogen receptor (ER)-positive (ER+), progesterone receptor (PR)-positive (PR+), and human epidermal growth factor receptor 2 (HER2)-positive (HER2+) tumors, whereas HR− tumors include the so-called triple-negative breast cancers (TNBCs), which lack the expression of the three receptor types (Kirkpatrick, 2009). Although the survival rate at 5 years for treated patients with early-stage BC is extremely high, some subgroups of patients with advanced-stage BC may have recurrence within 10 years of treatment. The recurrence rate for HR+ tumors appears to be lower (recurrence rate is 8%) compared with that of the subtypes overexpressing HER2 or that have a triple-negative phenotype (recurrence rate is 15%–20%) (Haffty et al., 2006, Millar et al., 2009, Nguyen et al., 2008).
The selective ER modulator (SERM) tamoxifen and aromatase inhibitors (anastrazole, letrozole) are used for the treatment of tumors expressing steroid HRs, while trastuzumab (Herceptin), a monoclonal antibody that targets HER2, is used for the treatment of HER2+ tumors. TNBCs are particularly dangerous, because they are associated with an unfavorable prognosis (Bauer et al., 2007, Carey et al., 2007, Haffty et al., 2006) and because patients with TNBC derive no benefit from molecularly targeted regimens employing endocrine-based therapy or trastuzumab (Kirkpatrick, 2009). Classical chemotherapy aims to block cell proliferation, which, however, does not discriminate cancer cells from rapidly dividing normal cells within the organism. Therefore, new therapeutic approaches resulting from the discovery of new compounds with antitumor activity against TNBCs are still highly desirable.
In this work, we report an unprecedented catalytic double cyclization process leading to a new class of bicyclic heterocycles (dihydrofurofuranone derivatives) with significant antitumor activity in vitro against BC cell lines, both HR+ (MCF-7) and TNBCs (MDA-MB-231 and MDAMB-468), while exhibiting practically no effects on normal MCF-10A (human mammary epithelial) and 3T3-L1 (murine fibroblasts) cells. These new compounds have therefore the potential to expand the therapeutic options against BC, in particular against its most aggressive forms (TNBCs).
Results and Discussion
Palladium-Catalyzed Carbonylative Oxidative Double Cyclization of 4-Yne-1,2-diols Leading to Dihydrofurofuranone Derivatives
Catalytic cyclization processes have recently attracted great interest in the chemistry scientific community owing to the possibility to synthesize in one step high-value-added molecules starting from simple building blocks, by their ordered sequential activation by the catalytic center (Alcaide and Almendros, 2014, Debrouwer et al., 2015, Guo et al., 2011, Krause and Winter, 2011, Tanaka and Tajima, 2012, Valera and Saa, 2016, Vlaar et al., 2011, Ye and Ma, 2014). Among the raw starting materials, carbon monoxide represents a simple and largely available C-1 unit, and its catalytic activation may allow to directly introduce a carbonyl functional group into an organic substrate in a highly atom-economical and efficient manner. In fact, carbonylation reactions are currently known to play a major role in the direct synthesis of carbonyl compounds both in industry and in the production of fine chemicals (Beller, 2006, Friis et al., 2016, Gabriele et al., 2012, Gadge and Bhanage, 2014, Gehrtz et al., 2016, Kalck and Urrutigoïty, 2015, Kollár, 2008, Liu et al., 2011, Omae, 2011, Peng et al., 2017, Shen and Wu, 2017, Wu and Neumann, 2012, Wu et al., 2013a, Wu et al., 2013b, Wu et al., 2014, Wu, 2016, Wu and Beller, 2016).
In this work, we have developed an unprecedented carbonylative oxidative double cyclization process, which allows synthesizing bioactive, high-value-added bicyclic heterocycles (dihydrofurofuranone derivatives 2) starting from readily available 4-yne-1,3-diols 1; CO; and O2 (Scheme 1). In this reaction, the alkynediol, carbon monoxide, and oxygen are sequentially activated through the catalytic action of a very simple system (consisting of PdI2 in conjunction with an excess of KI) (Gabriele et al., 2014, Mancuso et al., 2014, Mancuso et al., 2015, Mancuso et al., 2016, Veltri et al., 2015, Veltri et al., 2016a, Veltri et al., 2016b, Veltri et al., 2018), with the formation of two cycles and three new bonds (O-C, C-C, and C-O) in one single operation and in ordered sequence. It should be noted that, although an analogous process was reported for 4-ene-1,2-diols under different reaction conditions (Kapitán and Gracza, 2008), no previous example exists in the literature for the direct carbonylative double cyclization of 4-yne-1,3-diols leading to dihydrofurofuranones.
Scheme 1.
PdI2-Catalyzed Carbonylative Oxidative Double Cyclization of 4-Yne-1,3-diols Leading to Dihydrofurofuranones
We started our investigation by studying the reaction between 3-methylnon-4-yne-1,3-diol 1a and carbon monoxide using 2 mol % PdI2 and 10 mol % KI, under 40 atm of a 4:1 mixture of CO and air in MeOH as the solvent (0.05 mmol of 1a per mL of MeOH) at 100°C. After 15 hr reaction time, 3-butyl-6a-methyl-6,6a-dihydrofuro[3,2-b]furan-2(5H)-one 2a was isolated in 30% yield following chromatographic purification (unidentified heavy by-products accounted for substrate total conversion). The yield of 2a did not improve by changing the operative reaction conditions (data not shown); however, its formation confirmed the possibility to realize a previously unreported carbonylative double cyclization process starting from a 4-yne-1,3-diol in one step under catalytic conditions. The result was noteworthy, considering the different possible competitive pathways that could have been followed under carbonylation conditions by an alkynyldiol such as 1a (formation of cyclic carbonates, maleic esters, monocyclic β- or γ-lactones, and so on) (Beller, 2006, Kollár, 2008, Gabriele et al., 2012, Wu et al., 2013a, Wu and Beller, 2016). The catalytic process may be interpreted as occurring through an ordered sequence of mechanistic stages, involving (1) 5-exo-dig cyclization, via intramolecular nucleophilic attack of the hydroxyl group at C-1 to the triple bond coordinated to PdI2; (2) carbon monoxide insertion into the Pd-C bond of the ensuing vinylpalladium intermediate; (3) intramolecular trapping of the ensuing acylpalladium species by the hydroxyl at C-3, with further cyclization and nucleophilic displacement of palladium, leading to the final bicyclic product and Pd(0); and (4) reoxidation of Pd(0) back to PdI2 by oxygen in the presence of the 2 mol HI formally eliminated during the previous cyclization steps (Scheme 1; anionic iodide ligands are omitted for clarity).
We then tested the reactivity of a similar substrate, still bearing a butyl group on the triple bond, but with geminal methyl substituents at C-1. The reaction of 2,4-dimethyldec-5-yne-2,4-diol 1b led to the corresponding dihydrofurofuranone 2b in a higher isolated yield with respect to 2a, most probably due to the effect exerted by the geminal alkyl groups, which is known to favor cyclization processes (Jung and Piizi, 2005, Sammes and Weller, 1995). A 40% yield of 2c was obtained starting from alkynyldiol 1c, bearing a sterically demanding tert-butyl group on the triple bond. On the other hand, 2,4-dimethyl-6-phenylhex-5-yne-2,4-diol 1d, substituted with a phenyl group on the triple bond, afforded the corresponding dihydrofuranone 2d in 33% yield (Scheme 2).
Scheme 2.
Formation of Dihydrofurofuranones 2a–d by PdI2-Catalyzed Carbonylative Oxidative Double Cyclization of 4-Yne-1,3-diols 1a–d
Anionic iodide ligands are omitted for clarity. See Transparent Methods for experimental details.
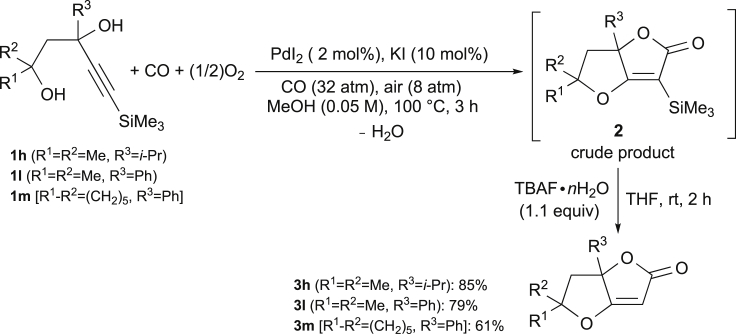
A significant increase in product yield was observed when the triple bond was substituted with a trimethylsilyl group. Thus, 3-methyl-5-(trimethylsilyl)pent-4-yne-1,3-diol 1e, under conditions similar to those previously employed for 1a, led to the dihydrofurofuranone 2e in 72% yield after 3 hr reaction time (Figure 1). Other differently substituted substrates 1f–m, still bearing the trimethylsilyl group (TMS) on the triple bond, behaved similarly, and afforded the corresponding dihydrofuranones 2f–m in fair to excellent yields (58%–94%, Figure 1). The structure of dihydrofurofuranone 2g was also confirmed by X-ray diffractometric analysis (for details see Figure S1, Table S1, Transparent Methods, and cif file for 2g, Data S1).
Figure 1.
Synthesis of Dihydrofurofuranones 2 by PdI2/KI-Catalyzed Carbonylative Oxidative Double Cyclization of 4-Yne-1,3-diols 1
Top: Reaction conditions: Alkynediol 1 (0.70 mmol), PdI2 (1.39 × 10−2 mmol), KI (6.95 × 10−2 mmol), CO (32 atm), air (8 atm), MeOH (14 mL), 100°C, 3–15 hr. In parentheses, isolated yields based on starting 1. See Transparent Methods for experimental details.
Bottom: Reaction time was 3 hr for 2e, 2f, 2g, 2h, 2l, and 2m.
Reaction time was 15 hr for 2i and 2j.
For 2i, the (5RS,6aSR)/(5RS,6aRS) diastereomeric ratio (DR) was ca. 1.9, determined by 1H NMR; starting 1i was a mixture of (2RS,4SR)/(2RS,4RS) diastereomers (DR ca. 2.2, determined by 1H NMR).
For 2j, the (5RS,6aSR)/(5RS,6aRS) DR was ca. 1.8, determined by 1H NMR; starting 1j was a mixture of (2RS,4SR)/(2RS,4RS) diastereomers (DR ca. 1.4, determined by 1H NMR).
Reaction time was 5 hr for 2k.
For 2k, the (5RS,6aRS)/(5RS,6aSR) DR was ca. 1.8, determined by 1H NMR; starting 1k was a mixture of (2RS,4SR)/(2RS,4RS) diastereomers (DR ca. 1.0, determined by 1H NMR). See Transparent Methods for experimental details. NMR, nuclear magnetic resonance.
In the case of alkynediols 1i–k, the substrates employed were a mixture of 2RS,4SR and 2RS,4RS diastereomers. To assess the different performances of the two diastereomers in the double cyclization process, we tested them independently after chromatographic separation. The reactions of the single diastereomers could also allow isolating the corresponding diastereomeric products, considering that the process is stereospecific. The results obtained are shown in Table 1. As can be seen from Table 1, the 2RS,4RS diastereomer was the most productive isomer (in terms of yield obtained for the corresponding dihydrofurofuranone) in the case of 1i and especially 1k, whereas in the case of 1j a slightly higher yield was observed starting from the 2RS,4SR diastereoisomer. The structure of (5RS,6aRS)-2j was unequivocally established by X-ray diffractometric analysis (for details, see Figure S2, Table S2, Transparent Methods, and cif file for 5RS,6aRS-2j, Data S2), which also permitted to corroborate (owing to the stereospecificity of the reaction) the 2RS,4RS stereochemistry of the starting material 1j.
Table 1.
Experiments with Single Diastereomers
| Entrya | 1 | Time (h) | 2 | Yield of 2b |
|---|---|---|---|---|
| 1 | (2RS,4SR)-1i | 8 | (5RS,6aSR)-2i | 52 |
| 2 | (2RS,4RS)-1i | 3 | (5RS,6aRS)-2i | 61 |
| 3 | (2RS,4SR)-1j | 15 | (5RS,6aSR)-2j | 68 |
| 4 | (2RS,4RS)-1j | 8 | (5RS,6aRS)-2j | 52 |
| 5 | (2RS,4SR)-1k | 5 | (5RS,6aSR)-2k | 40 |
| 6 | (2RS,4RS)-1k | 3 | (5RS,6aRS)-2k | 80 |
Reaction conditions: alkynediol 1 (0.25–0.5 mmol), PdI2 (2 mol%), KI (10 mol%), CO (32 atm), air (8 atm), MeOH (5–10 mL), 100°C, 3–15 hr. See Transparent Methods for details.
Isolated yields based on starting 1.
To further expand the synthetic scope of the reaction, we also attempted the desilylation of some representative silylated dihydrofurofuranones. Thus, crude products 2h, 2l, and 2m, deriving from carbonylation of 1h, 1l, and 1m, respectively, were treated, without further purification, with tetrabutylammonium fluoride (TBAF)۰nH2O in THF at room temperature for 2 hr. As shown in Scheme 3, the corresponding desilylated furofuranone derivatives were obtained in good to high yields over the two steps (85%, 79%, and 61% based on starting 1h, 1l, and 1m, respectively).
Scheme 3.
Synthesis of Desilylated Dihydrofurofuranones 3h, 3l, and 3m by PdI2-Catalyzed Carbonylative Double Cyclization of 4-Yne-1,3-diols 1h, 1l, and 1m, Respectively, Followed by TBAF-Induced Desilylation of the Crude Carbonylation Product
See Transparent Methods for experimental details.
Antiproliferative Activity In Vitro of the Newly Synthesized Dihydrofurofuranones on MCF-7, MDA-MB-231, and MDA-MB-468 Human Breast Cancer Cell Lines
Some of the newly synthesized dihydrofurofuranones have shown significant antiproliferative activity in vitro on human breast adenocarcinoma cell lines, including the most dangerous triple-negative ones (MDA-MB-231 and MDAMB-468). Although several furanone derivatives were previously described to possess antitumor properties (for a recent example, see Wu et al., 2017), the possible anticancer activity of bicyclic dihydrofurofuranones such as 2 has not been reported so far. The effects on tumor cell viability of different concentrations (1, 5, 10, 20, 40 μM) of five 6,6a-diidrofuro[3,2-b]furan-2-(5H) derivatives (2d, 2e, 2g, 2l, and 3l) have been evaluated against MCF-7, MDAMB-231, and MDAMB-468 BC cell lines (Figure 2); normal breast epithelial cells MCF-10A; and immortalized fibroblasts 3T3-L1 (Figure 3). In particular, for 2d a significant reduction in vitality of MCF-7 and MDA-MB231 was observed from 10 μM onward, whereas MDA-MB468 was slightly more sensitive and a significant reduction was obtained from the dose of 5 μM (Figure 2A). Compound 2g decreased the cell viability of all three cell lines from the lowest dose of 1 μM (Figure 2B). Compound 2e significantly affected the cell viability of TNBC cell lines from 5 μM, whereas it was effective from 10 μM on MCF-7 cells (Figure 2C). Product 2l also decreased MCF-7 (1–40 μM), MDA-MB-231, and MDA-MB-468 (10–40 μM) cell viability (Figure 2D). Dihydrofurofuranone 3l caused a modest but significant effect on MCF-7 (5–40 μM) and MDA-MB-231 (20–40 μM) cells, and was slightly more effective on MDA-MB-468, with the highest dose (40 μM) reaching almost a 40% inhibition (Figure 2E). These data indicate that among all tested cell lines MDA-MD-468 appears more sensitive to all compounds. This event could rely on a different pattern of gene expression present in these cells. A clear definition of target genes will help to explain such a difference.
Figure 2.
Dihydrofurofuranones 2d, 2e, 2g, 2l, and 3l Decrease Breast Cancer Cell Viability
(A–E) MCF-7, MDA-MB-231 and MDA-MB-468 cells were left untreated (0) or treated with different doses (1–40 μM) of 2d (A), 2g (B), 2e (C), 2l (D), and 3l (E) for 72 hr. Cell viability was evaluated by MTT (MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. Results were expressed as mean ± SD of three independent experiments each performed in triplicate. Statistically significant differences are indicated (*p < 0.05 versus basal). See Transparent Methods for experimental details. SD, standard deviation.
Figure 3.
Effects of Dihydrofurofuranones 2d, 2e, 2g, 2l, and 3l on MCF-10A and 3T3-L1 Cell Viability
(A–E) MCF-10A and 3T3-L1 cells were left untreated (0) or treated with different doses (1–40 μM) of 2d (A), 2g (B), 2e (C), 2l (D), and 3l (E) for 72 hr. Cell viability was evaluated by MTT ( 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide) assay. Results were expressed as mean ± SD of three independent experiments each performed in triplicate. Statistically significant differences are indicated (*p < 0.05 versus basal). See Transparent Methods for experimental details. SD, standard deviation.
To exclude the toxic effects of these compounds, experiments using normal breast epithelial cells MCF-10A and immortalized fibroblasts 3T3-L1 have been performed. As shown in Figure 3, the proliferative behavior of these cells is not affected by all doses of 2l (Figure 3D) and 3l (Figure 3E), whereas 2d (Figure 3A), 2g (Figure 3B), and 2e (Figure 3C) caused a reduction of cell viability in both MCF-10A and 3T3-L1 cells. In particular, in MCF-10A cells, 2d exerted a slight but statistically significant inhibitory effect at 20 and 40 μM (Figure 3A), whereas 2g was effective starting from 5 μM (Figure 3B) and 2e starting from 10 μM (Figure 3C). Moreover, in 3T3-L1, 2d (Figure 3A) and 2e (Figure 3C) decreased viability at 40 μM, whereas 2g (Figure 3B) was effective from 20 μM.
Taken together, the results obtained indicate that, among all compounds, 5,5-dimethyl-6a-phenyl-3-(trimethylsilyl)-6,6a-dihydrofuro[3,2-b]furan-2(5H)-one 2l is the one with the most significant inhibitory effects on the cell vitality of tumor cells, whereas it has little or no effect on the vitality of normal cells. This prompted us to investigate the molecular mechanism behind the 2l-dependent decrease of BC cell viability. To this aim, we performed assays aimed to investigate if 2l initiated an apoptotic mechanism in MCF-7 and MDA-MB-231 and MDA-MB-468 cells. Apoptosis is an energy-dependent process characterized by a number of hallmarks, such as membrane blebbing, nuclear chromatin condensation, cell shrinkage, internucleosomal DNA fragmentation, and protein cleavage (Bratton and Cohen, 2001). During apoptosis enzymes with cysteine protease activity designed as “caspases” (Chang and Yang, 2000) cleave several substrates including parp-1, a DNA nick sensor that catalyzes DNA repair (Duriez and Shah, 1997, Soldani and Scovassi, 2002). After cleavage, parp-1 loses the nick sensor function and is inactive toward DNA damage. Using western blot analysis, we showed that 2l treatment induced parp-1 cleavage in all three cell lines (Figures 4A–4C).
Figure 4.
Treatment with Dihydrofurofuranone 2l Induces parp-1 Cleavage in Breast Cancer Cells
(A–C) Cells were left untreated (-) or treated with 2l (20 μM) (+) for 48 hr. Western blot analyses of parp-1 in MCF-7 (A), MDA-MB-231 (B), and MDA-MB-468 (C) were performed on equal amounts of total proteins. GAPDH was used as a loading control. Blots are representative of three independent experiments with similar results. See Transparent Methods for experimental details.
In addition, we also investigated the effect of 2l on the activation of other apoptotic markers, in particular the bcl-2 family members, playing pivotal roles in regulating the mitochondrial apoptotic pathway. As can be seen from Figure 5, the presence of 2l decreased bcl-2 expression (Figure 5A) in all three BC cell lines. Cytosolic translocation of cytochrome c has been proposed to be an essential step in the mitochondria-dependent apoptotic pathway. In fact, cytochrome c release from mitochondria into the cytosol triggers caspase activation (Kuida et al., 1998, Wang, 2001, Wilson, 1998). Therefore, we examined if cytochrome c was released into the cytosol after treatment with 2l. Cytosolic protein fraction was isolated and analyzed by western blot analysis (Figure 5B). As reported in Figure 5B, cytochrome c levels increased in the cytosolic fraction of all three BC cell lines treated with 2l.
Figure 5.
Treatment with Dihydrofurofuranone 2l Modulates the Expression of Apoptotic Markers in Breast Cancer Cells
(A and B) MCF-7, MDA-MB-231, and MDA-MB-468 breast cancer cells were left untreated (bs, −) or treated with 2l (20 μM) (2l, +) for 48 hr. Western blot analyses of bcl-2 (A) and cytochrome c (cyt c) (B) were performed on equal amounts of total proteins. GAPDH was used as a loading control. Blots are representative of three independent experiments with similar results. (A and B, upper panels) Graphs represent means of normalized optical densities from three experiments, bars represent SD (*p < 0.05 versus untreated cells [−]). See Transparent Methods for experimental details. SD, standard deviation.
Conclusions
In conclusion, we have reported a novel carbonylative double cyclization approach to biologically active 6,6a-dihydrofuro[3,2-b]furan-2(5H)-ones 2 starting from simple and readily available starting materials (4-yne-1,3-diols 1, carbon monoxide, and oxygen). The process allows the formation of two cycles and three new bonds (O-C, C-C, and C-O) in one single operation and in ordered sequence. The dihydrofurofuranones thus synthesized have shown a significant antitumor activity against BC cell lines. In particular, 5,5-dimethyl-6a-phenyl-3-(trimethylsilyl)-6,6a-dihydrofuro[3,2-b]furan-2(5H)-one 2l showed the most significant inhibitory effects on the cell vitality of tumor cells, whereas it had practically no effect on the vitality of normal cells. For 2l, molecular events leading to cell death were further investigated, and the results evidenced that this molecule was able to activate an intrinsic apoptotic mechanism.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank the University of Calabria for financial support.
Author Contributions
Conceptualization, B.G., R.M, N.D.C., and V.P.; methodology, all authors; validation, R.M., I.Z., A.C., N.D.C., R.S.; investigation, I.Z., A.C., R.M., N.M.; writing, B.G. and V.P.; supervision, B.G.
Declaration of Interests
An Italian patent has been filed on July 13, 2017 (# 102017000078586), titled “Derivati 6,6a-diidrofuro[3,2-b]furan-2-(5H)onici, loro preparazione e uso nel trattamento dei tumori.” Inventors: Bartolo Gabriele, Adele Chimento, Raffaella Mancuso, Vincenzo Pezzi, Ida Ziccarelli, Rosa Sirianni; Applicant: University of Calabria, Italy.
Published: May 25, 2018
Footnotes
Supplemental Information includes Transparent Methods, 74 figures, 2 tables, and 2 data files and can be found with this article online at https://doi.org/10.1016/j.isci.2018.04.022.
Contributor Information
Raffaella Mancuso, Email: raffaella.mancuso@unical.it.
Bartolo Gabriele, Email: bartolo.gabriele@unical.it.
Supplemental Information
References
- Alcaide B., Almendros P. Gold-catalyzed cyclization reactions of allenol and alkynol derivatives. Acc. Chem. Res. 2014;47:939–952. doi: 10.1021/ar4002558. [DOI] [PubMed] [Google Scholar]
- Bauer K.R., Brown M., Cress R.D., Parise C.A., Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- Beller M., editor. Catalytic Carbonylation Reactions. Springer; 2006. [Google Scholar]
- Bratton S.B., Cohen G.M. Apoptotic death sensor: an organelle’s alter ego? Trends Pharmacol. Sci. 2001;22:306–315. doi: 10.1016/s0165-6147(00)01718-1. [DOI] [PubMed] [Google Scholar]
- Carey L.A., Dees E.C., Sawyer L., Gatti L., Moore D.T., Collichio F., Ollila D.W., Sartor C.I., Graham M.L., Perou C.M. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin. Cancer Res. 2007;13:2329. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- Chang H.Y., Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrouwer W., Heugebaert T.S.A., Roman B.I., Stevens C.V. Homogeneous gold-catalyzed cyclization reactions of alkynes with N- and S-nucleophiles. Adv. Synth. Catal. 2015;357:2975–3006. [Google Scholar]
- Duriez P., Shah G.M. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem. Cell Biol. 1997;75:337–349. [PubMed] [Google Scholar]
- Friis S.D., Lindhardt A.T., Skrydstrup T. The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions. Acc. Chem. Res. 2016;49:594–605. doi: 10.1021/acs.accounts.5b00471. [DOI] [PubMed] [Google Scholar]
- Gabriele B., Mancuso R., Salerno G. Oxidative carbonylation as a powerful tool for the direct synthesis of carbonylated heterocycles. Eur. J. Org. Chem. 2012:6825–6839. [Google Scholar]
- Gabriele B., Veltri L., Mancuso R., Carfagna C. Cascade reactions: a multicomponent approach to functionalized indane derivatives by a tandem palladium-catalyzed carbamoylation/carbocyclization process. Adv. Synth. Catal. 2014;356:2547–2558. [Google Scholar]
- Gadge S.T., Bhanage B.M. Recent developments in palladium catalysed carbonylation reactions. RSC Adv. 2014;4:10367–10389. [Google Scholar]
- Gehrtz P.H., Hirschbeck V., Ciszek B., Fleischer I. Carbonylations of alkenes in the total synthesis of natural compounds. Synthesis. 2016;48:1573–1576. [Google Scholar]
- Guo L.-N., Duan X.-H., Liang Y.-M. Palladium-catalyzed cyclization of propargylic compounds. Acc. Chem. Res. 2011;44:111–122. doi: 10.1021/ar100109m. [DOI] [PubMed] [Google Scholar]
- Haffty B.G., Yang Q., Reiss M., Kearney T., Higgins S.A., Weidhaas J., Harris L., Hait W., Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- Jung M.E., Piizi G. gem-disubstituent effect: theoretical basis and synthetic applications. Chem. Rev. 2005;105:1735–1766. doi: 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
- Kalck P., Urrutigoïty M. Recent improvements in the alkoxycarbonylation reactions catalyzed by transition metal complexes. Inorg. Chim. Acta. 2015;431:110–121. [Google Scholar]
- Kapitán P., Gracza T. Asymmetric intramolecular Pd(II)-catalysed oxycarbonylation of alkene-1,3-diols. Arkivoc. 2008;viii:8–17. [Google Scholar]
- Kirkpatrick P. Anticancer drugs: targeting triple-negative breast cancer. Nat. Rev. Drug Discov. 2009;8:21. [Google Scholar]
- Kollár L., editor. Modern Carbonylation Methods. Wiley-VCH; 2008. [Google Scholar]
- Krause N., Winter C. Gold-catalyzed nucleophilic cyclization of functionalized allenes: a powerful access to carbo- and heterocycles. Chem. Rev. 2011;111:1994–2009. doi: 10.1021/cr1004088. [DOI] [PubMed] [Google Scholar]
- Kuida K., Haydar T.F., Kuan C.Y., Gu Y., Taya C., Karasuyama H., Su M.S., Rakic P., Flavell R.A. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhang H., Lei A. Oxidative carbonylation reactions: organometallic compounds (R-M) or hydrocarbons (R-H) as nucleophiles. Angew. Chem. Int. Ed. 2011;50:10788–10799. doi: 10.1002/anie.201100763. [DOI] [PubMed] [Google Scholar]
- Mancuso R., Ziccarelli I., Armentano D., Marino N., Giofrè S.V., Gabriele B. Divergent palladium iodide catalyzed multicomponent carbonylative approaches to functionalized isoindolinone and isobenzofuranimine derivatives. J. Org. Chem. 2014;79:3506–3518. doi: 10.1021/jo500281h. [DOI] [PubMed] [Google Scholar]
- Mancuso R., Raut D.S., Della Ca’ N., Fini F., Carfagna C., Gabriele B. Catalytic oxidative carbonylation of amino moieties to ureas, oxamides, 2-oxazolidinones, and benzoxazolones. ChemSusChem. 2015;8:2204–2211. doi: 10.1002/cssc.201500343. [DOI] [PubMed] [Google Scholar]
- Mancuso R., Raut D.S., Marino N., De Luca G., Gordano C., Catalano S., Barone I., Andò S., Gabriele B. A palladium-catalyzed carbonylation approach to eight-membered lactam derivatives with antitumor activity. Chem. Eur. J. 2016;22:3053–3064. doi: 10.1002/chem.201504443. [DOI] [PubMed] [Google Scholar]
- Millar E.K.A., Graham P.H., O'Toole S.A., McNeil C.M., Browne L., Morey A.L., Eggleton S., Beretov J., Theocharous C., Capp A. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J. Clin. Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- Nguyen P.L., Taghian A.G., Katz M.S., Niemierko A., Abi Raad R.F., Boon W.L., Bellon J.R., Wong J.S., Smith B.L., Harris J.R. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J. Clin. Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- Omae I. Transition metal-catalyzed cyclocarbonylation in organic synthesis. Coord. Chem. Rev. 2011;255:139–160. [Google Scholar]
- Peng J.-B., Qi X., Wu X.-F. Recent achievements in carbonylation reactions: a personal account. Synlett. 2017;28:175–194. [Google Scholar]
- Sammes P.G., Weller D.J. Steric promotion of ring formation. Synthesis. 1995;1995:1205–1222. [Google Scholar]
- Shen C., Wu X.-F. Palladium-catalyzed carbonylative multicomponent reactions. Chem. Eur. J. 2017;23:2973–2987. doi: 10.1002/chem.201603623. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Soldani C., Scovassi A. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–328. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Tajima Y. Transition-metal-catalyzed cyclization of alkynal via ozametallacycle intermediates. Eur. J. Org. Chem. 2012:3715–3725. [Google Scholar]
- Valera J.A., Saa C. Metal-catalyzed cyclizations to pyran and oxazine derivatives. Synthesis. 2016;48:3470–3478. [Google Scholar]
- Veltri L., Mancuso R., Altomare A., Gabriele B. Divergent multicomponent tandem palladium-catalyzed aminocarnonylation-cyclization approaches to functionalized imidazothiazinones and imidazothiazoles. ChemCatChem. 2015;7:2206–2213. [Google Scholar]
- Veltri L., Paladino V., Plastina P., Gabriele B. A palladium iodide-catalyzed cyclocarbonylation approach to thiadiazafluorenones. J. Org. Chem. 2016;81:6106–6111. doi: 10.1021/acs.joc.6b01028. [DOI] [PubMed] [Google Scholar]
- Veltri L., Grasso G., Rizzi R., Mancuso R., Gabriele B. Palladium-catalyzed carbonylative multicomponent synthesis of functionalized benzimidazothiazoles. Asian J. Org. Chem. 2016;5:560–567. [Google Scholar]
- Veltri L., Giofrè S.V., Devo P., Romeo R., Dobbs A.P., Gabriele B. A palladium iodide-catalyzed oxidative aminocarbonylation–heterocyclization approach to functionalized benzimidazoimidazoles. J. Org. Chem. 2018;83:1680–1685. doi: 10.1021/acs.joc.7b03167. [DOI] [PubMed] [Google Scholar]
- Vlaar T., Ruijter E., Orru R.V.A. Recent advances in palladium-catalyzed cyclizations. Adv. Synth. Catal. 2011;353:809–841. [Google Scholar]
- Voduc K.D., Cheang M.C.U., Tyldesley S., Gelmon K., Nielsen T.O., Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J. Clin. Oncol. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Gene Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Wilson M.R. Apoptosis: unmasking the executioner. Cell Death Differ. 1998;5:646–652. doi: 10.1038/sj.cdd.4400394. [DOI] [PubMed] [Google Scholar]
- Wu X.-F., Neumann H. Ruthenium and rhodium-catalyzed carbonylation reactions. ChemCatChem. 2012;4:447–458. [Google Scholar]
- Wu X.-F., Neumann H., Beller M. Synthesis of heterocycles via palladium-catalyzed carbonylations. Chem. Rev. 2013;113:1–35. doi: 10.1021/cr300100s. [DOI] [PubMed] [Google Scholar]
- Wu X.-F., Neumann H., Beller M. Palladium-catalyzed oxidative carbonylation reactions. ChemSusChem. 2013;6:229–241. doi: 10.1002/cssc.201200683. [DOI] [PubMed] [Google Scholar]
- Wu L., Fang X., Liu Q., Jackstell R., Beller M., Wu X.-F. Palladium-catalyzed carbonylative transformation of C(sp3)–X bonds. ACS Catal. 2014;4:2977–2989. [Google Scholar]
- Wu X.-F. Palladium-catalyzed carbonylative transformation of aryl chlorides and aryl tosylates. RSC Adv. 2016;6:83831–83837. [Google Scholar]
- Wu X.-F., Beller M., editors. Vol. 42. Springer; 2016. (Transition Metal Catalyzed Carbonylative Synthesis of Heterocycles. Topics in Heterocyclic Chemistry). [Google Scholar]
- Wu Y.-C., Luo S.-H., Mei W.-J., Cao L., Wu H.-Q., Wang Z.-Y. Synthesis and biological evaluation of 4-biphenylamino-5-halo-2(5H)-furanones as potential anticancer agents. Eur. J. Med. Chem. 2017;139:84–94. doi: 10.1016/j.ejmech.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Ye Y., Ma S. Palladium-catalyzed cyclization reactions of allenes in the presence of unsaturated carbon-carbon bonds. Acc. Chem. Res. 2014;47:989–1000. doi: 10.1021/ar4002069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.