Key Points
Question
What are the clinical manifestations and viral loads of symptomatic infants, children, and adolescents infected with Zika virus?
Findings
In this cohort of 351 infants, children, and adolescents with Zika virus infection in Puerto Rico, most had fever, an often pruritic maculopapular rash, facial or neck erythema, and conjunctival hyperemia; presented for evaluation at fewer than 3 days after the onset of symptoms; and were discharged without hospitalization. Median viral loads obtained from the serum specimens differed significantly according to the number of days after the onset of symptoms.
Meaning
Symptomatic infants, children, and adolescents infected with Zika virus generally have a mild, often nonspecific illness not requiring hospitalization; and, although not significantly different according to age, sex, or disposition, Zika virus viral loads decreased with an increasing number of days since the onset of illness.
Abstract
Importance
Little information is available regarding Zika virus (ZIKV) infection in children.
Objective
To describe patients younger than 18 years who were infected with ZIKV and were enrolled in the Sentinel Enhanced Dengue and Acute Febrile Illness Surveillance System (SEDSS).
Design, Setting, and Participants
Children infected with ZIKV with 7 or fewer days of fever or emancipated minors aged 14 to 17 years with a generalized maculopapular rash, arthritis or arthralgia, or nonpurulent conjunctivitis were eligible for enrollment on or before December 31, 2016, in Puerto Rico. Patients were evaluated using ZIKV polymerase chain reaction testing at 7 or fewer days after the onset of symptoms. Available ZIKV polymerase chain reaction–positive specimens were evaluated to determine viral loads.
Exposures
Confirmed polymerase chain reaction–positive ZIKV infection.
Main Outcomes and Measures
Clinical characteristics and viral loads of symptomatic children with confirmed ZIKV infection.
Results
Of 7191 children enrolled in SEDSS on or before December 31, 2016, only those with confirmed ZIKV infection (351 participants) were included in this study. Participants who had confirmed ZIKV infection included 25 infants (7.1%), 69 children (19.7%) aged 1 to 4 years, 95 (27.1%) aged 5 to 9 years, and 162 (46.1%) aged 10 to 17 years. Among these, 260 patients (74.1%) presented for evaluation of ZIKV infection at fewer than 3 days after the onset of symptoms, 340 (96.9%) were discharged to home after evaluation, and 349 (99.4%) had fever, 280 (79.8%) had a rash, 243 (69.2%) had facial or neck erythema, 234 (66.7%) had fatigue, 223 (63.5%) had headache, 212 (60.4%) had chills, 206 (58.7%) had pruritus, and 204 (58.1%) had conjunctival hyperemia. Of 480 specimens collected (317 serum and 163 urine specimens) from 349 children, the median number of days after the onset of symptoms was lower for children who had serum specimens (1 day [interquartile range (IQR), 1-2 days]) than for children who had urine specimens (2 [1-3] days) (P < .001). Of 131 children who had both serum and urine specimens collected on the same day, the median viral load was higher in serum than in urine (median [IQR], 23 098 [8784-88 242] copies/mL for serum vs 9966 [2815-52 774] copies/mL for urine; P = .02). When a single serum sample from each of 317 patients was analyzed, there were no statistically significant differences in median viral loads according to age, sex, or disposition. However, the median serum viral load varied significantly according to the number of days after the onset of symptoms (0 days, 106 778 [IQR, 9772-1 571 718] copies/mL; 1 day, 46 299 [10 663-255 030] copies/mL; 2 days, 20 678 [8763-42 458] copies/mL; and ≥3 days, 15 901 [5135-49 248] copies/mL; P = .001).
Conclusions and Relevance
This study represents the largest study to date of ZIKV infection in the pediatric population. Most children infected with ZIKV had fever, rash, and conjunctival hyperemia. The children usually presented for evaluation at fewer than 3 days after the onset of symptoms. Viral loads for ZIKV were higher in serum vs urine specimens. Median viral loads in serum specimens differed significantly according to the number of days after the onset of symptoms.
This cohort study uses data from a national surveillance system to examine the viral load and clinical manifestations associated with Zika virus in infants, children, and adolescents in Puerto Rico.
Introduction
Zika virus (ZIKV) is a single-stranded RNA flavivirus (genus Flavivirus, family Flaviviridae) that is closely related to the dengue virus (DENV) and other flaviviruses (eg, West Nile virus, Japanese encephalitis, and yellow fever).1 Infections of ZIKV can be transmitted to humans by the bite of infected Aedes mosquitoes,2,3 from mother to child,4,5,6 and through sexual transmission,7,8 laboratory exposure,9 and transplantation or transfusion of blood or blood products.10 Zika virus has been detected in breast milk,6,11,12 but transmission of ZIKV through breastfeeding has not been confirmed. First discovered in 1947,13 ZIKV has been reported in more than 70 countries worldwide.14 From November 2015, through December 2016, more than 38 000 cases of ZIKV infection were reported in Puerto Rico.15 Infection with ZIKV is either asymptomatic or mildly symptomatic, and infection resulting in neurologic illness (eg, Guillain-Barré syndrome), hospitalization, or death is unusual.15 Infection during pregnancy may lead to severe adverse fetal and infant outcomes, including congenital Zika syndrome.16 Limited information is available regarding the clinical manifestations of ZIKV infection in the pediatric population or regarding concentrations of ZIKV in bodily fluids in any population. Active surveillance for acute febrile illnesses, including ZIKV infection, is ongoing through the Sentinel Enhanced Dengue and Acute Febrile Illness Surveillance System (SEDSS) in Ponce, Puerto Rico. Our objective was to describe the clinical manifestations and viral loads of pediatric patients who had symptomatic ZIKV infection and who were enrolled in SEDSS on or before December 31, 2016.
Methods
Since 2012, active enrollment in SEDSS has been conducted in Ponce, Puerto Rico, at the San Lucas Episcopal Hospital (SLEH) and, more recently (since April 2016), at Centro de Emergencia y Medicina Integrada, an affiliated clinic, in collaboration with the Ponce Health Sciences University. As a 425-bed, tertiary care teaching hospital and 1 of 4 hospitals serving almost 500 000 residents of Ponce and 11 neighboring municipalities, SLEH has approximately 50 000 emergency department visits and 11 000 hospital admissions each year. Patients presenting to the emergency department, to Centro de Emergencia y Medicina Integrada, or directly admitted to an inpatient ward at SLEH, were eligible for enrollment if they were febrile (oral temperature, ≥38.0°C; axillary temperature, ≥38.5°C) or had a fever for 7 or fewer days. In June 2016, the inclusion criteria were expanded to capture a higher number of suspected cases of ZIKV. Adult, and emancipated minors aged 14 to 20 years presenting with the acute onset of a generalized maculopapular rash, arthritis or arthralgia, or nonpurulent conjunctivitis became eligible for enrollment into SEDSS.
The institutional review boards at the Centers for Disease Control and Prevention (CDC) and the Ponce Health Sciences University approved the study protocol. Written informed consent was obtained from eligible individuals older than 20 years, emancipated minors aged 14 to 20 years, and the parent or guardian of children aged 7 years or younger. Written informed assent was obtained from nonemancipated minors aged 14 to 20 years, with written informed consent obtained from the parent or guardian. Verbal informed assent was obtained from children aged 7 to 13 years, with written informed consent obtained from the parent or guardian.
Symptoms of the current acute febrile illness, various exposures, and a history of chronic diseases were obtained from each participant at the time of enrollment. An independent physician examined the participants, and their vital signs were recorded. Signs and symptoms of the current illness were noted, and the clinical diagnosis was recorded. Participants discharged to home from the emergency department were asked to return 7 to 10 days after the onset of illness. Participants who were hospitalized had the medical record of their hospitalization summarized.
Following standard procedures, participants provided 2 samples of blood at enrollment: a 7-mL sample collected in a red, tiger-top serum separator tube and a 5-mL sample collected in a lavender-top tube. For participants younger than 4 years, the amount of blood drawn was based on weight, with the maximum amount of blood drawn not more than 2.5 mL/kg. Blood samples were processed on site where patients were evaluated (SLEH or Centro de Emergencia y Medicina Integrada). Nasopharyngeal and oropharyngeal secretions were collected separately with sterile, polyester specimen-collection swabs. These specimens were placed in a vial containing viral transport medium. A 5-mL sample of urine was also collected. Inoculated vials and blood, serum, and urine specimens were maintained at 4°C until they were transported to the Dengue Branch Laboratory of the CDC in San Juan, Puerto Rico.
Molecular diagnostic testing was performed on specimens collected 6 or fewer days after the onset of illness for the detection of ZIKV, DENV, chikungunya virus (CHIKV), adenovirus, metapneumovirus, influenza A virus, influenza B virus, respiratory syncytial virus, and parainfluenza viruses 1 and 3. Viral RNA was extracted from 200 μL of serum, urine, whole blood–EDTA, or nasopharyngeal or oropharyngeal specimens using an automated platform (MagNA Pure 96; Roche). The DENV, CHIKV, and ZIKV were detected with a reverse transcriptase–polymerase chain reaction (RT-PCR) assay (Trioplex Real-Time RT-PCR; CDC) approved by the US Food and Drug Administration under emergency use authorization, and respiratory viruses were detected using virus panel assays (Influenza and Non-influenza Respiratory Virus Panel assays; CDC). Viral loads of ZIKV were quantified using an RNA standard curve generated from the RT-PCR assay’s ZIKV target amplicons and were expressed as copies per milliliter. At the follow-up visit or at discharge from the hospital, blood and urine specimens were collected from the patient. Samples of serum collected 4 or more days after the onset of illness were tested for the presence of anti–DENV IgM, anti–CHIKV IgM, and anti–ZIKV IgM using enzyme-linked immunosorbent assays (ELISAs). The following assays were used to detect anti-DENV and anti-CHIKV IgM: DENV Detect IgM Capture MAC-ELISA (Inbios International Inc) and CHIKV IgM MAC-ELISA (CDC), respectively. Anti-ZIKV IgM was detected with the ZIKV IgM Capture MAC-ELISA (CDC) approved by the US Food and Drug Administration under emergency use authorization.
The study population for this analysis comprised individuals younger than 18 years who were enrolled in SEDSS on or before December 31, 2016, with confirmed ZIKV infection. A positive result for ZIKV on the CDC Trioplex Real-Time RT-PCR assay confirmed ZIKV infections. For patients enrolled more than once in SEDSS, only data from the initial enrollment in SEDSS were analyzed.
Descriptive analysis included calculation of frequencies of the demographic characteristics, the signs and symptoms, and the disposition for all participants by age group. Median and range results were calculated for continuous variables. The Cochran-Armitage test (for trend in proportions) and the Jonckheere-Terpstra test (for ordered alternatives in the medians) were used to assess trends. The χ2 test was used to measure association. For paired samples with viral load estimation, the Mann-Whitney–Wilcoxon rank sum test was used to compare medians of viral load by type of specimen. To identify clusters of signs and symptoms for the participants, the unsupervised grouping method of hierarchical cluster analysis was used. Clusters of signs and symptoms were determined for the patient population overall and by each age group using signs and symptoms reported by more than 50% of the participants. To determine the optimal number of clusters, the NbClust function incorporating Duda and Beale indices with a significance of .05 from the NbClust R-package was used.17
Similarities among signs and symptoms were measured using the sum of the absolute difference (Manhattan distance).18,19 Manhattan distance as well as other distances used in hierarchical clustering analysis do not have units. Manhattan distance is a special case of the Minkowski distance at m = 1. It is very sensitive to outliers, and it is used in clustering algorithms specially for the binary nature of vectors (absence or presence of symptoms). Signs and symptoms were grouped into the specified number of clusters by minimizing the total within-cluster variance (the Ward agglomeration method).20 Symbols at the termination of each dendrogram branch identify signs and symptoms that belong to the same cluster. Statistical analysis was performed using R, version 3.1 (R Foundation for Statistical Computing) and R-Studio Integrated Development Environment for R (R-Studio, Inc).
Results
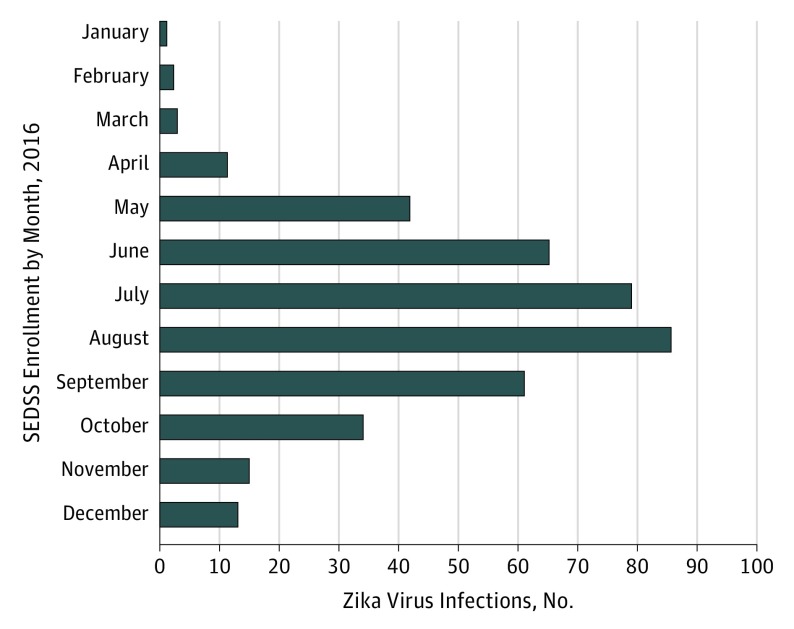
As of December 31, 2016, 7191 individuals younger than 18 years met the eligibility criteria and were enrolled in SEDSS. Of these 7191 pediatric patients, 351 had PCR-confirmed ZIKV infection. Two patients with confirmed infections of ZIKV were enrolled twice in SEDSS; only data from the first enrollment were analyzed. Figure 1 shows the distribution of all 351 pediatric patients enrolled into SEDSS according to the month of enrollment.
Figure 1. Symptomatic Zika Virus Infections in 351 Children Enrolled Into the Sentinel Enhanced Dengue and Acute Febrile Illness Surveillance System (SEDSS).
Characteristics of the 351 infants, children, and adolescents in the study population are shown in Table 1. The distribution by age was 25 infants (7.1%), 69 children (19.7%) aged 1 to 4 years, 95 (27.1%) aged 5 to 9 years, and 162 (46.1%) aged 10 to 17 years. Of 351 participants enrolled, 190 (54.1%) were boys and 161 (45.9%) were girls; this proportion did not vary significantly with age. Most (260 patients [74.1%]) presented at fewer than 3 days after the onset of symptoms; the median time was 2 days (range, 0-7 days), which increased as the age of the patient increased (median [range], 1 [0-5] days for patients aged <1 year vs 2 [0-6] days for patients aged 10-17 years; P = .004). Most (340 patients [96.9%]) were discharged to home from the emergency department, and only 10 children (2.8%) were hospitalized or transferred to another hospital. One 8-year-old girl left the facility against medical advice. There was a significant trend of decreased proportions of children being hospitalized as the age increased (3 of 25 patients [12.0%] for those aged <1 year vs 1 of 162 [0.6%] for those aged 10-17 years; P = .003). The median age of the children who were hospitalized was 3.5 years (range, 1 month to 11 years). The 10 children hospitalized were admitted because of vomiting, diarrhea, dehydration, and/or anorexia (n = 6); respiratory distress due to pneumonia or bronchiolitis (n = 3); and clinical sepsis (n = 1). No deaths were reported.
Table 1. Characteristics of 351 Infants, Children, and Adolescents in Puerto Rico With Symptomatic ZIKV Infection.
| Characteristic | Age, y | P Value for Trenda | ||||
|---|---|---|---|---|---|---|
| Overall (N = 351) | <1 (n = 25) | 1-4 (n = 69) | 5-9 (n = 95) | 10-17 (n = 162) | ||
| Sex, No. (%) | ||||||
| Male | 190 (54.1) | 17 (68.0) | 44 (63.8) | 45 (47.4) | 84 (51.9) | .09b |
| Female | 161 (45.9) | 8 (32.0) | 25 (36.2) | 50 (52.6) | 78 (48.1) | |
| Days after the onset of symptoms, median (range), No. | 2 (0-7) | 1 (0-5) | 1 (0-6) | 1 (0-7) | 2 (0-6) | .004 |
| Days after the onset of symptoms, No. (%) | ||||||
| 0-2 | 260 (74.1) | 19 (76.0) | 55 (79.7) | 75 (78.9) | 111 (68.5) | .42b |
| 3-5 | 83 (23.6) | 6 (24.0) | 12 (17.4) | 18 (18.9) | 47 (29.0) | |
| 6-7 | 8 (2.3) | 0 | 2 (2.9) | 2 (2.1) | 4 (2.5) | |
| Disposition, No. (%)c | ||||||
| Hospitalized | 10 (2.8) | 3 (12.0) | 5 (7.2) | 1 (1.1) | 1 (0.6) | .003b |
| Discharged to home | 340 (96.9) | 22 (88.0). | 64 (92.8) | 93 (97.9) | 161 (99.4) | |
| Signs and symptoms | ||||||
| Fever | 349 (99.4) | 25 (100) | 69 (100) | 95 (100) | 160 (98.8) | .87 |
| Maculopapular rash | 280 (79.8) | 18 (72.0) | 50 (72.5) | 77 (81.1) | 135 (83.3) | .26 |
| Facial or neck erythema | 243 (69.2) | 19 (76.0) | 47 (68.1) | 64 (67.4) | 113 (69.8) | .60 |
| Fatigue | 234 (66.7) | 17 (68.0) | 45 (65.2) | 54 (56.8) | 118 (72.8) | .11 |
| Headached | 223 (63.5) | 1 (4.0) | 22 (31.9) | 62 (65.3) | 138 (85.2) | .03 |
| Chillsd | 212 (60.4) | 10 (40.0) | 35 (50.7) | 54 (56.8) | 113 (69.8) | .97 |
| Pruritusd | 206 (58.7) | 4 (16.0) | 27 (39.1) | 54 (56.8) | 111 (68.5) | .81 |
| Conjunctival hyperemia | 204 (58.1) | 9 (36.0) | 38 (55.1) | 53 (55.8) | 104 (64.2) | .50 |
| General malaised | 176 (50.1) | 13 (52.0) | 32 (46.4) | 39 (41.1) | 92 (56.8) | .15 |
| Anorexia | 163 (46.4) | 11 (44.0) | 41 (59.4) | 39 (41.1) | 72 (44.4) | .07 |
| Petechiae | 155 (44.2) | 9 (36.0) | 29 (42.0) | 43 (45.3) | 74 (45.7) | .43 |
| Pharyngitisd | 133 (37.9) | 2 (8.0) | 17 (24.6) | 28 (29.5) | 86 (53.1) | .35 |
| Eye paind | 132 (37.6) | 0 | 14 (20.3) | 24 (25.3) | 94 (58.0) | .17 |
| Arthralgiad | 129 (36.8) | 1 (4.0) | 9 (13.0) | 34 (35.8) | 85 (52.5) | .21 |
| Myalgiad | 129 (36.8) | 0 | 14 (20.3) | 26 (27.4) | 89 (54.9) | .41 |
| Rhinorrhea | 129 (36.8) | 9 (36.0) | 36 (52.2) | 34 (35.8) | 50 (30.9) | .15 |
| Cough | 128 (36.5) | 13 (52.0) | 30 (43.5) | 39 (41.1) | 46 (28.4) | .66 |
| Bone paind | 119 (33.9) | 0 | 9 (13.0) | 27 (28.4) | 83 (51.2) | .81 |
| Irritability | 79 (22.5) | 14 (56.0) | 15 (21.7) | 16 (16.8) | 34 (21.0) | .005 |
| Chronic conditions | ||||||
| No | 271 (77.2) | 25 (100) | 58 (84.1) | 73 (76.8) | 115 (71.0) | <.001b |
| Yese | 80 (22.8) | 0 | 11 (15.9) | 22 (23.2) | 47 (29.0) | |
| Asthma | 69 (19.7) | 0 | 10 (14.5) | 19 (20.0) | 40 (24.7) | .12 |
Abbreviation: ZIKV, Zika virus.
Cochran-Armitage test for trend was used for proportions, and Jonckheere-Terpstra test for ordered alternatives in the medians was used to assess trends, unless another test was specified.
Calculated using the χ2 test to measure association.
One child left against medical advice.
Infants were excluded from the calculation of the P value for trend.
Other chronic diseases reported were diabetes, heart disease, hypertension, kidney disease, thyroid disease, and hypercholesterolemia.
Almost all patients (349 [99.4%]) had fever. Other signs and symptoms were reported: 280 patients (79.8%) had a rash, 243 (69.2%) had facial or neck erythema, 234 (66.7%) had fatigue, 223 (63.5%) had headache, 212 (60.4%) had chills, 206 (58.7%) had pruritus, and 204 (58.1%) had conjunctival hyperemia. The proportion of patients who had headache increased with age (22 of 69 patients [31.9%] for those aged 1-4 years vs 138 of 162 [85.2%] for those aged 10-17 years; P = .03), and the proportion of patients with irritability decreased with age (14 of 25 patients [56.0%] for those aged <1 year vs 34 of 162 [21.0%] for those aged 10-17 years; P = .005). Otherwise, there were no statistically significant differences regarding signs and symptoms in the proportion of children in each age group. The patient who left against medical advice had experienced 1 day of symptoms, which included fever, a pruritic rash, and cough. Most (271 patients, [77.2%]) had no underlying chronic conditions. Of the 80 patients with 1 or more chronic condition, 69 (86.2%) had asthma. The proportion of patients with a chronic disease increased with age (0 of 69 patients [31.9%] for those aged 1-4 years vs 47 of 162 [29.0%] for those aged 10-17 years; P < .001).
Four adolescent girls were pregnant at the time of enrollment into SEDSS, and all of the girls received pregnancy-related care at SLEH. A 17-year-old girl who was 4 weeks pregnant at the time of enrollment underwent ultrasonography at 12 weeks’ gestation; the results revealed fetal demise (intrauterine pregnancy of 9 weeks, 3 days). The other 3 adolescent girls (aged 14, 17, and 17 years) delivered full-term (38-39 weeks’ gestation), phenotypically normal newborns. All newborns were reported at birth to be without structural abnormalities; 2 newborns underwent postnatal cranial imaging and the results were without demonstrated pathologic features, and 1 newborn had been evaluated by in utero ultrasonography with interpreted results within normal limits.
Thirteen children (3.7%) had evidence of a concomitant infection. The detected coinfections were parainfluenza 3 (n = 4), adenovirus (n = 3), influenza B virus (n = 3), influenza A virus (n = 2), and human metapneumovirus (n = 1).
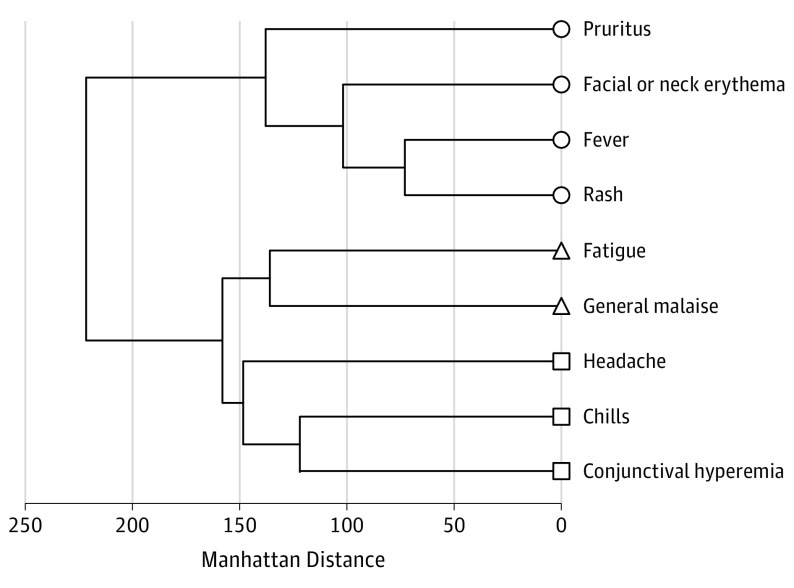
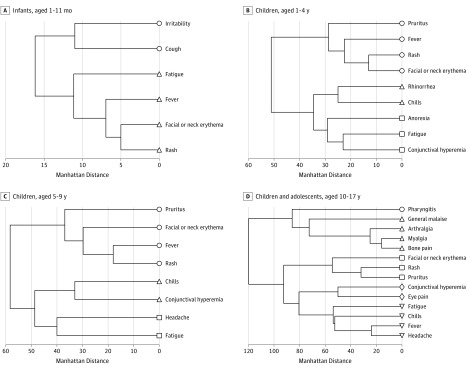
Clusters of signs and symptoms more likely to have occurred together were formed using hierarchical clustering, with similarities measured by distance as described above. The resulting cluster dendrograms are shown in Figure 2 and Figure 3, with distance shown on the x-axis. In the cohort of 351 infants, children, and adolescents (Figure 2), there were 3 clusters: 156 patients (44.4%) who had pruritus, facial or neck erythema, and fever with rash; 137 (39.0%) who had fatigue with general malaise; and 109 (31.0%) who had headache and chills with conjunctival hyperemia. Among 25 infants (Figure 3A), there were 2 clusters: 8 infants (32.0%) who had irritability with cough and 11 infants (44.0%) who had fatigue, fever, and facial or neck erythema with rash. Of 69 children aged 1 to 4 years (Figure 3B), there were 3 clusters: 29 children (42.0%) who had pruritus, fever, and rash with facial or neck erythema; 23 children (33.3%) who had rhinorrhea with chills; and 20 children (29.0%) who had anorexia and fatigue with conjunctival hyperemia. Among the 95 children aged 5 to 9 years (Figure 3C), there were 3 clusters: 42 children (44.2%) who had pruritus, facial or neck erythema, and fever with rash; 37 children (39.0%) who had chills with conjunctival hyperemia; and 38 children (40.0%) who had headache with fatigue. Of 162 children aged 10 to 17 years (Figure 3D), there were 5 clusters: 86 children (53.1%) who had pharyngitis; 51 (31.5%) who had general malaise, arthralgia, and myalgia with bone pain; 81 (50.0%) who had facial or neck erythema and rash with pruritus; 74 (45.7%) who had conjunctival hyperemia with eye pain; and 81 (50.0%) who had fatigue, chills, and fever with headache.
Figure 2. Analysis of Signs and Symptoms of 351 Children Infected With Zika Virus Overall.
Figure 3. Analysis of Signs and Symptoms of 351 Children Infected With Zika Virus by Age.
Of the 351 children in the study population, 349 had 1 or more specimens available for viral quantification (Table 2). Among the 480 specimens (317 serum and 163 urine specimens), the median viral load was 19 603 copies/mL (interquartile range, 5393-95 598 copies/mL). However, the median number of days after onset by specimen collection was lower (1 day [interquartile range, 1-2 days]) for children who submitted a serum specimen than for children who submitted a urine specimen (2 [1-3] days) (P < .001). When we analyzed only serum and urine specimens collected on the same day for 131 patients, the median viral load was higher in serum than in urine (median [interquartile range], 23 098 copies/mL [8784-88 242 copies/mL] for serum vs 9966 [2815-52 774] copies/mL for urine; P = .02). When we analyzed a single serum sample from each of the 317 patients, there were no statistically significant differences in the median viral loads according to age, sex, or disposition after evaluation. However, the median viral loads in serum varied significantly according to the number of days after the onset of illness (0 days, 106 778 copies/mL [interquartile range, 9772-1 571 718 copies/mL]; 1 day, 46 299 copies/mL [10 663-255 030]; 2 days, 20 678 copies/mL [8763-42 458]; and 3 or more days, 15 901 copies/mL [5135-49 248]; P = .001).
Table 2. Viral Load Results for 351 Children With PCR-Confirmed ZIKV Infection.
| Type of Specimen | Patients (Specimens), No. | Viral Load, Copies/mL | P Value for Comparison of Mediansa | ||||
|---|---|---|---|---|---|---|---|
| Minimum | First Quartile | Median | Third Quartile | Maximum | |||
| All | 349 (480)b | 608 | 5393 | 19 603 | 95 598 | 487 096 495 | |
| Serum and urine specimens collected on the same day | 131 (262) | ||||||
| Serum | 724 | 8784 | 23 098 | 88 242 | 487 096 495 | .02c | |
| Urine | 615 | 2815 | 9966 | 52 774 | 258 838 876 | ||
| Serum specimens, 1 per patient | 317 (317) | 608 | 8069 | 26 025 | 117 617 | 487 096 495 | |
| Days after onset of symptoms | |||||||
| 0 | 33 | 1664 | 9772 | 106 778 | 1 571 718 | 487 096 495 | .001 |
| 1 | 131 | 608 | 10 663 | 46 299 | 255 030 | 77 636 599 | |
| 2 | 77 | 772 | 8763 | 20 678 | 42 458 | 11 410 404 | |
| ≥3 | 76 | 724 | 5135 | 15 901 | 49 248 | 10 195 605 | |
| Age, y | |||||||
| <1 | 21 | 811 | 20 722 | 44 372 | 169 647 | 183 895 035 | .46 |
| 1-4 | 60 | 912 | 8652 | 26 781 | 110 807 | 10 195 605 | |
| 5-9 | 88 | 608 | 7318 | 22 828 | 90 799 | 487 096 495 | |
| 10-17 | 148 | 724 | 7727 | 27 683 | 121 131 | 92 278 283 | |
| Sex | |||||||
| Male | 171 | 608 | 7800 | 25 436 | 123 057 | 183 895 035 | .96 |
| Female | 146 | 724 | 9113 | 27 493 | 111 005 | 487 096 495 | |
| Dispositiond | |||||||
| Hospitalized | 6 | 10 282 | 79 515 | 99 771 | 320 963 | 10 195 605 | .09 |
| Discharged to home | 310 | 608 | 7853 | 25 647 | 116 365 | 487 096 495 | |
Abbreviations: PCR, polymerase chain reaction; ZIKV, Zika virus.
Jonckheere-Terpstra test for ordered differences in the median viral load (unless otherwise noted).
Of 480 specimens, 317 were serum and 163 were urine.
Mann-Whitney–Wilcoxon rank sum test.
One patient left against medical advice.
Discussion
In this cohort of 351 symptomatic individuals younger than 18 years with confirmed ZIKV infection, most presented for care fewer than 3 days after the onset of symptoms. Most were discharged to home from the emergency department; less than 3% were hospitalized or transferred to another hospital. Aside from fever, other signs and symptoms experienced by most patients were fatigue, headache, chills, pruritus, maculopapular rash, facial or neck erythema, and conjunctival hyperemia. The proportion of patients who had headache increased with the age of the child, whereas the proportion of patients with irritability decreased with the age of the child. Clustering of signs and symptoms varied by age; among infants, irritability with cough was prominent; among older children and adolescents, clusters of signs and symptoms with headache. Adolescents often reported only pharyngitis or clusters of signs or symptoms involving myalgia, arthralgia, bone pain, or eye pain. The median viral load was higher in serum than in urine specimens. In serum samples, there were no statistically significant differences in the median viral loads according to age, sex, or disposition after evaluation. However, the median viral load varied significantly according to days after the onset of illness, with a decrease in the viral load as the number of days after onset increased.
Our results are consistent with previous reports suggesting that the clinical manifestations of symptomatic ZIKV infection are generally mild and that severe illness is unusual. Between 1964 (when the initial case of natural ZIKV infection in humans was reported)9 and 2006, only 16 cases of human ZIKV infections were reported.9,21,22,23,24,25 Outbreaks occurred in different countries, including Yap State, Federated States of Micronesia, in 2007,26 and Brazil in 2015.27 Of 49 confirmed cases of ZIKV infection in a Micronesian population with a median age of 36 years, most of the 31 individuals who provided information reported symptoms of macular or papular rash (90%), fever (65%), arthritis or arthralgia (65%), and nonpurulent conjunctivitis (55%).26 Of 119 laboratory-confirmed ZIKV infections among a primarily adult population in Brazil, individuals presented with a macular or papular rash (97%), pruritus (79%), prostration (73%), headache (66%), arthralgia (63%), myalgia (61%), nonpurulent conjunctivitis (56%), and low back pain (51%).27 In a case series of 14 children aged 16 years or younger from Singapore,28 most children reported fever and rash, with myalgia, arthralgia, headache, conjunctivitis, and upper respiratory tract infections reported less frequently. Among 31 children aged 1 to 17 years with symptomatic ZIKV infection in Florida, all of the children reported a rash and 81% of them reported fever; only 29% had conjunctivitis, and 22% had arthralgia.29 Finally, of 158 children younger than 18 years with confirmed or probable ZIKV disease reported to the CDC from US states,30 fever and a rash were reported by most children, with conjunctivitis and arthralgia reported only in a minority of children.
Because few data have been published to date regarding the viral loads of patients infected with ZIKV, the viral load results for this pediatric cohort contribute significantly, to our knowledge, not only because of the overall characteristics of the viral load of patients infected with ZIKV but also because of factors associated with higher viral loads, including the type of specimen (serum) and number of days (fewer) after the onset of symptoms. In a report from the outbreak of ZIKV in Yap State, Micronesia, in 2007, viral load results were reported for 17 patients who tested positive for ZIKV.31 Among those patients, the estimated viral load values ranged from 930 to 625 280 copies/mL.
Our study of more than 300 infants, children, and adolescents with symptomatic ZIKV infection represents the largest study to date of ZIKV infection in the pediatric population. All cases had PCR-confirmed ZIKV infection identified through an ongoing acute febrile illness surveillance system (SEDSS) in Puerto Rico. Only 4% of pediatric patients infected with ZIKV in this study had a concomitant infection with a respiratory virus.
Limitations
A potential limitation of this study is that postnatal infection, as opposed to congenitally acquired infection, is presumed. Mothers of children were not necessarily tested for ZIKV infection during the index pregnancy, and data regarding maternal ZIKV infection is not collected as part of SEDSS. But, the ZIKV epidemic in Puerto Rico only began in late 2015, and thus only infants would be affected by this possible limitation. Only 25 (7.1%) of the patients in this cohort were infants; all but 1 infant (aged 1 month) were 5 months or older at the time of enrollment into SEDSS. In our study population, the proportion of children with different signs and symptoms did not vary by age with the exception of headache, which increased as the age of the patient increased, and irritability, which decreased as the age of the patient increased. Although infants were excluded from the more detailed evaluations because they could not articulate localization of pain or discomfort, it is still possible that young children could not clearly communicate the localization of pain or discomfort. Thus, the statistically significant trend observed for headache according to age may be an artifact. Similarly, irritability was observed to decrease as the age of the child increased; older children may be able to more clearly communicate the location of their symptoms, whereas younger children who appear uncomfortable may be characterized as irritable. Since SEDSS is a surveillance system of febrile illnesses, the eligibility criteria resulted in the enrollment of febrile cases of ZIKV primarily, and the clinical presentation of children included in this analysis may not be representative of all symptomatic Zika cases in pediatric patients.
Conclusions
Most children with symptomatic infection of ZIKV had relatively mild illness not requiring hospitalization. Although several signs and symptoms occurred, 4 signs experienced by most children included fever, facial or neck erythema, conjunctival hyperemia, and a maculopapular rash that was often pruritic. The children presented for care soon after the onset of illness (<3 days), and almost all patients were discharged to home from the emergency department. Zika virus viral loads were higher in serum specimens than in urine. Age, sex, and disposition after evaluation did not affect viral loads, but ZIKV viral loads decreased significantly with increasing number of days after the onset of symptoms.
References
- 1.Simmonds P, Becher P, Collet MS, et al. . Flaviviridae In: King AMQ, Adams MJ, Cartens EB, Lefkowitz EJ, eds. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Academic Press Elsevier; 2011:1008-1020. [Google Scholar]
- 2.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18(3):411-415. [DOI] [PubMed] [Google Scholar]
- 3.Grard G, Caron M, Mombo IM, et al. . Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8(2):e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martines RB, Bhatnagar J, Keating MK, et al. . Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(6):159-160. [DOI] [PubMed] [Google Scholar]
- 5.Martines RB, Bhatnagar J, de Oliveira Ramos AM, et al. . Pathology of congenital Zika syndrome in Brazil. Lancet. 2016;388(10047):898-904. [DOI] [PubMed] [Google Scholar]
- 6.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19(13):20751. [PubMed] [Google Scholar]
- 7.Foy BD, Kobylinski KC, Chilson Foy JL, et al. . Probable non-vector–borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011;17(5):880-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hills SL, Russell K, Hennessey M, et al. . Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(8):215-216. [DOI] [PubMed] [Google Scholar]
- 9.Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg. 1964;58:335-338. [PubMed] [Google Scholar]
- 10.Motta IJ, Spencer BR, Cordeiro da Silva SG, et al. . Evidence for transmission of Zika virus by platelet transfusion. N Engl J Med. 2016;375(11):1101-1103. [DOI] [PubMed] [Google Scholar]
- 11.Dupont-Rouzeyrol M, Biron A, O’Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet. 2016;387(10023):1051. [DOI] [PubMed] [Google Scholar]
- 12.Sotelo JR, Sotelo AB, Sotelo FJB, et al. . Persistence of Zika virus in breast milk after infection in late stage of pregnancy. Emerg Infect Dis. 2017;23(5):856-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dick GW, Kitchen SF, Haddow AJ. Zika virus, I: isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46(5):509-520. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Zika situation report, 2 February 2017. http://www.who.int/emergencies/zika-virus/situation-report/en/. Accessed January 31, 2018.
- 15.Puerto Rico Department of Health Weekly arboviral infection report [in Spanish]. Published January 12, 2017. http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Informes%20Arbovirales/Reporte%20ArboV%20semana%2052-53%202016.pdf. Accessed January 31, 2018.
- 16.Moore CA, Staples JE, Dobyns WB, et al. . Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatr. 2017;171(3):288-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw. 2014;61(6):1-36. [Google Scholar]
- 18.Shirkhorshidi AS, Aghabozorgi S, Wah TY. A comparison study of similarity and dissimilarity measures in clustering continuous data. PLoS One. 2015;10(12):e0144059. doi: 10.1371/journal.pone.0144059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss T, von Maltitz MJ. Generalizing Ward’s method for use with Manhattan distances. PLoS One. 2017;12(1):e0168288. doi: 10.1371/journal.pone.0168288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everitt BS, Landau S, Morven L, Stahl D. Cluster Analysis. 5th ed Chichester, United Kingdom: John Wiley & Sons; 2011. [Google Scholar]
- 21.Filipe AR, Martins CM, Rocha H. Laboratory infection with Zika virus after vaccination against yellow fever. Arch Gesamte Virusforsch. 1973;43(4):315-319. [DOI] [PubMed] [Google Scholar]
- 22.Berge T, ed. International Catalog of Arboviruses. 2nd ed Washington, DC: National Institute of Allergy and Infectious Diseases; Atlanta, GA: Centers for Disease Control; 1975. [Google Scholar]
- 23.Moore DL, Causey OR, Carey DE, et al. . Arthropod-borne viral infections of man in Nigeria, 1964-1970. Ann Trop Med Parasitol. 1975;69(1):49-64. [DOI] [PubMed] [Google Scholar]
- 24.Fagbami AH. Zika virus infections in Nigeria. J Hyg (Lond). 1979;83(2):213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg. 1981;75(3):389-393. [DOI] [PubMed] [Google Scholar]
- 26.Duffy MR, Chen TH, Hancock WT, et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360(24):2536-2543. [DOI] [PubMed] [Google Scholar]
- 27.Brasil P, Calvet GA, Siqueira AM, et al. . Zika virus outbreak in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2016;10(4):e0004636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Chong CY, Tan NW, Yung CF, Tee NW, Thoon KC. Characteristics of Zika virus disease in children: clinical, hematological, and virological findings from an outbreak in Singapore. Clin Infect Dis. 2017;64(10):1445-1448. [DOI] [PubMed] [Google Scholar]
- 29.Griffin I, Zhang G, Fernandez D, et al. . Epidemiology of pediatric Zika virus infections. Pediatrics. 2017;140(6):e20172044. doi: 10.1542/peds.2017-2044 [DOI] [PubMed] [Google Scholar]
- 30.Goodman AB, Dziuban EJ, Powell K, et al. . Characteristics of children aged <18 years with Zika virus disease acquired postnatally–U.S. states, January 2015–July 2016. MMWR Morb Mortal Wkly Rep. 2016;65(39):1082-1085. [DOI] [PubMed] [Google Scholar]
- 31.Lanciotti RS, Kosoy OL, Laven JJ, et al. . Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14(8):1232-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]