Abstract
The assembly of the blood–testis barrier (BTB) during postnatal development is crucial to support meiosis. However, the role of germ cells in BTB assembly remains unclear. Herein, KitW/KitWV mice were used as a study model. These mice were infertile, failing to establish a functional BTB to support meiosis due to c-Kit mutation. Transplantation of undifferentiated spermatogonia derived from normal mice into the testis of KitW/KitWV mice triggered functional BTB assembly, displaying cyclic remodeling during the epithelial cycle. Also, transplanted germ cells were capable of inducing Leydig cell testosterone production, which could enhance the expression of integral membrane protein claudin 3 in Sertoli cells. Early spermatocytes were shown to play a vital role in directing BTB assembly by expressing claudin 3, which likely created a transient adhesion structure to mediate BTB and cytoskeleton assembly in adjacent Sertoli cells. In summary, the positive modulation of germ cells on somatic cell function provides useful information regarding somatic–germ cell interactions.—Li, X.-Y., Zhang, Y., Wang, X.-X., Jin, C., Wang, Y.-Q., Sun, T.-C., Li, J., Tang, J.-X., Batool, A., Deng, S.-L., Chen, S.-R., Cheng, C. Y., Liu, Y.-X. Regulation of blood–testis barrier assembly in vivo by germ cells.
Keywords: tight junction, testosterone, claudin 3
Spermatogenesis is accomplished through a tight coordination between germ cells and somatic cells because these are the only cell types in the seminiferous epithelium. The blood–testis barrier (BTB), which mainly consists of extensive tight junctions (TJs) between somatic Sertoli cells, creates a unique immunologic and biochemical microenvironment required for meiosis (1). However, the BTB undergoes extensive restructure during VIII to IX of the seminiferous epithelial cycle in murine spermatogenesis, and large cysts of germ cells can be transported across this unique blood–tissue barrier without compromising its integrity even transiently (2). In brief, the old BTB is disassembled behind the preleptotene spermatocytes under transport while the new BTB is assembled. This thus allows the meiosis to proceed while preleptotene spermatocytes are being transported from the basal into the adlumenal compartment (3). However, whether germ cells are involved in this process remains unknown.
The BTB becomes fully functional through a considerable decline in the number of gap junctions with a concomitant increase in TJs, leading to the formation of a continuous beltlike ultrastructure at the base of the seminiferous tubule, composed mostly of TJs at the BTB, coinciding with the appearance of meiosis I/II during postnatal development (4, 5). c-Kit is a highly conserved gene among species that is known to control germ cell differentiation, and meiosis will be arrested if this gene is inactivated in the testis. Several mutations have been reported for this gene, including W, WV, and W35, which all lead to disruption of spermatogenesis (6, 7). Among these mutant mice, KitW/KitWV mice are alive except that the population of spermatogonia in the testis is considerably reduced, and the remaining few spermatogonia fail to enter meiosis. On the basis of these observations, we speculated that KitW/KitWV mice might be incapable of establishing a functional BTB. Thus, these mice could serve as a good research model to investigate the function of germ cells on BTB construction.
Androgen plays a role in BTB assembly by influencing the expression of claudin 3 (8), claudin 11 (9), and occludin (10). Claudin 3 is a 4-pass integral membrane protein and a component of both TJs and basal ectoplasmic specializations (ESs) (11, 12). Unlike other integral membrane proteins, claudin 3 was reported to transiently incorporate into newly formed TJs and then be subsequently replaced by claudin 11 (3, 8). Claudins in turn interact with zonula occludens (ZOs) at their C-terminal region and connect to actin filament (13). In short, claudin 3 is a marker and an important mediator of new BTB construction.
In this study, we first examined the structural and functional status of BTB in the KitW/KitWV testes because there are some controversies regarding the functional status of BTB in adult KitW/KitWV testes (14, 15). We sought to examine if these KitW/KitWV mutant mice had a functional BTB. If they did not, we sought to investigate if transplantation of undifferentiated spermatogonia that were enriched in spermatogonia stem cells (SSCs) or the presence of SSC-derived germ cells could induce the assembly of a functional BTB. We also used a bioinformatics approach for gene profiling to correlate transplanted exogenous germ cell differentiation status against time-dependent BTB assembly to assess if the progression of these 2 events was related.
MATERIALS AND METHODS
Animals
C57BL/6 and KitW/KitWV mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). The use of mice and all pertinent surgical procedures were approved by the Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences.
Biotin tracer studies
The BTB integrity in the testis in vivo was assessed by using biotin tracer as described earlier (8). In brief, mice were anesthetized and testes exposed, and ∼50 μl of EZ-Link Sulfo-NHS-LC-Biotin (10 mg/ml; Pierce, Rockford, IL, USA), freshly diluted in PBS (GE Healthcare, Parramatta, NSW, Australia) and containing 1 mM CaCl2, was administered under the tunica albuginea. The mice were humanely killed 30 min thereafter. Testes were removed and immediately embedded in Optimal Cutting Temperature compound (Sakura, Torrance, CA, USA). Frozen sections (8 μm thick) were prepared for further staining by streptavidin–FITC.
Immunofluorescence microscopy
Testes collected from KitW/KitWV mice with or without SSC transplantation at specific time points vs. C57BL/6 mice were fixed overnight in 4% paraformaldehyde and embedded in paraffin. Tissue sections (5 μm thick) were obtained in a microtome and mounted on glass slides. Sections were dewaxed and rehydrated, followed by antigen retrieval in 10 mM sodium citrate buffer. After blocking with 5% bovine serum albumin in PBS (wt/vol) for 1 h, the sections were incubated with primary antibody at 4°C overnight (Table 1). Secondary antibody conjugated with either FITC or tetramethylrhodamine isothiocyanate (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 1:200 dilution was used and was incubated for 1 h at room temperature. Slides were then stained with DAPI (blue) to visualize cell nuclei and mounted in prolonged antifade mounting medium. Fluorescence images were captured with an LSM780 confocal microscope (Carl Zeiss, Oberkochen, Germany). The immunofluorescent data shown are representative findings of an experiment that was repeated 3 to 4 times using different testes from 3 to 4 mice, and yielded similar results.
TABLE 1.
Antibodies used for different experiments
| Antibody | Vendor | Catalog no. | Host | Working dilution |
|---|---|---|---|---|
| Laminin | Abcam | Ab11575 | Rabbit | 1:300 |
| PARD6B | Santa Cruz Biotechnology | sc-67393 | Rabbit | 1:200 |
| Claudin 3 | Thermo Fisher Scientific | 187340 | Rabbit | 1:200 |
| Claudin 11 | Santa Cruz Biotechnology | sc-25711 | Rabbit | 1:200 |
| Tra 98 | Abcam | ab82527 | Rat | 1:300 |
| GFRα1 | R&D Systems | AF560 | Goat | 1:200 |
| c-Kit | Abcam | ab5506 | Rabbit | 1:200 |
| ZO-1 | Thermo Fisher Scientific | 617300 | Rabbit | 1:200 |
| SCP3 | Abcam | ab97672 | Mouse | 1:200 |
| β-Catenin | Santa Cruz Biotechnology | sc-1496 | Goat | 1:200 |
Abcam, Cambridge, United Kingdom; Santa Cruz Biotechnology, Dallas, TX, USA; Thermo Fisher Scientific, Waltham, MA, USA; R&D Systems, Minneapolis, MN, USA.
SSC culture transplantation and mating studies
Mouse SSC isolation, enrichment, and culture were performed as earlier described (16, 17) One-step enzymatic digestion with collagenase I and DNase I (Thermo Fisher Scientific, Waltham, MA, USA) was used for isolation of testicular cells from 5 to 7 d postpartum mice to small seminiferous tubule fragments. The fragments were plated on 100 mm dishes in mouse embryonic fibroblast medium, and loosely attached undifferentiated spermatogonia were collected with repetitive pipetting after 18 h; these undifferentiated spermatogonia were found to migrate into the culture dish. Thereafter, cells were transferred to a new, freshly prepared mouse embryonic fibroblast dish to enrich the SSC cultures. The enriched SSC cultures were passaged every 4 to 5 d at a dilution of 1:2 to 1:4, depending on the size of the cell clumps and the growth of the somatic cells, to remove testicular somatic cells in each passage. As such, relatively pure SSC cultures were routinely obtained after a total of ∼4 to 5 passages. Relatively pure SSC clumps, which have 93% GFRα-1-positive cells and 3% c-Kit–positive cells as reported earlier (16), were trypsinized into single cells and prepared at a concentration of 5 × 107 cells/ml for transplantation. Ten microliters of cell suspension was injected into each testis of 8 wk old KitW/KitWV mice via the efferent duct. Some recipient mice were used to mate with female C57BL/6 mice for further verification.
Electron microscopy
Electron microscopy analysis was performed as described previously (18) with some modifications. Testes were fixed with 2.5% glutaraldehyde in 0.2 M cacodylate buffer overnight. The testes were then cut into small pieces, ∼1 mm3, and immersed in 1% OsO4 in 0.2 M cacodylate buffer for 2 h at 4°C. After fixation, the samples were dehydrated through a graded ethanol series and embedded in resin. Ultrathin sections were cut on an ultramicrotome, stained with uranyl acetate and lead citrate, and then examined by a JEM-1400 transmission electron microscope (JEOL, Tokyo, Japan).
Hormone measurement
Hormone measurement analysis was performed as described previously (18) with some modifications. Blood samples were obtained from KitW/KitWV mice with or without SSC transplantation. Blood was allowed to clot at 4°C overnight, then was centrifuged at 1000g for 20 min at 4°C to obtain serum, which was then used for radioimmunoassay to quantify the level of testosterone. Testes were collected immediately after blood collection and euthanasia. The tunica albuginea were removed from the testes samples. The testes samples were then weighed, and homogenized in methanol using an IKA disperser (IKA Works, Wilmington, NC, USA). Homogenates were centrifuged at 1000g for 15 min at 4°C to remove cell debris, and the supernatant was blow-dried in N2. Then the remnants were redissolved in 0.1 ml of normal saline for radioimmunoassay. For each time point for hormone measurement, 3 to 4 mice were used.
RNA sequencing
We enriched fluorescent SSCs from green fluorescent protein (GFP)-labeled mice, and these cells were used for transplantation. These different tubules were then separated on the basis of light absorption patterns and fluorescence properties on the fifth week after transplantation when SSCs had differentiated into spermatocytes and round spermatids (Supplemental Fig. S3), or 3 mo after transplantation when offspring had been born. Three groups of seminiferous tubules were collected: empty tubes, tubes with spermatocytes and round spermatids, and tubes with complete spermatogenesis. There were 3 replicates in each group. RNA was extracted using the RNAprep pure Micro Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The cDNA libraries were obtained from these samples for deep sequencing using the digital gene expression profile method and the PE150 strategy (HiSeq4000; Illumina, San Diego, CA, USA) in Annoroad (Beijing, China). Gene expressions were calculated by the RPKM (reads per kilobase per million mapped reads) method using HTSeq 0.6.0 software (Python, https://pypi.python.org/pypi/HTSeq/0.6.0). Genes with P < 0.05 and |log2 ratio|≥1 were identified as differentially expressed genes (DEGs) using DESeq 1.16.0 software (Bioconductor, http://bioconductor.org/).
Statistical analysis
All experimental data were collected from a minimum of 3 mice. For culture experiments, experiments were repeated at least 3 times, which yielded similar results, and each culture dish had 3 or more replicates per experiment. Each data point represents mean ± sem. Statistical analyses were performed by 1-way ANOVA. P < 0.05 was considered statistically significant.
Accession numbers
All RNA sequencing (RNA-Seq) data have been deposited in the Gene Expression Omnibus database under accession number GEO: GSE93327.
RESULTS
There are no functional or typical BTB ultrastructures in the KitW/KitWV mouse testes
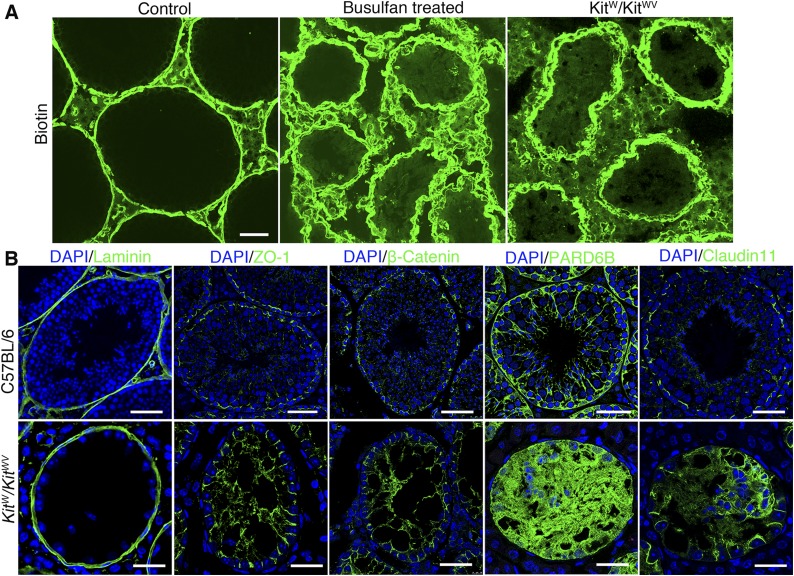
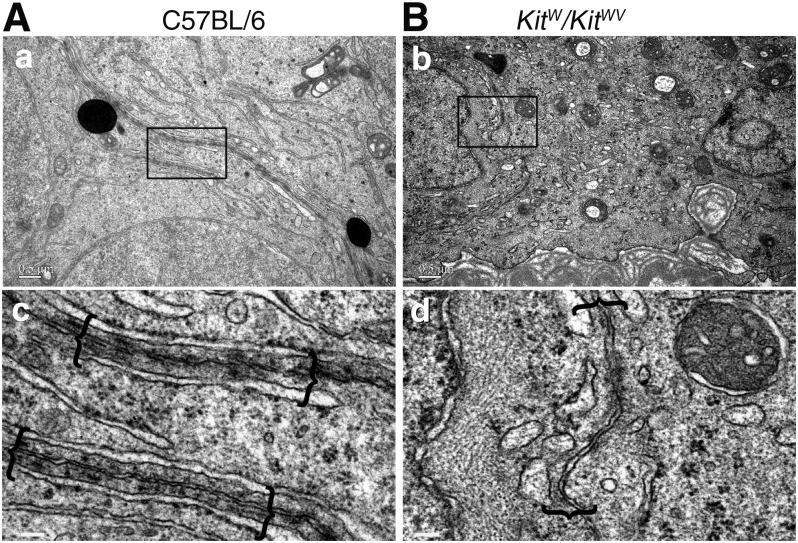
First, we used biotin tracer to explore the functional status of adult KitW/KitWV mice testes. Biotin was found to leak into the tubule lumen in these testes, similar to busulfan-treated mice, confirming that a functional BTB was absent in KitW/KitWV mouse testes (Fig. 1A). The distribution patterns of several TJ proteins (e.g., ZO-1 and claudin 11) and basal ES/BTB-associated proteins (e.g., β-catenin and Par6) vs. basement membrane protein laminin (Fig. 1B) also support the notion that the BTB in the adult KitW/KitWV mouse testis was not established. Compared to the age-matched wild-type (WT) control testes, the KitW/KitWV mouse testes had complete but thickened basement membrane, as illustrated by immunofluorescence localization of laminin (Fig. 1B). However, ZO-1, β-catenin, PARD6B, and claudin 11 displayed irregular distribution patterns at the BTB compared to control testes in C57BL/6 mice (Fig. 1B). For instance, ZO-1 was found in Sertoli cell plasma membrane that lay perpendicular to the basement membrane, similar to its distribution in immature testes before BTB was assembled (19). β-Catenin and claudin 11 were distributed similar to ZO-1 because these proteins were not tightly localized near the basement membrane as in WT control testes. PARD6B was also diffusely localized in the seminiferous epithelium, supporting the notion that there were defects in Sertoli cell polarity. In short, these BTB constituent proteins were mislocalized at the BTB, failing to support the assembly of a functional BTB structure in the KitW/KitWV mouse testes. A further study by electron microscopy confirmed the absence of typical BTB structures in the KitW/KitWV mouse testis vs. the corresponding WT mouse testis. For instance, some irregularly shaped vesicles were noted on both sides of the Sertoli cell plasma membrane, in marked contrast to the control testes (Fig. 2A vs. B).
Figure 1.
BTB functional status and abnormal expression patterns of BTB constituent proteins in KitW/KitWV mouse testes compared to age-matched control testes. A) BTB permeability was assessed by its ability to block diffusion of tracer biotin (green) from microvessels found in interstitium into seminiferous epithelium. In control mice, green fluorescence (biotin) was kept near base of seminiferous tubule consistent at their localization at BTB, but biotin was leaked into tubule lumen in KitW/KitWV mice and busulfan-treated mice, indicating BTB was compromised, which was also associated with loss of germ cells, as noted in DAPI staining (shown in B). Scale bars, 50 μm. B) Immunofluorescence staining of adult KitW/KitWV mouse testes wherein basement membrane was marked by laminin (green) and tubule appeared to be intact. However, BTB constitutive proteins ZO-1, β-catenin, PARD6B, and claudin 11 (all green) showed abnormal distribution in KitW/KitWV mouse testes compared to corresponding age-matched control testes. Scale bars, 50 μm (top panel), 25 μm (bottom panel).
Figure 2.
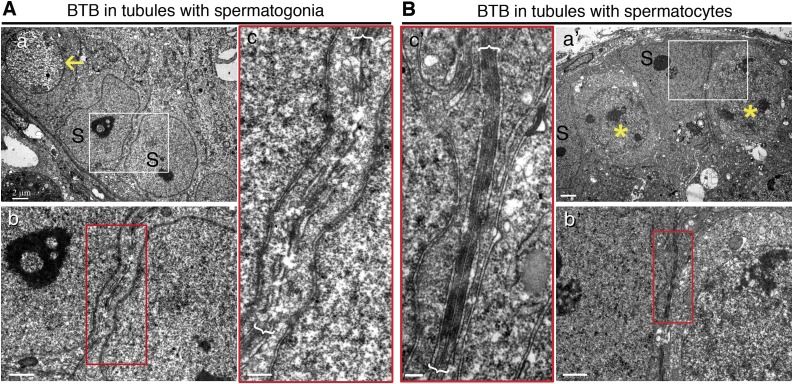
Structural status study of KitW/KitWV testes by electron microscopy. A) Electron micrographs illustrating presence of typical BTB ultrastructure (enclosed in brackets) in control C57BL/6 mice (a, c). B) No typical BTB ultrastructures were detected in seminiferous epithelium of KitW/KitWV mouse testes without germ cells; only some irregularly shaped vesicles were noted on inner sides of adjacent Sertoli cell membranes (b, d). Boxed areas were magnified and are shown in lower panel. Scale bars, 0.5 μm (a, b), 100 nm (c, d).
Transplantation of SSCs from C57BL/6 mice to KitW/KitWV mouse testes induces assembly of functional BTB
We next used these KitW/KitWV mice to serve as an animal model for our studies by transplanting SSCs derived from C57BL/6 mouse into the KitW/KitWV testis (Supplemental Fig. S1). In order to better assess the role of germ cells on BTB assembly, we used 8 wk old KitW/KitWV mice as the recipients. Thus, transplanted SSCs and their subsequent differentiation were the only variants in the recipient testis. When offspring of the recipient mice were obtained, the recipient testes were sampled for electron microscopic analysis. As anticipated, typical BTB structures were found in these recipient mouse testes, similar to control WT testes (Fig. 2A vs. Supplemental Fig. S1), confirming that the presence of developing germ cells was necessary for BTB assembly.
Germ cells determine extent of BTB assembly after SSC transplantation
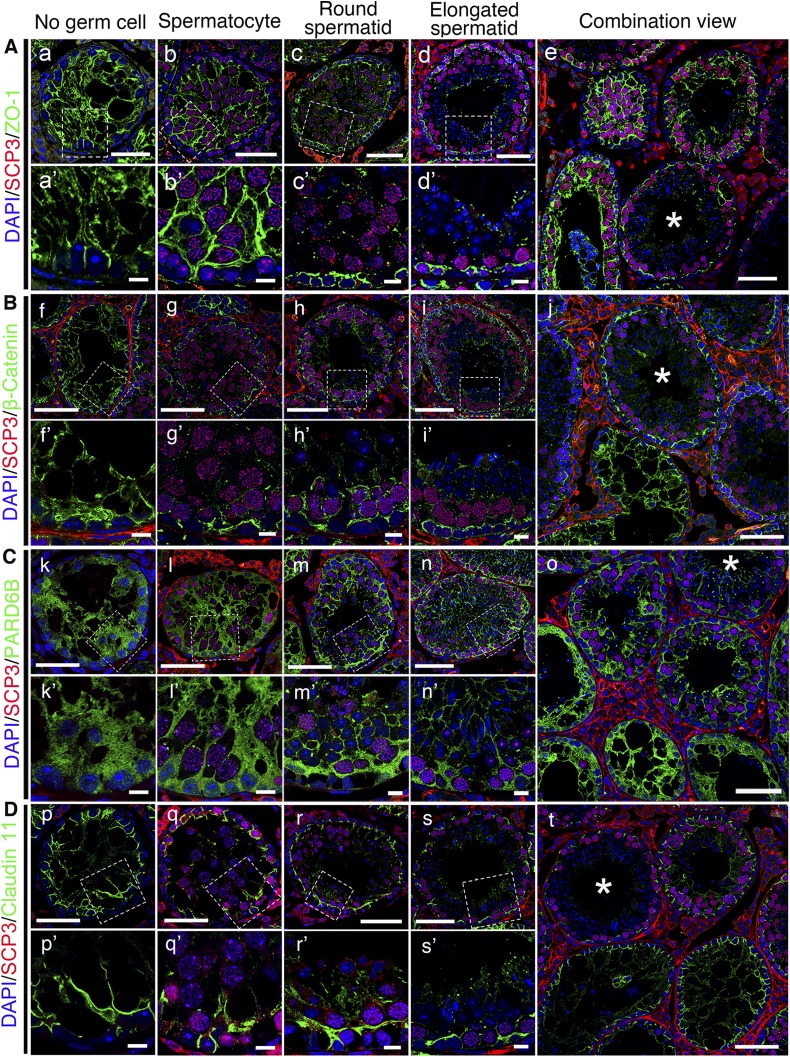
After transplantation, SSCs obtained from C57BL/6 mouse testes were found to home in on the basement membrane of KitW/KitWV testes, which proliferated and differentiated to establish spermatogenesis in the next 8 wk, similar to the normal mouse testis, consistent with an earlier report (20). We found 26.2% of tubules on testicular cross sections (n = 6) had normal claudin 11 distribution, which meant normal BTB had been assembled during this period (Supplemental Fig. S2). To examine the role of germ cells in BTB assembly, the recipient mice were humanely killed for examination by dual-labeled immunofluorescence analysis during this period so that the status of spermatogenesis in cross sections of seminiferous tubules could be carefully assessed. Surprisingly, the BTB assembly did not take place uniformly across the entire seminiferous epithelium in a tubule (Fig. 3). Instead, the establishment of a functional BTB was dependent on the availability of specific classes of germ cells, namely directly related to the differentiation stage of germ cells within specific domains of the seminiferous epithelium. In tubules without many germ cells, the distribution of ZO-1, β-catenin, PARD6B, and claudin 11 were diffusely localized and extended all the way to the tubule lumen, grossly different from control testes. However, these proteins were restrictively expressed to the site in the basal region of the seminiferous epithelium where SSCs had differentiated into spermatocytes and round spermatids, consistent with their location at the BTB in normal testes. In short, these findings illustrate that BTB was assembled only in some selected domains of the seminiferous epithelium wherein SSCs had differentiated into spermatocytes to support the formation of round spermatids via meiosis I/II.
Figure 3.
Changes in distribution of BTB constituent proteins after SSC transplantation, which is dependent on status of germ cell differentiation and spermatogenesis. Distribution of ZO-1, β-catenin, PARD6B, and claudin 11 (all green) were found to be different in different tubules, depending on status of spermatogenesis and differentiation status of germ cells. Abnormal distribution of these proteins in empty tubules devoid of germ cells (a, f, k, p) was obviously noted compared to tubules that gradually returned to normal after appearance of spermatocytes (b, g, l, q) and status of spermatogenesis was fully restored when round spermatid appeared (c, h, m, r). Spermatocytes were labeled by anti-SCP3 antibody (red); round spermatids and elongated spermatids were identified by corresponding nucleus shape (blue). Tubules with complete spermatogenesis are marked with an asterisk in e, j, o, t. Boxed areas in a–d, f–i, k–n, p–s are magnified and shown in a′–d′, f′–i′, k′–n′, p′–s′. Scale bars, 50 μm (a–t), 10 μm (a′–s′).
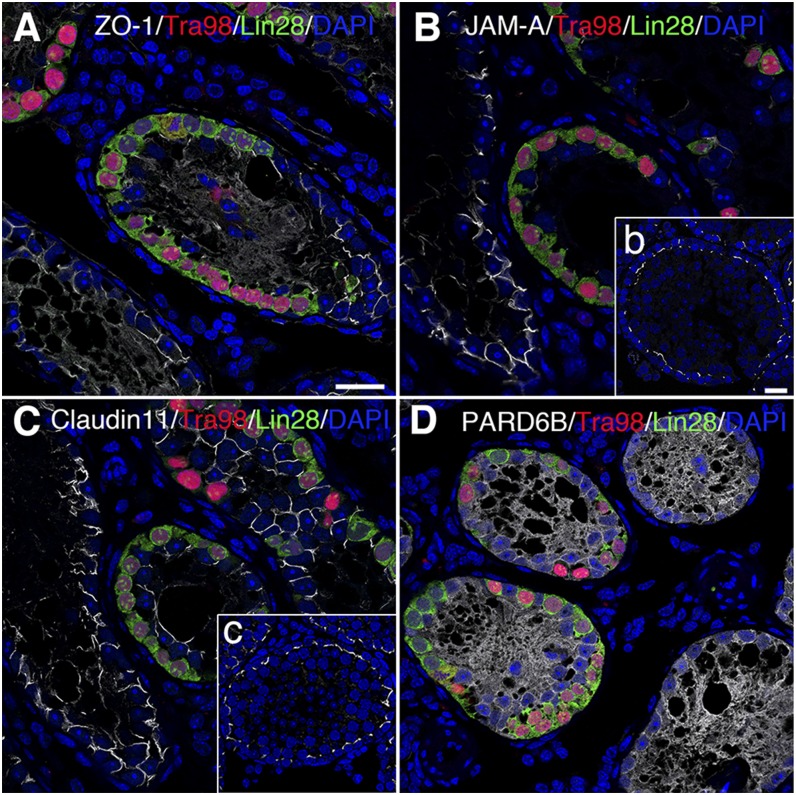
To assess if BTB assembly was strictly dependent on the presence of spermatocytes in the microdomains of the epithelium other than the more primitive germ cells, such as spermatogonia, SSCs obtained from KitW/KitWV mice were used for transplantation, wherein recipient testes were sampled after 2 mo for histologic analysis. Mutant SSCs were also found to home in on the basement membrane, and they were also capable of undergoing mitotic proliferation by encircling the base of the seminiferous tubule (Fig. 4). Unlike normal SSCs, the mutant SSCs that lacked the normal c-Kit gene failed to differentiate into spermatocytes and were positive for the spermatogonia marker lin28 (Fig. 4). The distribution of ZO-1, JAM-A, and claudin 11 between adjacent Sertoli cells displayed irregular localization across the entire length of the epithelium in tubules devoid of germ cells; further, they were notably down-regulated or nondetectable where SSCs resided (Fig. 4A–C). PARD6B remained diffusely localized well into the tubule lumen, illustrating polarity defects in Sertoli cells (Fig. 4D).
Figure 4.
Changes in expression pattern of BTB constituent proteins in seminiferous epithelium after mutant SSC transplantation. Mutant SSCs were capable of homing in on basement membrane and proliferated such that primitive germ cells encircle base of seminiferous tubules. Germ cells marked by Tra98 (red) were positive for spermatogonia marker Lin28 (green). A–C) Signals (silver-gray) of ZO-1, JAM-A, and claudin 11 between adjacent Sertoli cells disappeared after SSC transplantation. No right position pattern as shown in b, c could be seen in recipient testes (normal position pattern of ZO-1 and PARD6B shown in Fig. 1B). D) PARD6B was diffusely localized both in tubules with and without SSCs. Scale bars, 20 μm.
To further confirm the above observations, the BTB status of the 2 recipient samples—transplanted with mutant SSCs vs. normal mouse SSCs—were examined by electron microscopy 1 mo after transplantation wherein transplanted normal SSCs had differentiated into spermatocytes. For instance, ultrastructures (e.g., TJs, basal ESs) that are associated with intact BTB were noted in tubules where SSCs had differentiated into spermatocytes (Fig. 5B), but no such ultrastructures illustrating the presence of an intact BTB were detected in tubules where only undifferentiated spermatogonia homed in that failed to differentiate into spermatocytes (Fig. 5A). These findings thus support that notion that SSCs alone, without further differentiation into spermatocytes, failed to stimulate BTB assembly. Furthermore, BTB assembly is closely associated with the onset of meiosis I.
Figure 5.
Electron micrographs to confirm presence of BTB in seminiferous epithelium with spermatocytes vs. spermatogonia. A) Electron micrograph showed no typical BTB ultrastructure in tubules where only undifferentiated spermatogonia (yellow arrows) homed in on mouse testis after transplantation of mutant SSCs, displaying discontinuous and apparently truncated cell junctions. B) Typical BTB ultrastructures were detected in tubules where SSCs had differentiated into spermatocytes (yellow asterisk) in mice where normal SSCs had been transplanted. S, Sertoli cells. Boxed areas were magnified and are shown in lower or lateral panel. Scale bars, 2 μm (a, a′), 1 μm (b, b′), 0.4 μm (c, c′).
Transcriptome profiling using seminiferous tubules at different stages of spermatogenesis
The findings summarized above illustrate that the presence of differentiated germs cells influenced the behavior of adjacent Sertoli cells regarding the spatiotemporal expression of BTB-associated proteins to support BTB assembly. These findings also suggest that the presence of the signaling molecule or molecules necessary to mediate this event likely localize on or near the cell membrane through cell–cell contact. We next dissected seminiferous tubules with germ cells at different differentiation stages so we could analyze them and compare their gene expression profiles by RNA-Seq to explore any changes in cell–cell interactions.
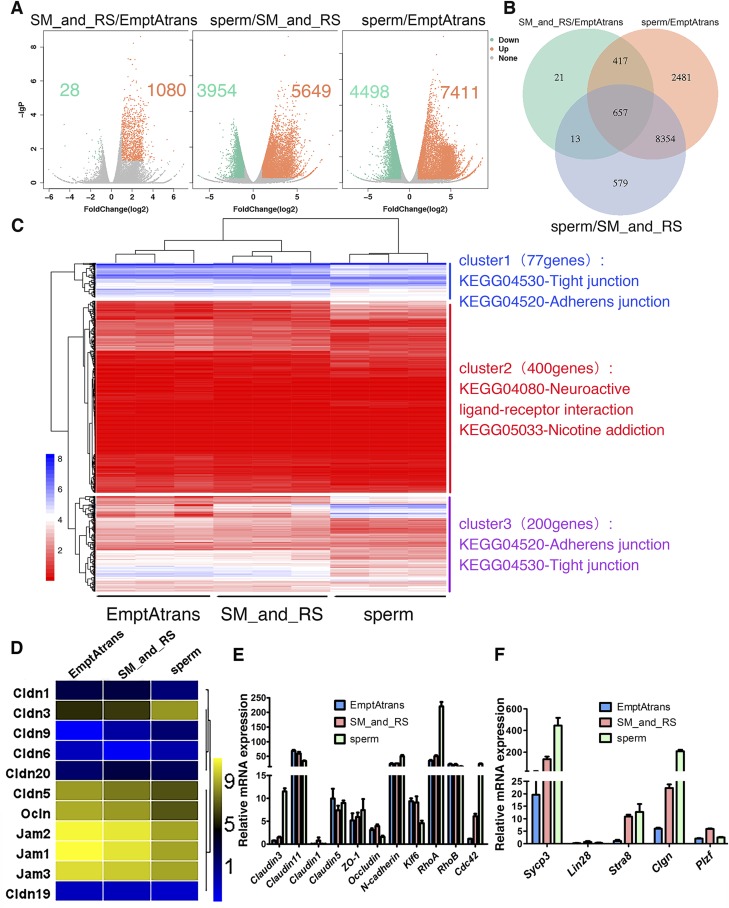
Three groups of samples were selected on the basis of the differentiation status of germ cells: 1) empty tubules devoid of germ cells after SSC transplantation, 2) tubules in which SSCs had differentiated into spermatocytes and round spermatids, and 3) fluorescent tubules that were taken from recipient mice that had earlier been shown to be fertile and capable of producing new offspring such that these tubules had functional spermatogenesis (Supplemental Fig. S3) for various analyses, as shown in Fig. 6A–D. The accuracy of the sampling method and RNA-Seq were validated by real-time RT-PCR (primers used in this part are listed in Table 2) using the remaining templates after RNA-Seq to identify selected genes known to be expressed during spermatogenesis (Fig. 6E, F). Expression of differentiating spermatogonial marker gene Stra8 and meiotic spermatocyte marker genes Sycp3 and Clgn were up-regulated in the more differential sample group (Fig. 6F), consistent with the RNA-Seq results. Pairwise comparisons were made among these 3 groups. The number of significantly DEGs increased with the differentiation status of germ cells (Fig. 6A). A total of 657 DEGs were shared by the 3 sample groups that were compared, and these genes significantly enriched the Gene Ontology (GO) biologic processes cilium assembly, spermatogenesis, and male gamete generation (Fig. 6B).
Figure 6.
Transcriptome profiles of seminiferous tubules at different stages of spermatogenesis. A) Scatterplot analysis for differential gene expression in pairwise comparison. B) Venn diagrams showing distribution of significant changed genes in 3 compared groups. C) Heat map of differentially expressed 677 genes that match GO cellular component subterm cell junction, combined with KEGG pathway analysis. D) Heat map analysis of all detected integral membrane proteins’ genes (from Fig. 4C) that are involved in TJ formation. Only Cldn3 showed considerable increase in expression. E, F) Validation of RNA-Seq results and sampling method by real-time reverse transcription–PCR. DEGs in E were enriched genes in KEGG pathway TJs, and Sycp3, Lin28, Stra8, Clgn, and PLZF are well known for their roles in spermatogenesis. Expression profiles of these genes confirmed RNA-Seq results and sampling method. All experiments were results of 3–4 independent experiments or 3–4 mouse testes.
TABLE 2.
Primer pairs used for quantitative real-time PCR to assess steady-state mRNA level of target genes
| Gene | GenBank accession no. | Sense | Antisense |
|---|---|---|---|
| Claudin-3 | NM_009902.4 | TTTTCCTGTTGGCGGCTCTG | CGAGGTTTCTTTGTCCATTCGG |
| Claudin-11 | NM_008770.3 | ATGGTAGCCACTTGCCTTCAG | AGTTCGTCCATTTTTCGGCAG |
| Occludin | NM_008756.2 | AAGAAAGATGGATCGGTAT | GGAGGCATGTCCTGTG |
| ZO-1 | NM_009386.2 | GCCCTCCGATCATTCC | TTAGACATTCGCTCTTCCTC |
| Claudin-1 | NM_016674.4 | CTTCTGGGTTTCATCCTGG | CAGATTCAGCAAGGAGTCG |
| Claudin-5 | NM_013805.4 | GCCAACATCGTAGTCCG | GTTCTTCTTGTCGTAATCGCC |
| N-cadherin | NM_007664.5 | CATGCTGAGCCACAGTACC | CGCTACTGGAGGAGTTGAGG |
| Lin28 | NM_145833.1 | AGACCAACCATTTGGAGTGC | AATCGAAACCCGTGAGACAC |
| PLZF | NM_001033324.2 | ATTTACTGGCTCATTCAGCG | CCAGTATGGGTCTGTCTGTG |
| Stra8 | NM_009292.1 | ACAAGAGTGAGGCCCAGCAT | CCTCTGGATTTTCTGAGTTGCA |
| Sycp3 | NM_011517.2 | ATGATGGAAACTCAGCAGCAAGAGA | TTGACACAATCGTGGAGAGAACAAC |
| Clgn | NM_009904.3 | CCAGGGTGTTGGACTATGTTTG | CCCCGAGGAAGGTTCATCTTTA |
| klf6 | NM_011803.2 | ACCCGACATGGATGTGCTCCCAAT | GCAGGGCTCACTCTGAAGATA |
| RhoA | NM_016802.5 | CCAAATGTGCCCATCATCCTAGTTG | TCCGTCTTTGGTCTTTGCTGAACAC |
| RhoB | NM_007483.2 | ATATTAGCGTGGGCACCGAG | GTAGGGGTGTAGGGAGTCGT |
| Cdc42 | NM_026514.2 | CCTTCCACAGCTCAGAGAAA | GCACCAGCGAGGACTGTT |
| Gapdh | NM_008084.3 | TGTGTCCGTCGTGGATCTGA | CCTGCTTCACCACCTTCTTGA |
In an attempt to identify key genes pertinent to BTB assembly, 677 DEGs that matched the GO cellular component subterm cell junction were selected from the thousands of DEGs by GO enrichment analysis. Hierarchical clustering was conducted on these DEGs, combined with Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis (Fig. 6C). The clustering tree was categorized into 3 clusters with different expression patterns. Then KEGG enrichment of the 3 clusters was implemented to further confirm the function of these DEGs. The 3 most significantly enriched pathways were listed on the heat map. DEGs in clusters 1 and 3 were enriched in pathways of adherens junctions and TJs, and were further analyzed.
Considering interactions that took place only between germ cells and the adjacent Sertoli cells, we speculated that germ cells likely transmitted signals to Sertoli cells at the cell–cell interface, so we examined integral membrane proteins that were likely involved in BTB formation. Combined with previous analysis, we filtered out 11 DEGs of all 3 kinds of integral membrane proteins coding genes related to the BTB formation: Jam, Ocln, and Cldn from clusters 1 and 3 (Fig. 6D). Through analysis of their expression trend, we noted that nearly all high abundantly expressed genes had reduced expression with germ cell differentiation, but only Cldn3 displayed an opposite trend (increased by 7.7-fold). The expression of Rho-family GTPases (RhoA and Cdc42) that are known to promote actin cytoskeleton assembly were significantly induced with the expression of claudin 3 (Fig. 6E), indicating that this might be a key gene mediating germ cell and Sertoli cell interactions.
Expression pattern of claudin 3 in recipient mice and its function in BTB assembly
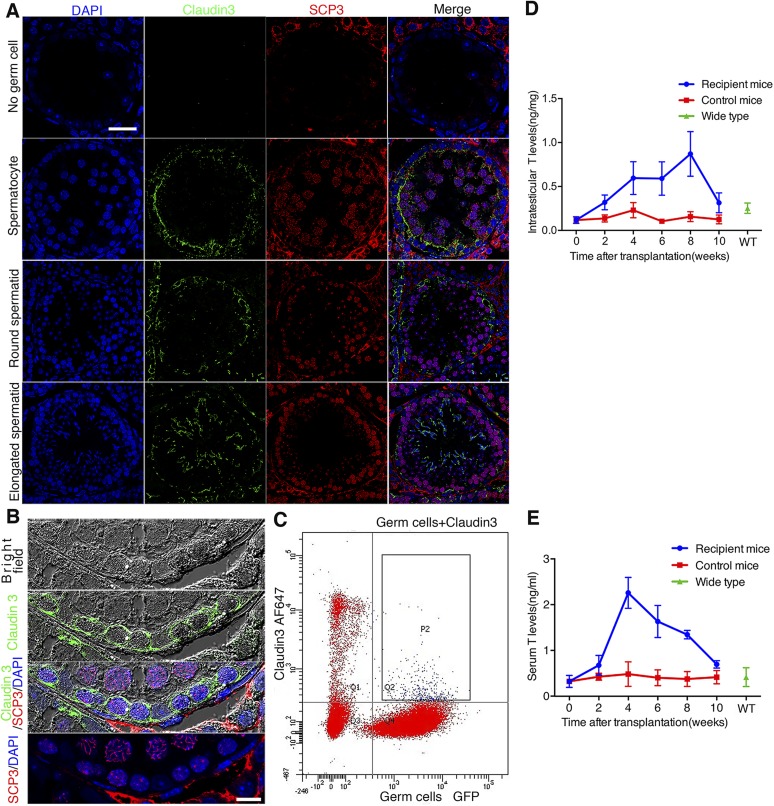
We detected the expression of claudin 3, a marker whose expression illustrates BTB assembly, in recipient testes 2 mo after SSC transplantation by immunofluorescence microscopy, consistent with the timing when BTB was being assembled. There was no obvious claudin 3 expression in tubules without germ cells. Furthermore, distinctive beltlike ultrastructures illustrating immunoreactivity of claudin 3 were detected in the seminiferous epithelium, consistent with its localization at the BTB near the base of the tubule in which spermatogonia had differentiated into spermatocytes, suggesting a new BTB was being assembled. The expression of claudin 3 considerably decreased with the subsequent differentiation of germ cells and almost disappeared at the BTB, but it was reexpressed in newly formed junction structures when spermatids emerged through meiosis (Fig. 7A).
Figure 7.
Expression pattern of claudin 3 and testosterone secretion in recipient testes. A) There was no obvious claudin 3 expression in tubules without germ cells. With appearance of spermatocytes marked by SCP3 (red), claudin 3 (green) began to express in abundance, marking assembly of BTB formation in recipient mouse; however, its expression was down-regulated at BTB after further germ cell differentiation, although it reexpressed in new junction structure where elongated spermatids emerged. Scale bar, 30 μm. B) High-magnification fluorescence micrographs of claudin 3 expression pattern, combining immunofluorescence with bright-field analysis. Signals of claudin 3 located around preleptotene/leptotene spermatocytes marked by SCP3. Scale bar, 10 μm. C) Flow cytometric analysis of cell types that express claudin 3. Germ cells were marked by GFP, and claudin 3 was marked by Alexa Fluor 647. Double-positive cells showed up in rectangle frame P2, indicating claudin 3 was expressed by both Sertoli cells and germ cells. D) Intratesticular testosterone level began to rise in first 2 wk after SSC transplantation, which kept rising until fourth week. Level stayed high until eighth week, when complete spermatogenesis had appeared. E) Serum testosterone level also increased after SSC transplantation as found in testes. WT, WT adult C57BL/6 mice. All experiments were results of 3–4 animals or 3–4 independent experiments.
In order to track down the cellular source of claudin 3, we monitored its expression pattern by confocal microscopy. Most claudin 3 signals overlapped with the membranes of preleptotene/leptotene spermatocytes (Fig. 7B), indicating that this TJ protein might not be exclusively expressed by Sertoli cells, as are other TJ proteins such as ZO-1 and claudin 11 (21). In order to verify this speculation, we analyzed the cell types that had claudin 3 expression by flow cytometry. GFP-labeled SSCs recipient testes were isolated and digested 2 mo after transplantation for flow cytometry analysis. AF647-conjugated claudin 3 antibody was used to mark cells that expressed claudin 3 in flow cytometric analysis. A total of 0.9% of cells were AF647 and GFP double positive and boxed in rectangle frame P2, which were germ cells that were expressing claudin 3 (Fig. 7C). Combining the microscopy and flow cytometry results, we confirmed that preleptotene/leptotene spermatocytes also expressed this molecule, consistent with an earlier report (22). Combined with the above results, the beltlike structure composed of claudin 3 as shown herein was derived from both Sertoli cells and preleptotene/leptotene spermatocytes, and it might not be a putative TJ structure but more like an unconventional adhesion structure between Sertoli cells and germ cells, which could activate actin cytoskeletal assembly in Sertoli cells to induce BTB assembly.
Germ cells promote claudin 3 expression by stimulating Leydig cell testosterone production
It has been reported that testosterone regulates the expression of claudin 3 through androgen receptors expressed in Sertoli cells (8). In addition to the unique spatiotemporal specific expression of claudin 3 in developing tubules after SSC transplantation, we next examined if there were any changes in testosterone levels in recipient mice. Interestingly, the level of testosterone found in blood samples and testis lysates indeed increased considerably within 4 wk after SSC transplantation (Fig. 7D, E). These high levels of testosterone were maintained until week 8, at which point the levels were then reduced considerably by week 10, when complete spermatogenesis was detected in the testis. However, in normal WT control KitW/KitWV animals without SSC transplantation, the testosterone level did not display such fluctuations (Fig. 7D, E). Taking collectively, these data suggest that the temporary expression of claudin 3 might be regulated by testosterone. It is also possible that germ cells are a contributing factor to stimulate Leydig cell testosterone production.
DISCUSSION
The biology of interactions between germ cells and somatic cells in the testis during spermatogenesis remains a rarely investigated topic in male reproductive physiology. Investigators have made great efforts by focusing on how somatic cells regulate germ cell development. However, the positive impact of germ cells on somatic cell function remains unexplored because germ cells, in particular postmeiotic spermatids, have been considered to be metabolically quiescent cells in the seminiferous epithelium. In the present study, we used a novel mouse model to investigate this subject by transplanting undifferentiated spermatogonia into KitW/KitWV testes without a functional BTB, thereby failing to support meiosis in adult mutant mice. In short, we investigated if the recipient testis could establish a functional BTB to support meiosis by transplanting SSCs into the mutant testis because Sertoli cells from these recipient mice failed to assemble a functional BTB. We next examined the BTB function in the recipient mouse testis when the transplanted SSCs were differentiating and developing into more advanced germ cells.
The evidence provided in this study demonstrates that germ cells could modulate Sertoli cell function as well as Leydig cell function. We identified the indispensable role of germ cells on BTB construction. However, some questions remain unanswered. For instance, would c-Kit expressed by the transplanted SSCs play a role in reestablishing a function BTB in the recipient mouse testis?
BTB assembly appears to be initiated after strong expression of claudin 3 from preleptotene/leptotene spermatocytes and Sertoli cells. It has been reported that claudin 3 expression is positively regulated by androgen (8). Herein, we have shown that high levels of androgen alone are insufficient to induce up-regulation and accumulation of claudin 3 at the BTB site. Also, there was no obvious claudin 3 expression in tubules without spermatocytes when the testosterone level had already increased. This integral membrane protein appeared to work well when produced by both Sertoli and germ cells near the basement membrane. This finding also sheds some light on the mechanism of coordinated BTB opening and closing during spermatogenesis. It has been reported that germ-line stem cells treated with claudin 3 short hairpin RNA significantly reduced clone formation efficiency after transplantation (15), but in this earlier study, any changes in the BTB assembly were not examined. Also, Cldn3-null mice had normal BTB and were fertile (23), thus illustrating that other molecules could supersede the function of claudin 3 after its deletion in the genetic model.
The repositioned and time-dependent expression of the BTB constitutive proteins during its assembly after SSC transplantation and subsequent development may be the most intriguing events that deserve to be better investigated. In our study, the signals provided by different classes of germ cells that induced Sertoli cell to work cooperatively to assemble the BTB remain unknown; this should be carefully evaluated in future investigation. Nonetheless, we have shown that the expression level of β-catenin, PARD6B (Fig. 1), JAM-A, and claudin 11 (Fig. 4) were considerably reduced with the homing of SSCs, suggesting that the signals control TJ proteins’ spatiotemporal expression and localization, and may originate from germ cells, which were absent in normal KitW/KitWV mice. It may be that the loss of necessary negative feedback signals caused overexpression of these proteins. However, this is different from the in vitro results (21), suggesting that interacting conditions in vivo are different from those in vitro. Haploid spermatids, which were not included in coculture models, may be a key reason for such an outcome.
The receptor tyrosine kinase KIT and its ligand are crucial to support gametogenesis (24). KIT expression in the adult testis is detectable in differentiating type A, intermediate, and type B spermatogonia, as well as preleptotene spermatocytes, but not in more differentiated germ cells (25). It has been reported that a functional KIT protein is not required for SSC self-renewal (26), which was also verified in this study. However, its unique expression pattern in germ cells led us to consider whether it played a negative role in BTB assembly. In order to answer this question, in future studies, we plan to establish a gene-modified SSC cell line that can express KIT in more differentiated germ cell types after transplantation. Such studies can then assess the role of KIT in spermatogenesis.
The mechanism or mechanisms by which germ cells induce Leydig cell testosterone secretion remain unknown. Studies in the literature have shown that hormones and paracrine factors released by somatic cells can modulate germ cell self-maintenance and differentiation, but few reports are found in the literature that examine the regulatory roles of germ cells on somatic cell function. Furthermore, there is no physical contact between germ cells and Leydig cells, so the signaling molecules and/or pathways that mediate communications between germ cells and Leydig cells remain to be identified. Nonetheless, Sertoli cells and myoid cells might serve as the agent to mediate Leydig cell and germ cell interactions. In addition, SSCs had differentiated into spermatocytes in the first month after transplantation. At this stage, no information is available regarding whether different classes of germ cells had the same or dissimilar influence on Leydig cell function. A systematic investigation is necessary to better define the interrelationship between germ cell and somatic cell function.
Changes in the cytoskeletal organization and the establishment of Sertoli cell polarity are important cellular processes during BTB assembly, which involve precise spatiotemporal expression and deposition of proteins. While we have now identified the involvement of Rho-family GTPases in BTB assembly based on a bioinformatics approach, the molecular mechanisms by which GTPases modulate cytoskeletal organization to support BTB assembly remain to be investigated. Nonetheless, our study provides an interesting model to understand the role of germ cells in BTB homeostasis and assembly.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by grants from Major Research Plan 973 Project (2012CB944702), Natural Science Foundation of China (31501161, 31501953, 31471352, 31471400, 81270662, and 31171380), Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC-C09-2015, LNCCC-D50-2015), and Academician Workstation Support (Shenyang, Changsha, and Shandong). The authors declare no conflicts of interest.
Glossary
- BTB
blood–testis barrier
- DEG
differentially expressed gene
- ES
ectoplasmic specialization
- GFP
green fluorescent protein
- GO
Gene Ontology
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- RNA-Seq
RNA sequencing
- SSC
spermatogonial stem cell
- TJ
tight junction
- WT
wild type
- ZO
zonula occludens
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X.-Y. Li performed the study design, performed most experiments, analyzed data, and wrote the article; Y. Zhang and X.-X. Wang performed some experiments; C. Y. Cheng and Y.-X. Liu participated in the study design and article modifications, and supervised the study; other colleagues participated in article modifications; and all authors read and approved the final article.
REFERENCES
- 1.Dym M., Cavicchia J. C. (1977) Further observations on the blood–testis barrier in monkeys. Biol. Reprod. 17, 390–403 10.1095/biolreprod17.3.390 [DOI] [PubMed] [Google Scholar]
- 2.Weber J. E., Russell L. D. (1987) A study of intercellular bridges during spermatogenesis in the rat. Am. J. Anat. 180, 1–24 10.1002/aja.1001800102 [DOI] [PubMed] [Google Scholar]
- 3.Smith B. E., Braun R. E. (2012) Germ cell migration across Sertoli cell tight junctions. Science 338, 798–802 10.1126/science.1219969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mruk D. D., Cheng C. Y. (2015) The mammalian blood–testis barrier: its biology and regulation. Endocr. Rev. 36, 564–591 10.1210/er.2014-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann M., Dierichs R. (1983) Postnatal formation of the blood–testis barrier in the rat with special reference to the initiation of meiosis. Anat. Embryol. (Berl.) 168, 269–275 10.1007/BF00315821 [DOI] [PubMed] [Google Scholar]
- 6.Nocka K., Tan J. C., Chiu E., Chu T. Y., Ray P., Traktman P., Besmer P. (1990) Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 9, 1805–1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta H., Tohda A., Nishimune Y. (2003) Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biol. Reprod. 69, 1815–1821 10.1095/biolreprod.103.019323 [DOI] [PubMed] [Google Scholar]
- 8.Meng J., Holdcraft R. W., Shima J. E., Griswold M. D., Braun R. E. (2005) Androgens regulate the permeability of the blood–testis barrier. Proc. Natl. Acad. Sci. USA 102, 16696–16700 10.1073/pnas.0506084102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaitu’u-Lino T. J., Sluka P., Foo C. F., Stanton P. G. (2007) Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction 133, 1169–1179 10.1530/REP-06-0385 [DOI] [PubMed] [Google Scholar]
- 10.Su L., Mruk D. D., Lee W. M., Cheng C. Y. (2010) Differential effects of testosterone and TGF-β3 on endocytic vesicle–mediated protein trafficking events at the blood–testis barrier. Exp. Cell Res. 316, 2945–2960 10.1016/j.yexcr.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitic L. L., Van Itallie C. M., Anderson J. M. (2000) Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G250–G254 [DOI] [PubMed] [Google Scholar]
- 12.Tsukita S., Furuse M., Itoh M. (2001) Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 10.1038/35067088 [DOI] [PubMed] [Google Scholar]
- 13.Mruk D. D., Cheng C. Y. (2004) Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- 14.Morrow C. M., Tyagi G., Simon L., Carnes K., Murphy K. M., Cooke P. S., Hofmann M. C., Hess R. A. (2009) Claudin 5 expression in mouse seminiferous epithelium is dependent upon the transcription factor ets variant 5 and contributes to blood–testis barrier function. Biol. Reprod. 81, 871–879 10.1095/biolreprod.109.077040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashima S., Kanatsu-Shinohara M., Tanaka T., Takehashi M., Morimoto H., Shinohara T. (2011) Rac mediates mouse spermatogonial stem cell homing to germline niches by regulating transmigration through the blood–testis barrier. Cell Stem Cell 9, 463–475 10.1016/j.stem.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Wang S., Wang X., Liao S., Wu Y., Han C. (2012) Endogenously produced FGF2 is essential for the survival and proliferation of cultured mouse spermatogonial stem cells. Cell Res. 22, 773–776 10.1038/cr.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S., Wang X., Ma L., Lin X., Zhang D., Li Z., Wu Y., Zheng C., Feng X., Liao S., Feng Y., Chen J., Hu X., Wang M., Han C. (2016) Retinoic acid is sufficient for the in vitro induction of mouse spermatocytes. Stem Cell Reports 7, 80–94 10.1016/j.stemcr.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen Q., Zheng Q. S., Li X. X., Hu Z. Y., Gao F., Cheng C. Y., Liu Y. X. (2014) Wt1 dictates the fate of fetal and adult Leydig cells during development in the mouse testis. Am. J. Physiol. Endocrinol. Metab. 307, E1131–E1143 10.1152/ajpendo.00425.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byers S., Graham R., Dai H. N., Hoxter B. Development of Sertoli cell junctional specializations and the distribution of tight-junction–associated protein ZO-1 in the mouse testis. (1991) Am. J. Anat. 191, 35–47 [DOI] [PubMed] [Google Scholar]
- 20.Nagano M., Avarbock M. R., Brinster R. L. (1999) Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 60, 1429–1436 10.1095/biolreprod60.6.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su L., Kopera-Sobota I. A., Bilinska B., Cheng C. Y., Mruk D. D. (2014) Germ cells contribute to the function of the Sertoli cell barrier. Spermatogenesis 3, e26460 10.4161/spmg.26460 [DOI] [Google Scholar]
- 22.Chihara M., Ikebuchi R., Otsuka S., Ichii O., Hashimoto Y., Suzuki A., Saga Y., Kon Y. (2013) Mice stage-specific claudin 3 expression regulates progression of meiosis in early stage spermatocytes. Biol. Reprod. 89, 3 10.1095/biolreprod.113.107847 [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty P., William Buaas F., Sharma M., Smith B. E., Greenlee A. R., Eacker S. M., Braun R. E. (2014) Androgen-dependent Sertoli cell tight junction remodeling is mediated by multiple tight junction components. Mol. Endocrinol. 28, 1055–1072 10.1210/me.2013-1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besmer P., Manova K., Duttlinger R., Huang E. J., Packer A., Gyssler C., Bachvarova R. F. (1993) The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev. Suppl., 1993, 125–137 [PubMed] [Google Scholar]
- 25.Schrans-Stassen B. H., van de Kant H. J., de Rooij D. G., van Pelt A. M. (1999) Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology 140, 5894–5900 10.1210/endo.140.12.7172 [DOI] [PubMed] [Google Scholar]
- 26.Kubota H., Avarbock M. R., Schmidt J. A., Brinster R. L. (2009) Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation. Biol. Reprod. 81, 293–301 10.1095/biolreprod.109.075960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.