Table 1.
Optimizations of Reaction Condition
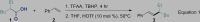 | ||
|---|---|---|
| Entry | Variation from the Standard Conditions | Yield(%)a,b |
| 1 | None | 88(75c) |
| 2 | Tf2O instead of HOTf | 78 |
| 3 | TsOH⋅H2O instead of HOTf | Trace |
| 4 | CF3COOH instead of HOTf | Trace |
| 5 | HOAc instead of HOTf | Trace |
| 6 | MeSO3H instead of HOTf | Trace |
| 7 | Room temperature instead of 50°C | 70 |
| 8 | Fresh distilled HOTf | 88 |
| 9 | In dark | 90 |
| 10 | Without HOTf | Trace |
Reaction conditions: First, 2-ethylhexanoic acid 1 (1.5 mmol), TBHP (1.5 mmol), and TFAA (1.5 mmol) at 0°C–rt for 4 hr, and then THF (2 mL), styrene 2 (0.5 mmol), and HOTf (0.05 mmol) were added. The mixture was stirred at 50°C for 8 hr.
1H NMR yield.
Yield of the isolated product.
