Abstract
Importance
The effect of neonatal and infant feeding practices on childhood obesity is unclear. The gut microbiome is strongly influenced by feeding practices and has been linked to obesity.
Objective
To characterize the association between breastfeeding, microbiota, and risk of overweight during infancy, accounting for the type and timing of supplementary feeding.
Design, Setting, and Participants
In this study of a subset of 1087 infants from the prospective CHILD pregnancy cohort, mothers were recruited between January 1, 2009, and December 31, 2012. Statistical analysis was performed from February 1 to December 20, 2017.
Main Outcomes and Measures
Feeding was reported by mothers and documented from hospital records. Fecal microbiota at 3 to 4 months (from 996 infants) and/or 12 months (from 821 infants) were characterized by 16S ribosomal RNA sequencing. Infants with a weight for length exceeding the 85th percentile were considered to be at risk for overweight.
Results
There were 1087 infants in the study (507 girls and 580 boys); at 3 months, 579 of 1077 (53.8%) were exclusively breastfed according to maternal report. Infants who were exclusively formula fed at 3 months had an increased risk of overweight in covariate-adjusted models (53 of 159 [33.3%] vs 74 of 386 [19.2%]; adjusted odds ratio, 2.04; 95% CI, 1.25-3.32). This association was attenuated (adjusted odds ratio, 1.33; 95% CI, 0.79-2.24) after further adjustment for microbiota features characteristic of formula feeding at 3 to 4 months, including higher overall richness and enrichment of Lachnospiraceae. A total of 179 of 579 infants who were exclusively breastfed (30.9%) received formula as neonates; this brief supplementation was associated with lower relative abundance of Bifidobacteriaceae and higher relative abundance of Enterobacteriaceae at 3 to 4 months but did not influence the risk of overweight. At 12 months, microbiota profiles differed significantly according to feeding practices at 6 months; among partially breastfed infants, formula supplementation was associated with a profile similar to that of nonbreastfed infants (higher diversity and enrichment of Bacteroidaceae), whereas the introduction of complementary foods without formula was associated with a profile more similar to that of exclusively breastfed infants (lower diversity and enrichment of Bifidobacteriaceae and Veillonellaceae). Microbiota profiles at 3 months were more strongly associated with risk of overweight than were microbiota profiles at 12 months.
Conclusions and Relevance
Breastfeeding may be protective against overweight, and gut microbiota may contribute to this effect. Formula feeding appears to stimulate changes in microbiota that are associated with overweight, whereas other complementary foods do not. Subtle microbiota differences emerge after brief exposure to formula in the hospital. These results identify important areas for future research and distinguish early infancy as a critical period when transient gut dysbiosis may lead to increased risk of overweight.
This cohort study characterizes the association between breastfeeding, microbiota, and risk of overweight during infancy, accounting for the type and timing of supplementary feeding.
Key Points
Question
How do infant feeding practices influence gut microbiota and risk of overweight?
Findings
Among 1087 infants from the Canadian Healthy Infant Longitudinal Development (CHILD) cohort, earlier cessation of breastfeeding and supplementation with formula (more so than complementary foods) were associated with a dose-dependent increase in risk of overweight by age 12 months; this association was partially explained by specific gut microbiota features at 3 to 4 months. Subtle but significant microbiota differences were observed after brief exposure to formula limited to the birth hospital stay, but these differences were not associated with overweight.
Meaning
Breastfeeding may contribute to protection against overweight by modifying the gut microbiota, particularly during early infancy.
Introduction
Obesity originates early in life,1 and breastfeeding appears to be protective against obesity.2 Hypothesized mechanisms for this protection include the promotion of self-regulation in breastfed infants and the lower protein content of breast milk compared with infant formula.3 Another potential mechanism involves modification of the developing gut microbiota, which contributes to nutrient acquisition, energy regulation, and fat storage.4 Microbiota shifts have been associated, albeit inconsistently,5 with obesity in adults, including lower diversity, enrichment of Ruminococcus gnavus,6 and a higher ratio of Firmicutes to Bacteroidetes.7 Microbiota transplant experiments in mice suggest that these associations are causal,8 and studies of children9,10,11,12 suggest that they originate early in life, although few studies have been conducted for infants. Breastfeeding is among the most influential factors shaping the infant gut microbiome because breast milk contains prebiotic oligosaccharides and probiotic microorganisms, including bifidobacteria.13
Despite this evidence, we do not fully understand how infant feeding practices affect the developing microbiota and influence weight gain. Studies often do not differentiate between partially breastfed infants receiving formula vs those receiving complementary foods, yet these forms of nutrition clearly provide very different substrates for microbiota. The definition of exclusive breastfeeding also varies, and few studies have accessed hospital records to confirm exclusivity in the neonatal period. To address these knowledge gaps, we characterized these specific infant feeding practices in the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort and examined their association with gut microbiota and risk of overweight in the first year of life.
Methods
Study Design
We accessed data from the CHILD birth cohort (http://childstudy.ca) of 3495 families across 4 sites in Canada.14 Women were recruited between January 1, 2009, and December 31, 2012, and remained eligible if they delivered a healthy, full-term infant. This study included 1087 infants enrolled in the general cohort at the Manitoba, Edmonton, and Vancouver sites. This subset is a representative selection of infants with fecal samples analyzed at 3 to 4 months (from 996 infants) and/or 12 months (from 821 infants), of which 730 infants had samples analyzed at both times (eFigure 1 in the Supplement). The rates of breastfeeding, overweight, and other demographics in this subset were similar to those of the general cohort (eTable 1 in the Supplement). The Human Research Ethics Boards at McMaster University, University of Manitoba, University of Alberta, University of Toronto, and University of British Columbia approved this study. Parents provided written consent at the time of enrollment.
Overweight
At 12 months of age (mean [SD] age, 12.4 [1.3] months), infants were weighed and measured by CHILD Study staff. Age- and sex-specific weight for length z (WFLz) scores were calculated according to World Health Organization standards.15 A WFLz score greater than the 97th percentile was considered overweight, and a WFLz score greater than the 85th percentile was considered at risk for overweight16; these 2 groups were combined into a composite outcome for logistic regression analyses.
Infant Feeding
Mothers completed questionnaires at 3, 6, and 12 months post partum, reporting on breastfeeding and the introduction of formula and complementary foods. At 3 months, breastfeeding status was classified as exclusive (breast milk only), partial (breast milk and formula), or none (formula only). Using hospital data, we further classified infants as exclusively breastfed after hospital discharge if they briefly received formula in the hospital but were exclusively breastfed after hospital discharge. At 6 months, feeding was defined as exclusively breastfed (breast milk only), partially breastfed with formula (breast milk and formula, with or without complementary foods), partially breastfed without formula (breast milk and complementary foods), or not breastfed (formula with or without complementary foods). The duration of breastfeeding was determined from the earliest report of cessation of breastfeeding. For microbiota analyses, breastfeeding status was determined on the date of collection of the fecal sample. In this study, breastfeeding refers to feeding the infant breast milk, whether at the breast or from a bottle.
Covariates
Mode of birth, parity, gestational diabetes, infant sex, birth weight, and hospital-administered antibiotics to the mother or neonate were documented from hospital records.17 Oral antibiotic use was reported by parents. As described previously,18 the quality of the maternal diet was estimated using the Healthy Eating Index,19 and the maternal prepregnancy body mass index was self-reported and validated against medical records. Data on maternal race/ethnicity, smoking status, educational level, and pet ownership were self-reported during pregnancy.
Fecal Microbiota Analysis
Fecal samples were collected at a home visit (3-4 months; mean [SD], 3.7 [1.0] months) and a clinic visit (12 months; mean [SD], 12.3 [1.2] months); DNA was extracted using the QIAamp DNA Stool Mini Kit (Qiagen); and the 16S ribosomal RNA gene, hypervariable region V4, was amplified and sequenced by Illumina MiSeq (eAppendix in the Supplement). Using QIIME, version 1.8.0,20 reads were assembled, demultiplexed, filtered against the Greengenes reference database, version 13.8,21 and clustered at 97% similarity. After filtering, a total of 265 095 597 reads were retained (median, 235 623 per sample [range, 13 134-833 392]), representing 939 unique operational taxonomic units. For subsequent analyses, data were rarefied to 13 000 sequences per sample and summarized at the family taxonomic level.
Statistical Analysis
Statistical analysis was performed from February 1 to December 20, 2017. Covariates were tabulated against feeding and overweight and compared by use of the χ2 test. Multivariable regression was used to investigate associations between feeding and overweight. Models were adjusted for suspected confounders selected a priori or identified in univariate analyses, grouped as maternal body mass index, other maternal factors (educational level, smoking status, ethnicity, and study site), and microbiota-related factors (cesarean delivery, dog ownership, infant sex, and antibiotics). Sensitivity analyses were conducted to adjust for birth weight, exclude never-breastfed infants, and evaluate continuous WFLz scores as an alternative outcome. Results are presented as crude odds ratios (ORs) and adjusted ORs (aORs) or differences in WFLz scores (SDs with 95% CIs). Multiple imputation (20 imputed data sets) was performed for all covariates using the R package mice.22 Microbiota alpha diversity was assessed using the abundance-based coverage estimator and Chao1 indices of species richness and the Simpson and Shannon indices of diversity. Microbiota measures were compared between feeding or weight status groups by use of nonparametric Kruskal-Wallis tests and post hoc Dunn tests with false discovery rate (FDR) correction for multiple comparisons. Microbiota community structures were compared by permutational analysis of variance (PERMANOVA) on UniFrac23 distance matrices and visualized by principal coordinate analysis. Microbiota composition and diversity (classified in quartiles) were further investigated in multivariable logistic regression models to evaluate their influence on the association between breastfeeding and risk of overweight. All analyses were performed in R, version 3.3.3 (R Development Core Team). P < .05 (2-sided) after FDR correction was considered significant.
Results
Study Population
Most mothers were white (817 of 1078 [75.8%]) and delivered vaginally (790 of 1064 [74.2%]); 408 of 1025 mothers (39.8%) were overweight or obese (eTable 1 in the Supplement). The breastfeeding initiation rate was 95.5% (1032 of 1081) (eTable 2 in the Supplement). At 3 months, 53.8% of infants (579 of 1077) were exclusively breastfed, including 37.1% (400 of 1077) who were exclusively breastfed since birth and 16.6% (179 of 1077) who briefly received formula in the hospital. The remaining infants were partially breastfed (323 of 1077 [30.0%]) or not breastfed (175 of 1077 [16.2%]). By 6 months, the rate of exclusive breastfeeding had decreased to 17.6% (183 of 1040), and partial breastfeeding had increased to 54.6% (593 of 1087), including 28.2% (307 of 1087) who received formula with or without food and 26.3% (286 of 1087) who received food but not formula. At 12 months, 42.2% of infants (459 of 1087) were still breastfeeding; the mean (SD) WFLz score was 0.29 (1.08), and 22.9% of infants (249 of 1087) were overweight or at risk for overweight.
Infant Feeding and Risk of Overweight
Breastfeeding was associated with a lower risk of overweight at 12 months, with dose responses observed according to breastfeeding exclusivity and duration (Table 1). Among infants who were exclusively breastfed at 3 months, 19.2% (74 of 386) were overweight or at risk of overweight by 12 months compared with 27.6% of infants (84 of 304) who were partially breastfed (OR, 1.61; 95% CI, 1.13-2.30) and 33.3% of infants (53 of 159) who were not breastfed (ie, exclusively formula fed) (OR, 2.11; 95% CI, 1.39-3.19). There was no increase in risk of overweight among exclusively breastfed infants who briefly received formula in the hospital (35 of 171 [20.5%] at risk; OR, 1.09; 95% CI, 0.68-1.69). These associations were largely unaffected by adjustment for maternal body mass index, education, smoking, and other potential confounders (eTable 3 in the Supplement) (partial breastfeeding: aOR, 1.63; 95% CI, 1.09-2.44; exclusive formula feeding: aOR, 2.02; 95% CI, 1.18-3.45; exclusive breastfeeding after hospital discharge: aOR, 1.13; 95% CI, 0.68-1.89) (Table 1).
Table 1. Crude and Adjusted Association of Infant Feeding Practices With Infant Weight Status at 12 Months.
| Breastfeeding Exposure | Prevalence of Overweight, No. (%) | Crude OR (95% CI) (n = 1020) | Adjusted OR (95% CI) With Multiple Imputation of Missing Data (N = 1087)a |
|---|---|---|---|
| Breastfeeding at 3 mo | |||
| None (formula only) | 53/159 (33.3) | 2.11 (1.39-3.19) | 2.02 (1.18-3.45) |
| Partial (breast milk and formula) | 84/304 (27.6) | 1.61 (1.13-2.30) | 1.63 (1.09-2.44) |
| Exclusive after hospital discharge | 35/171 (20.5) | 1.09 (0.68-1.69) | 1.13 (0.68-1.89) |
| Exclusive (breast milk only) | 74/386 (19.2) | 1 [Reference] | 1 [Reference] |
| Breastfeeding at 6 mo | (n = 1001) | ||
| None (formula with or without food) | 77/249 (30.9) | 2.11 (1.33-3.42) | 1.59 (0.92-2.74) |
| Partial with formula (breast and formula with or without food) | 81/296 (27.4) | 1.77 (1.13-2.85) | 1.43 (0.87-2.37) |
| Partial without formula (breast milk and food) | 55/279 (19.7) | 1.16 (0.71-1.90) | 0.96 (0.57-1.64) |
| Exclusive (breast milk only) | 31/177 (17.5) | 1 [Reference] | 1 [Reference] |
| Breastfeeding duration | (n = 978) | ||
| <6 mob | 68/219 (31.1) | 2.02 (1.39-2.93) | 1.64 (1.06-2.52) |
| 6 to <12 mo | 85/309 (27.5) | 1.70 (1.21-2.41) | 1.47 (0.99-2.18) |
| ≥12 mo | 82/450 (18.2) | 1 [Reference] | 1 [Reference] |
Abbreviation: OR, odds ratio.
Adjusted for maternal body mass index, smoking, postsecondary education, race/ethnicity, cesarean delivery, dog in household, infant sex, any oral antibiotics between 0 and 12 mo, and study site.
Excludes infants who were never breastfed. Breastfeeding refers to breast milk feeding regardless of feeding mode (at the breast or from a bottle).
At 6 months, partial breastfeeding supplemented with formula was associated with an increased risk of overweight when adjusting individually for maternal body mass index (aOR, 1.60; 95% CI, 1.01-2.59), other maternal factors (aOR, 1.65; 95% CI, 1.03-2.68), or microbiota-related factors (aOR, 1.64; 95% CI, 1.02-2.70), although statistical significance was lost in the fully adjusted model (aOR, 1.43; 95% CI, 0.87-2.37) (Table 1). In contrast, partial breastfeeding without formula (ie, with foods only) was not associated with risk of overweight (aOR, 0.96; 95% CI, 0.57-1.64). Earlier cessation of breastfeeding was associated with an increased risk of overweight (before 6 months: aOR, 1.64; 95% CI, 1.06-2.52; between 6 and 12 months: aOR, 1.47; 95% CI, 0.99-2.18 compared with 12 months or longer). Sensitivity analyses using the WFLz score as a continuous outcome, adjusting for infant birth weight or excluding infants who never received breast milk, followed similar patterns of association (eTable 4 in the Supplement).
Infant Feeding and Gut Microbiota
As expected, breastfeeding was strongly associated with the richness, diversity, and composition of gut microbiota at 3 to 4 months, with clear dose responses according to exclusivity (Figure 1 and eTables 5 and 6 in the Supplement). The richness and diversity of microbiota were highest in infants who were not breastfed, lower in partially breastfed infants, and lowest in exclusively breastfed infants (Figure 1A). The community structure of microbiota also differed significantly (overall P = .001, pseudo F, 10.9 [unweighted UniFrac]; P = .001, pseudo F, 12.4 [weighted UniFrac], determined by use of PERMANOVA; eTable 6 in the Supplement), with principal coordinate analysis (Figure 1D and eFigure 2A and B in the Supplement) showing clear separation between the exclusively breastfed and nonbreastfed groups. The group that briefly received formula in the hospital overlapped almost completely with the exclusively breastfed group (P = .24, pseudo F, 0.24, determined by use of pairwise PERMANOVA) (Figure 1D and eTable 6 in the Supplement), indicating similar microbiota community structures.
Figure 1. Infant Gut Microbiota at 3 to 4 Months According to Breastfeeding (BF) Status.
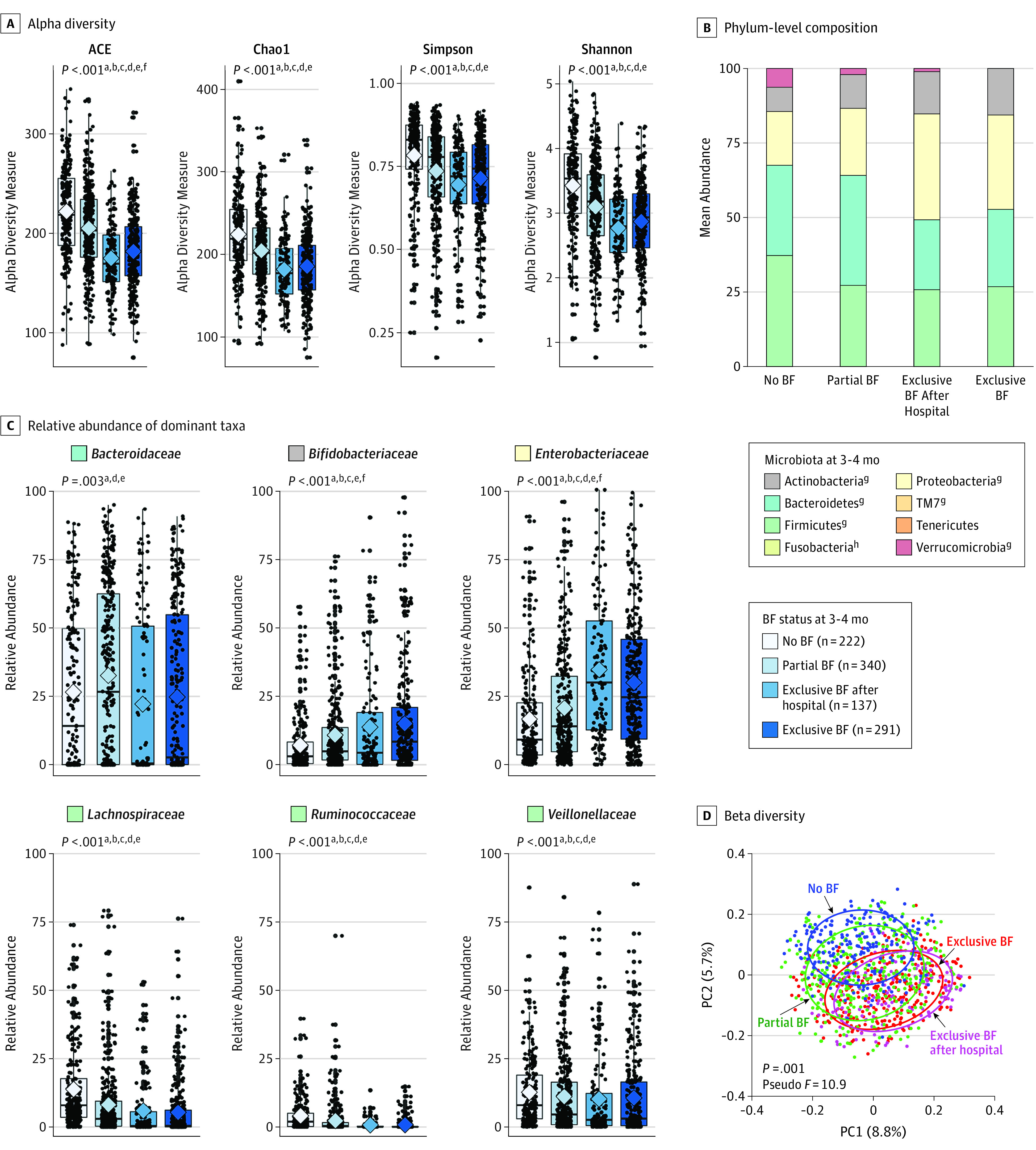
A, Alpha diversity evaluated by richness (abundance-based coverage estimator [ACE] and Chao1) and diversity (Simpson and Shannon). Median estimates are compared across feeding groups using the Kruskal-Wallis test (nonparametric analysis of variance) and Dunn post hoc tests for multiple comparisons. Boxes indicate interquartile range, lines indicate medians, diamonds indicate means, and whiskers represent range. B, Mean phylum-level composition. C, Relative abundance of dominant taxa across feeding groups. Breastfeeding (BF) status is assessed at the time of sample collection. Breastfeeding refers to breast milk feeding regardless of feeding mode (at the breast or from a bottle). D, Principal coordinate analysis (PC1 and PC2) based on unweighted UniFrac distances, with community structure differences tested by permutational analysis of variance with 999 permuations.
P values represent false discovery rate–corrected P values testing for overall differences across the 4 feeding groups. Significant pairwise comparisons:
aNo BF/partial BF;
bNo BF/exclusive BF after hospital;
cNo BF/exclusive BF;
dPartial BF/exclusive BF after hospital;
ePartial BF/exclusive BF;
fExclusive BF after hospital/exclusive BF.
gP < .001.
hP < .05.
Nearly all phyla and families demonstrated disproportional abundances across breastfeeding groups, and significant dose responses were observed with particular taxa (Figure 1B and C and eTable 5 in the Supplement). Increasing exclusivity of breastfeeding was associated with increasing relative abundance of Bifidobacteriaceae and Enterobacteriaceae and decreasing relative abundance of Lachnospiraceae, Veillonellaceae, and Ruminococcaceae. Although most taxa were similarly abundant between infants who were exclusively breastfed from birth and those exclusively breastfed after hospital discharge, the relative abundance of Bifidobacteriaceae was significantly lower after brief exposure to formula in the hospital (median, 4.3% vs 8.3% of total microbiota; FDR P = .03) and the relative abundance of Enterobacteriaceae was higher (29.8% vs 24.5% of total microbiota; FDR P = .05) (Figure 1C and eTable 5 in the Supplement).
Twelve-month microbiota profiles were more homogeneous overall, but significant differences were still detectable according to dietary exposures at 6 months (Figure 2A-D, eFigure 2C and 2D, and eTables 6 and 7 in the Supplement). Richness was significantly higher among formula-fed infants (whether or not they were also receiving breast milk) compared with breastfed infants (whether or not they were receiving complementary foods) (Figure 2A). The relative abundances of Actinobacteria and Proteobacteria were highest in exclusively breastfed infants and lowest in nonbreastfed infants (Figure 2B). Several differences were observed between the partial breastfeeding groups, including significantly higher relative abundance of Bifidobacteriaceae and Veillonellaceae in those receiving complementary foods without formula (Figure 2C). Overall, the microbiota of partially breastfed infants who did not receive formula were similar to the microbiota of exclusively breastfed infants (no significant differences by 12 months; P = .78, pseudo F = 0.40, determined by use of pairwise PERMANOVA), whereas the microbiota of those who received formula were more similar to the microbiota of nonbreastfed infants (Figure 2D and eTable 6 in the Supplement).
Figure 2. Infant Gut Microbiota at 12 Months According to Diet at 6 Months.
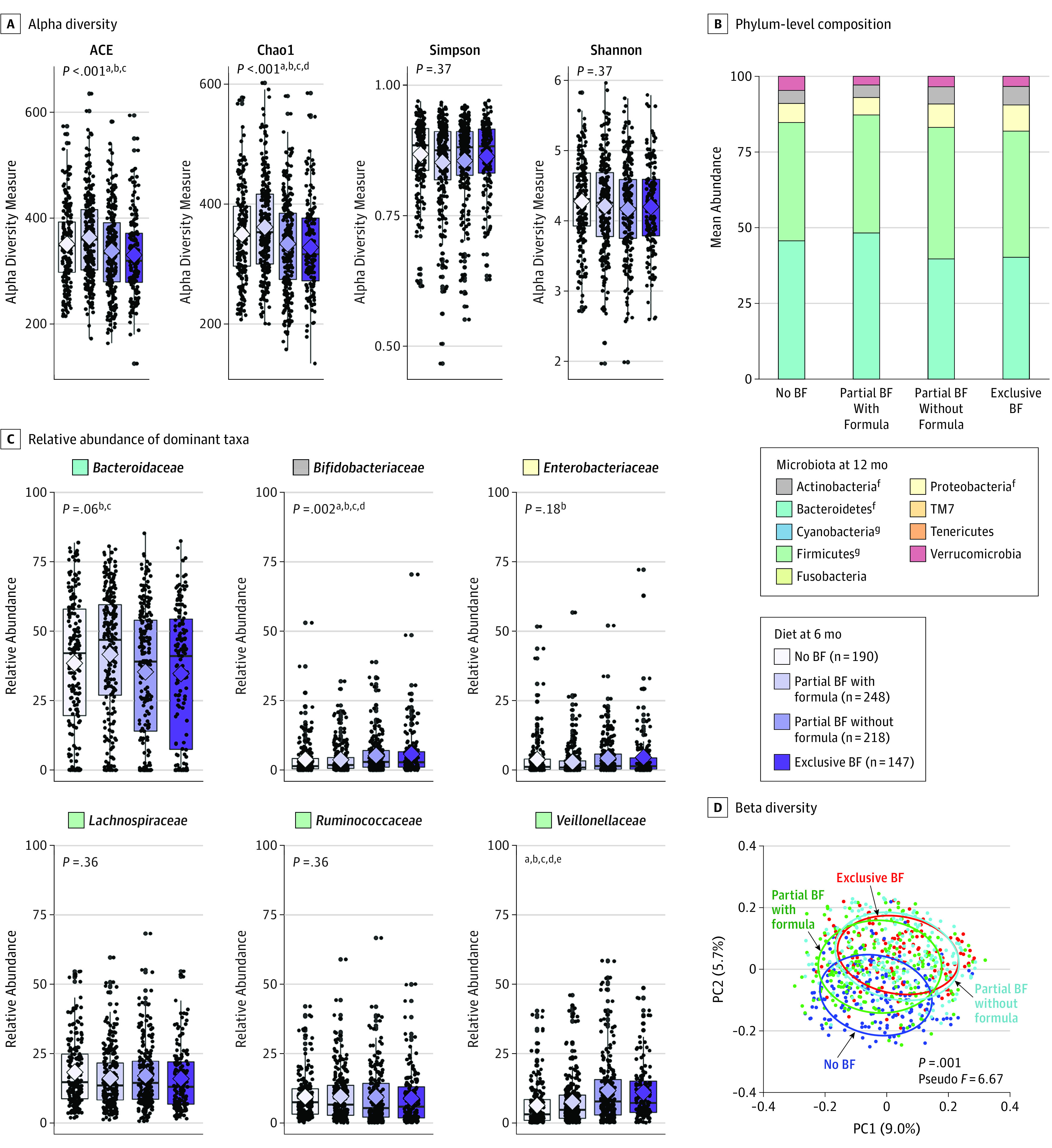
A, Alpha diversity evaluated by richness (abundance-based coverage estimator [ACE] and Chao1) and diversity (Simpson and Shannon). Median estimates are compared across feeding groups using the Kruskal-Wallis test and Dunn post hoc tests for multiple comparisons. Boxes indicate interquartile range, lines indicate medians, diamonds indicate means, and whiskers represent range. B, Mean phylum-level composition. C, Relative abundance of dominant taxa across feeding groups. Breastfeeding (BF) refers to breast milk feeding regardless of feeding mode (at the breast or from a bottle). D, Principal coordinate analysis (PC1 and PC2) based on unweighted UniFrac distances, with community structure differences tested by permutational analysis of variance with 999 permuations.
P values represent false discovery rate–corrected P values testing for overall differences across the 4 feeding groups. Significant pairwise comparisons:
aNo BF/exclusive BF;
bPartial BF with formula/partial BF without formula;
cPartial BF with formula/exclusive BF; no significant differences observed between partial BF without formula and exclusive BF;
dNo BF/partial BF without formula;
eNo BF/partial BF with formula.
fP < .01.
gP < .05.
The duration of breastfeeding was also associated with gut microbiota at 12 months (eFigure 3 and eTable 8 in the Supplement). Richness and diversity were lowest among infants who were still breastfeeding at 12 months and highest among those who had weaned before 6 months. Bifidobacteriaceae, Veillonellaceae, and Proteobacteria were enriched among infants who were still breastfeeding and depleted among infants who had never been breastfed. In contrast, Lachnospiraceae, Ruminococcaceae, and Porphyromonadaceae were enriched among infants who were not breastfeeding at 12 months.
Gut Microbiota and Overweight
Infants who were overweight or at risk of overweight at 12 months had significantly higher richness of microbiota by 3 to 4 months of age (Figure 3A); significant differences in composition were also detected (Figure 3B and C and eTable 9 in the Supplement). The strongest association was the enrichment of Lachnospiraceae among infants who subsequently became overweight (median relative abundance, 5.9% of total microbiota) or at risk for overweight (median relative abundance, 4.7% of total microbiota) by 12 months compared with normal-weight infants (median relative abundance, 1.9% of total microbiota; FDR P = .01). We also observed significantly higher relative abundance of Coriobacteriaceae, Erysipelotrichaceae, and Ruminococcaceae at 3 to 4 months among infants who became overweight. The Firmicutes to Bacteroidetes ratio was highest in infants who became overweight at 1 year, although this difference was not significant. By 12 months, few differences in microbiota were observed according to weight status (eFigure 4 and eTable 9 in the Supplement).
Figure 3. Infant Gut Microbiota Characterization at 3 Months According to Infant Weight Status at 12 Months.
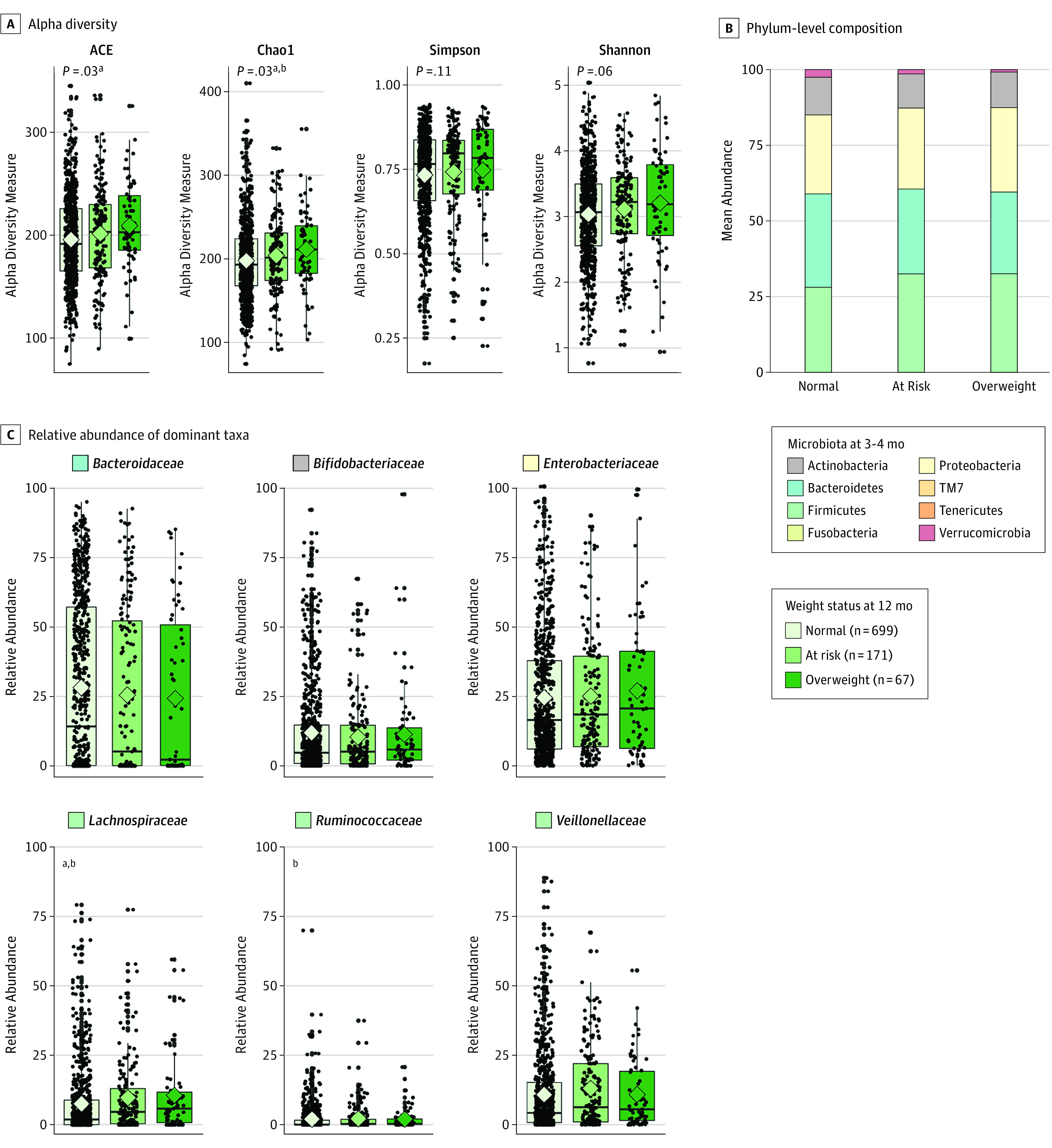
A, Alpha diversity evaluated by richness (abundance-based coverage estimator [ACE] and Chao1) and diversity (Simpson and Shannon). Median estimates are compared across weight status using the Kruskal-Wallis test and Dunn post hoc tests for multiple comparisons. Boxes indicate interquartile range, lines indicate medians, diamonds indicate means, and whiskers represent range. B, Mean phylum-level composition. C, Relative abundance of dominant taxa across weight status groups. Breastfeeding refers to breast milk feeding regardless of feeding mode (at the breast or from a bottle).
Significant pairwise comparisons:
aNormal/overweight;
bNormal/at risk.
To further explore the association of weight status at 12 months with the composition and diversity of gut microbiota, we classified candidate microbiota measures in quartiles and conducted logistic regression analyses (eFigure 5 in the Supplement). At 3 to 4 months, higher relative abundance of Lachnospiraceae (above vs below median) were associated with an 89% increase in risk of overweight by 12 months (OR, 1.89; 95% CI, 1.40-2.56). Each quartile increase in the Firmicutes to Bacteroidetes ratio was associated with a 12% increase in the risk of overweight (OR, 1.12; 95% CI, 0.98-1.28). The richness of gut microbiota was also positively associated with the risk of overweight by 12 months (OR, 1.24 per quartile increase; 95% CI, 1.09-1.42 per quartile increase), as was the diversity of gut microbiota (OR, 1.21 per quartile increase; 95% CI, 1.06-1.38 per quartile increase). No comparable associations were detected for microbiota measures at 12 months.
Contribution of Gut Microbiota to Association of Infant Feeding Practices and Overweight
To examine whether gut microbiota contribute to the increased risk of overweight associated with formula feeding and shorter duration of breastfeeding, we tested these associations in mutually adjusted models. Adjustment for richness of microbiota, diversity of microbiota, or relative abundance of Lachnospiraceae substantially attenuated the effect estimate for cessation of breastfeeding before 3 months (Table 2). Simultaneous adjustment for richness of microbiota and Lachnospiraceae attenuated this estimate from 2.04 (95% CI, 1.25-3.32) to 1.33 (95% CI, 0.79-2.24). In contrast, associations between infant feeding and weight status were largely unaffected by adjustment for concurrent microbiota measures at 12 months.
Table 2. Association of Infant Feeding and Key Microbiota Measures at 3 and 12 Months With Weight Status at 12 Months.
| Breastfeeding and Microbiota Exposure | OR (95% CI) for Overweight or at Risk of Overweight (WFLz score >85th Percentile) at 12 mo | |||||
|---|---|---|---|---|---|---|
| Adjusted for Covariates Plus Feeding or Microbiota (Individually)a | Mutually Adjusted | |||||
| For Covariates, Feeding, and Chao1 | For Covariates, Feeding, and Shannon | For Covariates, Feeding, and Lachnospiraceae | For Covariates, Feeding, and F/B Ratio | For Covariates, Feeding, and Selected Microbiota Measuresb | ||
| Breastfeeding status at 3 mo (n = 795) | ||||||
| None (formula only) | 1.79 (1.09-2.93) | 1.56 (0.93-2.59) | 1.63 (0.98-2.70) | 1.47 (0.87-2.45) | 1.77 (1.07-2.91) | 1.33 (0.79-2.24) |
| Partial (breast milk and formula) | 1.49 (0.98-2.26) | 1.37 (0.90-2.09) | 1.41 (0.93-2.16) | 1.37 (0.90-2.09) | 1.52 (1.00-2.32) | 1.28 (0.83-2.97) |
| Exclusive after hospital discharge | 1.00 (0.58-1.69) | 1.02 (0.59-1.73) | 1.02 (0.59-1.73) | 1.00 (0.58-1.69) | 0.93 (0.53-1.58) | 1.02 (0.59-1.73) |
| Exclusive (breast milk only) | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Microbiota measures at 3 mo (n = 795) | ||||||
| Chao1 (per quartile increase) | 1.25 (1.08-1.46) | 1.20 (0.59-1.73) | NA | NA | NA | 1.16 (0.99-1.37) |
| Shannon (per quartile increase) | 1.18 (1.02-1.38) | NA | 1.13 (0.97-1.32) | NA | NA | NA |
| High Lachnospiraceae (above median)c | 1.82 (1.29-2.57) | NA | NA | 1.66 (1.16-2.39) | NA | 1.58 (1.10-2.28) |
| F/B ratio (per quartile increase) | 1.17 (1.00-1.38) | NA | NA | NA | 1.20 (1.02-1.42) | NA |
| Breastfeeding duration at 12 mo (n = 695) | ||||||
| <6 mo | 1.99 (1.23-3.22) | 1.97 (1.21-3.18) | 1.95 (1.20-3.15) | 1.98 (1.22-3.20) | 2.02 (1.25-3.27) | 1.96 (1.21-3.16) |
| 6 to <12 mo | 1.59 (1.02-2.48) | 1.53 (0.98-2.39) | 1.57 (1.00-2.45) | 1.57 (1.00-2.45) | 1.60 (1.02-2.50) | 1.52 (0.97-2.38) |
| ≥12 mo | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Microbiota measures at 12 mo (n = 695) | ||||||
| Chao1 (per quartile increase) | 1.15 (0.97-1.36) | 1.13 (0.96-1.35) | NA | NA | NA | 1.13 (0.95-1.34) |
| Shannon (per quartile increase) | 1.18 (1.00-1.40) | NA | 1.17 (0.99-1.39) | NA | NA | NA |
| High Lachnospiraceae (above median)c | 1.27 (0.87-1.85) | NA | NA | 1.24 (0.85-1.81) | NA | 1.21 (0.83-1.78) |
| F/B ratio (per quartile increase) | 1.06 (0.90-1.26) | NA | NA | NA | 1.08 (0.91-1.28) | NA |
Abbreviations: F/B ratio, Firmicutes to Bacteroidetes ratio; NA, not applicable; OR, odds ratio; WFLz, weight for length z.
Adjusted for maternal race/ethnicity, educational level, body mass index, smoking, cesarean delivery, dogs in household, infant sex, antibiotic exposure between 0 and 12 mo, and study site.
The final model is adjusted for Chao1 and Lachnospiraceae because these were the strongest individual microbiota variables associated with risk of overweight; Shannon and F/B ratio were omitted to avoid multicollinearity because Shannon and Chao1 are highly correlated with each other (as 2 measures of alpha diversity), as are the F/B ratio and Lachnospiraceae relative abundance (Lachnospiraceae is a family in the Firmicutes phylum). Breastfeeding refers to breast milk feeding, regardless of feeding mode (at the breast or from a bottle). There were 795 infants for the 3-mo analyses and 695 infants for the 12-mo analyses.
High relative abundance of Lachnospiraceae.
Discussion
Our findings demonstrate a strong inverse and dose-dependent association between breastfeeding and the risk of overweight in the first year of life that is partially explained by gut microbiota. Although the effect of breast milk on the development of the gut microbiome is well known,24,25,26,27 our findings address important nuances that, to our knowledge, have not been explored in previous studies, identifying differences according to the type and timing of supplemental feeding. We also report novel longitudinal associations between the composition of gut microbiota at 3 to 4 months of age and weight status at 12 months of age.
Similar to previous studies,28,29 we found a 63% increased risk of overweight among infants who were partially vs exclusively breastfed at 3 months and a 102% increased risk among exclusively formula-fed infants. As others have reported,25,27,30 we detected significantly lower bacterial richness and diversity in breastfed infants, accompanied by enrichment of several taxa (eg, Bifidobacteriaceae, Pasteurellaceae, and Enterobacteriaceae) and depletion of others (eg, Bacteroidaceae and Lachnospiraceae), with dose effects according to the degree of breastfeeding exclusivity. These findings are consistent with evidence that human milk oligosaccharides function as selective substrates for particular groups of microorganisms, including Bifidobacteriaceae.31,32,33,34
Building on previous studies of adults,35,36 children,9,10,11,12 and infants,37,38,39,40,41,42 our study provides new evidence linking gut microbiota with the risk of overweight in the first year of life. Prior research of infants has reported reduced relative abundance of Bifidobacteria and enrichment of streptococci and Bacteroides fragilis to be associated with overweight later in childhood.37,38,39,40,41,42 Although we did not observe these particular trends, perhaps owing to cohort differences in age, geography, or feeding practices (eg, extremely high rates of initiation of breastfeeding in the CHILD Study), we identified several novel associations. Although few associations were detected between microbiota and overweight measured concurrently at 12 months, several microbiota features associated with overweight were identified at 3 to 4 months. For example, while Lachnospiraceae were similarly abundant in normal-weight and overweight infants at 12 months, they were significantly enriched among overweight infants at 3 to 4 months. Lachnospiraceae has been associated with maternal obesity and is enriched in meconium from neonates born to mothers with diabetes.43 In our study, enrichment of Lachnospiraceae was associated with exposure to formula in a dose-dependent manner, along with the richness and diversity of microbiota; adjustment for these microbiota features partially explained the association between exposure to formula and the risk of overweight.
Taken together, our results suggest that the transient perturbation of microbiota in early infancy (related to feeding practices or other exposures) may influence weight gain and body composition, which may ultimately influence the risk of metabolic disease risk later in life.44 This hypothesis (eFigure 6 in the Supplement) is consistent with studies of mice showing that the disruption of gut microbiota limited to early life has permanent metabolic effects, including elevated adiposity, despite “recovery” of the microbiota.45 Other important mechanisms linking gut microbiota and obesity include microbial metabolites influencing levels of and sensitivity to the satiety hormone leptin.46,47
To our knowledge, this is the first study to evaluate the potential association of brief exposure to formula during the neonatal period as it pertains to the development of microbiota and the risk of overweight. These are clinically important questions since many neonates receive formula in the hospital, often without medical indication,48 yet the effect of this brief intervention on the developing microbiota (and related clinical outcomes) is not known. In our cohort, 179 of 579 infants (30.9%) reported by their mothers as exclusively breastfed actually received some formula in the hospital. Overall, we found no difference in the risk of overweight among these infants. However, while their microbiota profiles at 3 to 4 months were clearly more similar to those of exclusively breastfed than partially or nonbreastfed infants, some significant differences were detected. The richness and diversity of the microbiota were lower, as was the relative abundance of Bifidobacteriaceae, suggesting that even brief exposure to formula may disrupt normal colonization of the infant gut. We have likely underestimated this disruption, since our first sample was not collected until 3 to 4 months after hospital discharge. It is possible that the reason for formula supplementation contributed to the observed microbiota differences, but this possibility could not be directly examined in our study because we did not systematically document reasons for supplementation.
Multiple studies have investigated the effects of breast milk on the gut microbiome24,25,26,34,49,50; however, many of these studies did not distinguish between partial breastfeeding mixed with formula vs mixed with foods. We found that breastfed infants supplemented with formula were more similar to nonbreastfed infants, whereas breastfed infants given complementary foods (without formula) were more similar to exclusively breastfed infants. These differences might explain why mixed feeding with (but not without) formula was associated with an increased risk of overweight, although more research is needed to characterize these complex associations.
Strengths and Limitations
The strengths of our study include the detailed description of infant feeding practices, repeated analysis of microbiota, and adjustment for multiple confounders. However, we lacked information about the reasons for supplementation and did not address the mode of breast milk feeding, type of formula, quantity of breast milk or formula intake, or breast milk composition. Finally, a limitation of 16S ribosomal RNA analysis is that it cannot quantify or accurately resolve individual bacterial species.
Conclusions
Our findings indicate that breastfeeding is protective against overweight and suggest that the gut microbiota contribute to this effect. Formula feeding was associated with higher microbiota diversity and enrichment of Lachnospiraceae at 3 to 4 months, and these microbiota features partially explained the increased risk of overweight among nonbreastfed infants. Subtle but statistically significant differences in the microbiota were observed after brief exposure to formula in the hospital, although the clinical implications of these changes are unclear. Together, these results identify important areas for future research and emphasize the importance of early infancy as a critical period during which transient gut dysbiosis is associated with the subsequent risk of overweight.
eFigure 1. Flow Diagram Summarizing Selection of CHILD Study Infants Included in the Current Analysis
eFigure 2. Microbial Community Structure of 3-Month and 12-Month Microbiota Based on Breastfeeding Status at 3-4 Months and Infant Diet at 6 months, Respectively, as Measured by Beta-Diversity
eFigure 3. Infant Gut Microbiota at 12 Months According to Breastfeeding (BF) Duration
eFigure 4. Infant Gut Microbiota Characterization at 12 Months According to Infant Weight Status at 12 Months
eFigure 5. Association of Key Microbiota Measures at 3 and 12 Months With Infant Weight Status at 12 Months
eFigure 6. Associations and Hypothesized Mechanisms Linking Infant Feeding Practices, Gut Microbiota and Obesity
eTable 1. Characteristics of Participants Included in the Current Study and the General CHILD Cohort
eTable 2. Infant Feeding and Weight Variables Among Participants in the Subcohort
eTable 3. Prevalence of Potential Confounders and Associations With Breastfeeding and Overweight Risk
eTable 4. Sensitivity Analyses: Association of Infant Feeding Practices With Infant Weight Status at 12 Months
eTable 5. Median Relative Abundance of Abundant Taxa in Gut Microbiota at 3-4 Months According to Feeding Status
eTable 6. Pairwise PERMANOVA Analyses of Infant Microbiota According to Feeding Status at 3-4 Months and 6 Months
eTable 7. Median Relative Abundance of Abundant Taxa in Fecal Microbiota of Infants at 12 Months According to Feeding Status at 6 Months
eTable 8. Median Relative Abundance of Abundant Taxa in Fecal Microbiota of Infants at 12 Months According to Breastfeeding (BF) Duration
eTable 9. Median Relative Abundance of Abundant Taxa in Fecal Microbiota of Infants at 3-4 and 12 Months According to Infant Weight Status at 12 Months
eAppendix. Detailed Methods
References
- 1.Ojha S, Budge H. Early origins of obesity and developmental regulation of adiposity. In: Symonds ME, ed. Adipose Tissue Biology. New York, NY: Springer International Publishing; 2017:427-456. [Google Scholar]
- 2.Wang L, Collins C, Ratliff M, Xie B, Wang Y. Breastfeeding reduces childhood obesity risks. Child Obes. 2017;13(3):197-204. [DOI] [PubMed] [Google Scholar]
- 3.Marseglia L, Manti S, D’Angelo G, et al. Obesity and breastfeeding: the strength of association. Women Birth. 2015;28(2):81-86. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum M, Knight R, Leibel RL. The gut microbiota in human energy homeostasis and obesity. Trends Endocrinol Metab. 2015;26(9):493-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5-11. [DOI] [PubMed] [Google Scholar]
- 6.Le Chatelier E, Nielsen T, Qin J, et al. ; MetaHIT Consortium . Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541-546. [DOI] [PubMed] [Google Scholar]
- 7.Koliada A, Syzenko G, Moseiko V, et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027-1031. [DOI] [PubMed] [Google Scholar]
- 9.Riva A, Borgo F, Lassandro C, et al. Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol. 2017;19(1):95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgo F, Verduci E, Riva A, et al. Relative abundance in bacterial and fungal gut microbes in obese children: a case control study. Child Obes. 2017;13(1):78-84. [DOI] [PubMed] [Google Scholar]
- 11.Ignacio A, Fernandes MR, Rodrigues VAA, et al. Correlation between body mass index and faecal microbiota from children. Clin Microbiol Infect. 2016;22(3):258.e1-258.e8. [DOI] [PubMed] [Google Scholar]
- 12.Lemas DJ, Yee S, Cacho N, et al. Exploring the contribution of maternal antibiotics and breastfeeding to development of the infant microbiome and pediatric obesity. Semin Fetal Neonatal Med. 2016;21(6):406-409. [DOI] [PubMed] [Google Scholar]
- 13.Cerdó T, Ruiz A, Campoy C. Human gut microbiota and obesity during development. In: Gordeladze J, ed. Adiposity—Omics and Molecular Understanding. London, United Kingdom: InTechOpen; 2017:265-285. [Google Scholar]
- 14.Subbarao P, Anand SS, Becker AB, et al. ; CHILD Study investigators . The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax. 2015;70(10):998-1000. [DOI] [PubMed] [Google Scholar]
- 15.WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 16.Corby L, Secker D. Growth monitoring of infants and children using the 2006 World Health Organization [WHO] Child Growth Standards and 2007 WHO growth references: practice-based evidence in nutrition. Acta Paediatr. 2006;(suppl 450):76-85. [Google Scholar]
- 17.Azad MB, Konya T, Persaud RR, et al. ; CHILD Study Investigators . Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. 2016;123(6):983-993. [DOI] [PubMed] [Google Scholar]
- 18.Azad MB, Sharma AK, de Souza RJ, et al. ; Canadian Healthy Infant Longitudinal Development Study Investigators . Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr. 2016;170(7):662-670. [DOI] [PubMed] [Google Scholar]
- 19.Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 23.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228-8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paolella G, Vajro P. Childhood obesity, breastfeeding, intestinal microbiota, and early exposure to antibiotics: what is the link? JAMA Pediatr. 2016;170(8):735-737. [DOI] [PubMed] [Google Scholar]
- 25.Pannaraj PS, Li F, Cerini C, et al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171(7):647-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmerman HM, Rutten NBMM, Boekhorst J, et al. Intestinal colonisation patterns in breastfed and formula-fed infants during the first 12 weeks of life reveal sequential microbiota signatures. Sci Rep. 2017;7(1):8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milani C, Duranti S, Bottacini F, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017;81(4):e00036-e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung H, Leff M, Rhee KE. Effect of exclusive breastfeeding among overweight and obese mothers on infant weight-for-length percentile at 1 year. Breastfeed Med. 2017;12:39-47. [DOI] [PubMed] [Google Scholar]
- 29.Bider-Canfield Z, Martinez MP, Wang X, et al. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr Obes. 2017;12(2):171-178. [DOI] [PubMed] [Google Scholar]
- 30.Castanys-Muñoz E, Martin MJ, Vazquez E. Building a beneficial microbiome from birth. Adv Nutr. 2016;7(2):323-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sela DA, Mills DA. Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010;18(7):298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcobal A, Barboza M, Froehlich JW, et al. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58(9):5334-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azad MB, Konya T, Maughan H, et al. ; CHILD Study Investigators . Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;185(5):385-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laursen MF, Andersen LBB, Michaelsen KF, et al. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere. 2016;1(1):e00069-e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016;8(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes. 2014;4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koleva PT, Bridgman SL, Kozyrskyj AL. The infant gut microbiome: evidence for obesity risk and dietary intervention. Nutrients. 2015;7(4):2237-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheepers LE, Penders J, Mbakwa CA, Thijs C, Mommers M, Arts ICW. The intestinal microbiota composition and weight development in children: the KOALA Birth Cohort Study. Int J Obes (Lond). 2015;39(1):16-25. [DOI] [PubMed] [Google Scholar]
- 39.White RA, Bjørnholt JV, Baird DD, et al. Novel developmental analyses identify longitudinal patterns of early gut microbiota that affect infant growth. PLoS Comput Biol. 2013;9(5):e1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vael C, Verhulst SL, Nelen V, Goossens H, Desager KN. Intestinal microflora and body mass index during the first three years of life: an observational study. Gut Pathog. 2011;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534-538. [DOI] [PubMed] [Google Scholar]
- 42.Korpela K, Zijlmans MAC, Kuitunen M, et al. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome. 2017;5(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh S, Karagas MR, Mueller NT. Charting the maternal and infant microbiome: what is the role of diabetes and obesity in pregnancy? Curr Diab Rep. 2017;17(2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy SM, Spivack JG, Faith MS, et al. Infant BMI or weight-for-length and obesity risk in early childhood. Pediatrics. 2016;137(5):e20153492-e20153492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cox LM, Yamanishi S, Sohn J, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cani PD, Lecourt E, Dewulf EM, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90(5):1236-1243. [DOI] [PubMed] [Google Scholar]
- 47.Schéle E, Grahnemo L, Anesten F, Hallén A, Bäckhed F, Jansson JO. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology. 2013;154(10):3643-3651. [DOI] [PubMed] [Google Scholar]
- 48.Chantry CJ, Dewey KG, Peerson JM, Wagner EA, Nommsen-Rivers LA. In-hospital formula use increases early breastfeeding cessation among first-time mothers intending to exclusively breastfeed. J Pediatr. 2014;164(6):1339-1345.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amarri S, Benatti F, Callegari ML, et al. Changes of gut microbiota and immune markers during the complementary feeding period in healthy breast-fed infants. J Pediatr Gastroenterol Nutr. 2006;42(5):488-495. [DOI] [PubMed] [Google Scholar]
- 50.Qasem W, Azad MB, Hossain Z, et al. Assessment of complementary feeding of Canadian infants: effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017;17(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flow Diagram Summarizing Selection of CHILD Study Infants Included in the Current Analysis
eFigure 2. Microbial Community Structure of 3-Month and 12-Month Microbiota Based on Breastfeeding Status at 3-4 Months and Infant Diet at 6 months, Respectively, as Measured by Beta-Diversity
eFigure 3. Infant Gut Microbiota at 12 Months According to Breastfeeding (BF) Duration
eFigure 4. Infant Gut Microbiota Characterization at 12 Months According to Infant Weight Status at 12 Months
eFigure 5. Association of Key Microbiota Measures at 3 and 12 Months With Infant Weight Status at 12 Months
eFigure 6. Associations and Hypothesized Mechanisms Linking Infant Feeding Practices, Gut Microbiota and Obesity
eTable 1. Characteristics of Participants Included in the Current Study and the General CHILD Cohort
eTable 2. Infant Feeding and Weight Variables Among Participants in the Subcohort
eTable 3. Prevalence of Potential Confounders and Associations With Breastfeeding and Overweight Risk
eTable 4. Sensitivity Analyses: Association of Infant Feeding Practices With Infant Weight Status at 12 Months
eTable 5. Median Relative Abundance of Abundant Taxa in Gut Microbiota at 3-4 Months According to Feeding Status
eTable 6. Pairwise PERMANOVA Analyses of Infant Microbiota According to Feeding Status at 3-4 Months and 6 Months
eTable 7. Median Relative Abundance of Abundant Taxa in Fecal Microbiota of Infants at 12 Months According to Feeding Status at 6 Months
eTable 8. Median Relative Abundance of Abundant Taxa in Fecal Microbiota of Infants at 12 Months According to Breastfeeding (BF) Duration
eTable 9. Median Relative Abundance of Abundant Taxa in Fecal Microbiota of Infants at 3-4 and 12 Months According to Infant Weight Status at 12 Months
eAppendix. Detailed Methods


