Abstract
Abnormalities of the endosomal–lysosomal network (ELN) are a signature feature of Alzheimer’s disease (AD). These include the earliest known cytopathology that is specific to AD and that affects endosomes and induces the progressive failure of lysosomes, each of which are directly linked by distinct mechanisms to neurodegeneration. The origins of ELN dysfunction and β-amyloidogenesis closely overlap, which reflects their common genetic basis, the established early involvement of endosomes and lysosomes in amyloid precursor protein (APP) processing and clearance, and the pathologic effect of certain APP metabolites on ELN functions. Genes that promote β-amyloidogenesis in AD (APP, PSEN1/2, and APOE4) have primary effects on ELN function. The importance of primary ELN dysfunction to pathogenesis is underscored by the mutations in more than 35 ELN-related genes that, thus far, are known to cause familial neurodegenerative diseases even though different pathogenic proteins may be involved. In this article, I discuss growing evidence that implicates AD gene–driven ELN disruptions as not only the antecedent pathobiology that underlies β-amyloidogenesis but also as the essential partner with APP and its metabolites that drive the development of AD, including tauopathy, synaptic dysfunction, and neurodegeneration. The striking amelioration of diverse deficits in animal AD models by remediating ELN dysfunction further supports a need to integrate APP and ELN relationships, including the role of amyloid-β, into a broader conceptual framework of how AD arises, progresses, and may be effectively therapeutically targeted.—Nixon, R. A. Amyloid precursor protein and endosomal–lysosomal dysfunction in Alzheimer’s disease: inseparable partners in a multifactorial disease.
Keywords: autophagy, cholinergic neurodegeneration, presenilin, apoliprotein E, Down Syndrome
Analyses of neurofibrillary tangles and neuritic plaques have continuously informed our notions of the molecular pathogenesis of Alzheimer’s disease (AD). More recent recognition of signature AD cytopathology that reflects the progressive dysfunction of the endosomal–lysosomal network (ELN; Fig. 1) has offered further clues to AD development as well as to other adult-onset neurodegenerative diseases. The pathobiology of amyloid precursor protein (APP) and ELN in AD are intertwined at various levels: the genes and other risk factors that promote APP mismetabolism also dysregulate the ELN that drives APP mismetabolism and, in sporadic AD, may be the antecedent pathologic events that are essential to initiate the pathogenic effects of APP (Fig. 2). Exemplifying this APP–ELN interplay, dysfunctional endolysosomal compartments, which are induced by APP–β-carboxyl terminal fragment (βCTF), have been identified as organelles within which the first traces of intracellular amyloid-β (Aβ) collect years before β-amyloid is deposited. These inter-relationships and their consequences for the development of key features of AD pathology, including neuritic dystrophy, impaired Aβ clearance, tauopathy, and neuron death, are further detailed in this article. Here, a multifactorial disease model is proposed in which neither β-amyloidogenesis or ELN is the sole cause of the other or of AD, and, instead, it is proposed that both are essential to the cause and progression of AD.
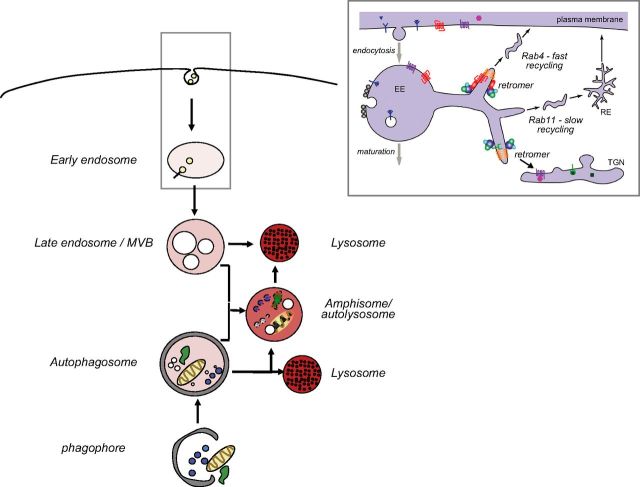
Figure 1.
The ELN network. The term, network, emphasizes that lysosomes degrade cargoes that are delivered by both endocytic and autophagic routes and that, in neurons especially, these pathways merge when endosomes frequently fuse with autophagosomes before lysosome fusion. Network also refers to the extensive crosstalk between endocytic and autophagic pathways in terms of regulatory mechanisms and involvement of endocytic pathway components (e.g., rab5, rab9, rab7, endosomal sorting complex required for transport components, etc.) in the formation, function, and maturation of autophagosomes. Endocytic pathway to lysosomes: endocytosis, reviewed in detail elsewhere (1–5), involves internalization of extracellular material and plasma membrane via clathrin-dependent and -independent routes and additional cargoes from the Golgi, which are delivered to an early endosome with both signaling and cargo-sorting properties (inset). Sorting of cargo includes recycling of proteins and lipids to the plasma membrane in fast- or slow-recycling endosomes that are regulated, in part, by Rab4 or Rab11 and the retromer, respectively (11, 13). The retromer also traffics vesicle-bound constituents, notably APP, back and forth from the Golgi. Additional trafficking signals commit other early endosome cargoes to downstream sorting or degradation via late endosomes (LEs) and lysosomes. During maturation to LE (6, 30), an LE/multivesicular body (MVB) is created by the inward budding of the surface membrane to form a collection of internal vesicles (102). This process reduces endosome volume and provides access of the internalized membranes to hydrolases while also creating a population of exosomes that can be released extracellularly rather than degraded internally. LEs/MVBs fuse with lysosomes (endolysosomes) or, in neurons, more commonly fuse with autophagosomes to form amphisomes before fusing with lysosomes (autolysosomes). Autophagic routes to the lysosome include macroautophagy: structures/cytoplasm targeted for degradation are sequestered via a phagophore that forms a double membrane-limited autophagosome that fuses with lysosomes; chaperone-mediated autophagy, which directs select proteins that carry the pentapeptide KFERQ-like sequence directly into lysosomes via chaperones (e.g., Hsc70) and translocation machinery (e.g., lysosome-associated membrane protein-2a); and microautophagy (not shown) involving bulk or chaperone-facilitated internalization of cytoplasmic substrates into an endolysosomal compartment for degradation.
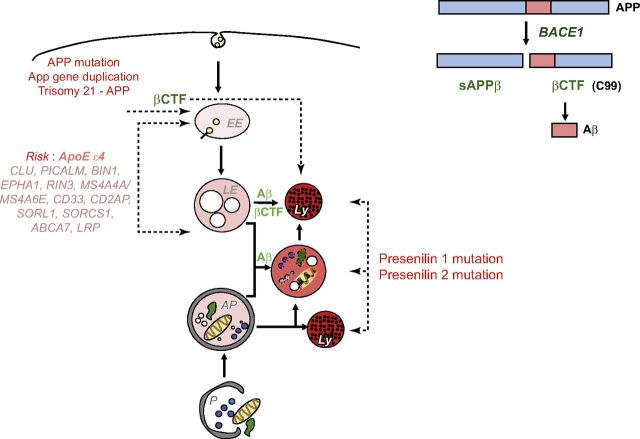
Figure 2.
Genetics of AD implicate both APP and ELN in AD pathogenesis. AD-related causative and risk factor genes and aging disrupt ELN function directly and promote β-amyloidogenesis by altering APP cleavage and turnover directly or by modifying APP metabolism via ELN dysfunction. APP and ELN biology are intertwined via the biologic actions of genes that are responsible for AD risk. The critical roles of βCTF (C99) and Aβ are indicated and discussed further in the text. Of note, the Icelandic APP mutation that lowers risk for AD is believed to act by reducing BACE1 cleavage of APP (216), thus reducing levels of APP–βCTF and Aβ. EE, early endosome; LE, late endosome.
THE ELN OF NEURONS
The ELN is a system of dynamically communicating vesicles that sort and traffic internalized extracellular materials (nutrients, trophic factors, etc.) to sites throughout the cell (Fig. 1). Detailed recent reviews are available (1–5). Endocytosis and endosome recycling back to the cell surface control the abundance and topography of surface receptors, including those that support neurotransmission and synaptic plasticity. Equally important are the cell signaling functions of each ELN compartment (discussed in the following section) that convey vast information from the surface and external environment to the nucleus and various cellular effectors. The rab family of small GTPases, of note, rab5 and rab7, and resident or recruited rab effectors/adaptors, including multiple kinases, signaling molecules, and motor complexes, regulate endosome motility, maturation, and recycling. Changes in the intralumenal ionic fluxes and surface polypeptide composition in each compartment mediate additional intervesicular interactions (e.g., vesicle fusion/fission events, exchange of lumenal contents) and other cell signaling cascades, which are activated by pH shifts. Finally, ELN compartments undergo varying forms of exocytosis for the purposes of intercellular communication (6–8), repairing cell membranes (9), or eliminating unwanted material as a back-up to lysosomal degradation (7, 8). Limited proteolytic processing of certain proteins, including APP, occurs along this pathway, though the complete degradation of unneeded cargos is initiated in late endosomes and is completed in lysosomes (10).
ENDOSOMOPATHY: THE EARLIEST AD PATHOBIOLOGY AND THE KEY TO EARLY CHOLINERGIC NEURODEGENERATION
Endosome structural anomalies are the earliest-appearing neuropathology thus far identified that is specific to AD. The endosomopathy of AD encompasses morphologic and functional abnormalities of endosomes that stem principally from the pathologic overactivation of the small GTPase, rab5. These include the up-regulated expression of endocytic genes, accelerated endocytosis, impaired transport of enlarged endosomes, and neurodegenerative events that are associated with aberrant cell signaling from these abnormal endosomes. Accompanying the anomalous functioning of the rab5 endosome are additional abnormalities of the trafficking routes to and from sorting endosomes. Of note, the retromer, which is linked by mutation to Parkinson’s disease, misfunctions in AD, as evidenced by early deficiency of its Vps35 and -36 components (11, 12). The defect is associated with diminished sorting of endosomal cargoes, including APP, to the trans-Golgi network and the cell surface via recycling endosomes—a process that is further dysregulated by deficient rab11a in AD (13).
The signature enlargement of rab5 endosomes that has been observed in AD is not a feature of normal brain aging (14) or other major neurodegenerative diseases thus far studied and is distinct from changes in late endosome size that are induced experimentally to model other diseases (15). A curious exception is Neimann-Pick type C1 (NPC1), a congenital lysosomal storage disease that shares similar endosome anomalies and other AD-associated lesions (e.g., tauopathy, small amyloid deposits, and cholinergic neurodegeneration) with AD (16). Pyramidal neurons of the AD neocortex exhibit endosomopathy at a disease stage when amyloid deposition and tauopathy are restricted to the hippocampus (Braak stage 2) (14, 17). Endosomopathy first appears at perinatal ages in Down syndrome (DS; trisomy 21) (14), a cause of early-onset AD that has been linked to an extra copy of APP (18, 19). Rab5 is aberrantly overactivated on endosomes in AD and DS (20, 21). Expected consequences of rab5 overactivation are seen in DS fibroblasts, including markedly accelerated endocytosis rates, increased fusion and swelling of early endosomes (17, 22), and pathologic endosomal signaling (20, 23–25). These anomalies can be reversed by down-regulating rab5 (22) and induced in normal fibroblasts (18) and neurons (20) by rab5 overexpression. Moreover, transgenic mice that overexpress rab5 in neurons exhibit deficits in synaptic plasticity and memory (Kim and Ohno, unpublished results), as predicted from the known synaptic actions of rab5.
APP–βCTF directly interacts with an APPL1-rab5 complex on endosomes to mediate AD-related endosomopathy
Emergence of endosomopathy at the earliest stage of AD implies a mechanism that is linked to the most upstream molecular/genetic origins of the disease. Indeed, elevated levels of APP–βCTF—independently of Aβ—cause AD-related endosomopathy via direct interaction of APP–βCTF with APPL1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif) that is recruited to the rab5 complex on endosomes (Fig. 3) (20). APP–βCTF levels are elevated in the AD brain (20) despite normal APP levels, and APP–βCTF levels in DS brain, patient fibroblasts, and DS mouse models are elevated beyond levels that are expected from the extra copy of the APP gene in DS (20, 26). These increases accord with the elevated levels of sAPPβ and BACE1 (β-site amyloid precursor protein cleaving enzyme 1) activity in AD (27–30). Increased BACE1 activity is known to arise in various ways in AD (31–42). For example, shifts in the localization of BACE1 and APP, which are further promoted by ApoE4 overexpression (43) and bridging integrator 1 loss of function (44), bring these 2 proteins together within acidic microdomains (45).
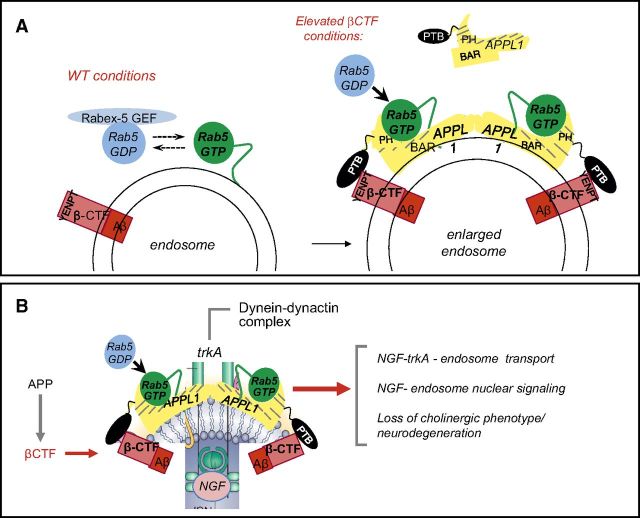
Figure 3.
APP–βCTF mediates pathologic activation of rab5 on endosomes in AD, which leads to compromise of cholinergic neurons. A) Recruited APPL1 binds via its phosphotyrosine binding (PTB) domain to the YENPT domain of APP–βCTF. APPL1 dimerization via BAR domains facilitates vesicle curvature and binding of GTP-rab5 via the PH domain stabilizes rab5 in this activated state on endosomes. B) Rab5 hyperactivation slows endosome transport and diminishes TrkA signaling, which leads to the loss of trophic support for cholinergic neurons.
APP–βCTF is directly linked to rab5 overactivation via a common ligand, the adaptor protein, APPL1. A specific binding partner and effector of rab5 via its N-terminal BAR (Bin1/amphiphysin/RVS167) domain and a PH (pleckstrin homology) domain (46, 47), APPL1 stabilizes the activated (GTP) form of rab5 on endosomes, thereby slowing its cycling to GDP-rab5 and amplifying rab5 endosome signaling (46, 47). When elevated, APP–βCTF recruits APPL1 to rab5 endosomes by binding the phosphotyrosine binding domain of APPL1. This recruitment can be readily observed on enlarged endosomes in the AD and DS brain (20). Binding to βCTF relative to other APP polypeptides within lipid rafts may be enhanced by the known selective interaction of βCTF with cholesterol (48, 49) and additional proteins within the endosomal rab5 complex. Moreover, these lipid rafts have increased BACE1 activity and decreased α-secretase activity (50).
APPL1 serves various signaling roles n APPL1 endosomes but its abnormal recruitment to rab5 endosomes by APP–βCTF, which induces overactivation of rab5 signaling, is deleterious to endosome motility, nuclear signaling, synaptic plasticity, and endolysosomal cargo processing, including that of APP. In addition, diversion of APPL1 to rab5 endosomes may also antagonize its normal signaling, which is mediated by a distinct population of APPL1 endosomes (51–54). Knockdown of APPL1 expression, such as APP–βCTF or rab5 knockdown, in fibroblasts of patients with DS fully corrects the endosomopathy (20), including elevated nuclear translocation of p65/RelA and activation of NF-κB signaling that are mediated by rab5 endosomes (55). Additional roles for APPL1 that are potentially relevant to AD include cellular signaling via insulin (55, 56), Akt (57), phosphoinositides (58), epidermal growth factor, and tropomoysin receptor kinase (Trk)A and TrkB receptors (59, 60). Of note, APPL1 localizes prominently to dendritic spines and synapses (61, 62), mediates the synaptic activity–dependent activation of PI3K-Akt signaling by coupling this pathway to NMDA receptor–PSD95 complexes (57), and activates the phosphatidylinositol 3,4,5 trisphosphate pathway in response to long-term potentiation (LTP) induction (63). In the brains of patients with AD, but not in those of controls, APPL1 colocalizes with glutamate receptor 2 and ubiquitin in nonplaque dystrophic dendrites (61).
Rab5 overactivation causes synaptic dysfunction related to memory and learning
Pathologic Rab5 activation that drives endocytic dysfunction in AD negatively impacts LTP and long-term depression (LTD), 2 aspects of synaptic plasticity that are closely associated with learning and memory (64). LTD induction requires surface removal of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors driven by their Rab5-dependent internalization (65). Conversely, deletion of the neuronal rab5 guanine nucleotide exchange factor, rin1, reduces rab5 activation, increases LTP induction in the amygdala, and enhances fear learning and memory, most likely by increasing surface levels of AMPA receptors (66). Most Rab5 in the hippocampus is present in its inactive GDP-bound conformation under basal conditions, but upon NMDA receptor activation, Rab5-GTP accumulates rapidly and transiently at pre-established endocytic hotspots and produces a transient endocytic wave of AMPA receptor removal. Thus, endocytic machinery driven by rab5 is a regulated component in the signaling cascade that underlies LTD (65). Recycling endosomes in dendrites also contain a reserve pool of AMPA receptors that are mobilized during LTP by a process that requires Rab11a (67), levels of which are lowered in AD via an APP-βCTF mechanism (13).
Synaptic removal of AMPA receptors is necessary and sufficient to produce a loss of dendritic spines and synaptic NMDA responses (68), which leads to long-lasting depression of synaptic strength (i.e., LTD) (69). Much attention has focused on the ability of Aβ or Aβ oligomers to drive the loss of NMDA-type glutamate receptors, endocytosis of synaptic AMPA receptors, and LTD (65). More recently, this effect has been tied to the heightened activity of neurons that causes increased generation and release of Aβ as an early response to stress before neurodegenerative events (70–72). It is important to note, however, that activity-dependent increases in Aβ production also imply a commensurate increase in APP–βCTF generation and rab5 activation; therefore, an additional scenario for suppression of synaptic strength (i.e., LTD) in AD could be neuronal activity–dependent rab5 activation, which is a requirement for endocytosis and LTD and an event that is already known to be overactivated by APP–βCTF in AD. Indeed, in AD mouse models, APP–βCTF accumulates in neurons before intracellular Aβ appears and this is associated with neuronal hyperactivity (73, 74), Aβ-independent LTP deficits (75–80), and cognitive impairment (74, 78, 79, 81). Directly administered βCTF causes similar effects (82, 83). In Tg2576 mice, lateral entorhinal cortex hyperactivity arises by age 3 mo, which correlates with elevated intracellular APP–βCTF and soluble Aβ in the anterior piriform cortex, a major afferent input of the lateral entorhinal cortex (73). In phase III trials in patients with AD, γ-secretase inhibitors, which inhibit Aβ production and elevate βCTF levels, worsen cognition (84)—a predicted outcome of βCTF–mediated endosomal signaling anomalies at synapses.
Aberrant rab5 endosome signaling causes loss of neurotrophic support and cholinergic neurodegeneration
Nerve growth factor (NGF) signaling is critical for the survival of basal forebrain cholinergic neurons (BFCNs) (85, 86), which are among the earliest anatomic targets of AD, the loss of which may be a basis for cognitive decline (87, 88). Loss of NGF signaling is a feature of early AD and DS (89–91). Signaling by NGF is transduced by endocytosis and the retrograde trafficking of an rab5 signaling endosome that contains the NGF receptor, TrkA (21) (Fig. 3). Both APP–βCTF–mediated rab5 hyperactivation and impaired rab11a endosome recycling (13) promote the fusion of these endosomes, increasing their size and impeding their retrograde transport and signaling (Fig. 3) (20, 21). NGF trophic support to BFCNs is thus disrupted, which induces atrophy of these neurons and cholinergic deficits in DS mouse models (21, 92) that can be rescued by raising NGF levels (93). Elevating βCTF levels directly or by inhibiting γ-secretase also induces rab5-dependent atrophy of cultured BFCNs (21). Conversely, reducing APP–βCTF levels in a similar DS mouse model in vivo by deleting one BACE1 allele prevents endosomopathy and loss of choline acetyltransferase–positive medial septal neurons. Of note, brain Aβ40 and Aβ42 peptide levels were unaltered, which is in accordance with the lack of change in Aβ levels when a single copy of BACE1 is deleted in nontransgenic mice (94) or in young PDAPP mice before Aβ deposition (95).
Beyond the effects on NGF, chronic rab5 hyperactivation promotes neurodegeneration in vulnerable neuronal populations in other ways. Overactive rab5 signaling is linked to cell survival/death, in part, via the nuclear delivery of transcription factors via rab5 endosomes (20). Elevated APP–BP1, another ligand of rab5, is implicated in apoptosis induced by FAD mutant APP (V642I)—independently of Aβ—by accentuating Rab5 or dynamin activity (24). Additional proteins that are known to play roles in both endocytosis regulation and cell survival decisions link AD-related endosome dysregulation to signaling of cell death cascades (96–100). Moreover, sAPPβ is implicated in synaptic pruning and apoptosis (101).
ApoE4 allele and cholesterol accelerate rab5-mediated endosomopathy
The E4 allele of APOE4 is the most genetically influential risk factor for sporadic AD (102). Its inheritance not only accelerates disease onset but also increases the severity of rab5 endosome enlargement in AD (14, 17). Although APOE4 influences Aβ deposition, the relevance of plaque amyloid burden to dementia severity has become controversial (103). Other actions of the ApoE4 allele on neurons are considered potentially more important (104). Relative to ApoE3/2, ApoE4 disproportionately disrupts processes ranging from lipoprotein trafficking to synaptic plasticity and neurite outgrowth (104), some of which are likely also related to ApoE4 effects in the promotion of endosomopathy. In this regard, the ApoE4 isoform increases endocytosis of APP, ApoE2, and BACE1 (105), and promotes colocalization of APP and BACE1 in early endosomes (45) while delaying endosome recycling (106, 107), all of which are expected to raise endosomal APP–βCTF levels.
Of particular relevance to AD, lipoprotein receptors mediate glia-derived cholesterol transport within the CNS, which is essential to form and maintain synapses (108). Cholesterol, a highly specific ligand of APP–βCTF (48, 49), directs βCTF to lipid rafts (109) and influences its access to other secretases, hence βCTF levels within rafts. In this regard, high dietary LDL cholesterol and overexpression of its receptor ApoE, (particularly ApoE4, elevate βCTF and BACE1 levels and enlarge rab5 endosomes, even when APP is normally expressed (110, 111). Pathobiologic overlaps between AD and the lysosomal storage disorder, NPC1, including the added risk that is conferred by APOE4 inheritance (112), implicate cholesterol in a common mechanism. Treatment of PC12 cells with the cholesterol modulator, U18666A, a cellular NPC1 model, produces a phenotype that includes enlargement of TrkA endosomes, reduced TrkA recycling, altered NGF signaling, τ hyperphosphorylation, and cell death (6, 16).
In addition to APOE, other genes that are associated with high susceptibility to late-onset AD are closely involved with cholesterol/lipid metabolism and trafficking, including CLU, SORL1, SORCS1, ABCA7, PICALM, and BIN1 (113). Most of these and other genome-wide association study–identified AD risk factors—CD2AP, EPHA1—have known roles in endocytosis or lysosomal or autophagy function (113), which underscores the significance of the ELN as a genetic risk hot spot in AD (Fig. 2).
ENDOLYSOSOMAL DYSFUNCTION AND THE GENESIS OF NEURITIC DYSTROPHY
A considerable proportion of endocytosed cargo in neurons enters the autophagic pathway before reaching lysosomes (10, 114), which provides insight into one way that endocytic dysfunction can also impact autophagy. To prevent traffic jams in axons, transport and autophagy must be efficient, which is normally the case; however, when endolysosomal proteolysis is impeded, autophagic waste rapidly builds up within neuritic swellings (115–117). Dystrophic neurites in AD brain and AD mouse models are almost entirely filled (>80%) with autophagic vacuoles (118), which implies a selective deficit in organelle axonal transport, rather than a global failure of transport (10). Similar autophagic vacuole–filled dystrophic swellings with AD-like local cytoskeletal protein hyperphosphorylation and ubiquitin immunolabeling (10) can be induced in cortical neurons by blocking endolysosomal acidification or cysteine cathepsins (10). Presenilin 1 (PSEN1) deletion elevates endolysosomal pH by disrupting vATPase assembly (119), and, in these neurons, retrograde transport of amphisomes and endolysosomes is selectively slowed as a result of a pH-dependent release of calcium from these organelles via TRPML1 (transient receptor potential cation channel mucolipin subfamily member 1) channels, which, in turn, inhibits dynein activity (unpublished results). In this regard, loss-of-function PSEN1 mutations in fAD, which cause similar endolysosomal abnormalities (119), greatly accelerate the onset of neuritic dystrophy and associated pathologies in the brain (120).
Both intraneuronal and extracellular factors may contribute to neuritic dystrophy in AD. In 3×Tg-AD mice, axonal pathology that is associated with putative APP–βCTF and BACE1 immunoreactive accumulations precedes diffuse plaque formation (121). Inhibiting endolysosomal proteolysis or acidification can induce neuritic dystrophy in the absence of amyloid deposition in nontransgenic mice (10, 122) and accentuate neuritic plaque development in AD mouse models (123, 124). Endosomes, multivesicular bodies, and amphisomes that accumulate in neuritic swellings are enriched in APP substrate, BACE1, and γ-secretase, and are major reservoirs of intraneuronal Aβ (125, 126) and APP–βCTF (127). These organelles, including amphisomes, are potentially capable of exocytosis (11, 12, 128). Cathepsins and lysosomal matrix proteins are detectable in extracellular plaque deposits, which suggests that exocytic or degenerative release of accumulated autophagic materials, such as Aβ and heparin sulfate proteoglycans, could seed extracellular amyloid formation in neuritic plaques (129, 130). In addition, the regulated release of late endosome/MVB compartment constituents into the extracellular space via exosomes (8) is another potential source of plaque constituents (8). Further recruitment of inflammatory cells and other sources of oxidative stress, including amyloid itself, exacerbate the process, as evidenced by the attenuation of dystrophy with amyloid-lowering treatments (131). Other evidence suggests that neuritic dystrophy may be initiated by extracellular Aβ or Aβ oligomers that dissociate from plaques (131–133). The appearance of diffuse plaques followed by neuritic plaques suggests a cause and effect relationship; however, the evidence is correlational. In this regard, Aβ that is administered directly into the brains of wild-type rodents does not induce neuritic dystrophy, even in the presence of diffuse amyloid deposits (134, 135).
LYSOSOME FAILURE IN AD: CATALYST FOR HALLMARK AD PATHOLOGY AND NEURONAL CELL DEATH
Early lysosomal biogenesis is followed by progressive corruption of lysosome function in AD
Lysosomal biogenesis is up-regulated at early stages of the AD brain and AD models in response to rab5-mediated acceleration of endocytosis (136–138), as evidenced by activation of the microphthalmia-associated transcription factor family transcription factors that regulate lysogenesis (TFEB, TFE3), expression of lysosomal gene targets (138, 139), and expansion of the lysosomal population. Lysosomes later become dysfunctional as reflected by their enlargement as they accumulate autophagic and endocytic substrates. Most enlarged lysosomes, in fact, are autolysosomes and amphisomes, which become lipofuscin granules as hydrolysis further declines (22, 138, 140, 141). Reduced specific activity of cathepsin D and certain other hydrolases is additional evidence of lysosomal dysfunction in the AD brain and AD models (119, 138, 142–144). Such decrease results functionally in slower turnover rates of endocytic or autophagic substrates (22, 119, 142, 145). Progressive dysfunction of lysosomes in AD is multifactorial, involving AD-related genes, substrate overload from accelerated endocytosis and autophagy, aging- and disease-related oxidative damage that creates more hydrolase-resistant substrates, and generation of free radicals from peroxidation of cholesterol and other lipids (146, 147), as reviewed in more detail elsewhere (unpublished results). Together, these factors modify and compromise lysosomal hydrolases and acidification machinery and promote the accumulation of toxic molecules and peptides that can destabilize lysosomal membranes and initiate cell death programs (148–152).
AD-related genetic factors disrupt lysosomal function
APP–βCTF
APP–βCTF levels are elevated in sporadic AD and this rise is promoted by multiple AD risk factors, including APP duplication in FAD and DS, PSEN1 FAD, and APOE4 inheritance, and high dietary cholesterol. Curiously, APP–βCTF degradation involves varied lysosomal mechanisms, which suggests that an efficient termination of APP–βCTF signaling is important. Delivery to lysosomes is facilitated via specialized autophagic routes (153, 154), and lysosomal metabolism of βCTF is highly vulnerable to the inhibition of cysteine proteases (155) and by crosstalk between endoplasmic reticulum-associated degradation and ubiquitin-independent lysosomal degradation (156); exosomes are another elimination route for βCTF from cells (7).
Rab5 overactivation that is induced by APP–βCTF in DS fibroblasts, DS mouse model neurons, and APP overexpressing cells (Colacurcio, unpublished results) has recently been shown to impair lysosomal acidification, cathepsin D maturation, and protease activities, as well as to promote autophagic substrate accumulation in enlarged endolysosomes (145). This phenotype can be induced in normal fibroblasts by overexpressing APP, βCTF, γ-secretase inhibitors, or Rab5, which supports its dependence on APP–βCTF, but not Aβ, and can be rescued by APP small interfering RNA knockdown or reacidification with lysosome-targeted acidic nanoparticles. Experimental manipulations of cell and transgenic mouse models of AD that increase production of APP–βCTF—independently of changes in Aβ levels—induce lysosomal βCTF accumulation, marked derangement of lysosome morphologies and proteolysis, and massive buildup of autophagic substrates (75, 76). Furthermore, aggregated C99 accumulates within the membranes of ELN vesicles in these AD models, which suggests that perturbation of membrane integrity may underlie the observed neuronal necrosis and release of aggregated C99 (and C99-derived C83) into the extracellular space in the brains of these mice, as observed earlier in a transgenic Aβ42-expressing Drosophila model (157). These early APP–βCTF changes synergize with those of Aβ, which also accumulates in endolysosomal compartments.
Presenilins
Presenilins (PSEN1 and PSEN2), as catalytic subunits of the γ-secretase complex, carry out intramembrane proteolysis on diverse proteins, terminating the biologic action of some and generating bioactive fragments from others (158). PSEN1 FAD mutations reduce APP γ- cleavage, thereby lowering Aβ production but yielding a slight shift toward a high Aβ42 to Aβ40 ratio, which has been considered critical for Aβ toxicity in AD (159, 160). However, a recent study of several dozen PSEN1 mutations in patient fibroblast lines demonstrated that the shift in the Aβ42/40 ratio is inconsistent across different mutations and does not correlate with clinical disease onset (161). Less often considered to be another outcome of γ-secretase loss of function in PSEN1 fAD is the substantial and consistent elevated level of APP–βCTF (162, 163), which accumulates in lysosomes and disrupts their functions. In addition, both presenilins have secretase-independent roles as uncleaved polypeptides (119, 142, 164, 165).
Of note, both PSEN1 and PSEN2 proteins are implicated in AD-related actions that directly impact lysosomal function (166). Consistent with playing key roles in lysosomal proteolytic clearance, each presenilin is abundant in endolysosomal/autophagic compartments and multiprotein complexes. Each performs intramembrane proteolysis with broad substrate specificity and under relatively limited regulation. Lysosomes are the principal location of PSEN2 (167) and of a second catalytically essential γ-secretase component, nicastrin (168, 169). When autophagy is induced, as occurs in the AD brain, autophagic vacuoles become the cellular site with the highest γ-secretase activity (125).
Consistent with a broad role of PSEN in lysosomal function, PSEN1 holoprotein supports lysosome acidification by facilitating the glycosylation, stabilization, and lysosomal assembly of the V0a1 subunit of v-ATPase, which is an established PSEN1 ligand (170). Under PSEN1 loss-of-function conditions, poorly glycosylated V0a1 subunit is more rapidly degraded by endoplasmic reticulum-associated degradation, which causes marked deficiency of the vATPase complex and its proton pumping activity in lysosomes. The resulting deacidification of the lysosomal lumen causes myriad deficits in lysosomal hydrolytic and signaling functions and substrate accumulation (119, 142), which are reversed by selectively reacidifying lysosomes. Of importance, fibroblasts from individuals with PSEN-FAD exhibit a similar range of lysosomal deficits, which provides a basis for potentiated autophagic-lysosomal and amyloid pathology and accelerated neuronal cell death in the brains of these individuals (120). Since 2010, six familial degenerative diseases have been shown to arise from mutations in vATPase complex components (171), which highlights the close relationship between vATPase dysfunction and loss of neuronal resiliency (172). Cellular aging, the sine qua non for AD and other late-onset diseases, critically involves declines of autophagic flux and lysosomal function. Recent evidence more directly implicates the decline of lysosomal acidification in the reduction of longevity (173), reversal of which has been achieved in yeast by overexpressing v-ATPase components (173, 174) or restricting calories or methionine (173, 175).
ApoE
Intracellular Aβ degradation of Aβ, in part, is mediated by neprilysin. Expression of the ApoE4 allele, but not ApoE3, in mice that received neprilysin inhibitor increases Aβ immunoreactivity in lysosomes and causes neurodegeneration of hippocampal CA1, entorhinal, and septal neurons (176). ApoE4, which traffics to lysosomes more readily than ApoE3, promotes the leakage of acid hydrolases and induces apoptosis in cultured neuronal cells by forming membrane-damaging intermediates in the low-pH environment of the lysosome (177).
Consequences of failed lysosomal function
Lysosomes and tauopathy
Abundant neurofibrillary tangles are rarely observed, except in AD and a few aging-related tauopathies; however, they are prominent in at least 2 lysosomal disorders, NPC and mucopolysaccharidosis type IIB (178, 179). Molecular relationships have recently been uncovered that link lysosomal dysfunction to tauopathy. Decreased lysosomal acidification in PSEN1 FAD and likely in APP-FAD and DS induces the activation of calpains and cdk5 (via p25 generation), both of which are implicated in tauopathy in AD (180) and the P301L τ mouse model of frontotemporal dementia (181). Moreover, asparagine endopeptidase, a lysosomal cysteine proteinase that is up-regulated during aging and activated in AD (182), is released from lysosomes of neurons that have been compromised by brain ischemia and hypoxia. This enzyme and I2PP2A translocate from neuronal lysosomes and the nucleus, respectively, to the cytoplasm where they interact and are associated with hyperphosphorylated τ in the AD brain (183). Asparagine endopeptidase inhibition ameliorates tauopathy and associated deficits in τ P301S-transgenic mice (182), which underscores the pathogenic relevance of this lysosomal enzyme. τ is metabolized by the ubiquitin-proteasome system and autophagy and includes a motif that targets it for chaperone-mediated autophagy. τ is a macroautophagy substrate, at least when overexpressed (184). Incomplete chaperone-mediated autophagy of τ generates fragments that aggregate and are cleared by macroautophagy (185). Moreover, autophagy preferentially degrades a caspase-cleaved fragment of τ that is been implicated in τ neurotoxicity (186). Consistent with these findings, autophagy induction reduces τ pathology in the triple transgenic AD mouse model (187), whereas, conversely, autophagic-lysosomal dysfunction amplifies τ pathology and τ neurotoxicity in other models (188, 189).
Protein clearance failure and β-amyloidogenesis
Endocytosis and autophagy, major pathways of APP processing and Aβ generation, are both up-regulated in AD (22, 138, 190). Autophagic vacuoles are also enriched in APP substrates and secretases, and, during autophagy, Aβ peptide is generated from APP (125), though it is subsequently degraded in lysosomes under normal circumstances (191–193). Lysosomes are a major degradative route for Aβ in APP-transgenic mouse models of AD (116, 194), and the progressive compromise of lysosomal function can account for endolysosomal/amphisomal vesicles becoming the major site of Aβ/βCTF accumulation in AD and AD models (125, 195). Among Aβ-degrading proteases (196, 197), multiple cathepsins have been implicated (194, 198, 199), and human neurons may be particularly dependent on this pathway (200). Autophagy induction (201) or enhancement of lysosomal proteolysis selectively (202, 203) can markedly diminish Aβ levels and amyloid load in APP transgenic mice, which underscores the importance of lysosomal clearance of Aβ (204–206).
Lysosomal-mediated neurodegeneration
The close connection between lysosomal network dysfunction and mechanisms of neurodegeneration is well documented (207–210). The fundamental importance of efficient lysosomal function to survival, particularly of postmitotic neurons, is underscored by the prominent and often predominant neurodegenerative phenotype that has been observed in a majority of the >50 primary lysosomal storage disorders that affect lysosomes throughout the body. In the AD brain, the death process is complex and varies in different neuronal populations and among neurons of the same population that are captured at different stages of disease. Lysosomal cell death is considered a common form of mixed apoptotic-necrotic cell death. Compromised lysosomes can leak cathepsins into the cytoplasm, which ultimately activates death programs that are mediated by caspases, calpains, and released lysosomal hydrolases. Lysosome compromise can derange neuronal functions either subtly or catastrophically and may potentially account for a broad range of phenotypic features in a given neurodegenerative disease (211, 212). Triggers of neuronal death in AD are not clear but are likely to include intralysosomal accumulation of not only Aβ (16, 26, 75, 150, 206, 213) but also of βCTF (75, 76), other APP fragments, and membrane-destabilizing oxidized lipids and proteins.
Remediating lysosomal network dysfunction as AD therapy
Recent evidence, including the aforementioned examples, supports the value of targeting autophagy efficiency as a possible therapeutic strategy for AD. Peripheral administration of rapamycin to strongly stimulate autophagy substantially reduces amyloid deposition and τ pathology in both APP and triple transgenic mouse models of AD pathology (187, 201, 214). Autophagy induction also has beneficial effects in transgenic models of several aging-related neurodegenerative diseases (215). Stimulating lysosomal proteolytic efficiency in an FAD-APP mouse model by deleting an endogenous inhibitor of lysosomal cysteine proteases (cystatin B) rescues lysosomal pathology, eliminates abnormal autolysosomal accumulation of autophagy substrates, including Aβ, decreases Aβ and amyloid deposition, and ameliorates learning and memory deficits (140). Similar therapeutic effects, including a restoration of synaptic functions, are observed in APP mouse models after deleting cystatin C (203) by overexpressing cathepsin B (198), or enhancing its activity (199, 217), or by stimulating lysosomal biogenesis by TFEB over expression. Collectively, these observations support the pathogenic significance of autophagic-lysosomal dysfunction in AD and, specifically, the importance of deficient lysosomal proteolysis. The range of possible ways that endosomes and lysosome function may become corrupted in disease states is just now being appreciated and should be fertile ground for the identification of innovative therapeutics for AD and other neurodegenerative diseases.
ACKNOWLEDGMENTS
Research from R.A.N. laboratories is supported by the U.S. National Institutes of Health, National Institute on Aging. Contributions of Martin Berg (Center for Dementia Research, Nathan S. Kline Institute) to manuscript preparation are gratefully acknowledged.
Glossary
- Aβ
amyloid-β
- βCTF
β carboxyl-terminal fragment
- AD
Alzheimer’s disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- APP
amyloid precursor protein
- APPL1
adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif
- BACE1
β-site amyloid precursor protein cleaving enzyme 1
- BFCN
basal forebrain cholinergic neuron
- DS
Down syndrome
- ELN
endosomal–lysosomal network
- LTD
long-term depression
- LTP
long-term pontentiation
- NGF
nerve growth factor
- NPC
Neimann-Pick type C
- PSEN
presenilin
- Trk
tropomyosin receptor kinase
REFERENCES
- 1.Bissig C., Gruenberg J. (2013) Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harb. Perspect. Biol. , a016816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Settembre C., Ballabio A. (2014) Lysosomal adaptation: how the lysosome responds to external cues. Cold Spring Harb. Perspect. Biol. , a016907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosker K. E., Segal R. A. (2014) Neuronal signaling through endocytosis. Cold Spring Harb. Perspect. Biol. , a020669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Fiore P. P., von Zastrow M. (2014) Endocytosis, signaling, and beyond. Cold Spring Harb. Perspect. Biol. , a016865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bright N. A., Davis L. J., Luzio J. P. (2016) Endolysosomes are the principal intracellular sites of acid hydrolase activity. Curr. Biol. , 2233–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H., Ren D. (2015) Lysosomal physiology. Annu. Rev. Physiol. , 57–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Gonzalez R., Gauthier S. A., Kumar A., Levy E. (2012) The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J. Biol. Chem. , 43108–43115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eitan E., Suire C., Zhang S., Mattson M. P. (2016) Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. , 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng X., Zhang X., Yu L., Xu H. (2015) Calcium signaling in membrane repair. Semin. Cell Dev. Biol. , 24–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S., Sato Y., Nixon R. A. (2011) Lysosomal proteolysis inhibition selectively disrupts axonal transport of degradative organelles and causes an Alzheimer’s-like axonal dystrophy. J. Neurosci. , 7817–7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Small S. A., Petsko G. A. (2015) Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat. Rev. Neurosci. , 126–132 [DOI] [PubMed] [Google Scholar]
- 12.Muhammad A., Flores I., Zhang H., Yu R., Staniszewski A., Planel E., Herman M., Ho L., Kreber R., Honig L. S., Ganetzky B., Duff K., Arancio O., Small S. A. (2008) Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc. Natl. Acad. Sci. USA , 7327–7332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodruff G., Reyna S. M., Dunlap M., Van Der Kant R., Callender J. A., Young J. E., Roberts E. A., Goldstein L. S. (2016) Defective transcytosis of APP and lipoproteins in human iPSC-derived neurons with familial Alzheimer’s disease mutations. Cell Rep. , 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cataldo A. M., Peterhoff C. M., Troncoso J. C., Gomez-Isla T., Hyman B. T., Nixon R. A. (2000) Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. , 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urwin H., Ghazi-Noori S., Collinge J., Isaacs A. (2009) The role of CHMP2B in frontotemporal dementia. Biochem. Soc. Trans. , 208–212 [DOI] [PubMed] [Google Scholar]
- 16.Cabeza C., Figueroa A., Lazo O. M., Galleguillos C., Pissani C., Klein A., Gonzalez-Billault C., Inestrosa N. C., Alvarez A. R., Zanlungo S., Bronfman F. C. (2012) Cholinergic abnormalities, endosomal alterations and up-regulation of nerve growth factor signaling in Niemann-Pick type C disease. Mol. Neurodegener. , 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cataldo A. M., Barnett J. L., Pieroni C., Nixon R. A. (1997) Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer’s disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J. Neurosci. , 6142–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y., Mullaney K. A., Peterhoff C. M., Che S., Schmidt S. D., Boyer-Boiteau A., Ginsberg S. D., Cataldo A. M., Mathews P. M., Nixon R. A. (2010) Alzheimer’s-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc. Natl. Acad. Sci. USA , 1630–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israel M. A., Yuan S. H., Bardy C., Reyna S. M., Mu Y., Herrera C., Hefferan M. P., Van Gorp S., Nazor K. L., Boscolo F. S., Carson C. T., Laurent L. C., Marsala M., Gage F. H., Remes A. M., Koo E. H., Goldstein L. S. (2012) Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature , 216–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S., Sato Y., Mohan P. S., Peterhoff C., Pensalfini A., Rigoglioso A., Jiang Y., Nixon R. A. (2016) Evidence that the rab5 effector APPL1 mediates APP-betaCTF-induced dysfunction of endosomes in Down syndrome and Alzheimer’s disease. Mol. Psychiatry , 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W., Weissmiller A. M., White J. A. II, Fang F., Wang X., Wu Y., Pearn M. L., Zhao X., Sawa M., Chen S., Gunawardena S., Ding J., Mobley W. C., Wu C. (2016) Amyloid precursor protein-mediated endocytic pathway disruption induces axonal dysfunction and neurodegeneration. J. Clin. Invest. , 1815–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cataldo A. M., Mathews P. M., Boiteau A. B., Hassinger L. C., Peterhoff C. M., Jiang Y., Mullaney K., Neve R. L., Gruenberg J., Nixon R. A. (2008) Down syndrome fibroblast model of Alzheimer-related endosome pathology: accelerated endocytosis promotes late endocytic defects. Am. J. Pathol. , 370–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McPhie D. L., Coopersmith R., Hines-Peralta A., Chen Y., Ivins K. J., Manly S. P., Kozlowski M. R., Neve K. A., Neve R. L. (2003) DNA synthesis and neuronal apoptosis caused by familial Alzheimer disease mutants of the amyloid precursor protein are mediated by the p21 activated kinase PAK3. J. Neurosci. , 6914–6927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laifenfeld D., Patzek L. J., McPhie D. L., Chen Y., Levites Y., Cataldo A. M., Neve R. L. (2007) Rab5 mediates an amyloid precursor protein signaling pathway that leads to apoptosis. J. Neurosci. , 7141–7153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y., Huang X., Zhang Y. W., Rockenstein E., Bu G., Golde T. E., Masliah E., Xu H. (2012) Alzheimer’s β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid. J. Neurosci. , 11390–11395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi J. H., Kaur G., Mazzella M. J., Morales-Corraliza J., Levy E., Mathews P. M. (2013) Early endosomal abnormalities and cholinergic neuron degeneration in amyloid-β protein precursor transgenic mice. J. Alzheimers Dis. , 691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan X.-X. C., Zhang X.-M., Macklin N., Cai H., Luo X.-G., Struble R. G., Rose G. M., Patrylo P. R. (2011) BACE1 elevation is involved in amyloid plaque pathogenesis in the triple transgenic model of Alzheimer’s disease: profiling an early-onset axonal accumulation of putative APP b-carboxyl terminal fragments. Neuroscience 2011: 41st Annual Neuroscience Meeting, presentation 354.317, Society for Neuroscience, Washington, DC [Google Scholar]
- 28.Nistor M., Don M., Parekh M., Sarsoza F., Goodus M., Lopez G. E., Kawas C., Leverenz J., Doran E., Lott I. T., Hill M., Head E. (2007) Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiol. Aging , 1493–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holsinger R. M., McLean C. A., Beyreuther K., Masters C. L., Evin G. (2002) Increased expression of the amyloid precursor beta-secretase in Alzheimer’s disease. Ann. Neurol. , 783–786 [DOI] [PubMed] [Google Scholar]
- 30.Fukumoto H., Cheung B. S., Hyman B. T., Irizarry M. C. (2002) Beta-secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. , 1381–1389 [DOI] [PubMed] [Google Scholar]
- 31.Tesco G., Koh Y. H., Kang E. L., Cameron A. N., Das S., Sena-Esteves M., Hiltunen M., Yang S. H., Zhong Z., Shen Y., Simpkins J. W., Tanzi R. E. (2007) Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron , 721–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liang B., Duan B. Y., Zhou X. P., Gong J. X., Luo Z. G. (2010) Calpain activation promotes BACE1 expression, amyloid precursor protein processing, and amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. , 27737–27744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeates E. F., Tesco G. (2016) The endosome-associated deubiquitinating enzyme USP8 regulates BACE1 enzyme ubiquitination and degradation. J. Biol. Chem. , 15753–15766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas R. S., Henson A., Gerrish A., Jones L., Williams J., Kidd E. J. (2016) Decreasing the expression of PICALM reduces endocytosis and the activity of β-secretase: implications for Alzheimer’s disease. BMC Neurosci. , 50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song W. J., Son M. Y., Lee H. W., Seo H., Kim J. H., Chung S. H. (2015) Enhancement of BACE1 activity by p25/Cdk5-mediated phosphorylation in Alzheimer’s disease. PLoS One , e0136950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Natunen T., Takalo M., Kemppainen S., Leskelä S., Marttinen M., Kurkinen K. M., Pursiheimo J. P., Sarajärvi T., Viswanathan J., Gabbouj S., Solje E., Tahvanainen E., Pirttimäki T., Kurki M., Paananen J., Rauramaa T., Miettinen P., Mäkinen P., Leinonen V., Soininen H., Airenne K., Tanzi R. E., Tanila H., Haapasalo A., Hiltunen M. (2016) Relationship between ubiquilin-1 and BACE1 in human Alzheimer’s disease and APdE9 transgenic mouse brain and cell-based models. Neurobiol. Dis. , 187–205 [DOI] [PubMed] [Google Scholar]
- 37.Devi L., Ohno M. (2014) PERK mediates eIF2α phosphorylation responsible for BACE1 elevation, CREB dysfunction and neurodegeneration in a mouse model of Alzheimer’s disease. Neurobiol. Aging , 2272–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He X., Li F., Chang W. P., Tang J. (2005) GGA proteins mediate the recycling pathway of memapsin 2 (BACE). J. Biol. Chem. , 11696–11703 [DOI] [PubMed] [Google Scholar]
- 39.Schnöder L., Hao W., Qin Y., Liu S., Tomic I., Liu X., Fassbender K., Liu Y. (2016) Deficiency of neuronal p38α MAPK attenuates amyloid pathology in Alzheimer disease mouse and cell models through facilitating lysosomal degradation of BACE1. J. Biol. Chem. , 2067–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okada H., Zhang W., Peterhoff C., Hwang J. C., Nixon R. A., Ryu S. H., Kim T. W. (2010) Proteomic identification of sorting nexin 6 as a negative regulator of BACE1-mediated APP processing. FASEB J. , 2783–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araki W. (2016) Post-translational regulation of the β-secretase BACE1. Brain Res. Bull. , 170–177 [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Li R., Shen Y. (2013) β-Secretase: its biology as a therapeutic target in diseases. Trends Pharmacol. Sci. , 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhinn H., Fujita R., Qiang L., Cheng R., Lee J. H., Abeliovich A. (2013) Integrative genomics identifies APOE ε4 effectors in Alzheimer’s disease. Nature , 45–50 [DOI] [PubMed] [Google Scholar]
- 44.Calafate S., Flavin W., Verstreken P., Moechars D. (2016) Loss of bin1 promotes the propagation of tau pathology. Cell Rep. , 931–940 [DOI] [PubMed] [Google Scholar]
- 45.Das U., Wang L., Ganguly A., Saikia J. M., Wagner S. L., Koo E. H., Roy S. (2016) Visualizing APP and BACE-1 approximation in neurons yields insight into the amyloidogenic pathway. Nat. Neurosci. , 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miaczynska M., Christoforidis S., Giner A., Shevchenko A., Uttenweiler-Joseph S., Habermann B., Wilm M., Parton R. G., Zerial M. (2004) APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell , 445–456 [DOI] [PubMed] [Google Scholar]
- 47.Zhu G., Chen J., Liu J., Brunzelle J. S., Huang B., Wakeham N., Terzyan S., Li X., Rao Z., Li G., Zhang X. C. (2007) Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J. , 3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beel A. J., Mobley C. K., Kim H. J., Tian F., Hadziselimovic A., Jap B., Prestegard J. H., Sanders C. R. (2008) Structural studies of the transmembrane C-terminal domain of the amyloid precursor protein (APP): does APP function as a cholesterol sensor? Biochemistry , 9428–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beel A. J., Sakakura M., Barrett P. J., Sanders C. R. (2010) Direct binding of cholesterol to the amyloid precursor protein: an important interaction in lipid-Alzheimer’s disease relationships? Biochim. Biophys. Acta , 975–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ehehalt R., Keller P., Haass C., Thiele C., Simons K. (2003) Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J. Cell Biol. , 113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J. R., Hahn H. S., Kim Y. H., Nguyen H. H., Yang J. M., Kang J. S., Hahn M. J. (2011) Adaptor protein containing PH domain, PTB domain and leucine zipper (APPL1) regulates the protein level of EGFR by modulating its trafficking. Biochem. Biophys. Res. Commun. , 206–211 [DOI] [PubMed] [Google Scholar]
- 52.Danson C., Brown E., Hemmings O. J., McGough I. J., Yarwood S., Heesom K. J., Carlton J. G., Martin-Serrano J., May M. T., Verkade P., Cullen P. J. (2013) SNX15 links clathrin endocytosis to the PtdIns3P early endosome independently of the APPL1 endosome. J. Cell Sci. , 4885–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flores-Rodriguez N., Kenwright D. A., Chung P. H., Harrison A. W., Stefani F., Waigh T. A., Allan V. J., Woodman P. G. (2015) ESCRT-0 marks an APPL1-independent transit route for EGFR between the cell surface and the EEA1-positive early endosome. J. Cell Sci. , 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalaidzidis I., Miaczynska M., Brewińska-Olchowik M., Hupalowska A., Ferguson C., Parton R. G., Kalaidzidis Y., Zerial M. (2015) APPL endosomes are not obligatory endocytic intermediates but act as stable cargo-sorting compartments. J. Cell Biol. , 123–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hupalowska A., Pyrzynska B., Miaczynska M. (2012) APPL1 regulates basal NF-κB activity by stabilizing NIK. J. Cell Sci. , 4090–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryu J., Galan A. K., Xin X., Dong F., Abdul-Ghani M. A., Zhou L., Wang C., Li C., Holmes B. M., Sloane L. B., Austad S. N., Guo S., Musi N., DeFronzo R. A., Deng C., White M. F., Liu F., Dong L. Q. (2014) APPL1 potentiates insulin sensitivity by facilitating the binding of IRS1/2 to the insulin receptor. Cell Rep. , 1227–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Lu W., Chen L., Zhang P., Qian T., Cao W., Luo J. (2016) Serine 707 of APPL1 is critical for the synaptic NMDA receptor-mediated akt phosphorylation signaling pathway. Neurosci. Bull. , 323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bohdanowicz M., Balkin D. M., De Camilli P., Grinstein S. (2012) Recruitment of OCRL and Inpp5B to phagosomes by Rab5 and APPL1 depletes phosphoinositides and attenuates Akt signaling. Mol. Biol. Cell , 176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu X., Yang Y., Xu C., Niu Y., Chen T., Zhou Q., Liu J. J. (2011) Retrolinkin cooperates with endophilin A1 to mediate BDNF-TrkB early endocytic trafficking and signaling from early endosomes. Mol. Biol. Cell , 3684–3698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin D. C., Quevedo C., Brewer N. E., Bell A., Testa J. R., Grimes M. L., Miller F. D., Kaplan D. R. (2006) APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol. Cell. Biol. , 8928–8941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ogawa A., Yamazaki Y., Nakamori M., Takahashi T., Kurashige T., Hiji M., Nagano Y., Yamawaki T., Matsumoto M. (2013) Characterization and distribution of adaptor protein containing a PH domain, PTB domain and leucine zipper motif (APPL1) in Alzheimer’s disease hippocampus: an immunohistochemical study. Brain Res. , 118–124 [DOI] [PubMed] [Google Scholar]
- 62.Majumdar D., Nebhan C. A., Hu L., Anderson B., Webb D. J. (2011) An APPL1/Akt signaling complex regulates dendritic spine and synapse formation in hippocampal neurons. Mol. Cell. Neurosci. , 633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernández-Monreal M., Sánchez-Castillo C., Esteban J. A. (2016) APPL1 gates long-term potentiation through its plekstrin homology domain. J. Cell Sci. , 2793–2803 [DOI] [PubMed] [Google Scholar]
- 64.Kessels H. W., Malinow R. (2009) Synaptic AMPA receptor plasticity and behavior. Neuron , 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown T. C., Tran I. C., Backos D. S., Esteban J. A. (2005) NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron , 81–94 [DOI] [PubMed] [Google Scholar]
- 66.Dhaka A., Costa R. M., Hu H., Irvin D. K., Patel A., Kornblum H. I., Silva A. J., O’Dell T. J., Colicelli J. (2003) The RAS effector RIN1 modulates the formation of aversive memories. J. Neurosci. , 748–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Park M., Penick E. C., Edwards J. G., Kauer J. A., Ehlers M. D. (2004) Recycling endosomes supply AMPA receptors for LTP. Science , 1972–1975 [DOI] [PubMed] [Google Scholar]
- 68.Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. (2006) AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron , 831–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koffie R. M., Hyman B. T., Spires-Jones T. L. (2011) Alzheimer’s disease: synapses gone cold. Mol. Neurodegener. , 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laurén J., Gimbel D. A., Nygaard H. B., Gilbert J. W., Strittmatter S. M. (2009) Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature , 1128–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Renner M., Lacor P. N., Velasco P. T., Xu J., Contractor A., Klein W. L., Triller A. (2010) Deleterious effects of amyloid beta oligomers acting as an extracellular scaffold for mGluR5. Neuron , 739–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cirrito J. R., Kang J. E., Lee J., Stewart F. R., Verges D. K., Silverio L. M., Bu G., Mennerick S., Holtzman D. M. (2008) Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron , 42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu W., Fitzgerald S., Nixon R. A., Levy E., Wilson D. A. (2015) Early hyperactivity in lateral entorhinal cortex is associated with elevated levels of AβPP metabolites in the Tg2576 mouse model of Alzheimer’s disease. Exp. Neurol. , 82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamm V. B., Bott J. B., Heraud C., Herbeaux K., Strittmatter C., Cassel J. C., Goutagny R. (2014) β-CTF induced early behavioral and electrophysiological alterations in transgenic TgCRND8 mouse model of Alzheimer’s disease. Neuroscience 2014: 44th Annual Neuroscience Meeting, presentation 307.318, Society for Neuroscience, Washington, DC [Google Scholar]
- 75.Lauritzen I., Pardossi-Piquard R., Bauer C., Brigham E., Abraham J. D., Ranaldi S., Fraser P., St-George-Hyslop P., Le Thuc O., Espin V., Chami L., Dunys J., Checler F. (2012) The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J. Neurosci. , 16243–16255a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lauritzen I., Pardossi-Piquard R., Bourgeois A., Pagnotta S., Biferi M. G., Barkats M., Lacor P., Klein W., Bauer C., Checler F. (2016) Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta. Neuropathol. , 257–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mondragon-Rodriguez S. M., Manseau F., Gu N., Boyce R., Williams S. (2014) Early brain network alterations are correlated with β-CTF in Alzheimer’s transgenic mouse model. Neuroscience 2014: 44th Annual Neuroscience Meeting, presentation 40.09, Society for Neuroscience, Washington, DC [Google Scholar]
- 78.Tamayev R., Matsuda S., Giliberto L., Arancio O., D’Adamio L. (2011) APP heterozygosity averts memory deficit in knockin mice expressing the Danish dementia BRI2 mutant. EMBO J. , 2501–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tamayev R., D’Adamio L. (2012) Inhibition of γ-secretase worsens memory deficits in a genetically congruous mouse model of Danish dementia. Mol. Neurodegener. , 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tamayev R., Matsuda S., Arancio O., D’Adamio L. (2012) β- but not γ-secretase proteolysis of APP causes synaptic and memory deficits in a mouse model of dementia. EMBO Mol. Med. , 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simón A. M., Schiapparelli L., Salazar-Colocho P., Cuadrado-Tejedor M., Escribano L., López de Maturana R., Del Río J., Pérez-Mediavilla A., Frechilla D. (2009) Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Abeta levels. Neurobiol. Dis. , 369–378 [DOI] [PubMed] [Google Scholar]
- 82.Choi S. H., Park C. H., Koo J. W., Seo J. H., Kim H. S., Jeong S. J., Lee J. H., Kim S. S., Suh Y. H. (2001) Memory impairment and cholinergic dysfunction by centrally administered Abeta and carboxyl-terminal fragment of Alzheimer’s APP in mice. FASEB J. , 1816–1818 [DOI] [PubMed] [Google Scholar]
- 83.Kim H. S., Kim E. M., Kim N. J., Chang K. A., Choi Y., Ahn K. W., Lee J. H., Kim S., Park C. H., Suh Y. H. (2004) Inhibition of histone deacetylation enhances the neurotoxicity induced by the C-terminal fragments of amyloid precursor protein. J. Neurosci. Res. , 117–124 [DOI] [PubMed] [Google Scholar]
- 84.Imbimbo B. P., Giardina G. A. (2011) γ-secretase inhibitors and modulators for the treatment of Alzheimer’s disease: disappointments and hopes. Curr. Top. Med. Chem. , 1555–1570 [DOI] [PubMed] [Google Scholar]
- 85.Holtzman D. M., Li Y., Parada L. F., Kinsman S., Chen C. K., Valletta J. S., Zhou J., Long J. B., Mobley W. C. (1992) p140trk mRNA marks NGF-responsive forebrain neurons: evidence that trk gene expression is induced by NGF. Neuron , 465–478 [DOI] [PubMed] [Google Scholar]
- 86.Sofroniew M. V., Galletly N. P., Isacson O., Svendsen C. N. (1990) Survival of adult basal forebrain cholinergic neurons after loss of target neurons. Science , 338–342 [DOI] [PubMed] [Google Scholar]
- 87.Grothe M., Heinsen H., Teipel S. J. (2012) Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol. Psychiatry , 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmitz T. W., Nathan Spreng R.; Alzheimer’s Disease Neuroimaging Initiative (2016) Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. , 13249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Muth K., Schönmeyer R., Matura S., Haenschel C., Schröder J., Pantel J. (2010) Mild cognitive impairment in the elderly is associated with volume loss of the cholinergic basal forebrain region. Biol. Psychiatry , 588–591 [DOI] [PubMed] [Google Scholar]
- 90.Mufson E. J., Ma S. Y., Dills J., Cochran E. J., Leurgans S., Wuu J., Bennett D. A., Jaffar S., Gilmor M. L., Levey A. I., Kordower J. H. (2002) Loss of basal forebrain P75(NTR) immunoreactivity in subjects with mild cognitive impairment and Alzheimer’s disease. J. Comp. Neurol. , 136–153 [DOI] [PubMed] [Google Scholar]
- 91.Mufson E. J., Bothwell M., Kordower J. H. (1989) Loss of nerve growth factor receptor-containing neurons in Alzheimer’s disease: a quantitative analysis across subregions of the basal forebrain. Exp. Neurol. , 221–232 [DOI] [PubMed] [Google Scholar]
- 92.Salehi A., Delcroix J. D., Belichenko P. V., Zhan K., Wu C., Valletta J. S., Takimoto-Kimura R., Kleschevnikov A. M., Sambamurti K., Chung P. P., Xia W., Villar A., Campbell W. A., Kulnane L. S., Nixon R. A., Lamb B. T., Epstein C. J., Stokin G. B., Goldstein L. S., Mobley W. C. (2006) Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron , 29–42 [DOI] [PubMed] [Google Scholar]
- 93.Granholm A. C., Sanders L. A., Crnic L. S. (2000) Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp. Neurol. , 647–663 [DOI] [PubMed] [Google Scholar]
- 94.Nishitomi K., Sakaguchi G., Horikoshi Y., Gray A. J., Maeda M., Hirata-Fukae C., Becker A. G., Hosono M., Sakaguchi I., Minami S. S., Nakajima Y., Li H. F., Takeyama C., Kihara T., Ota A., Wong P. C., Aisen P. S., Kato A., Kinoshita N., Matsuoka Y. (2006) BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J. Neurochem. , 1555–1563 [DOI] [PubMed] [Google Scholar]
- 95.McConlogue L., Buttini M., Anderson J. P., Brigham E. F., Chen K. S., Freedman S. B., Games D., Johnson-Wood K., Lee M., Zeller M., Liu W., Motter R., Sinha S. (2007) Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic Mice. J. Biol. Chem. , 26326–26334 [DOI] [PubMed] [Google Scholar]
- 96.Gout I., Middleton G., Adu J., Ninkina N. N., Drobot L. B., Filonenko V., Matsuka G., Davies A. M., Waterfield M., Buchman V. L. (2000) Negative regulation of PI 3-kinase by Ruk, a novel adaptor protein. EMBO J. , 4015–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen B., Borinstein S. C., Gillis J., Sykes V. W., Bogler O. (2000) The glioma-associated protein SETA interacts with AIP1/Alix and ALG-2 and modulates apoptosis in astrocytes. J. Biol. Chem. , 19275–19281 [DOI] [PubMed] [Google Scholar]
- 98.Missotten M., Nichols A., Rieger K., Sadoul R. (1999) Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. , 124–129 [DOI] [PubMed] [Google Scholar]
- 99.Vito P., Lacanà E., D’Adamio L. (1996) Interfering with apoptosis: Ca2+-binding protein ALG-2 and Alzheimer’s disease gene ALG-3. Science , 521–525 [DOI] [PubMed] [Google Scholar]
- 100.Vito P., Pellegrini L., Guiet C., D’Adamio L. (1999) Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J. Biol. Chem. , 1533–1540 [DOI] [PubMed] [Google Scholar]
- 101.Nikolaev A., McLaughlin T., O’Leary D. D., Tessier-Lavigne M. (2009) APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature , 981–989 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Huang Y. (2010) Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol. Med. , 287–294 [DOI] [PubMed] [Google Scholar]
- 103.Nelson P. T., Alafuzoff I., Bigio E. H., Bouras C., Braak H., Cairns N. J., Castellani R. J., Crain B. J., Davies P., Del Tredici K., Duyckaerts C., Frosch M. P., Haroutunian V., Hof P. R., Hulette C. M., Hyman B. T., Iwatsubo T., Jellinger K. A., Jicha G. A., Kövari E., Kukull W. A., Leverenz J. B., Love S., Mackenzie I. R., Mann D. M., Masliah E., McKee A. C., Montine T. J., Morris J. C., Schneider J. A., Sonnen J. A., Thal D. R., Trojanowski J. Q., Troncoso J. C., Wisniewski T., Woltjer R. L., Beach T. G. (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. , 362–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lane-Donovan C., Philips G. T., Herz J. (2014) More than cholesterol transporters: lipoprotein receptors in CNS function and neurodegeneration. Neuron , 771–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He X., Cooley K., Chung C. H. Y., Dashti N., Tang J. (2007) Apolipoprotein receptor 2 and X11 α/β mediate apolipoprotein E-induced endocytosis of amyloid-β precursor protein and β-secretase, leading to amyloid-β production. J. Neurosci. , 4052–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Heeren J., Grewal T., Laatsch A., Becker N., Rinninger F., Rye K. A., Beisiegel U. (2004) Impaired recycling of apolipoprotein E4 is associated with intracellular cholesterol accumulation. J. Biol. Chem. , 55483–55492 [DOI] [PubMed] [Google Scholar]
- 107.Rellin L., Heeren J., Beisiegel U. (2008) Recycling of apolipoprotein E is not associated with cholesterol efflux in neuronal cells. Biochim. Biophys. Acta , 232–238 [DOI] [PubMed] [Google Scholar]
- 108.Mauch D. H., Nägler K., Schumacher S., Göritz C., Müller E. C., Otto A., Pfrieger F. W. (2001) CNS synaptogenesis promoted by glia-derived cholesterol. Science , 1354–1357 [DOI] [PubMed] [Google Scholar]
- 109.Barrett P. J., Song Y., Van Horn W. D., Hustedt E. J., Schafer J. M., Hadziselimovic A., Beel A. J., Sanders C. R. (2012) The amyloid precursor protein has a flexible transmembrane domain and binds cholesterol. Science , 1168–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji Z. S., Müllendorff K., Cheng I. H., Miranda R. D., Huang Y., Mahley R. W. (2006) Reactivity of apolipoprotein E4 and amyloid beta peptide: lysosomal stability and neurodegeneration. J. Biol. Chem. , 2683–2692 [DOI] [PubMed] [Google Scholar]
- 111.Cossec J. C., Marquer C., Panchal M., Lazar A. N., Duyckaerts C., Potier M. C. (2010) Cholesterol changes in Alzheimer’s disease: methods of analysis and impact on the formation of enlarged endosomes. Biochim. Biophys. Acta , 839–845 [DOI] [PubMed] [Google Scholar]
- 112.Fu R., Yanjanin N. M., Elrick M. J., Ware C., Lieberman A. P., Porter F. D. (2012) Apolipoprotein E genotype and neurological disease onset in Niemann-Pick disease, type C1. Am. J. Med. Genet. A. , 2775–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karch C. M., Goate A. M. (2015) Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry , 43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larsen K. E., Sulzer D. (2002) Autophagy in neurons: a review. Histol. Histopathol. , 897–908 [DOI] [PubMed] [Google Scholar]
- 115.Ivy G. O., Kanai S., Ohta M., Smith G., Sato Y., Kobayashi M., Kitani K. (1989) Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. Adv. Exp. Med. Biol. , 31–45, discussion 45–47 [DOI] [PubMed] [Google Scholar]
- 116.Nixon R. A. (2013) The role of autophagy in neurodegenerative disease. Nat. Med. , 983–997 [DOI] [PubMed] [Google Scholar]
- 117.Felbor U., Kessler B., Mothes W., Goebel H. H., Ploegh H. L., Bronson R. T., Olsen B. R. (2002) Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc. Natl. Acad. Sci. USA , 7883–7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nixon R. A., Wegiel J., Kumar A., Yu W. H., Peterhoff C., Cataldo A., Cuervo A. M. (2005) Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. , 113–122 [DOI] [PubMed] [Google Scholar]
- 119.Lee J. H., Yu W. H., Kumar A., Lee S., Mohan P. S., Peterhoff C. M., Wolfe D. M., Martinez-Vicente M., Massey A. C., Sovak G., Uchiyama Y., Westaway D., Cuervo A. M., Nixon R. A. (2010) Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell , 1146–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cataldo A. M., Peterhoff C. M., Schmidt S. D., Terio N. B., Duff K., Beard M., Mathews P. M., Nixon R. A. (2004) Presenilin mutations in familial Alzheimer disease and transgenic mouse models accelerate neuronal lysosomal pathology. J. Neuropathol. Exp. Neurol. , 821–830 [DOI] [PubMed] [Google Scholar]
- 121.Cai Y., Zhang X. M., Macklin L. N., Cai H., Luo X. G., Oddo S., Laferla F. M., Struble R. G., Rose G. M., Patrylo P. R., Yan X. X. (2012) BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer’s disease: differential Aβ antibody labeling of early-onset axon terminal pathology. Neurotox. Res. , 160–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boland B., Kumar A., Lee S., Platt F. M., Wegiel J., Yu W. H., Nixon R. A. (2008) Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer’s disease. J. Neurosci. , 6926–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang D. S., Kumar A., Stavrides P., Peterson J., Peterhoff C. M., Pawlik M., Levy E., Cataldo A. M., Nixon R. A. (2008) Neuronal apoptosis and autophagy cross talk in aging PS/APP mice, a model of Alzheimer’s disease. Am. J. Pathol. , 665–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamashita N., Kuruvilla R. (2016) Neurotrophin signaling endosomes: biogenesis, regulation, and functions. Curr. Opin. Neurobiol. , 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu W. H., Cuervo A. M., Kumar A., Peterhoff C. M., Schmidt S. D., Lee J. H., Mohan P. S., Mercken M., Farmery M. R., Tjernberg L. O., Jiang Y., Duff K., Uchiyama Y., Näslund J., Mathews P. M., Cataldo A. M., Nixon R. A. (2005) Macroautophagy--a novel beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol. , 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mony V. K., Benjamin S., O’Rourke E. J. (2016) A lysosome-centered view of nutrient homeostasis. Autophagy , 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoon S. Y., Choi J. E., Yoon J. H., Huh J. W., Kim D. H. (2006) BACE inhibitor reduces APP-beta-C-terminal fragment accumulation in axonal swellings of okadaic acid-induced neurodegeneration. Neurobiol. Dis. , 435–444 [DOI] [PubMed] [Google Scholar]
- 128.Villaseñor R., Kalaidzidis Y., Zerial M. (2016) Signal processing by the endosomal system. Curr. Opin. Cell Biol. , 53–60 [DOI] [PubMed] [Google Scholar]
- 129.Cheng F., Cappai R., Lidfeldt J., Belting M., Fransson L. A., Mani K. (2014) Amyloid precursor protein (APP)/APP-like protein 2 (APLP2) expression is required to initiate endosome-nucleus-autophagosome trafficking of glypican-1-derived heparan sulfate. J. Biol. Chem. , 20871–20878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nguyen K., Rabenstein D. L. (2016) Interaction of the heparin-binding consensus sequence of β-amyloid peptides with heparin and heparin-derived oligosaccharides. J. Phys. Chem. B , 2187–2197 [DOI] [PubMed] [Google Scholar]
- 131.Brendza R. P., Bacskai B. J., Cirrito J. R., Simmons K. A., Skoch J. M., Klunk W. E., Mathis C. A., Bales K. R., Paul S. M., Hyman B. T., Holtzman D. M. (2005) Anti-Abeta antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. J. Clin. Invest. , 428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Novotny R., Langer F., Mahler J., Skodras A., Vlachos A., Wegenast-Braun B. M., Kaeser S. A., Neher J. J., Eisele Y. S., Pietrowski M. J., Nilsson K. P., Deller T., Staufenbiel M., Heimrich B., Jucker M. (2016) Conversion of synthetic Aβ to in vivo active seeds and amyloid plaque formation in a hippocampal slice culture model. J. Neurosci. , 5084–5093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yang T., Li S., Xu H., Walsh D. M., Selkoe D. J. (2017) Large soluble oligomers of amyloid β-protein from Alzheimer brain are far less neuroactive than the smaller oligomers to which they dissociate. J. Neurosci. , 152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Frautschy S. A., Horn D. L., Sigel J. J., Harris-White M. E., Mendoza J. J., Yang F., Saido T. C., Cole G. M. (1998) Protease inhibitor coinfusion with amyloid beta-protein results in enhanced deposition and toxicity in rat brain. J. Neurosci. , 8311–8321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frautschy S. A., Yang F., Calderón L., Cole G. M. (1996) Rodent models of Alzheimer’s disease: rat A beta infusion approaches to amyloid deposits. Neurobiol. Aging , 311–321 [DOI] [PubMed] [Google Scholar]
- 136.Cataldo A. M., Barnett J. L., Berman S. A., Li J., Quarless S., Bursztajn S., Lippa C., Nixon R. A. (1995) Gene expression and cellular content of cathepsin D in Alzheimer’s disease brain: evidence for early up-regulation of the endosomal-lysosomal system. Neuron , 671–680 [DOI] [PubMed] [Google Scholar]
- 137.Cataldo A. M., Hamilton D. J., Barnett J. L., Paskevich P. A., Nixon R. A. (1996) Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer’s disease. J. Neurosci. , 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bordi M., Berg M. J., Mohan P. S., Peterhoff C. M., Alldred M. J., Che S., Ginsberg S. D., Nixon R. A. (2016) Autophagy flux in CA1 neurons of Alzheimer hippocampus: increased induction overburdens failing lysosomes to propel neuritic dystrophy. Autophagy , 2467–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nixon R. A., Cataldo A. M. (2006) Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. (3 Suppl), 277–289 [DOI] [PubMed] [Google Scholar]
- 140.Yang D. S., Stavrides P., Mohan P. S., Kaushik S., Kumar A., Ohno M., Schmidt S. D., Wesson D., Bandyopadhyay U., Jiang Y., Pawlik M., Peterhoff C. M., Yang A. J., Wilson D. A., St George-Hyslop P., Westaway D., Mathews P. M., Levy E., Cuervo A. M., Nixon R. A. (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain , 258–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang D. S., Stavrides P., Saito M., Kumar A., Rodriguez-Navarro J. A., Pawlik M., Huo C., Walkley S. U., Saito M., Cuervo A. M., Nixon R. A. (2014) Defective macroautophagic turnover of brain lipids in the TgCRND8 Alzheimer mouse model: prevention by correcting lysosomal proteolytic deficits. Brain , 3300–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee J. H., McBrayer M. K., Wolfe D. M., Haslett L. J., Kumar A., Sato Y., Lie P. P., Mohan P., Coffey E. E., Kompella U., Mitchell C. H., Lloyd-Evans E., Nixon R. A. (2015) Presenilin 1 maintains lysosomal Ca2+ homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. , 1430–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xue X., Wang L. R., Sato Y., Jiang Y., Berg M., Yang D. S., Nixon R. A., Liang X. J. (2014) Single-walled carbon nanotubes alleviate autophagic/lysosomal defects in primary glia from a mouse model of Alzheimer’s disease. Nano Lett. , 5110–5117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang D. S., Stavrides P., Kumar A., Jiang Y., Mohan P. S., Ohno M., Dobrenis K., Davidson C. D., Saito M., Pawlik M., Huo C., Walkley S. U., Nixon R. A. (2017) Cyclodextrin has conflicting actions on autophagy flux in vivo in brains of normal and Alzheimer model mice. Hum. Mol. Genet. , 843–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nixon R. A., Jiang Y., Sato Y., Im E., Bordi M., Colacurcio D., Pensalfini A., Rigoglioso A., Mohan P. (2017) APP-β CTF-mediated pathological endosome signaling also disrupts lysosomal function in Down syndrome (DS). AD/PD 2017: 13th International Congress on Alzheimer’s and Parkinson’s Diseases, presentation A01.f.009 [Google Scholar]
- 146.Giudetti A. M., Romano A., Lavecchia A. M., Gaetani S. (2016) The role of brain cholesterol and its oxidized products in Alzheimer’s disease. Curr. Alzheimer Res. , 198–205 [DOI] [PubMed] [Google Scholar]
- 147.Gamba P., Testa G., Gargiulo S., Staurenghi E., Poli G., Leonarduzzi G. (2015) Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front. Aging Neurosci. , 119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kavčič N., Pegan K., Turk B. (2016) Lysosomes in programmed cell death pathways: from initiators to amplifiers. [E-pub ahead of print] Biol. Chem. doi: 10.1515/hsz-2016-0252 [DOI] [PubMed] [Google Scholar]
- 149.Yamashima T. (2016) Can ‘calpain-cathepsin hypothesis’ explain Alzheimer neuronal death? Ageing Res. Rev. , 169–179 [DOI] [PubMed] [Google Scholar]
- 150.Gómez-Sintes R., Ledesma M. D., Boya P. (2016) Lysosomal cell death mechanisms in aging. Ageing Res. Rev. , 150–168 [DOI] [PubMed] [Google Scholar]
- 151.Boya P., Kroemer G. (2008) Lysosomal membrane permeabilization in cell death. Oncogene , 6434–6451 [DOI] [PubMed] [Google Scholar]
- 152.Aits S., Jäättelä M. (2013) Lysosomal cell death at a glance. J. Cell Sci. , 1905–1912 [DOI] [PubMed] [Google Scholar]
- 153.Tian Y., Chang J. C., Greengard P., Flajolet M. (2014) The convergence of endosomal and autophagosomal pathways: implications for APP-CTF degradation. Autophagy , 694–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tian Y., Chang J. C., Fan E. Y., Flajolet M., Greengard P. (2013) Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc. Natl. Acad. Sci. USA , 17071–17076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Siman R., Mistretta S., Durkin J. T., Savage M. J., Loh T., Trusko S., Scott R. W. (1993) Processing of the beta-amyloid precursor. Multiple proteases generate and degrade potentially amyloidogenic fragments. J. Biol. Chem. , 16602–16609 [PubMed] [Google Scholar]
- 156.Bustamante H. A., Rivera-Dictter A., Cavieres V. A., Muñoz V. C., González A., Lin Y., Mardones G. A., Burgos P. V. (2013) Turnover of C99 is controlled by a crosstalk between ERAD and ubiquitin-independent lysosomal degradation in human neuroglioma cells. PLoS One , e83096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ling D., Magallanes M., Salvaterra P. M. (2014) Accumulation of amyloid-like Aβ1-42 in AEL (autophagy-endosomal-lysosomal) vesicles: potential implications for plaque biogenesis. ASN Neuro , e00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kopan R., Ilagan M. X. (2004) Gamma-secretase: proteasome of the membrane? Nat. Rev. Mol. Cell Biol. , 499–504 [DOI] [PubMed] [Google Scholar]
- 159.Kumar-Singh S., Theuns J., Van Broeck B., Pirici D., Vennekens K., Corsmit E., Cruts M., Dermaut B., Wang R., Van Broeckhoven C. (2006) Mean age-of-onset of familial alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum. Mutat. , 686–695 [DOI] [PubMed] [Google Scholar]
- 160.Kuperstein I., Broersen K., Benilova I., Rozenski J., Jonckheere W., Debulpaep M., Vandersteen A., Segers-Nolten I., Van Der Werf K., Subramaniam V., Braeken D., Callewaert G., Bartic C., D’Hooge R., Martins I. C., Rousseau F., Schymkowitz J., De Strooper B. (2010) Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. , 3408–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun L., Zhou R., Yang G., Shi Y. (2017) Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Aβ42 and Aβ40 peptides by γ-secretase. Proc. Natl. Acad. Sci. USA , E476–E485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pitsi D., Octave J. N. (2004) Presenilin 1 stabilizes the C-terminal fragment of the amyloid precursor protein independently of gamma-secretase activity. J. Biol. Chem. , 25333–25338 [DOI] [PubMed] [Google Scholar]
- 163.De Jonghe C., Esselens C., Kumar-Singh S., Craessaerts K., Serneels S., Checler F., Annaert W., Van Broeckhoven C., De Strooper B. (2001) Pathogenic APP mutations near the gamma-secretase cleavage site differentially affect Abeta secretion and APP C-terminal fragment stability. Hum. Mol. Genet. , 1665–1671 [DOI] [PubMed] [Google Scholar]
- 164.Duggan S. P., McCarthy J. V. (2016) Beyond γ-secretase activity: the multifunctional nature of presenilins in cell signalling pathways. Cell. Signal. , 1–11 [DOI] [PubMed] [Google Scholar]
- 165.Jayne T., Newman M., Verdile G., Sutherland G., Münch G., Musgrave I., Moussavi Nik S. H., Lardelli M. (2016) Evidence for and against a pathogenic role of reduced γ-secretase activity in familial Alzheimer’s disease. J. Alzheimers Dis. , 781–799 [DOI] [PubMed] [Google Scholar]
- 166.Schröder B., Saftig P. (2016) Intramembrane proteolysis within lysosomes. Ageing Res. Rev. , 51–64 [DOI] [PubMed] [Google Scholar]
- 167.Sannerud R., Esselens C., Ejsmont P., Mattera R., Rochin L., Tharkeshwar A. K., De Baets G., De Wever V., Habets R., Baert V., Vermeire W., Michiels C., Groot A. J., Wouters R., Dillen K., Vints K., Baatsen P., Munck S., Derua R., Waelkens E., Basi G. S., Mercken M., Vooijs M., Bollen M., Schymkowitz J., Rousseau F., Bonifacino J. S., Van Niel G., De Strooper B., Annaert W. (2016) Restricted location of PSEN2/γ-secretase determines substrate specificity and generates an intracellular Aβ pool. Cell , 193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bagshaw R. D., Pasternak S. H., Mahuran D. J., Callahan J. W. (2003) Nicastrin is a resident lysosomal membrane protein. Biochem. Biophys. Res. Commun. , 615–618 [DOI] [PubMed] [Google Scholar]
- 169.Pasternak S. H., Bagshaw R. D., Guiral M., Zhang S., Ackerley C. A., Pak B. J., Callahan J. W., Mahuran D. J. (2003) Presenilin-1, nicastrin, amyloid precursor protein, and gamma-secretase activity are co-localized in the lysosomal membrane. J. Biol. Chem. , 26687–26694 [DOI] [PubMed] [Google Scholar]
- 170.Merkulova M., Păunescu T. G., Azroyan A., Marshansky V., Breton S., Brown D. (2015) Mapping the H+ (V)-ATPase interactome: identification of proteins involved in trafficking, folding, assembly and phosphorylation. Sci. Rep. , 14827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Colacurcio D. J., Nixon R. A. (2016) Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. , 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Williamson W. R., Hiesinger P. R. (2010) On the role of v-ATPase V0a1-dependent degradation in Alzheimer disease. Commun. Integr. Biol. , 604–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hughes A. L., Gottschling D. E. (2012) An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature , 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ruckenstuhl C., Netzberger C., Entfellner I., Carmona-Gutierrez D., Kickenweiz T., Stekovic S., Gleixner C., Schmid C., Klug L., Sorgo A. G., Eisenberg T., Büttner S., Mariño G., Koziel R., Jansen-Dürr P., Fröhlich K. U., Kroemer G., Madeo F. (2014) Lifespan extension by methionine restriction requires autophagy-dependent vacuolar acidification. PLoS Genet. , e1004347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Molin M., Demir A. B. (2014) Linking peroxiredoxin and vacuolar-ATPase functions in calorie restriction-mediated life span extension. Int. J. Cell Biol. , 913071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Belinson H., Lev D., Masliah E., Michaelson D. M. (2008) Activation of the amyloid cascade in apolipoprotein E4 transgenic mice induces lysosomal activation and neurodegeneration resulting in marked cognitive deficits. J. Neurosci. , 4690–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ji Z. S., Miranda R. D., Newhouse Y. M., Weisgraber K. H., Huang Y., Mahley R. W. (2002) Apolipoprotein E4 potentiates amyloid beta peptide-induced lysosomal leakage and apoptosis in neuronal cells. J. Biol. Chem. , 21821–21828 [DOI] [PubMed] [Google Scholar]
- 178.Ohmi K., Kudo L. C., Ryazantsev S., Zhao H. Z., Karsten S. L., Neufeld E. F. (2009) Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc. Natl. Acad. Sci. USA , 8332–8337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ryazantsev S., Yu W. H., Zhao H. Z., Neufeld E. F., Ohmi K. (2007) Lysosomal accumulation of SCMAS (subunit c of mitochondrial ATP synthase) in neurons of the mouse model of mucopolysaccharidosis III B. Mol. Genet. Metab. , 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McBrayer M., Nixon R. A. (2013) Lysosome and calcium dysregulation in Alzheimer’s disease: partners in crime. Biochem. Soc. Trans. , 1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rao M. V., McBrayer M. K., Campbell J., Kumar A., Hashim A., Sershen H., Stavrides P. H., Ohno M., Hutton M., Nixon R. A. (2014) Specific calpain inhibition by calpastatin prevents tauopathy and neurodegeneration and restores normal lifespan in tau P301L mice. J. Neurosci. , 9222–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang Z., Song M., Liu X., Kang S. S., Kwon I. S., Duong D. M., Seyfried N. T., Hu W. T., Liu Z., Wang J. Z., Cheng L., Sun Y. E., Yu S. P., Levey A. I., Ye K. (2014) Cleavage of tau by asparagine endopeptidase mediates the neurofibrillary pathology in Alzheimer’s disease. Nat. Med. , 1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Basurto-Islas G., Grundke-Iqbal I., Tung Y. C., Liu F., Iqbal K. (2013) Activation of asparaginyl endopeptidase leads to tau hyperphosphorylation in Alzheimer disease. J. Biol. Chem. , 17495–17507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Berger Z., Ravikumar B., Menzies F. M., Oroz L. G., Underwood B. R., Pangalos M. N., Schmitt I., Wullner U., Evert B. O., O’Kane C. J., Rubinsztein D. C. (2006) Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum. Mol. Genet. , 433–442 [DOI] [PubMed] [Google Scholar]
- 185.Wang Y., Martinez-Vicente M., Krüger U., Kaushik S., Wong E., Mandelkow E. M., Cuervo A. M., Mandelkow E. (2009) Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum. Mol. Genet. , 4153–4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dolan P. J., Johnson G. V. (2010) A caspase cleaved form of tau is preferentially degraded through the autophagy pathway. J. Biol. Chem. , 21978–21987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Caccamo A., Majumder S., Richardson A., Strong R., Oddo S. (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and tau: effects on cognitive impairments. J. Biol. Chem. , 13107–13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Khurana V., Elson-Schwab I., Fulga T. A., Sharp K. A., Loewen C. A., Mulkearns E., Tyynelä J., Scherzer C. R., Feany M. B. (2010) Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet. , e1001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hamano T., Gendron T. F., Causevic E., Yen S. H., Lin W. L., Isidoro C., Deture M., Ko L. W. (2008) Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur. J. Neurosci. , 1119–1130 [DOI] [PubMed] [Google Scholar]
- 190.Ginsberg S. D., Alldred M. J., Counts S. E., Cataldo A. M., Neve R. L., Jiang Y., Wuu J., Chao M. V., Mufson E. J., Nixon R. A., Che S. (2010) Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer’s disease progression. Biol. Psychiatry , 885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bahr B. A., Bendiske J. (2002) The neuropathogenic contributions of lysosomal dysfunction. J. Neurochem. , 481–489 [DOI] [PubMed] [Google Scholar]
- 192.Florez-McClure M. L., Hohsfield L. A., Fonte G., Bealor M. T., Link C. D. (2007) Decreased insulin-receptor signaling promotes the autophagic degradation of beta-amyloid peptide in C. elegans. Autophagy , 569–580 [DOI] [PubMed] [Google Scholar]
- 193.Heinrich M., Wickel M., Schneider-Brachert W., Sandberg C., Gahr J., Schwandner R., Weber T., Saftig P., Peters C., Brunner J., Krönke M., Schütze S. (1999) Cathepsin D targeted by acid sphingomyelinase-derived ceramide. EMBO J. , 5252–5263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nixon R. A. (2007) Autophagy, amyloidogenesis and Alzheimer disease. J. Cell Sci. , 4081–4091 [DOI] [PubMed] [Google Scholar]
- 195.Takahashi R. H., Milner T. A., Li F., Nam E. E., Edgar M. A., Yamaguchi H., Beal M. F., Xu H., Greengard P., Gouras G. K. (2002) Intraneuronal Alzheimer Abeta42 accumulates in multivesicular bodies and is associated with synaptic pathology. Am. J. Pathol. , 1869–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guénette S. Y. (2003) Mechanisms of Abeta clearance and catabolism. Neuromolecular Med. , 147–160 [DOI] [PubMed] [Google Scholar]
- 197.Eckman E. A., Eckman C. B. (2005) Abeta-degrading enzymes: modulators of Alzheimer’s disease pathogenesis and targets for therapeutic intervention. Biochem. Soc. Trans. , 1101–1105 [DOI] [PubMed] [Google Scholar]
- 198.Mueller-Steiner S., Zhou Y., Arai H., Roberson E. D., Sun B., Chen J., Wang X., Yu G., Esposito L., Mucke L., Gan L. (2006) Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer’s disease. Neuron , 703–714 [DOI] [PubMed] [Google Scholar]
- 199.Butler D., Hwang J., Estick C., Nishiyama A., Kumar S. S., Baveghems C., Young-Oxendine H. B., Wisniewski M. L., Charalambides A., Bahr B. A. (2011) Protective effects of positive lysosomal modulation in Alzheimer’s disease transgenic mouse models. PLoS One , e20501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.LeBlanc A. C., Goodyer C. G. (1999) Role of endoplasmic reticulum, endosomal-lysosomal compartments, and microtubules in amyloid precursor protein metabolism of human neurons. J. Neurochem. , 1832–1842 [DOI] [PubMed] [Google Scholar]
- 201.Tian Y., Bustos V., Flajolet M., Greengard P. (2011) A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. , 1934–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang D. S., Stavrides P., Mohan P. S., Kaushik S., Kumar A., Ohno M., Schmidt S. D., Wesson D. W., Bandyopadhyay U., Jiang Y., Pawlik M., Peterhoff C. M., Yang A. J., Wilson D. A., St George-Hyslop P., Westaway D., Mathews P. M., Levy E., Cuervo A. M., Nixon R. A. (2011) Therapeutic effects of remediating autophagy failure in a mouse model of Alzheimer disease by enhancing lysosomal proteolysis. Autophagy , 788–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sun B., Zhou Y., Halabisky B., Lo I., Cho S. H., Mueller-Steiner S., Devidze N., Wang X., Grubb A., Gan L. (2008) Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron , 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Napolitano G., Ballabio A. (2016) TFEB at a glance. J. Cell Sci. , 2475–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Martini-Stoica H., Xu Y., Ballabio A., Zheng H. (2016) The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neurosci. , 221–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fraldi A., Klein A. D., Medina D. L., Settembre C. (2016) Brain disorders due to lysosomal dysfunction. Annu. Rev. Neurosci. , 277–295 [DOI] [PubMed] [Google Scholar]
- 207.Bellettato C. M., Scarpa M. (2010) Pathophysiology of neuropathic lysosomal storage disorders. J. Inherit. Metab. Dis. , 347–362 [DOI] [PubMed] [Google Scholar]
- 208.Cherra S. J. III, Dagda R. K., Chu C. T. (2010) Review: autophagy and neurodegeneration: survival at a cost? Neuropathol. Appl. Neurobiol. , 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.McCray B. A., Taylor J. P. (2008) The role of autophagy in age-related neurodegeneration. Neurosignals , 75–84 [DOI] [PubMed] [Google Scholar]
- 210.Nixon R. A., Yang D. S., Lee J. H. (2008) Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy , 590–599 [DOI] [PubMed] [Google Scholar]
- 211.Kroemer G., Jäättelä M. (2005) Lysosomes and autophagy in cell death control. Nat. Rev. Cancer , 886–897 [DOI] [PubMed] [Google Scholar]
- 212.Syntichaki P., Tavernarakis N. (2003) The biochemistry of neuronal necrosis: rogue biology? Nat. Rev. Neurosci. , 672–684 [DOI] [PubMed] [Google Scholar]
- 213.Mullins C., Bonifacino J. S. (2001) The molecular machinery for lysosome biogenesis. BioEssays , 333–343 [DOI] [PubMed] [Google Scholar]
- 214.Spilman P., Podlutskaya N., Hart M. J., Debnath J., Gorostiza O., Bredesen D., Richardson A., Strong R., Galvan V. (2010) Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One , e9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.García-Arencibia M., Hochfeld W. E., Toh P. P., Rubinsztein D. C. (2010) Autophagy, a guardian against neurodegeneration. Semin. Cell Dev. Biol. , 691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Maloney J. A., Bainbridge T., Gustafson A., Zhang S., Kyauk R., Steiner P., van der Brug M., Liu Y., Ernst J. A., Watts R. J., Atwal J. K. (2014) Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J. Biol. Chem. , 30990–31000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Polito V. A., Li H., Martini-Stoica H., Wang B., Yang Li., Swartzlander D. B., Palmieri M., Di Ronza A., V. M.-Y., Sardiello M., Ballabio A., Zheng H. (2014) Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. Embo. Mol. Med. , 1105–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]