Summary
The master circadian pacemaker in mammals resides in the hypothalamic suprachiasmatic nuclei (SCN) and is synchronized to ambient light/dark cycles (i.e., photoentrainment). Melanopsin (Opn4) and classical rod-cone photoreceptors are believed to provide all the photic input necessary for circadian photoentrainment. Although the UVA-sensitive photopigment Opn5 is known to be expressed in retinal ganglion cells, its physiological role remains unclear and a potential role for Opn5 in the photoentrainment of the master clock has not been addressed. Here we report impaired photoentrainment and phase shifting to UVA light in Opn5-null mice. However, triple-knockout mice lacking all known functional circadian photoreceptors (i.e., rods, cones, and melanopsin) failed to entrain to UVA-light/dark cycles, despite the presence of Opn5, demonstrating that Opn5 alone is not sufficient for photoentrainment of the SCN clock. Since Opn5 is involved in the regulation of the retinal circadian clock, disrupted retinal function may cause impaired circadian photoentrainment in Opn5-null mice.
Subject Areas: Molecular Mechanism of Behavior, Sensory Neuroscience
Graphical Abstract

Highlights
-
•
Opn5-null mice show impaired photoentrainment to UVA-light/dark (LD) cycles
-
•
Mice lacking melanopsin and functional rods/cones fail to entrain to UVA-LD cycles
-
•
Opn5 alone is not sufficient for circadian photoentrainment
-
•
Retinal circadian clocks play a possible role in circadian photoentrainment
Molecular Mechanism of Behavior; Sensory Neuroscience
Introduction
Circadian rhythms are approximately 24-hr, cell-autonomous biological rhythms observed in virtually all living organisms. The mammalian circadian pacemaker resides in the hypothalamic suprachiasmatic nuclei (SCN). Daily physiological and behavioral activity patterns are regulated by the SCN, which is synchronized to ambient light/dark (LD) cycles via direct connections from retinal ganglion cells in the retina. Thus, circadian photoentrainment enables organisms to enhance their fitness by anticipating daily changes in food availability and predator activity. Melanopsin (Opn4) and classical rod-cone photoreceptive systems are widely believed to provide all photic input required for circadian photoentrainment (Hattar et al., 2003, Panda et al., 2003), and a potential role for other photopigments has not been addressed. Opn5 is a deep brain photopigment that regulates seasonal reproduction in birds (Nakane et al., 2010, Nakane et al., 2014, Yamashita et al., 2010). In mammals, Opn5 is expressed in selected retinal ganglion cells and exhibits an absorption maximum in the UVA range (λmax = 380 nm) (Kojima et al., 2011, Yamashita et al., 2014). Although mammalian Opn5 has been demonstrated to be involved in the photoentrainment of local circadian clocks in the retina and the cornea, its involvement in photoentrainment of the SCN remains unclear (Buhr et al., 2015).
Results and Discussion
Generation of Mice Deficient in Opn5 and Classical Rod-Cone Photoreceptors
To assess the physiological function of mammalian Opn5 in vivo, we generated Opn5-null (Opn5−/−) mice (Figures 1A–1D). Since mice also possess a UVA-sensitive cone opsin (Opn1sw; λmax = 360 nm), we generated mice deficient in Opn5 and classical rod-cone photoreceptors (Opn5−/−; rd1/rd1) by breeding Opn5-null mice with CBA/J mice harboring the retinal degeneration (rd1) mutation. Because the genetic background of Opn5-null mice was C57BL/6J, F2 hybrids of the parental strains were used to minimize the effect of genetic background, as suggested by the Jackson Laboratory. To examine the effects of Opn5 deficiency and the rd1 mutation on retinal morphology, we performed immunohistochemistry and analyzed the expression of different opsins. Information about the antibodies used is given in Table S1. The retina of Opn5-null mice was indistinguishable from that of wild-type (WT) mice, and immunopositive signals for rod (Rho) and cone (Opn1sw; Opn1mw) opsins and melanopsin (Opn4) were observed (Figure 1E). By contrast, although the rd1 mutation caused severe retinal degeneration and abolished the rod-cone photoreceptor layer, the retinal ganglion cell layer was intact and melanopsin expression was clearly observed in both rd1/rd1 and Opn5−/−; rd1/rd1 mutants (Figure 1E). Note that we have generated several antibodies against Opn5, but none of them were specific; therefore, we were unable to examine the expression of Opn5 in the different mutant mice.
Figure 1.
Generation of Opn5−/− Mice, and Histological Analysis of the Retina
(A) Physical map of the wild-type Opn5 locus, targeting construct, targeted allele before Cre-mediated recombination, and disrupted Opn5 locus. The targeting vector was designed to excise a part of Opn5 exon 1 and insert EGFP in-frame with the start codon of Opn5, followed by an SV40 poly(A) tail. The SV40 poly(A) tail halts the transcription of the full-length Opn5 transcript, resulting in Opn5 deficiency. The 5′ and -3′ probes used for screening targeted embryonic stem (ES) clones and mouse genotypes are represented by the black bars. PCR primers used for mouse genotyping (p1–3) are shown as arrowheads. E, EcoRV; Neo, neomycin resistance gene; DTA, diphtheria toxin.
(B) Southern blot analysis of genomic DNA from Opn5+/+, Opn5+/−, and Opn5−/− mice, hybridized with indicated probes. If homologous recombination was successful, the fragment corresponding to the wild-type Opn5 allele (11 kb) is replaced by a smaller fragment representing the recombined mutant allele (7.9 or 4.1 kb).
(C) PCR genotyping of genomic DNA. Amplicons corresponding to the wild-type (191 bp) and targeted allele (251 bp) are indicated.
(D) Absence of Opn5 mRNA in the retina was confirmed by RT-PCR.
(E) Immunohistochemistry of photopigments in the retina of WT, Opn5−/−, rd1/rd1, and Opn5−/−; rd1/rd1 mice, respectively. Arrows indicate immunopositive signals. RPE, retinal pigment epithelium; PRL, photoreceptor layer; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar: 50 μm.
Impaired Re-Entrainment to UVA-LD Cycles in Opn5-Null Mice
We then compared photoentrainment to UVA-LD cycles in WT mice, Opn5−/− and rd1/rd1 single mutant mice, and Opn5−/−; rd1/rd1 double mutant mice. Absorption spectra of the retinal photopigments present in these mice are illustrated in Figure S1A. Mice were initially kept under white-LD cycles and then transferred to UVA-LD cycles (λp = 365 nm, Figure S1B); wheel-running activity was recorded to measure entrainment (Figure 2A). The intensity of the UVA light was decreased every 2–10 weeks, concomitant with a 2-hr advance of the LD cycle, as described previously (Butler and Silver, 2011). Entrainment to the advanced UVA-LD cycles was determined by a transient interval of a free-running rhythm with a period shorter than 24 hr, which was then followed by a 24-hr rhythm with a stable phase angle of entrainment.
Figure 2.
Impaired Circadian Photoentrainment to UVA-LD Cycles in Opn5−/−, rd1/rd1, and Opn5−/−; rd1/rd1 Mice
(A) Representative double-plotted actograms of WT, Opn5−/−, rd1/rd1, and Opn5−/−; rd1/rd1 mice. The vertical and horizontal axes indicate the number of recorded days and the time of day over 2 days, respectively. Yellow and purple represent white and UVA light, respectively. The log photon flux of UVA light (log photons cm−2 s−1) is indicated on the right.
(B) Days required for re-entrainment to a new LD cycle. *, WT versus Opn5−/−; rd1/rd1. §, WT versus Opn5−/−. †, rd1/rd1 versus Opn5−/−; rd1/rd1. Values represent the mean ± SEM. Single symbol, p < 0.05; double symbol, p < 0.01 (one-way ANOVA followed by Scheffé’s post-hoc test; n = 4–13).
(C) Percentage of animals entrained at each step. To calculate the half-maximal intensity (I50), a three-parameter logistic function (sigmoidal dose-response) was fitted to intensity-response relationships using GraphPad Prism.
(D) I50 for the entrainment of each genotype.
See also Figures S1 and S2.
Double mutant mice (Opn5−/−; rd1/rd1) required more days for re-entrainment to new UVA-LD cycles than WT mice (Figure 2B), whereas the single mutants (Opn5−/− and rd1/rd1) exhibited intermediate phenotypes (Figure 2B) (e.g., WT: ∼12 days, rd1/rd1: ∼20 days, Opn5−/−: ∼23 days, Opn5−/−; rd1/rd1: ∼30 days at a log photon flux of 12.7). Half-maximal intensities (I50) for entrainment were higher for single and double mutant mice than for WT mice (Figures 2C and 2D). Although differences in the free-running period can affect the duration and light intensity required for re-entrainment, the free-running period of all 4 genotypes did not differ significantly (Figure S2). Together, these results demonstrated that Opn5−/− and rd1/rd1 mice exhibit significantly slower rates of re-entrainment to UVA-LD cycles than WT mice.
Although Opn5−/−; rd1/rd1 mice exhibited severe impairment in circadian photoentrainment, they were able to entrain to the higher intensity UVA-LD cycles, suggesting the involvement of additional photopigments. In the rd1/rd1 retina, most rods degenerate rapidly (within the first week after birth) and all rods disappear completely by about 7 weeks of age. By contrast, cones degenerate much more slowly, with some surviving for at least 18 months (Carter-Dawson et al., 1978). The involvement of UVA-sensitive cones in circadian photoentrainment is well established (van Oosterhout et al., 2012, Yoshimura and Ebihara, 1998). Therefore, it is possible that the remaining UVA-sensitive cone opsins and/or melanopsin may mediate UVA-dependent circadian photoentrainment in Opn5−/−; rd1/rd1 mice.
Impaired Phase Shifting and Fos Induction to UVA Light Pulses in Opn5-Null Mice
To further evaluate the ability of Opn5-null mice to respond to light for circadian functions, we examined the effect of phase-shifting light pulses under constant darkness (Figure 3). A single 15-min light pulse was given 4 hr after activity onset (i.e., circadian time 16). Retinal degenerate (rd1/rd1) mice showed decreased phase shifts to both UVA and orange light pulses (λp = 602 nm, Figure S1B) compared with WT mice (Figures 3A–3D). This is most likely due to the loss of classical photoreceptors, whose contribution to irradiance encoding and regulation of circadian rhythms is well known (Altimus et al., 2010, Lall et al., 2010, van Diepen et al., 2013, van Oosterhout et al., 2012, Yoshimura and Ebihara, 1996, Yoshimura and Ebihara, 1998). Although Opn5−/− mice showed impaired phase shifting to UVA light pulses (Figures 3A and 3C), they showed normal phase shifting to orange light pulses (Figures 3B and 3D), indicating that impaired photoresponses observed in Opn5−/− mice are UVA light specific.
Figure 3.
Impaired Phase Shifting and Fos Induction to a UVA Light Pulse in Opn5−/−, rd1/rd1, and Opn5−/−; rd1/rd1 Mice
(A and B) Effects of a single 15-min UVA light pulse (purple filled circles, λp = 365 nm, 12.3 log photons cm−2 s−1) (A) and orange-light pulse (orange filled circles, λp = 602 nm, 13.7 log photons cm−2 s−1) (B) on wheel-running activity rhythms. Representative double-plotted actograms of each genotype are shown. Eye-fitted lines were drawn using activity onset as a reference point.
(C and D) Irradiance-response relationship of circadian phase shifts by UVA (C) and orange-light (D) pulses. The magnitude of the phase shift was determined by eye-fitted lines as shown in (A) and (B). *, WT versus Opn5−/−; rd1/rd1. §, WT versus Opn5−/−. Values represent the mean ± SEM. Single symbol, p < 0.05; double symbols, p < 0.01 (one-way ANOVA followed by Scheffé’s post-hoc test; n = 5–9).
(E) Densitometric quantification of Fos mRNA expression in the SCN induced by a single UVA light pulse at the intensity indicated by the black arrow in (C) (12.3 log photons cm−2 s−1 for 15-min). Values represent the mean ± SEM. p < 0.05 (one-way ANOVA followed by Scheffé’s post-hoc test; n = 4–5). Representative autoradiograms are shown at the top.
See also Figures S1 and S2.
We also examined the induction of neuronal activation marker, Fos mRNA, in the SCN by UVA light (single 15-min pulse at a log photon flux of 12.3 cm−2 s−1). These results showed that UVA-dependent Fos induction was significantly decreased in Opn5−/− and rd1/rd1 single mutant mice, as well as in Opn5−/−; rd1/rd1 double mutants (Figure 3E). However, the phenotype was much more severe in double mutant mice (Opn5−/−; rd1/rd1) than in the single mutants (Opn5−/− and rd1/rd1) and correlates well with our phase shifting results in Figure 3C. All together, these results demonstrated that Opn5−/− mice have decreased photosensitivity to UVA light.
Opn4−/−; Gnat1−/−; Gnat2−/− Mice Lack the Ability to Photoentrain to UVA-LD Cycles
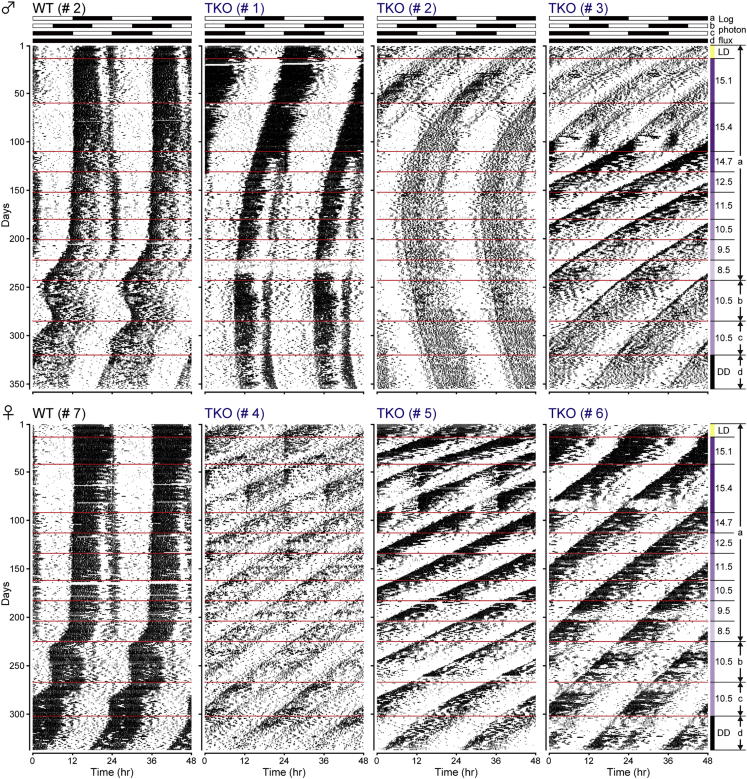
Previous studies have shown that mice deficient in melanopsin and rod-cone photoreceptors (e.g., Opn4−/−; rd1/rd1 double mutant mice and Opn4−/−; Gnat1−/−; Cnga3−/− [melanopsin; guanine nucleotide-binding protein α transducin 1; cyclic nucleotide-gated channel α3, respectively] triple-knockout [TKO] mice) failed to entrain to white-LD cycles; therefore, melanopsin and classical rod-cone photoreceptive systems are widely believed to account for all photic input required for circadian photoentrainment (Hattar et al., 2003, Panda et al., 2003). However, these studies used relatively dim white light (100–800 lx) (Hattar et al., 2003, Panda et al., 2003). The illuminance under direct sunlight is approximately 100,000 lx, whereas that of sunrise or sunset on a clear day is approximately 400 lx. Thus, it is possible that the illuminance (i.e., 100–800 lx) and/or wavelengths (i.e., white light but not UVA light) used in these previous studies were not sufficient to stimulate Opn5. We therefore examined circadian photoentrainment to UVA-LD cycles in Opn4−/−; Gnat1−/−; Gnat2−/− TKO mice (Gnat1/2: guanine nucleotide-binding protein α transducin 1/2), which have mutations in all known circadian photoreceptor pathways (Figure S1A). WT and TKO mice were initially kept under white-LD cycles and then transferred to UVA-LD cycles. The intensity of UVA light was adjusted every 3–7 weeks. WT mice could entrain to white- and UVA-LD cycles, except at very low intensities of UVA light (log photon flux of 8.5–9.5 cm−2 s−1); however, TKO mice failed to entrain to LD cycles of white light and all intensities of UVA light (Figure 4). Although 2 of 6 TKO mice (#1 and #2) showed free-running periods close to 24 hr, they failed to entrain to advanced UVA-LD cycles (Figure 4). These results demonstrated that the UVA-sensitive photopigment Opn5 alone is not sufficient for photoentrainment of the circadian pacemaker.
Figure 4.
Photoentrainment Analysis of Opn4−/−; Gnat1−/−; Gnat2−/− Mice
Representative double-plotted actograms of WT and Opn4−/−; Gnat1−/−; Gnat2−/− TKO mice. The vertical and horizontal axes indicate the number of recorded days and the time of day over 2 days, respectively. Yellow, purple, and black bars on the right indicate different lighting conditions: white-LD cycles, UVA-LD cycles, and constant darkness (DD), respectively. The log photon flux of UVA light (log photons cm−2 s−1) is also marked on the right. The timing of different LD cycles is illustrated at the top, and the assigned letter (a–d) for each LD cycle corresponds to the letter shown on the right. The red lines indicate when lighting conditions were changed. See also Figure S1.
Conclusions
In the present study, we demonstrated impaired photoentrainment to UVA light in Opn5-null mice. However, Opn4−/−; Gnat1−/−; Gnat2−/− TKO mice that lack melanopsin and functional rod-cone photoreceptors, but still possess Opn5, failed to photoentrain to LD cycles of both white and UVA light. This means that Opn5 is required for maximum UVA sensitivity, but it alone is not sufficient for entrainment to UVA-LD cycles. Although it is possible that Gnat1 and/or Gnat2 are involved in the phototransduction of Opn5, this seems unlikely, since they are thought to be expressed in different cell types, with Opn5 being expressed in retinal ganglion cells and Gnat1/2 in rod and cone photoreceptors, respectively (Buhr et al., 2015). The mammalian retina itself has a local circadian clock (Buhr and Van Gelder, 2014, Tosini and Menaker, 1996), which is photoentrainable in vivo and ex vivo and does not require the SCN. Photoentrainment of the retinal clock is mediated by Opn5 and not by melanopsin or rod-cone photoreceptors (Buhr et al., 2015). Thus, the retinal circadian clock is likely to be disrupted in Opn5−/− mice. Many retinal functions are known to be under the control of the circadian clock. For example, photoreceptors undergo daily cycles of renewal and shedding of outer segment disc membranes, which are fundamental for the maintenance of photoreceptor health (La Vail, 1976). Rod and cones are anatomically coupled by gap junctions, and the retinal circadian clock controls the extent and strength of rod-cone electrical coupling, leading to changes in retinal sensitivity between the daytime and nighttime (Ribelayga et al., 2008). Furthermore, disruption of the retinal circadian clock has been demonstrated to result in abnormal retinal transcriptional responses to light and defective inner retinal electrical responses to light (Storch et al., 2007). It is therefore plausible that the impaired photoentrainment we observed in Opn5-null mice is caused by disruption of the retinal circadian clock.
Although Opn5 is expressed in retinal ganglion cells, it alone is not sufficient for photoentrainment of the circadian pacemaker in the SCN. However, remarkably, Opn5 still contributes to circadian light responses even in the presence of functional rods, cones, and melanopsin light responses.
Limitations of the Study
Our study focused primarily on behavioral analysis of circadian locomotor activity of various mutant mice (except Figures 1 and 3E). Therefore, we do not fully understand the underlying mechanism of impaired photoentrainment and phase shifting in Opn5-null mice. Further molecular analysis of the retina of Opn5-null mice might be required in future studies to characterize this mechanism.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Kathy Tamai for comments on the manuscript, Mr. Kousuke Okimura for technical support, Dr. Willem J. DeGrip for the Rho antibody, and Dr. Keisuke Ikegami for helpful discussions. We also thank the Nagoya University Radioisotope Center for use of their facilities. This work was supported by the Funding Program for Next Generation World Leading Researchers (NEXT Program) initiated by the Council for Science and Technology Policy (CSTP) (LS055), JSPS KAKENHI “Grant-in-Aid for Specially Promoted Research” (Grant Number 26000013), Human Frontier Science Program (RGP0030/2015), Grant-in-Aid for JSPS Fellows (14J03915), and Program for Leading Graduate Schools “Integrative Graduate Education and Research in Green Natural Sciences (IGER),” MEXT, Japan. WPI-ITbM is supported by World Premier International Research Center Initiative (WPI), MEXT, Japan.
Author Contributions
T.Y. conceived and directed the work; W.O. and Y.N. performed research; S.H. provided new materials; W.O. and T.Y. analyzed data; all authors discussed the results and commented on the manuscript; W.O. and T.Y. wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: August 31, 2018
Footnotes
Supplemental Information includes Transparent Methods, two figures, and one table and can be found with this article online at https://doi.org/10.1016/j.isci.2018.08.010.
Supplemental Information
References
- Altimus C.M., Güler A.D., Alam N.M., Arman A.C., Prusky G.T., Sampath A.P., Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat. Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr E.D., Van Gelder R.N. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc. Natl. Acad. Sci. USA. 2014;111:8625–8630. doi: 10.1073/pnas.1323350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr E.D., Yue W.W.S., Ren X., Jiang Z., Liao H.R., Mei X., Vemaraju S., Nguyen M.T., Reed R.R., Lang R.A. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc. Natl. Acad. Sci. USA. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M.P., Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proc. Biol. Sci. 2011;278:745–750. doi: 10.1098/rspb.2010.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson L.D., LaVail M.M., Sidman R.L. Differential effect of the rd mutation on rods and cones in the mouse retina. Invest. Ophthalmol. Vis. Sci. 1978;17:489–498. [PubMed] [Google Scholar]
- Hattar S., Lucas R.J., Mrosovsky N., Thompson S., Douglas R.H., Hankins M.W., Lem J., Biel M., Hofmann F., Foster R.G. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima D., Mori S., Torii M., Wada A., Morishita R., Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6:e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vail M.M. Survival of some photoreceptor cells in albino rats following long-term exposure to continuous light. Invest. Ophthalmol. 1976;15:64–70. [PubMed] [Google Scholar]
- Lall G.S., Revell V.L., Momiji H., Al Enezi J., Altimus C.M., Güler A.D., Aguilar C., Cameron M.A., Allender S., Hankins M.W. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y., Ikegami K., Ono H., Yamamoto N., Yoshida S., Hirunagi K., Ebihara S., Kubo Y., Yoshimura T. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. USA. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y., Shimmura T., Abe H., Yoshimura T. Intrinsic photosensitivity of a deep brain photoreceptor. Curr. Biol. 2014;24:R596–R597. doi: 10.1016/j.cub.2014.05.038. [DOI] [PubMed] [Google Scholar]
- Panda S., Provencio I., Tu D.C., Pires S.S., Rollag M.D., Castrucci A.M., Pletcher M.T., Sato T.K., Wiltshire T., Andahazy M. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Ribelayga C., Cao Y., Mangel S.C. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch K.F., Paz C., Signorovitch J., Raviola E., Pawlyk B., Li T., Weitz C.J. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosini G., Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- van Diepen H.C., Ramkisoensing A., Peirson S.N., Foster R.G., Meijer J.H. Irradiance encoding in the suprachiasmatic nuclei by rod and cone photoreceptors. FASEB J. 2013;27:4204–4212. doi: 10.1096/fj.13-233098. [DOI] [PubMed] [Google Scholar]
- van Oosterhout F., Fisher S.P., van Diepen H.C., Watson T.S., Houben T., VanderLeest H.T., Thompson S., Peirson S.N., Foster R.G., Meijer J.H. Ultraviolet light provides a major input to non-image-forming light detection in mice. Curr. Biol. 2012;22:1397–1402. doi: 10.1016/j.cub.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Ohuchi H., Tomonari S., Ikeda K., Sakai K., Shichida Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc. Natl. Acad. Sci. USA. 2010;107:22084–22089. doi: 10.1073/pnas.1012498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Ono K., Ohuchi H., Yumoto A., Gotoh H., Tomonari S., Sakai K., Fujita H., Imamoto Y., Noji S. Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation. J. Biol. Chem. 2014;289:3991–4000. doi: 10.1074/jbc.M113.514075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Ebihara S. Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J. Comp. Physiol. A. 1996;178:797–802. doi: 10.1007/BF00225828. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Ebihara S. Decline of circadian photosensitivity associated with retinal degeneration in CBA/J-rd/rd mice. Brain Res. 1998;779:188–193. doi: 10.1016/s0006-8993(97)01122-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.