Abstract
The thioredoxin system plays a role in a variety of physiological functions, including cell growth, differentiation, apoptosis, tumorigenesis, and immunity. We previously confirmed that butaselen (BS), a novel thioredoxin reductase inhibitor, can inhibit the growth of various human cancer cell lines, yet the underlying mechanism remains elusive. In this study, we investigated the anti-tumor effect of BS in vivo through regulating the immune system of KM mice. We found that BS inhibits tumor proliferation by promoting the activation of splenic lymphocytes in mice. BS can elevate the percentage of CD4 −CD8 + T lymphocytes and the secretion of downstream cytokines in mice via down-regulating the expression of programmed death-ligand 1 (PD-L1) on the tumor cells’ surface in vivo. Further study in HepG2 and BEL-7402 cells showed that decrease of PD-L1 level after BS treatment was achieved by inhibiting signal transducer and activator of transcription 3 (STAT3) phosphorylation. Taken together, our results suggest that BS has a role in promoting the immune response by reducing PD-L1 expression via the STAT3 pathway, and subsequently suppresses tumorigenesis.
Keywords: Butaselen, Signal transducer and activator of transcription 3 (STAT3), Programmed death-ligand 1 (PD-L1), Immunity, Thioredoxin reductase
1. Introduction
Cancer is a pivotal global public health problem and is one of the major causes of deaths in both developed and developing countries. Primary liver cancer (hereinafter referred to as liver cancer), originating in liver cells or the intrahepatic bile duct, is one of the most common clinical malignancies, and the most frequent primary liver cancer (70%–90%) in the world is hepatocellular carcinoma (Torre et al., 2015). In the United States, the incidence of liver cancer is still increasing rapidly, by about 3% per year in women and 4% per year in men (Siegel et al., 2017). About 782 500 patients with liver cancer and 745 500 deaths newly occurred in 2012, and 50% of the cases and deaths happened in China (Torre et al., 2015).
Thioredoxin reductase (TrxR), an NADPH-dependent dimeric selenase, is a member of the pyridine nucleotide-disulfide oxidoreductase family (Lu and Holmgren, 2014). TrxR, together with thioredoxin and NADPH, composes the thioredoxin system, which is a broad-spectrum thiol reduction system and plays an important role in maintaining the redox balance within the cells. The thioredoxin system is over-expressed in a variety of tumors, including lung cancer, liver cancer, colorectal cancer, and gastric cancer, and is of great importance in promoting the proliferation, survival, and malignant transformation of abnormal cells in tumorigenesis (Nakamura et al., 2000; Mohler et al., 2002; Fernandes et al., 2009). Hence, the thioredoxin system can be used as a drug target for cancer treatment (Mahmood et al., 2013).
Butaselen (1,2-[bis(1,2-benzisoselenazolone-3(2H)-ketone)]butane, BS) is a TrxR inhibitor independently designed by our research group, and the half maximal inhibitory concentration (IC50) of TrxR inhibitory activity in vitro is (1.03±0.05) μmol/L (He et al., 2012). Our previous study confirmed the inhibitory effect of BS on TrxR activity in human colon cancer cell lines. However, the biological mechanism of tumor inhibition induced by BS remains unknown.
T cell-mediated cellular immunity is an important mechanism of anti-tumor. Cytotoxic T cells can directly kill tumor cells with allergens. The programmed cell death protein 1 (PD-1) serves as a key part in the regulation of the self-balance between positive and negative impacts of T cell-mediated immunity. PD-1 binds to its ligand such as the programmed death-ligand 1 (PD-L1), which is highly expressed on the cell surface in many categories of tumors including liver cancer, and leads to the decrease in immune cell activity in the microenvironment of tumor cells, allowing tumor cells to escape from immune damage (Uçeyler et al., 2010; Hassan et al., 2015; Meng et al., 2015; Ahmad et al., 2016; Li et al., 2016). The expression of PD-L1 is regulated by sophisticated mechanisms, including the extracellular signal-regulated kinase (ERK) pathway, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway (PI3K/Akt pathway), hypoxia-inducible factor-1 (HIF-1), signal transducer and activator of transcription 3 (STAT3), nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), etc. (Chen et al., 2016). STAT3 can bind to the PD-L1 promoter and enhance its expression, and inhibition of phosphorylated STAT3 (p-STAT3) by Janus kinase 3 (JAK3) inhibitor CP-690550 can reduce the expression of PD-L1 (Fang et al., 2014). In addition, the expression of STAT3 can also be modulated by the ERK and PI3K/Akt pathways (O'Sullivan et al., 2016).
Given the role of the thioredoxin system in regulation of the PI3K/Akt pathway (Meuillet et al., 2004; Kaimul Ahsan et al., 2005), we aimed to determine whether the TrxR inhibitor BS has a role in regulating the expression of STAT3 and PD-L1 and thus enhances the immunological function of the organism. In order to verify our conjecture, we studied the effect of BS on the immune system of the KM mouse using a tumor transplantation model, and carried out molecular and cellular experiments in vitro to investigate the effects of BS on the expression of STAT3 and PD-L1. Our study suggests that BS has a role in promoting the immune response by down-regulating the expression of PD-L1 by blocking STAT3 pathway, and finally leads to tumor inhibition.
2. Materials and methods
2.1. Animal treatment
The experiments were subject to the requirements of Animal Ethics from Peking University Health Science Center Ethics Committee, Beijing, China. Twenty-four KM male mice (four weeks old, 18–22 g; Peking University Medical Laboratory Animal Center, China) were randomly divided into four groups (n=6 in each group): BS low-dose group (90 mg/kg, intragastrical administration (i.g.), quāque diē (q.d.); BSL), BS medium-dose group (180 mg/kg, i.g., q.d.; BSM), BS high-dose group (360 mg/kg, i.g., q.d.; BSH), and control group (5 g/L carboxymethyl cellulose sodium (CMC-Na), i.g., q.d.). H22 tumor cell suspension (cell survival rate greater than 95%) was injected into KM male mice, 106 cells each. Treatments were performed on the 2nd day after tumor inoculation. Basic status and the tumor volume (length×width2×π/6) were recorded every 2 d. The mice were sacrificed on the 9th day of our observation, and a blood test was then carried out.
2.2. Cell culture
Mouse hepatocellular carcinoma cell line H22 was provided by the researcher Jia-chun FANG from Peking University Cancer Institute as a gift. Human hepatocellular carcinoma cell lines HepG2 and BEL-7402 were purchased from the Cell Resource Center at Peking Union Medical College, Beijing, China. HepG2 and BEL-7402 cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Macgene, Beijing, China) supplemented with 10% fetal bovine serum (Biological Industries, Israel) at 37 °C with 5% CO2.
2.3. Preparation of mouse spleen and tumor single cell suspension
The spleen and tumor tissues were isolated using elbow tweezers and gently grinding with a 150-mesh cell filter to get single cell suspension liquid. The cell suspension liquid was centrifuged at 1200 r/min for 5 min, discarding the supernatant and adding 4 ml of erythrocyte lysate (Beyotime Bio, Shanghai, China). After 4-min stewing, the liquid was centrifuged at 1200 r/min for 5 min, discarding the supernatant to get the spleen cells. The cells were then prepared as previously described (Godoy-Ramirez et al., 2000). Finally, 5×106 cells of extracted single spleen cell suspension from each group were stimulated with 20 ng/ml phorbol 12-myristate 13-acetate (PMA) and 20 ng/ml ionomycin calcium (MultiScience, Hangzhou, China) for 48 h. The cells were then centrifuged to collect the supernatant for enzyme-linked immunosorbent assay (ELISA) analysis.
2.4. Flow cytometry analysis
Total 1×106 cells from the single spleen cell suspension were stained with fluorescence marked surface antibodies for 30 min. Total 1×106 cells from the single tumor cell suspension and HepG2, BEL-7402 cell lines were stained with antibodies for 1 h. Then cells were incubated with fluorescence secondary antibody for 30 min. The antibodies used were the following: anti-mouse CD8-PE, anti-mouse CD4-FITC (MultiScience, Hangzhou, China); CD274 antibody (Enogene, Nanjing, China); anti-human CD274 (B7-H1) purified (eBioscience Inc., USA); DyLight 488 AffiniPure goat anti-rabbit IgG (Abbkine, USA). Mean fluorescence intensity (MFI) was used to quantify the protein expression level.
2.5. ELISA analysis for the assessment of IL-2, IFN-γ, and TNF-α in the supernatant
The concentrations of interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) in the supernatant were determined using mouse cytokine ELISA kits (Peak Albert Biotechnology, Beijing, China).
2.6. Cell viability assay
The effect of BS (designed and synthetized by our laboratory, patent cooperation treaty (PCT): 100202) on the proliferation of HepG2 cells was determined under sulforhodamine B (SRB) assay (Vichai and Kirtikara, 2006). Cells were seeded in 96-well plates (3600 per well) and treated with BS after attachment for 24, 48, and 72 h.
2.7. Apoptosis analysis
The influence of BS on the apoptosis of HepG2 cells was determined after treatment for 48 h. Cells were collected and stained with Annexin V-FITC and propidium iodide (PI) using the Annexin V-FITC apoptosis kit (BioScience, Shanghai, China).
2.8. In vitro TrxR activity assay
5,5-Dithio-bis-(2-nitrobenzoic acid) (DTNB) reduction assay was used to determine the TrxR activity in cell extracts. The HepG2 and BEL-7402 cells treated with different concentrations of BS were cracked by radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime Bio, Shanghai, China) containing protease inhibitors and protein concentration was measured by bicinchoninic acid (BCA) assay (Thermo, USA). Fifty micrograms of proteins dissolved in 80 μl reaction buffer (0.1 mol/L phosphate-buffered saline (PBS), 0.2 mg/ml bovine serum albumin (BSA), 10 mmol/L ethylenediaminetetraacetic acid (EDTA), pH 7.0–7.4) were incubated for 30 min at 37 °C. After that 20 μl 2 mmol/L NADPH (Genview, league City, TX, USA) and 100 μl 10 mmol/L DTNB (Sigma-Aldrich, St. Louis, MO, USA) were added to the mixture to start the reaction. Both NADPH and DTNB were dissolved in the reaction buffer. TrxR activity was quantified by the linear increase of absorption values at 405 nm in the first 7.5 min on a microplate reader (Thermo Fisher Scientific, USA).
2.9. Western blot analysis
HepG2 and BEL-7402 cells were treated with 0, 10, 20, 30, 40, and 50 μmol/L BS for 48 h and harvested into RIPA lysis buffer with protease and phosphatase inhibitors. Thirty micrograms of proteins from diverse groups were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). The antibodies used are as follows: anti-β-actin (ZSGB-Bio, Beijing, China), anti-TrxR1 (Abcam, Cambridge, MA, USA), anti-p-STAT3, anti-STAT3, anti-CD274 (Enogene, Nanjing, China), and horseradish peroxidase-conjugated secondary antibodies (ZSGB-Bio, Beijing, China). The signal was detected using the ECL Western blotting detection reagent (Advansta, Menlo Park, CA, USA) and visualized with a ChampGel 5000 Imager (Sagecreation, Beijing, China). The optical density of the protein bands was quantified using ImageJ software.
2.10. Statistical analysis
Statistical analysis was carried out using SPSS 17.0 software (USA). All results were expressed as mean±standard deviation (SD) and analyzed by the two-tailed Student’s t-test. P<0.05 was considered statistically significant.
3. Results
3.1. Influence of BS on tumor volume of KM mice
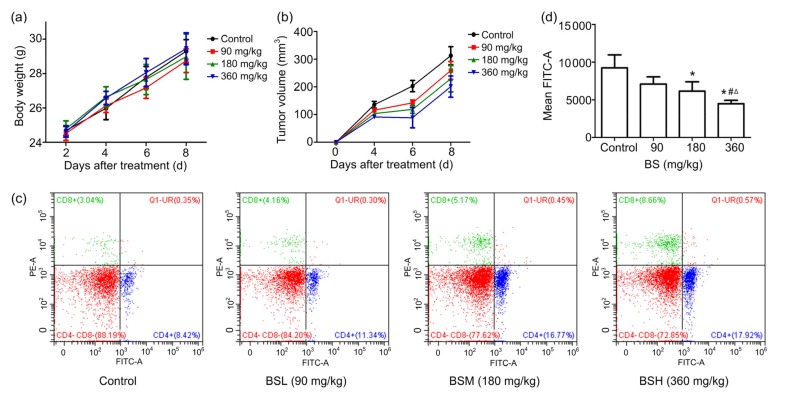
First, we evaluated the influence of BS on the immune system of KM mice. As shown in Fig. 1a, there appeared to be no significant difference between the control and the BS treatment group on the body weights, while the tumor growth in the control group was much faster than that in the BS treatment group in a dose-dependent manner (Fig. 1b). We found that the tumor growth rate was significantly slower than that of the control group on Days 4‒6, illustrating that BS may be more effective in the early stage of tumorigenesis.
Fig. 1.
Influence of BS on KM mice
(a) Influence of BS on weight of KM mice. The body weight was measured every 2 d (n=6). (b) Influence of BS on tumor volume of KM mice. The tumor volume was measured every 2 d (n=6). (c) The percentages of CD4 +CD8 − and CD4 −CD8 + T lymphocyte in mice. (d) BS has a negative effect on the expression of PD-L1 on tumor cells (n=3). (a, b, d) Values are represented as the mean±SD. * P<0.05 vs. control; # P<0.01 vs. BSL; ∆ P<0.05 vs. BSM. BSL, BS low-dose group (90 mg/kg); BSM, BS medium-dose group (180 mg/kg); BSH, BS high-dose group (360 mg/kg)
3.2. Influences of BS on organ relative weight, the numbers of red blood cells, white blood cells, and platelet of KM mice
The organs thymus, spleen, liver, and kidney were observed and weighed at the endpoint of the experiment. The numbers of white blood cells, erythrocytes, and platelets in peripheral blood were also measured. Table 1 shows that the spleen relative weight, thymus relative weight, and the number of white blood cells of the BS-treated group were significantly higher than those of the control group, indicating that BS may have a certain immune-enhancing function. At the same time, there was no significant difference in liver or kidney relative weight between the control and the BS treatment groups, showing that there was no obvious liver or kidney toxicity induced by BS.
Table 1.
Influences of BS on organ relative weight and the numbers of RBC, WBC, and PLT of KM mice
| Group | Organ relative weight (%) |
RBC (×1012 L−1) | WBC (×109 L−1) | PLT (×109 L−1) | |||
| Spleen | Liver | Renal | Thymus | ||||
| Control | 0.50±0.10 | 6.67±0.65 | 1.51±0.22 | 0.15±0.02 | 9.23±0.77 | 7.92±2.99 | 641.5±165.7 |
| BSL | 0.61±0.07* | 6.66±0.54 | 1.39±0.12 | 0.27±0.07** | 9.11±1.33 | 12.18±2.99* | 503.2±198.6 |
| BSM | 0.64±0.11* | 6.92±0.79 | 1.53±0.21 | 0.22±0.04** | 8.90±1.37 | 14.42±2.48** | 617.3±134.3 |
| BSH | 0.73±0.11** | 7.06±0.52 | 1.42±0.17 | 0.24±0.05** | 8.96±1.46 | 10.37±2.12 | 462.8±185.0* |
Values are represented as the mean±SD (n=6)
P<0.05
P<0.01 vs. control
BSL, BS low-dose group (90 mg/kg); BSM, BS medium-dose group (180 mg/kg); BSH, BS high-dose group (360 mg/kg); RBC, red blood cell; WBC, white blood cell; PLT, platelet
3.3. BS elevates percentage of CD4−CD8+ T lymphocytes in mice
After confirming the tumor inhibition effect of BS in vivo, we then tried to evaluate the influence of BS on the immune system on KM mice. The isolated spleen lymphocytes in mice were stained with FITC-labeled CD4+ and PE-labeled CD8+ antibody, and then detected by flow cytometry (Fig. 1c). The percentage of CD4+CD8− T lymphocyte in BSL group and the percentage of CD4−CD8+ T lymphocyte in BSM and BSH groups were significantly higher than those in the control group (Table 2), indicating that BS mainly affected CD4−CD8+ T cells in the immune system.
Table 2.
Influences of BS on percentages of CD4 +, CD8 + T lymphocytes of KM mice
| Group | Dosage (mg/kg) | CD4 +CD8 − (%) | CD4 −CD8 + (%) | |||
| Control | 0 | 11.93±0.02 | 3.67±0.01 | |||
| BSL | 90 | 17.25±0.03** | 4.33±0.01 | |||
| BSM | 180 | 13.05±0.03 | 5.46±0.00** | |||
| BSH | 360 | 12.09±0.05 | 6.25±0.02* |
Values are represented as the mean±SD (n=3)
P<0.05
P<0.01 vs.control. BSL
BS low-dose group (90 mg/kg); BSM, BS medium-dose group (180 mg/kg); BSH, BS high-dose group (360 mg/kg)
3.4. BS down-regulates the expression of PD-L1 protein on cell surface
We then tried to elucidate the possible mechanism by which BS regulates T cells. We first examined the changes of expression of PD-L1 protein on H22 tumor cells by flow cytometry after staining to investigate the effects of BS. As shown in Fig. 1d, the PD-L1 expression on the surface of H22 tumor cells decreased in a dose-dependent manner. When the treatment concentration of BS was 360 mg/kg, the expression of PD-L1 was reduced by 51.41%, compared with the control group. Thus BS can significantly down-regulate the PD-L1 expression on the surface of tumor cells in vivo.
As previously reported, PD-L1 down-regulates the expression of various inflammatory cytokines when it binds to PD-1, such as IL-2, IFN-γ, and TNF-α (Nomi et al., 2007; Lee et al., 2009; Grzywnowicz et al., 2015). In order to confirm the effects of BS on immune system, IL-2, IFN-γ, and TNF-α were targeted in our study. As shown in Table 3, we found that IL-2, IFN-γ, and TNF-α displayed significant up-regulation in KM mice with BS administration, after stimulation by PMA and ionomycin calcium for 48 h. Taken together, the data above indicate that BS regulates the expression of PD-L1, thereby promoting the secretion of cytokines and enhancing the immune function.
Table 3.
Influences of BS on IFN-γ, IL-2, and TNF-α of KM mice
| Group | Dosage (mg/kg) | IFN-γ (ng/L) | IL-2 (pg/ml) | TNF-α (ng/L) |
| Control | 0 | 280.14±55.68 | 4.52±0.41 | 122.07±24.11 |
| BSL | 90 | 285.57±64.29 | 4.69±1.44 | 123.96±34.36 |
| BSM | 180 | 299.38±129.43 | 4.98±1.63 | 128.95±30.68 |
| BSH | 360 | 369.00±83.10* # Δ | 5.45±1.50* | 151.25±54.08* |
Values are represented as the mean±SD (n=3)
P<0.05 vs. control
P<0.01 vs. BSL
P<0.05 vs. BSM
BS low-dose group (90 mg/kg); BSM, BS medium-dose group (180 mg/kg); BSH, BS high-dose group (360 mg/kg)
3.5. BS down-regulates PD-L1 expression through the STAT3 pathway in hepatocellular carcinoma cells
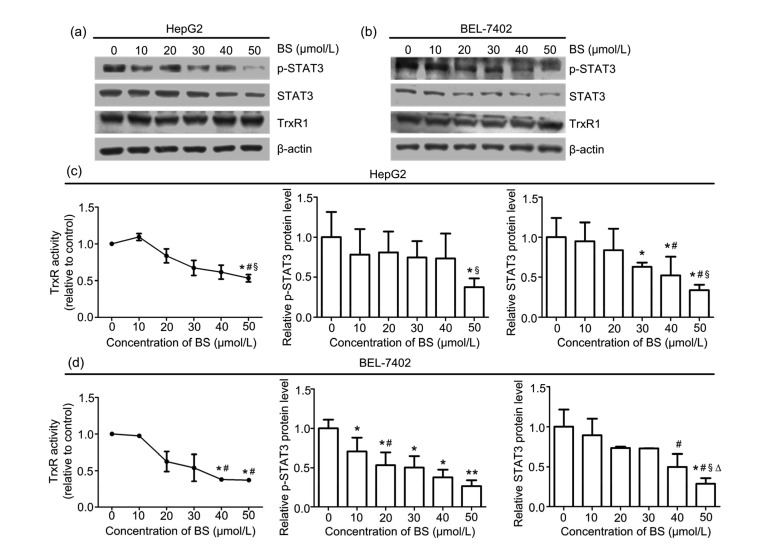
Our previous work has indicated that TrxR can deactivate PI3K/Akt and ERK pathways, two cascades both regulating STAT3. Therefore, we suppose that BS may regulate the STAT3 pathway through TrxR. We first explored the effect of BS on the TrxR level. Compared with the control, the expression of TrxR was not altered significantly after treatment with BS for 48 h (Figs. 2a and 2b). Then we conducted an experiment to determine the influence of BS on intracellular TrxR activity using DTNB assay. HepG2 and BEL-7402 cells were incubated with BS (0–50 μmol/L) for 48 h. As shown in Figs. 2c and 2d, TrxR activity in HepG2 and BEL-7402 cell lines decreased under high concentration of BS. TrxR activity was 53.15% and 37.23% of the control group after 50 μmol/L BS treatment for 48 h in HepG2 and BEL-7402 cells, respectively. Taken together, the regulation function of BS on HepG2 and BEL-7402 cell lines is mediated by inhibition of TrxR activity, rather than TrxR protein expression.
Fig. 2.
Expression of TrxR1, p-STAT3, STAT3, and β-actin protein levels, and TrxR activity after BS treatment for 48 h
Western blot analyses of TrxR1, p-STAT3, STAT3, and β-actin protein levels in HepG2 (a) and BEL-7402 (b) cells. BS decreased the intracellular TrxR activity and p-STAT3 and STAT3 protein levels in HepG2 (c) and BEL-7402 (d) cells. Values are represented as the mean±SD (n≥3). * P<0.05, ** P<0.01 vs. control; # P<0.05 vs. 10 μmol/L BS; Δ P<0.05 vs. 20 μmol/L BS; § P<0.05 vs. 30 μmol/L BS
Next we verified the influence of BS on the STAT3 pathway. Western blot analysis illustrated that the protein levels of p-STAT3 and STAT3 in HepG2 and BEL-7402 cells decreased after exposure to BS for 48 h (Figs. 2a and 2b). The relative protein levels of p-STAT3 and STAT3 in HepG2 cells under 50 μmol/L BS treatment were only 37.68% and 33.70% of that in the control group, while in BEL-7402 cells were 30.89% and 26.70%, respectively. Thus, these data suggest that BS could down-regulate the STAT3 pathway.
3.6. BS decreases PD-L1 expression in hepatocellular carcinoma cells
As STAT3 is able to regulate the expression of PD-L1 (Chen et al., 2016), after confirming the effect of BS on STAT3 pathway, we investigated the effect of BS on PD-L1 expression. As shown in Figs. 3a and 3b, BS markedly down-regulated PD-L1 expression in HepG2 and BEL-7402 cells after 48 h treatment in a dose-dependent manner. Furthermore, we investigated the expression of PD-L1 protein on the surface of HepG2 and BEL-7402 cells after 20 μmol/L BS treatment for 5 d using flow cytometry analysis (Figs. 3c and 3d). The expression of PD-L1 protein on the surface of HepG2 and BEL-7402 cells was significantly lower than that of the control group, which was consistent with the change of protein level. Together, the data suggest that BS could down-regulate PD-L1 expression by inhibiting the STAT3 pathway.
Fig. 3.
Effect of BS on PD-L1 expression in hepatocellular carcinoma cells
Western blot analyses of PD-L1 and β-actin protein levels in HepG2 (a) and BEL-7402 (b) cells (top panel); relative PD-L1 protein level in HepG2 (a) and BEL-7402 (b) cells (down panel). The expression of PD-L1 protein level on the surface of HepG2 (c) and BEL-7402 (d) cells under 20 μmol/L BS treatment for 5 d. Values are represented as the mean±SD (n≥3). * P<0.05, ** P<0.01 vs. control; # P<0.05 vs. 10 μmol/L BS; ∆ P<0.05 vs. 20 μmol/L BS
3.7. BS inhibits the survival and proliferation, and induces apoptosis of HepG2 cells
SRB assay and Annexin-V/PI staining were then employed to examine the cytotoxic effect and apoptosis induction of BS on the HepG2 cells. In SRB assay, HepG2 cells were treated with 0–100 μmol/L BS for 24, 48, and 72 h. The results indicated that BS inhibited cell proliferation in a dose-and time-dependent manner (Fig. 4a). Specially, the IC50 of BS on HepG2 cells was (25.42±5.36) μmol/L for 24 h, (18.67±3.50) μmol/L for 48 h, and (15.96±1.03) μmol/L for 72 h. In the apoptosis analysis, HepG2 cells were treated with 0, 20, 30, and 40 μmol/L BS for 48 h. Fig. 4c shows the typical results. As displayed in Fig. 4b, the total apoptosis rates of 20, 30, and 40 μmol/L BS were 15.1%, 58.6%, and 66.3%, respectively. The results showed that BS induced apoptosis of HepG2 cells in a dose-dependent manner. These data further confirm that BS also exerts significant inhibitory and pro-apoptotic effects on the HepG2 cells.
Fig. 4.
Effects of BS on the survival, proliferation, and apoptosis of HepG2 cells
(a) HepG2 cells were treated with 0, 10, 15, 20, 30, 50, and 100 μmol/L BS for 24, 48, and 72 h. (b) The percentage of total apoptosis of HepG2 cells after treatment for 48 h. (c) Pro-apoptotic effect of BS on HepG2 cells after treatment for 48 h with 0 (control), 20, 30, or 40 μmol/L BS. Upper left quadrant: dead cells; Upper right quadrant: late apoptotic cells; Lower left quadrant: viable cells; Lower right quadrant: early apoptotic cells. Values are represented as the mean±SD (n≥3). * P<0.05 vs. control; # P<0.01 vs. 20 μmol/L BS; ∆ P<0.05 vs. 30 μmol/L BS
4. Discussion
We found that the TrxR inhibitor BS markedly decreased the expression of PD-L1 protein level by inhibiting the expression of total and phosphorylated STAT3. BS could down-regulate the expression of PD-L1 protein on the surface of tumor cells in vivo and in vitro. The reduced PD-L1 protein level on the surface of tumor cells can decrease its binding to PD-1 on T lymphocytes, thus enhancing the function of lymphocytes and the secretion of cytokines, and this was confirmed by the ELISA analyses of IL-2, IFN-γ, and TNF-α in the supernatant of KM mice. In addition, BS inhibited the survival and proliferation, and induced apoptosis of HepG2 cells, indicating that BS can kill tumor cells and inhibit PD-L1 expression to trigger immune response simultaneously.
The thioredoxin system plays a significant role in immune function. Thioredoxin is shown to be a growth promoting factor in transformed T-and B-cells (Mahmood et al., 2013). However, little is known about how the thioredoxin system is involved in immunity. In the present study, we demonstrated that BS, as a selective TrxR inhibitor, could down-regulate the expression of PD-L1 on tumor cells via the STAT3 pathway to promote an immune response, indicating that the combinational use of TrxR inhibitor and other chemotherapeutic agents might become potential effective anticancer strategies on cancers which are resistant to chemotherapy because of immune escape.
The tumor microenvironment is a complex one on which tumor cells rely, mainly composed of a variety of different extracellular matrix and stromal cells. Components of the tumor microenvironment and tumor cells can stimulate each other, thereby promoting tumor progression and tumor cell metastasis. The immune checkpoints on tumor-infiltrating T cells including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), PD-1, inducible T-cell co-stimulator (ICOS) and so on can be influenced by tumor cells to enhance suppressive activity. In our work, we investigated the influence of BS on spleen lymphocytes, not the tumor-infiltrating cells. Therefore, we may get more reliable evidence by exploring the influence of BS on lymphocytes in the tumor microenvironment.
In summary, we provided experimental evidence that BS is able to suppress the expression of PD-L1 protein through the STAT3 pathway. Given the advantage of BS compared with other current clinical anti-tumor drugs against liver cancer, it is reasonable to hypothesize that BS could potentially become a promising agent for tumor treatment. Still, more experiments need to be performed to delineate the intrinsic mechanism by which the thioredoxin system interacts with immunity.
Footnotes
Project supported by the National Natural Science Foundation of China (No. 81372266) and the National Science and Technology Major Project (No. 2011zx09101-001-03), China
Compliance with ethics guidelines: Qiao ZOU, Yi-fan CHEN, Xiao-qing ZHENG, Suo-fu YE, Bin-yuan XU, Yu-xi LIU, and Hui-hui ZENG declare that they have no conflict of interest.
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, that there is no professional nor other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Ahmad SM, Borch TH, Hansen M, et al. PD-L1-specific T cells. Cancer Immunol Immunother. 2016;65(7):797–804. doi: 10.1007/s00262-015-1783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Jiang CC, Jin L, et al. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27(3):409–416. doi: 10.1093/annonc/mdv615. [DOI] [PubMed] [Google Scholar]
- 3.Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5(23):12189–12202. doi: 10.18632/oncotarget.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes AP, Capitanio A, Selenius M, et al. Expression profiles of thioredoxin family proteins in human lung cancer tissue: correlation with proliferation and differentiation. Histopathology. 2009;55(3):313–320. doi: 10.1111/j.1365-2559.2009.03381.x. [DOI] [PubMed] [Google Scholar]
- 5.Godoy-Ramirez K, Franck K, Gaines H. A novel method for the simultaneous assessment of natural killer cell conjugate formation and cytotoxicity at the single-cell level by multi-parameter flow cytometry. J Immunol Methods. 2000;239(1-2):35–44. doi: 10.1016/S0022-1759(00)00161-7. [DOI] [PubMed] [Google Scholar]
- 6.Grzywnowicz M, Karczmarczyk A, Skorka K, et al. Expression of programmed death 1 ligand in different compartments of chronic lymphocytic leukemia. Acta Haematol. 2015;134(4):255–262. doi: 10.1159/000430980. [DOI] [PubMed] [Google Scholar]
- 7.Hassan SS, Akram M, King EC, et al. PD-1, PD-L1 and PD-L2 gene expression on T-cells and natural killer cells declines in conjunction with a reduction in PD-1 protein during the intensive phase of tuberculosis treatment. PLoS ONE. 2015;10(9):e0137646. doi: 10.1371/journal.pone.0137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Li D, Xiong K, et al. Inhibition of thioredoxin reductase by a novel series of bis-1,2-benzisoselenazol-3 (2H)-ones: organoselenium compounds for cancer therapy. Bioorg Med Chem. 2012;20(12):3816–3827. doi: 10.1016/j.bmc.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Kaimul Ahsan M, Nakamura H, Tanito M, et al. Thioredoxin-1 suppresses lung injury and apoptosis induced by diesel exhaust particles (DEP) by scavenging reactive oxygen species and by inhibiting DEP-induced downregulation of Akt. Free Radic Biol Med. 2005;39(12):1549–1559. doi: 10.1016/j.freeradbiomed.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Zhuang Y, Wei X, et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J Mol Cell Cardiol. 2009;46(2):169–176. doi: 10.1016/j.yjmcc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Li F, Jiang F, et al. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway & translational blockade of immune checkpoints. Int J Mol Sci. 2016;17(7):1151. doi: 10.3390/ijms17071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood DF, Abderrazak A, El Hadri K, et al. The thioredoxin system as a therapeutic target in human health and disease. Antioxid Redox Signal. 2013;19(11):1266–1303. doi: 10.1089/ars.2012.4757. [DOI] [PubMed] [Google Scholar]
- 14.Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41(10):868–876. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Meuillet EJ, Mahadevan D, Berggren M, et al. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN’s lipid phosphatase activity and membrane binding: a mechanism for the functional loss of PTEN’s tumor suppressor activity. Arch Biochem Biophys. 2004;429(2):123–133. doi: 10.1016/j.abb.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Mohler JL, Morris TL, Ford OH 3rd, et al. Identification of differentially expressed genes associated with androgen-independent growth of prostate cancer. Prostate. 2002;51(4):247–255. doi: 10.1002/pros.10086. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura H, Bai J, Nishinaka Y, et al. Expression of thioredoxin and glutaredoxin, redox-regulating proteins, in pancreatic cancer. Cancer Detect Prev. 2000;24(1):53–60. [PubMed] [Google Scholar]
- 18.Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 19.O'Sullivan KE, Breen EP, Gallagher HC, et al. Understanding STAT3 signaling in cardiac ischemia. Basic Res Cardiol. 2016;111(3):27. doi: 10.1007/s00395-016-0543-8. [DOI] [PubMed] [Google Scholar]
- 20.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 21.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 22.Uçeyler N, Göbel K, Meuth SG, et al. Deficiency of the negative immune regulator B7-H1 enhances inflammation and neuropathic pain after chronic constriction injury of mouse sciatic nerve. Exp Neurol. 2010;222(1):153–160. doi: 10.1016/j.expneurol.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1(3):1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]