Abstract
The substantial rise in the prevalence of nonalcoholic steatohepatitis (NASH), an advanced form of nonalcoholic fatty liver disease, and the strong association between NASH and the development of hepatocellular carcinoma indicate the urgent need for a better understanding of the underlying mechanisms. In the present study, by using the Stelic animal model of NASH and NASH-derived liver carcinogenesis, we investigated the role of the folate-dependent 1-carbon metabolism in the pathogenesis of NASH. We demonstrated that advanced NASH and NASH-related liver carcinogenesis are characterized by a significant dysregulation of 1-carbon homeostasis, with diminished expression of key 1-carbon metabolism genes, especially a marked inhibition of the S-adenosylhomocysteine hydrolase (Ahcy) gene and an increased level of S-adenosyl-l-homocysteine (SAH). The reduction in Ahcy expression was associated with gene-specific cytosine DNA hypermethylation and enrichment of the gene promoter by trimethylated histone H3 lysine 27 and deacetylated histone H4 lysine 16, 2 main transcription-inhibiting markers. These results indicate that epigenetically mediated inhibition of Ahcy expression may be a driving force in causing SAH elevation and subsequent downstream disturbances in transsulfuration and transmethylation pathways during the development and progression of NASH.—Pogribny, I. P., Dreval, K., Kindrat, I., Melnyk, S., Jimenez, L., de Conti, A., Tryndyak, V., Pogribna, M., Ortega, J. F., James, S. J., Rusyn, I., Beland, F. A. Epigenetically mediated inhibition of S-adenosylhomocysteine hydrolase and the associated dysregulation of 1-carbon metabolism in nonalcoholic steatohepatitis and hepatocellular carcinoma.
Keywords: hepatocarcinogenesis, mouse, epigenetics
The prevalence of nonalcoholic fatty liver disease (NAFLD), which encompasses a spectrum of chronic liver pathologic states ranging from simple steatosis (e.g., nonalcoholic fatty liver or NAFL) to nonalcoholic steatohepatitis (NASH), an advanced form of the disease with hepatocellular injury and inflammation, is increasing worldwide (1–4), especially in adolescents and young adults (5). In addition, a wealth of epidemiologic data has established convincingly an association between NASH and the development of hepatocellular carcinoma (HCC) in humans (6). Currently, NASH is becoming one of the most prevalent causes of HCC, replacing viral hepatitis B and C viruses and alcohol-related chronic liver diseases as a major etiological risk factor for HCC (7).
Uncomplicated NAFL is generally considered to be a benign form of NAFLD and has a favorable outcome; however, ∼30% of individuals diagnosed with simple NAFL develop NASH (1). NASH and NASH-related cirrhosis are associated with increased morbidity and mortality, and the percentage of patients with NASH and NASH-related HCC awaiting liver transplantation is rising. Currently, NASH is the second most prevalent cause of liver transplantation in the United States (8, 9). These statistics indicate an urgent need for a better understanding of the mechanisms and factors that mediate progression of the disease. This understanding is critical for discovering and identifying biomarkers to enhance the diagnosis of NASH and monitor its progression and for use in disease treatment and prevention (10).
The development of NASH and NASH-related liver carcinogenesis has been associated with genetic, genomic, and metabolic abnormalities, with alterations in hepatic lipid homeostasis being one of the most prominent (11). In our studies, using a mouse model of NAFLD induced by a choline- and folate-deficient (CFD) (12–14) or methionine-deficient (15) diet, we found that the development of NASH is characterized by a profound and interconnected dysregulation in hepatic folate-dependent 1-carbon and lipid metabolism. Specifically, feeding C57BL/6J and DBA/2J mice a CFD diet for 18 wk resulted in an extensive hepatic lipid accumulation, prominent alterations in methyl donor metabolism, and development of NASH (12). In a subsequent study, using a panel of 7 genetically diverse inbred mouse strains (14), we demonstrated that the development of a strain-dependent NASH-like liver injury induced by a 12-wk dietary choline- and folate deficiency is paralleled by a prominent dysregulation in lipid and 1-carbon metabolism. Similar findings were reported in the livers of mice fed a methionine-deficient diet for 10 wk (15).
Alterations in 1-carbon metabolism have also been reported in experimental alcohol-induced steatohepatitis (16) and hepatic steatosis induced by a high-fat diet (17). Furthermore, several clinical studies have demonstrated an increased plasma level of homocysteine, a pathophysiological consequence and indicator of 1-carbon metabolism dysregulation (18), in patients with alcoholic steatohepatitis (19) and NASH (20–22); however, there is insufficient knowledge to clarify the role, mechanism, and functional outcomes of 1-carbon dysregulation in the pathogenesis of NASH and its progression to NASH-associated fibrosis and HCC.
Based on these considerations, in the present study, we investigated alterations in 1-carbon metabolism during NASH-associated liver carcinogenesis using a Stelic Animal Model (STAM), the first animal model to resemble the development of NASH and NASH-derived HCC in humans (23).
MATERIALS AND METHODS
Animals, experimental design, and mouse models of NASH
In the present study, we used liver tissue samples from male C57BL/6J mice subjected to two different models of NASH induced by feeding: a high-fat diet [Stelic animal model (STAM)] and a CFD diet (CFD model). STAM is the first mouse model of NASH that depicts the sequential development of clinical and pathomorphologic features of NASH in diabetic patients (23). In brief, 2-d-old male C57BL/6J mice were injected with streptozotocin (200 μg/mouse). Starting from 4 wk of age, mice were continuously fed a high-fat diet (HFD-32; Clea, Tokyo, Japan; Supplemental Table 1) throughout the study. Control mice were maintained on standard animal chow for the duration of the study. Liver samples from male STAM mice in the steatotic (6 wk), NASH fibrotic (12 wk), and full-fledged (20 wk) HCC stages of liver carcinogenesis and from age-matched C57BL/6J mice were purchased from SMC Laboratories (Tokyo, Japan). These experimental procedures were performed according to the Japanese Pharmacological Society Guidelines and the experimental protocols were approved by the Research Animal Care and Use Committee of SMC Laboratories, Inc.
The CFD model of NASH is characterized by uniform morphologic features of human NASH (24) and is an ideal model for studying the subgroup of NASH patients with histologically advanced NASH (25). The in-life portion of the CFD study, mouse treatment, tissue sample collection, and liver histopathology are detailed in Pogribny et al. (12). In brief, mice in the CFD experimental group were maintained on a diet low in methionine (0.17% w/w) and lacking in choline and folic acid (diet no. 519541, CFD, iron-supplemented, and l-amino acid-defined diet; Dyets, Bethlehem, PA, USA; Supplemental Table 1) for 12 wk. Mice in the control group were fed the same diet supplemented with 0.4% methionine, 0.3% choline bitartrate, and 2 mg/kg folic acid. Mice from both groups were euthanized by exsanguination following deep isoflurane anesthesia 12 wk after diet initiation. These experimental procedures were reviewed and approved by the National Center for Toxicological Research Animal Care and Use Committee.
In addition to liver tissue samples from mice subjected to NASH-related liver carcinogenesis, HCC tissue samples were obtained from male C57BL/6J mice subjected to fibrosis- and inflammation-associated hepatocarcinogenesis induced by N,N-diethylnitrosamine (DEN) and carbon tetrachloride (CCl4), as detailed in Uehara et al. (26). These animal experiments were approved by the University of North Carolina at Chapel Hill Animal Care and Use Committee.
RNA extraction and gene expression analysis using microarray technology
Total RNA was extracted from liver tissue samples of STAM mice and mice fed the CFD diet (n = 5/group per treatment) using miRNeasy Mini kits (Qiagen, Germantown, MD, USA), according to the manufacturer’s instructions. Whole-genome hepatic gene expression profiles were determined with an Agilent-074809 SurePrint G3 Mouse GE v2 8x60K Microarray in the STAM mice and an Agilent-014868 Whole Mouse Genome 4x44K Microarray (Agilent Technologies, Santa Clara, CA, USA) in mice fed the CFD diet, as described in detail elsewhere (13, 27). The microarray gene expression data have been deposited in the National Center for Biotechnology Information (NCBI; Bethesda, MD, USA) Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/; accession numbers GSE83596 and GSE96936).
S-Adenosylhomocysteine hydrolase gene expression data
S-Adenosylhomocysteine hydrolase (AHCY) gene expression data in liver biopsy samples from patients with NASH were downloaded from the Gene Expression Omnibus database (accession number GSE37031) and analyzed.
Gene expression and enrichment analysis
The mouse and human gene expression datasets were analyzed using the limma R/Bioconductor software package (28). Benjamini-Hochberg adjusted P values (29) were calculated; an adjusted P value cut off of 0.05 and a fold-change threshold of 2.0 were used to identify significantly altered gene transcripts at each time point in the mice. To characterize pathways that were statistically enriched in relation to lipid metabolism, the lists of differentially expressed genes in the livers of control, STAM, and CFD diet-fed mice containing gene identifiers and their corresponding expression values were uploaded into the clusterProfiler package of the Bioconductor project (30). The enrichment analysis was performed based on the subontologies of molecular functions, cellular components, and biologic processes. Gene sets were considered significantly enriched when the false-discovery rate was <5%.
Quantitative RT-PCR
Total RNA (2 μg) from liver tissues of control and STAM mice (n = 5/group/time interval) was reverse transcribed with random primers and High Capacity cDNA Reverse Transcription kits (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. cDNA was analyzed in a 96-well plate PCR assay format on a QuantStudio 7 Flex Real-Time PCR System (Thermo Fisher Scientific). Each plate contained experimental genes and a housekeeping gene (TATA box binding protein; Tbp). All primers for the gene expression analysis were obtained from Thermo Fisher Scientific and are listed in Supplemental Table 2. The relative amount of each mRNA transcript was determined by using the 2−ΔΔCt method (31). Negative template controls were run on each plate. All samples were analyzed in triplicate; the values are expressed as the means ± sd of 3 runs.
Determination of tissue contents
The levels of methionine, S-adenosyl-l-methionine (SAM), S-adenosyl-l-homocysteine (SAH), homocysteine, and glutathione (GSH) were measured in the livers and plasma of control and STAM mice by HPLC, coupled with coulometric electrochemical detection (32, 33).
Methylated DNA immunoprecipitation analyses
Genomic DNA was isolated from liver tissues of control and STAM mice with DNeasy Blood and Tissue kits (Qiagen), according to the manufacturer’s instructions.
Methylated DNA immunoprecipitation (MeDIP) was performed with MethylMiner Methylated DNA Enrichment kits (Thermo Fisher Scientific) according to the manufacturer’s instructions. The methylation status of the CpG island located within the 5′-UTR of the Ahcy and cystathionine β-synthase (Cbs) genes was determined by MeDIP-quantitative PCR (MeDIP-qPCR), with the following primer sets: Ahcy forward, 5′-CTCCGCCCCTAAGTTTCAG-3′, and reverse, 5′-GCTTCCGCTAGGACTGATGA-3′; and Cbs forward, 5′-GCTCTCCATCCTAGCACAGG-3′, and reverse, 5′-GCGAGGATCTCGCTGTGT-3′. The results were normalized to the amount of input DNA and presented as fold change for each DNA in the livers of STAM mice relative to age-matched control mice.
Chromatin immunoprecipitation and qPCR
Chromatin immunoprecipitation was performed with a Chromatin Immunoprecipitation Assay kit (Millipore-Sigma, Billerica, MA, USA) according to the manufacturer’s protocol. The liver tissue lysates were incubated with primary antibodies against trimethylated histone H3 lysine 27 (H3K27me3; Abcam, Cambridge, UK) and acetylated histone H4 lysine 16 (H4K16ac; Cell Signaling Technology, Danvers, MA, USA). Purified DNA from immunoprecipitated and input DNA was analyzed by qPCR with the same Ahcy gene primer set as was used in the MeDIP-qPCR analysis. The results were normalized to the amount of input DNA and presented as fold change for each DNA in the livers of STAM mice relative to age-matched control mice.
Western blot analysis
The levels of the maintenance DNA methyltransferase (DNMT)-1, de novo DNA methyltransferases DNMT3A and -3B, and enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) protein were determined in the livers of STAM mice by Western blot analysis (27).
Statistical analyses
Results are presented as means ± sd (n = 5). Significant differences between groups were evaluated with an unpaired 2-tailed Student’s t test. When necessary, the data were natural log transformed before conducting the analyses to maintain a more equal variance or normal data distribution. Statistical significance was set at P < 0.05.
RESULTS
Expression of lipid metabolism–related genes in the livers of STAM mice and mice fed a CFD diet
In another study (27), we demonstrated that NASH-related liver carcinogenesis in STAM mice is characterized by extensive molecular abnormalities in the livers, among which dysregulation of hepatic lipid metabolism is one of the most significant and exhibited progressive alterations during the carcinogenic process. This corresponds to recent reports of altered hepatic lipid profiles (34) and proteins involved in lipid metabolism (35) in NASH liver tissues of STAM mice. In view of this finding, we performed an in-depth analysis of lipid metabolism dysregulation in the livers of STAM mice.
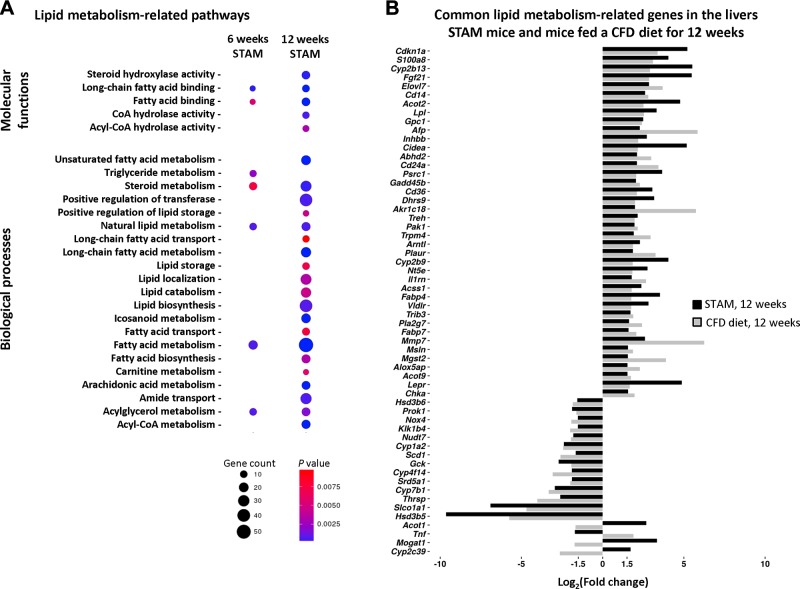
The development of NASH in STAM mice was associated with a progressive increase in both the number of dysregulated lipid metabolism–related pathways and the number of differentially expressed genes in affected pathways (Fig. 1A). Despite different etiological factors that caused the development of NASH in STAM mice and mice fed a CFD diet, there was a substantial overlap in dysregulated hepatic lipid metabolism–related pathways in these 2 models of NASH. This overlap was evidenced by the fact that of the 144 differentially expressed lipid metabolism genes in the livers of STAM mice and the 197 differentially expressed lipid metabolism genes in the livers of mice fed a CFD diet, 54 of the genes were present in common in the 2 different NASH models (Fig. 1B).
Figure 1.
Expression of lipid metabolism–related genes in the livers of STAM mice and mice fed a CFD diet, as determined by whole-genome microarrays. A) Gene enrichment of lipid metabolism pathways in STAM mice at 6 and 12 wk. The size and color of the circles indicate the number of genes and their significance. B) Common differentially expressed lipid metabolism–related genes in the livers of STAM mice and mice fed a CFD diet for 12 wk.
Alterations in hepatic 1-carbon metabolism during NASH development in STAM mice
In another study, using a mouse model of NASH induced by feeding mice a CFD diet, we demonstrated significant aberrations in hepatic 1-carbon metabolism (14). Based on our findings in similarities in lipid metabolism alterations in the livers of STAM mice and mice fed a CFD diet (Fig. 1), we investigated whether the development of NASH in STAM mice induced by a high-fat diet is also characterized by the dysregulation of hepatic 1-carbon metabolism.
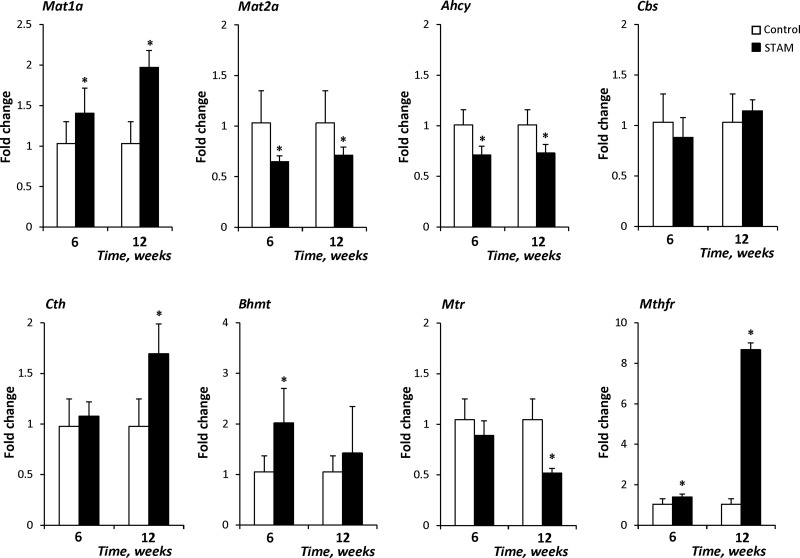
First, we analyzed the expression of 1-carbon metabolism–related genes and demonstrated that the methionine adenosyltransferase 1-α (Mat1a) and methylene tetrahydrofolate reductase (Mthfr) genes were overexpressed in steatotic (6 wk) and NASH fibrotic (12 wk) liver tissues, whereas the expression of Mat2a and Ahcy was reduced (Fig. 2). In addition to these changes, 2 other genes were altered in NASH fibrotic (12 wk) liver tissue: the expression of cystathionase (cystathionine γ-lyase) (Cth) was increased, whereas the level of Mtr was reduced (Fig. 2). The altered expression of 1-carbon metabolism–related genes had been observed in the livers of C57BL/6J mice fed a CFD diet for 12 wk (14). A similar reduction in Ahcy expression and increase in Mthfr expression was observed in these 2 different models of NASH (Supplemental Fig. 1).
Figure 2.
Expression of 1-carbon metabolism–related genes in the livers of STAM mice. The expression of Mat1a, Mat2a, Ahcy, Cbs, Cth, Bhmt, Mtr, and Mthfr transcripts was determined by qRT-PCR. The results are presented as the mean fold change in the expression of each gene in the livers of STAM mice relative to that of age-matched control mice, which were assigned a value of 1. Data are means ± sd (n = 5). *P < 0.05 vs. age-matched control mice.
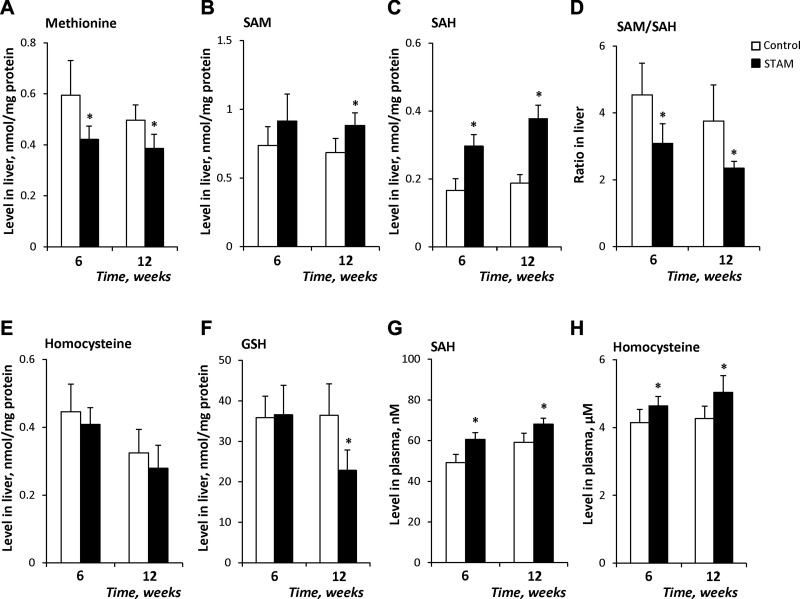
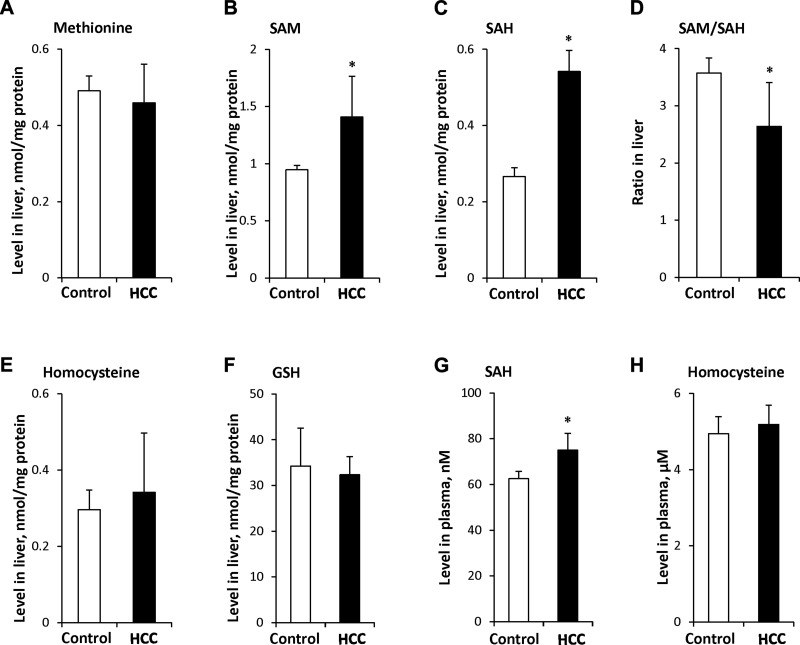
Next, we evaluated the levels of the 1-carbon cycle metabolites methionine, SAM, SAH, homocysteine, and GSH in the livers of control and STAM mice during NASH development. The levels of methionine in steatotic (6 wk) and NASH fibrotic (12 wk) liver tissue samples decreased by 28 and 24%, respectively, relative to control levels (Fig. 3A). In contrast, the level of SAM was elevated by 30% in NASH fibrotic liver tissue (Fig. 3B). The most prominent alterations were found in the level of SAH, which was increased 79 and 101%, respectively, in steatotic and NASH fibrotic livers of STAM mice (Fig. 3C). Despite a great increase in the level of SAH, the level of homocysteine did not change (Fig. 3E), which may be attributable to the inhibition of Ahcy expression and activity, with a corresponding reduction in homocysteine synthesis. As a consequence of the marked increase of hepatic SAH, the hepatic SAM/SAH decreased in steatotic and NASH fibrotic liver tissues by 32 and 38%, respectively (Fig. 3D). In contrast, the level of GSH was reduced by 37% in NASH fibrotic liver tissue (Fig. 3F), which may be attributed to an impaired hepatic SAH metabolism.
Figure 3.
Levels of 1-carbon metabolites in liver and plasma of STAM mice: methionine (A), SAM (B), and SAH (C), SAM:SAH ratio (D), homocysteine (E), and GSH (F) in liver and SAH (G) and homocysteine (H) in plasma. Data are means ± sd (n = 5). *P < 0.05 vs. age-matched control mice.
The results of several experimental and clinical studies have demonstrated that hyperhomocysteinemia is one of the pathophysiological features of alcoholic steatohepatitis and NASH (19–22). Hence, the plasma levels of homocysteine and SAH were measured in the STAM mice. A slight, but significant, increase in the level of SAH and homocysteine was found at 6 and 12 wk (Fig. 3G, H).
Alterations in 1-carbon metabolism in NASH-derived HCC
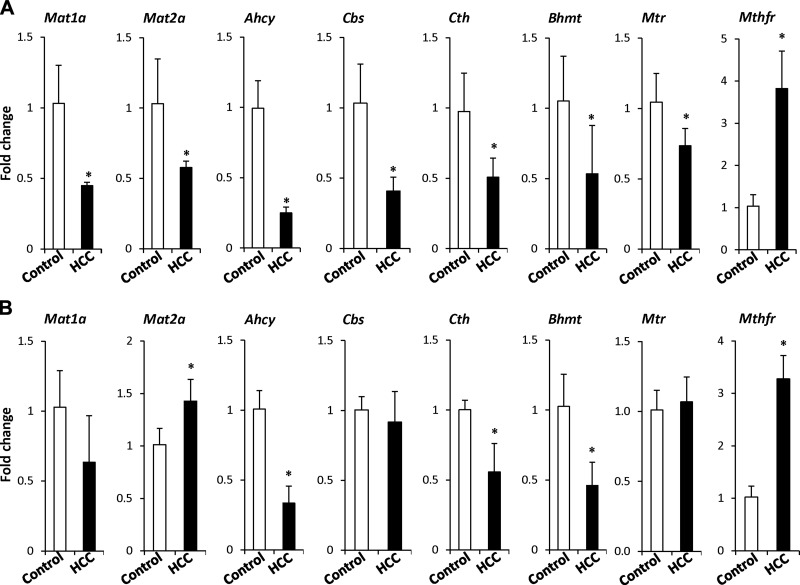
To determine whether the abnormalities in 1-carbon homeostasis observed in the NASH fibrotic livers are also present in NASH-derived HCC, we investigated the status of 1-carbon metabolism in HCC tissue samples. The major alterations in the expression of 1-carbon metabolism–related genes in NASH-derived HCC were characterized by a reduced expression of the Mat1a, Mat2a, Ahcy, Cbs, Cth, betaine-homocysteine methyltransferase (Bhmt), and Mtr genes (Fig. 4A). A diminished expression of these genes has been reported in experimental and human HCC (36–38). In contrast, the expression of Mthfr was 4-fold greater. A similar pattern in the expression of Ahcy, Cth, Bhmt, and Mthfr genes was found in fibrosis- and inflammation-associated HCC induced by a combined DEN and CCl4 treatment (Fig. 4B). Ahcy was the most down-regulated gene, whereas Mthfr was the most up-regulated gene in both models of hepatocarcinogenesis.
Figure 4.
Expression of 1-carbon metabolism–related genes in HCC. The expression of Mat1a, Mat2a, Ahcy, Cbs, Cth, Bhmt, Mtr, and Mthfr transcripts was determined by qRT-PCR in NASH-derived HCC (A) and fibrosis- and inflammation-associated HCC (B). The data are means ± sd (n = 5). *P < 0.05, denotes a significant difference from the age-matched control mice.
To determine the functional consequences of these gene expression alterations, we analyzed the methionine, SAM, SAH, homocysteine, and GSH content in the livers of mice with NASH-derived HCC. The level of SAM and, especially, SAH in HCC was 1.5- and 2.0-times greater than in the livers of age-matched control mice (Fig. 5B, C), whereas the levels of methionine, homocysteine, and GSH did not differ (Fig. 5A, E, F). A marked increase in the level of SAH resulted in a decreased hepatic SAM:SAH ratio (Fig. 5D) and elevation of plasma SAH in HCC-bearing mice (Fig. 5G). In contrast, the plasma level of homocysteine did not differ from that in control mice (Fig. 5H).
Figure 5.
Levels of 1-carbon metabolites in HCC and plasma of tumor-bearing STAM mice: methionine (A), SAM (B), and SAH (C), SAM:SAH ratio (D), homocysteine (E), and GSH (F) in liver and SAH (G) and homocysteine (H) in plasma. Data are means ± sd (n = 5). *P < 0.05 vs. age-matched control mice.
Elevation of hepatic SAH is associated with the hypermethylation-mediated silencing of Ahcy
The striking elevation of SAH in the livers of STAM mice, especially in NASH fibrotic and NASH-derived HCC, may be attributed to inhibition of the expression of the Ahcy gene, which encodes SAH hydrolase. SAH hydrolase is the only mammalian enzyme known to catalyze the conversion of SAH to homocysteine and adenosine in a reversible reaction (39). Therefore, down-regulation of Ahcy could impair SAH hydrolysis resulting in its elevation. Indeed, decreased Ahcy expression was associated with increased levels of SAH, in contrast to no changes in homocysteine (Supplemental Fig. 2). Down-regulation of AHCY expression was also found in liver biopsies from patients with NASH (Supplemental Fig. 3).
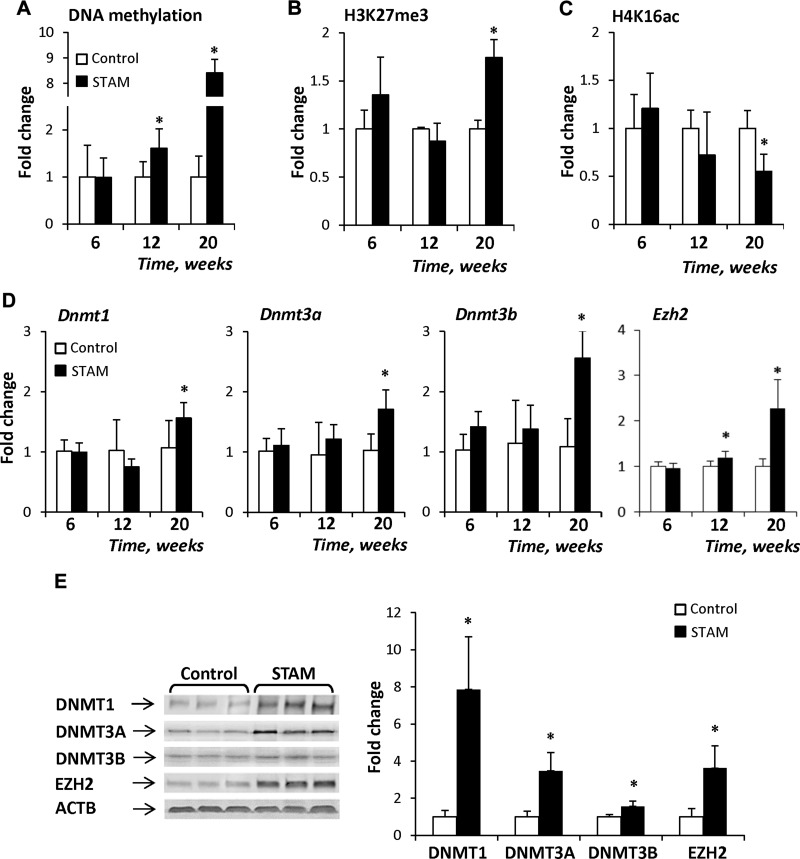
The expression and function of the Ahcy gene may be controlled genetically (e.g., single nucleotide polymorphism) or epigenetically (e.g., cytosine DNA methylation). Analysis of the promoter region of the mouse Ahcy gene (NM_016661.3) identified the presence of a strong CpG island, based upon well-established criteria: greater than 500 bp in length, G+C >55%, and an observed CpG/expected CpG ratio of >0.65 (40). Therefore, we investigated the role of epigenetic mechanisms in the inhibition of Ahcy expression. A time-dependent increase was noted in Ahcy gene promoter methylation in livers of STAM mice, with the greatest increase being found in NASH-derived HCC at 20 wk (Fig. 6A). In addition to gene-specific cytosine DNA hypermethylation, there were substantial alterations in the levels of histone H3K27me3 and H4K16ac, two histone modification markers associated with gene transcription. Specifically, the level of histone H3K27me3, a transcription-repressing marker, was increased in NASH-derived HCC (Fig. 6B), whereas the level of histone H4K16ac, a transcription-activating marker, was decreased in the livers of STAM mice, reaching the lowest level in NASH-derived HCC (Fig. 6C). In contrast, the extent of promoter methylation and the levels of histone H3K27me3 and H4K16ac in the promoter of the down-regulated Cbs gene did not differ from that in the normal livers (Supplemental Fig. 4).
Figure 6.
Epigenetic alterations in the Ahcy gene in the livers of STAM mice. A) The extent of gene-specific DNA methylation (A), H3K27me3 enrichment (B), and H4K16ac enrichment (C) in the promoter region of the Ahcy gene in the livers of STAM mice. D) Expression of the Dnmt1, -3a, and -3b and Ezh2 genes in the livers of STAM mice. E) Western blot analysis of DNMT1, -3A, -3B, and EZH2 proteins in NASH-derived HCC. Representative Western blot images from control (n = 3) and STAM (n = 3) mice are shown. The results are presented as mean fold change in the livers of STAM mice (n = 5) relative to that in age-matched control mice (n = 5), which were assigned a value of 1 after normalization to input DNA. Data are means ± sd. *P < 0.05 vs. age-matched control mice.
The increase in the extent of cytosine DNA methylation and histone H3K27me3 in the Ahcy gene promoter was accompanied by up-regulation of the DNA methyltransferase genes, Dnmt1, -3a, and -3b, and histone H3K27me3 methyltransferase Ezh2 (Fig. 6D), with the highest values being detected in NASH-derived HCC. This finding was further confirmed by the Western blot analysis showing a substantial increase in the levels of the DNMT1, -3A, - and 3B and EZH2 proteins (Fig. 6E). Similarly, the loss of the Ahcy-specific histone H4K16 acetylation was in good agreement with our prior findings of global and gene-specific H4K16 deacetylation in the livers of STAM mice, which was mediated by a profound down-regulation of histone H4K16 acetyltransferase K(lysine) acetyltransferase-8 (27).
DISCUSSION
The alarming increase in NAFLD over the past decade indicates the urgent need to understand better the pathogenesis of its progression and to develop novel mechanism-based screening measures and approaches to the clinical management and prevention of NAFLD. However, there continue to be important knowledge gaps in our understanding of the underlying molecular mechanisms of the disease.
In this study, advanced NASH and NASH-related HCC were characterized by a significant dysregulation of hepatic lipid metabolism and 1-carbon homeostasis. The development of NASH in STAM mice and mice fed a CFD diet showed a high degree of similarity in dysregulated hepatic lipid metabolism–related pathways. During the development of NASH and NASH-related liver carcinogenesis in STAM mice, there were substantial perturbations of hepatic 1-carbon metabolism, especially a marked inhibition of Ahcy expression and increased hepatic SAH content. Similar alterations in the levels of SAH and Ahcy expression were reported in the livers of C57BL/6J mice fed a methyl-deficient diet (14, 41) that causes advanced NASH-like liver injury. On the basis of the findings that two different etiological factors for NASH, a high-fat diet and methyl donor-deficient diet, caused corresponding 1-carbon metabolism alterations, we hypothesize that an elevation in hepatic SAH driven by Ahcy inhibition would be a significant cause of the pathogenesis of NASH. This suggestion is in a good agreement with the previous reports by Matthews et al. (42) and Pacana et al. (38). Specifically, Matthews et al. (42) have demonstrated that reduced expression of the Ahcy gene induced by a causative mutation resulted in the development of hepatic steatosis and liver degeneration in zebrafish. These histopathological changes were accompanied by elevated hepatic SAH content and reduced SAM:SAH ratio. In a more recent report, Pacana et al. (38) reported similar findings of an elevation of hepatic SAH and reduction of SAH hydrolase protein in an isogenic cross between C57BL/6J and 129S1/SvImJ female mice fed a high-fat, high-cholesterol diet. These results indicate that the alterations are independent of species, sex, and genetic background. An inhibition of AHCY expression has also been reported in patients with NASH (43). In addition, several reports showed a link between a human AHCY deficiency and liver pathologies (44, 45), including the development of HCC in a young woman (46).
SAH hydrolase is a key member of the transmethylation pathway and the only enzyme in mammalian cells capable of hydrolyzing SAH. An elevation of SAH caused by genetic or environmental inhibition of Ahcy may have a major negative effect on cell metabolism, in general, and the functioning of downstream anti-inflammatory transsulfuration pathways (Supplemental Fig. 5; 47), in particular, especially in conditions when the expression of Cbs and Bhmt is also inhibited. Other studies have demonstrated the aberrant expression of several key 1-carbon metabolism–related genes, including Mat1a, glycine N-methyltransferse (Gnmt), Bhmt, and Cbs, and the dysregulation of 1-carbon homeostasis, including an elevation of hepatic SAH, during the development of NASH and HCC (36–38). This finding has led to the suggestion that dietary supplementation with methyl-group donor metabolites prevents, corrects, or lessens the pathophysiological consequences associated with 1-carbon metabolism dysregulation, including SAH–homocysteine axis alterations. Indeed, several studies have demonstrated a positive effect of methyl-group donor supplementation on correcting 1-carbon metabolism perturbations (48, 49); however, when these disturbances were caused by an impairment of the remethylation homocysteine to methionine and conversion of homocysteine to glutathione in the transsulfuration pathway, methyl donor supplementation was not effective. For example, it has been reported that the SAH–homocysteine disturbances caused by Bhmt- or Cbs-deficiency are independent of dietary methyl donor supplementation (50, 51).
The results of the present study demonstrated that diet-induced epigenetic mechanisms play a major role in the inhibition of Ahcy expression, as evidenced by progressive cytosine DNA hypermethylation of the Ahcy gene promoter region and gene-specific histone H4K16 deacetylation in the livers of STAM mice during hepatocarcinogenesis. In addition, the level of histone H3K27me3 was greatly increased in NASH-derived HCC. It is well established that these epigenetic alterations are associated with gene silencing. Mechanistically, these Ahcy gene-specific epigenetic alterations were associated with increased levels of DMNT and EZH2 proteins. Hypermethylation-mediated inhibition of Ahcy expression has been reported in NASH induced by a methyl-deficient diet (14, 41) and in patients with histologically confirmed NASH fibrosis (43).
In summary, we demonstrated that dysregulated 1-carbon metabolism, characterized by diminished expression of key 1-carbon metabolism genes, especially Ahcy, and elevation of hepatic SAH, is one of the key metabolic dysregulations in the pathogenesis and progression of NASH. Mechanistically, the down-regulation of Ahcy expression was associated with significant cytosine DNA hypermethylation, an enrichment of H3K27me3, and a decrease in histone H4K16ac in the upstream region of the Ahcy gene. These data indicate that the down-regulation of Ahcy expression may be a driving force causing SAH elevation and subsequent down-stream 1-carbon metabolism disturbances in NASH.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was partly supported by appointment (of K.D., I.K., and J.F.O.) to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education. The views expressed in this article do not necessarily represent those of the U.S. Food and Drug Administration. The authors declare no conflicts of interest.
Glossary
- Ahcy
S-adenosylhomocysteine hydrolase
- Bhmt
betaine-homocysteine methyltransferase
- Cbs
cystathionine β-synthase
- CFD
choline- and folate-deficient
- Cth
cystathionase (cystathionine γ-lyase)
- Dnmt1
DNA methyltransferase
- EZH2
enhancer of zeste 2 polycomb repressive complex 2 subunit
- FDR
false discovery rate
- GEO
Gene Expression Omnibus
- Gnmt
glycine N-methyltransferase
- GSH
glutathione
- H3K27me3
histone H3 lysine 27 trimethylation
- H4K16ac
histone H4 lysine 16 acetylation
- HCC
hepatocellular carcinoma
- KAT8
K(lysine) acetyltransferase 8
- Mat
methionine adenosyltransferase
- MeDIP
methylated DNA immunoprecipitation
- Mthfr
5,10-methylenetetrahydrofolate reductase
- Mtr
5-methyltetrahydrofolate-homocysteine methyltransferase
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- qPCR
quantitative PCR
- SAH
S-adenosyl-l-homocysteine
- SAM
S-adenosyl-l-methionine
- STAM
Stelic animal model
- Tbp
TATA box binding protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
I. P. Pogribny and F. A. Beland designed the study; K. Dreval, I. Kindrat, S. Melnyk, L. Jimenez, A. de Conti, V. Tryndyak, M. Pogribna, and J. F. Ortega performed the research; I. P. Pogribny, S. J. James, I. Rusyn, and F. A. Beland wrote the paper; and all authors analyzed the data, edited, and approved the final version of the article.
REFERENCES
- 1.Younossi Z. M., Koenig A. B., Abdelatif D., Fazel Y., Henry L., Wymer M. (2016) Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 2.Satapathy S. K., Sanyal A. J. (2015) Epidemiology and natural history of nonalcoholic fatty liver disease. Semin. Liver Dis. 35, 221–235 10.1055/s-0035-1562943 [DOI] [PubMed] [Google Scholar]
- 3.Neuschwander-Tetri B. A. (2017) Non-alcoholic fatty liver disease. BMC Med. 15, 45 10.1186/s12916-017-0806-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellentani S. (2017) The epidemiology of non-alcoholic fatty liver disease. Liver Int. 37(Suppl 1), 81–84 10.1111/liv.13299 [DOI] [PubMed] [Google Scholar]
- 5.Doycheva I., Watt K. D., Alkhouri N. (2017) Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology 65, 2100–2109 10.1002/hep.29068 [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z. M., Otgonsuren M., Henry L., Venkatesan C., Mishra A., Erario M., Hunt S. (2015) Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62, 1723–1730 10.1002/hep.28123 [DOI] [PubMed] [Google Scholar]
- 7.Marengo A., Rosso C., Bugianesi E. (2016) Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu. Rev. Med. 67, 103–117 10.1146/annurev-med-090514-013832 [DOI] [PubMed] [Google Scholar]
- 8.Goldberg D., Ditah I. C., Saeian K., Lalehzari M., Aronsohn A., Gorospe E. C., Charlton M. (2017) Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 152, 1090–1099 10.1053/j.gastro.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong R. J., Aguilar M., Cheung R., Perumpail R. B., Harrison S. A., Younossi Z. M., Ahmed A. (2015) Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 148, 547–555 10.1053/j.gastro.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V., Goodman Z., Sanyal A. (2015) Current efforts and trends in the treatment of NASH. J. Hepatol. 62(1 Suppl), S65–S75 10.1016/j.jhep.2015.02.041 [DOI] [PubMed] [Google Scholar]
- 11.Cheung O., Sanyal A. J. (2008) Abnormalities of lipid metabolism in nonalcoholic fatty liver disease. Semin. Liver Dis. 28, 351–359 10.1055/s-0028-1091979 [DOI] [PubMed] [Google Scholar]
- 12.Pogribny I. P., Tryndyak V. P., Bagnyukova T. V., Melnyk S., Montgomery B., Ross S. A., Latendresse J. R., Rusyn I., Beland F. A. (2009) Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J. Hepatol. 51, 176–186 10.1016/j.jhep.2009.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tryndyak V., de Conti A., Kobets T., Kutanzi K., Koturbash I., Han T., Fuscoe J. C., Latendresse J. R., Melnyk S., Shymonyak S., Collins L., Ross S. A., Rusyn I., Beland F. A., Pogribny I. P. (2012) Interstrain differences in the severity of liver injury induced by a choline- and folate-deficient diet in mice are associated with dysregulation of genes involved in lipid metabolism. FASEB J. 26, 4592–4602 10.1096/fj.12-209569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogribny I. P., Kutanzi K., Melnyk S., de Conti A., Tryndyak V., Montgomery B., Pogribna M., Muskhelishvili L., Latendresse J. R., James S. J., Beland F. A., Rusyn I. (2013) Strain-dependent dysregulation of one-carbon metabolism in male mice is associated with choline- and folate-deficient diet-induced liver injury. FASEB J. 27, 2233–2243 10.1096/fj.12-227116 [DOI] [PubMed] [Google Scholar]
- 15.Aissa A. F., Tryndyak V., de Conti A., Melnyk S., Gomes T. D. U. H., Bianchi M. L. P., James S. J., Beland F. A., Antunes L. M. G., Pogribny I. P. (2014) Effect of methionine-deficient and methionine-supplemented diets on the hepatic one-carbon and lipid metabolism in mice. Mol. Nutr. Food Res. 58, 1502–1512 10.1002/mnfr.201300726 [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya M., Ji C., Kosyk O., Shymonyak S., Melnyk S., Kono H., Tryndyak V., Muskhelishvili L., Pogribny I. P., Kaplowitz N., Rusyn I. (2012) Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology 56, 130–139 10.1002/hep.25641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubio-Aliaga I., de Roos B., Sailer M., McLoughlin G. A., Boekschoten M. V., van Erk M., Bachmair E.-M., van Schothorst E. M., Keijer J., Coort S. L., Evelo C., Gibney M. J., Daniel H., Muller M., Kleemann R., Brennan L. (2011) Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol. Genomics 43, 408–416 10.1152/physiolgenomics.00179.2010 [DOI] [PubMed] [Google Scholar]
- 18.Yi P., Melnyk S., Pogribna M., Pogribny I. P., Hine R. J., James S. J. (2000) Increase in plasma homocysteine associated with parallel increases in plasma S-adenosylhomocysteine and lymphocyte DNA hypomethylation. J. Biol. Chem. 275, 29318–29323 10.1074/jbc.M002725200 [DOI] [PubMed] [Google Scholar]
- 19.Medici V., Peerson J. M., Stabler S. P., French S. W., Gregory J. F., III, Virata M. C., Albanese A., Bowlus C. L., Devaraj S., Panacek E. A., Rahim N., Richards J. R., Rossaro L., Halsted C. H. (2010) Impaired homocysteine transsulfuration is an indicator of alcoholic liver disease. J. Hepatol. 53, 551–557 10.1016/j.jhep.2010.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leach N. V., Dronca E., Vesa S. C., Sampelean D. P., Craciun E. C., Lupsor M., Crisan D., Tarau R., Rusu R., Para I., Grigorescu M. (2014) Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur. J. Intern. Med. 25, 762–767 10.1016/j.ejim.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 21.Pastore A., Alisi A., di Giovamberardino G., Crudele A., Ceccarelli S., Panera N., Dionisi-Vici C., Nobili V. (2014) Plasma levels of homocysteine and cysteine increased in pediatric NAFLD and strongly correlated with severity of liver damage. Int. J. Mol. Sci. 15, 21202–21214 10.3390/ijms151121202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H., Wang W., Tang X., Chen R., Chen Z., Lu Y., Yuan H. (2016) Association between homocysteine and non-alcoholic fatty liver disease in Chinese adults: a cross-sectional study. Nutr. J. 15, 102 10.1186/s12937-016-0221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii M., Shibazaki Y., Wakamatsu K., Honda Y., Kawauchi Y., Suzuki K., Arumugam S., Watanabe K., Ichida T., Asakura H., Yoneyama H. (2013) A murine model for non-alcoholic steatohepatitis showing evidence of association between diabetes and hepatocellular carcinoma. Med. Mol. Morphol. 46, 141–152 10.1007/s00795-013-0016-1 [DOI] [PubMed] [Google Scholar]
- 24.Maher J. J. (2011) New insights from rodent models of fatty liver disease. Antioxid. Redox Signal. 15, 535–550 10.1089/ars.2010.3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Machado M. V., Michelotti G. A., Xie G., de Almeida T. A., Boursier J., Bohnic B., Guy C. D., Diehl A. M. (2015) Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One 10, e0127991 10.1371/journal.pone.0127991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uehara T., Ainslie G. R., Kutanzi K., Pogribny I. P., Muskhelishvili L., Izawa T., Yamate J., Kosyk O., Shymonyak S., Bradford B. U., Boorman G. A., Bataller R., Rusyn I. (2013) Molecular mechanisms of fibrosis-associated promotion of liver carcinogenesis. Toxicol. Sci. 132, 53–63 10.1093/toxsci/kfs342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Conti A., Dreval K., Tryndyak V., Orisakwe O. E., Ross S. A., Beland F. A., Pogribny I. P. (2017) Inhibition of the cell death pathway in nonalcoholic steatohepatitis (NASH)-related hepatocarcinogenesis is associated with histone H4 lysine 16 deacetylation. Mol. Cancer Res. 15, 1163–1172 10.1158/1541-7786.MCR-17-0109 [DOI] [PubMed] [Google Scholar]
- 28.Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., Smyth G. K. (2015) limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y., Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300 [Google Scholar]
- 30.Yu G., Wang L.-G., Han Y., He Q.-Y. (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 32.Melnyk S., Pogribna M., Pogribny I., Hine R. J., James S. J. (1999) A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J. Nutr. Biochem. 10, 490–497 10.1016/S0955-2863(99)00033-9 [DOI] [PubMed] [Google Scholar]
- 33.Melnyk S., Pogribna M., Pogribny I. P., Yi P., James S. J. (2000) Measurement of plasma and intracellular S-adenosylmethionine and S-adenosylhomocysteine utilizing coulometric electrochemical detection: alterations with plasma homocysteine and pyridoxal 5′-phosphate concentrations. Clin. Chem. 46, 265–272 [PubMed] [Google Scholar]
- 34.Saito K., Uebanso T., Maekawa K., Ishikawa M., Taguchi R., Nammo T., Nishimaki-Mogami T., Udagawa H., Fujii M., Shibazaki Y., Yoneyama H., Yasuda K., Saito Y. (2015) Characterization of hepatic lipid profiles in a mouse model with nonalcoholic steatohepatitis and subsequent fibrosis. Sci. Rep. 5, 12466 10.1038/srep12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kakehashi A., Stefanov V. E., Ishii N., Okuno T., Fujii H., Kawai K., Kawada N., Wanibuchi H. (2017) Proteome characteristics of non-alcoholic steatohepatitis liver tissue and associated hepatocellular carcinomas. Int. J. Mol. Sci. 18, 434 10.3390/ijms18020434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avila M. A., Berasain C., Torres L., Martín-Duce A., Corrales F. J., Yang H., Prieto J., Lu S. C., Caballería J., Rodés J., Mato J. M. (2000) Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J. Hepatol. 33, 907–914 10.1016/S0168-8278(00)80122-1 [DOI] [PubMed] [Google Scholar]
- 37.Frau M., Feo F., Pascale R. M. (2013) Pleiotropic effects of methionine adenosyltransferases deregulation as determinants of liver cancer progression and prognosis. J. Hepatol. 59, 830–841 10.1016/j.jhep.2013.04.031 [DOI] [PubMed] [Google Scholar]
- 38.Pacana T., Cazanave S., Verdianelli A., Patel V., Min H.-K., Mirshahi F., Quinlivan E., Sanyal A. J. (2015) Dysregulated hepatic methionine metabolism drives homocysteine elevation in diet-induced nonalcoholic fatty liver disease. PLoS One 10, e0136822 10.1371/journal.pone.0136822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner M. A., Yang X., Yin D., Kuczera K., Borchardt R. T., Howell P. L. (2000) Structure and function of S-adenosylhomocysteine hydrolase. Cell Biochem. Biophys. 33, 101–125 10.1385/CBB:33:2:101 [DOI] [PubMed] [Google Scholar]
- 40.Takai D., Jones P. A. (2002) Comprehensive analysis of CpG islands in human chromosomes 21 and 22. Proc. Natl. Acad. Sci. USA 99, 3740–3745 10.1073/pnas.052410099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tryndyak V. P., Han T., Muskhelishvili L., Fuscoe J. C., Ross S. A., Beland F. A., Pogribny I. P. (2011) Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Mol. Nutr. Food Res. 55, 411–418 10.1002/mnfr.201000300 [DOI] [PubMed] [Google Scholar]
- 42.Matthews R. P., Lorent K., Mañoral-Mobias R., Huang Y., Gong W., Murray I. V. J., Blair I. A., Pack M. (2009) TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase. Development 136, 865–875 10.1242/dev.027565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy S. K., Yang H., Moylan C. A., Pang H., Dellinger A., Abdelmalek M. F., Garrett M. E., Ashley-Koch A., Suzuki A., Tillmann H. L., Hauser M. A., Diehl A. M. (2013) Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology 145, 1076–1087 10.1053/j.gastro.2013.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barić I., Fumić K., Glenn B., Ćuk M., Schulze A., Finkelstein J. D., James S. J., Mejaški-Bošnjak V., Pažanin L., Pogribny I. P., Radoš M., Sarnavka V., Šćukanec-Špoljar M., Allen R. H., Stabler S., Uzelac L., Vugrek O., Wagner C., Zeisel S., Mudd S. H. (2004) S-adenosylhomocysteine hydrolase deficiency in a human: a genetic disorder of methionine metabolism. Proc. Natl. Acad. Sci. USA 101, 4234–4239 10.1073/pnas.0400658101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buist N. R. M., Glenn B., Vugrek O., Wagner C., Stabler S., Allen R. H., Pogribny I., Schulze A., Zeisel S. H., Barić I., Mudd S. H. (2006) S-Adenosylhomocysteine hydrolase deficiency in a 26-year-old man. J. Inherit. Metab. Dis. 29, 538–545 10.1007/s10545-006-0240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stender S., Chakrabarti R. S., Xing C., Gotway G., Cohen J. C., Hobbs H. H. (2015) Adult-onset liver disease and hepatocellular carcinoma in S-adenosylhomocysteine hydrolase deficiency. Mol. Genet. Metab. 116, 269–274 10.1016/j.ymgme.2015.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barroso M., Kao D., Blom H. J., Tavares de Almeida I., Castro R., Loscalzo J., Handy D. E. (2016) S-adenosylhomocysteine induces inflammation through NFkB: a possible role for EZH2 in endothelial cell activation. Biochim. Biophys. Acta 1862, 82–92 10.1016/j.bbadis.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwahn B. C., Laryea M. D., Chen Z., Melnyk S., Pogribny I., Garrow T., James S. J., Rozen R. (2004) Betaine rescue of an animal model with methylenetetrahydrofolate reductase deficiency. Biochem. J. 382, 831–840 10.1042/BJ20030822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deminice R., da Silva R. P., Lamarre S. G., Kelly K. B., Jacobs R. L., Brosnan M. E., Brosnan J. T. (2015) Betaine supplementation prevents fatty liver induced by a high-fat diet: effects on one-carbon metabolism. Amino Acids 47, 839–846 10.1007/s00726-014-1913-x [DOI] [PubMed] [Google Scholar]
- 50.Teng Y.-W., Cerdena I., Zeisel S. H. (2012) Homocysteinemia in mice with genetic betaine homocysteine S-methyltransferase deficiency is independent of dietary folate intake. J. Nutr. 142, 1964–1967 10.3945/jn.112.166835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S., Wang L., Kruger W. D. (2016) Betaine supplementation is less effective than methionine restriction in correcting phenotypes of CBS deficient mice. J. Inherit. Metab. Dis. 39, 39–46 10.1007/s10545-015-9883-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.