Key Points
Question
What technique is best for surgical management of gallstone disease with biliary duct calculi?
Findings
In this systematic review and network meta-analysis of 20 randomized clinical trials that included 2489 unique patients and 4 surgical techniques combining laparoscopic cholecystectomy with a second technique, the rendezvous approach (laparoscopic cholecystectomy plus intraoperative cholangiopancreatography) was associated with the highest rates of safety and success compared with the other approaches.
Meaning
The rendezvous approach should be the first choice for patients with gallstone disease and biliary duct calculi.
Abstract
Importance
Several techniques are used for surgical treatment of gallstone disease with biliary duct calculi, but the safety and efficacy of these approaches have not been compared.
Objectives
To compare the efficacy and safety of 4 surgical approaches to gallstone disease with biliary duct calculi.
Data Sources
MEDLINE, Scopus, and ISI-Web of Science databases, articles published between 1950 and 2017 and searched from August 12, 2017, to September 14, 2017. Search terms used were LCBDE, LC, preoperative, ERCP, postoperative, period, cholangiopancreatography, endoscopic, retrograde, rendezvous, intraoperative, one-stage, two-stage, single-stage, gallstone, gallstones, calculi, stone, therapy, treatment, therapeutics, surgery, surgical, procedures, clinical trials as topic, random, and allocation in several logical combinations.
Study Selection
Randomized clinical trials comparing at least 2 of the following strategies: preoperative endoscopic retrograde cholangiopancreatography (PreERCP) plus laparoscopic cholecystectomy (LC); LC with laparoscopic common bile duct exploration (LCDBE); LC plus intraoperative endoscopic retrograde cholangiopancreatography (IntraERCP); and LC plus postoperative ERCP (PostERCP).
Data Extraction and Synthesis
A frequentist random-effects network meta-analysis was performed. The surface under the cumulative ranking curve (SUCRA) was used to show the probability that each approach would be the best for each outcome.
Main Outcomes and Measures
Primary outcomes were the safety to efficacy ratio using overall mortality and morbidity rates as the main indicators of safety and the success rate as an indicator of efficacy. Secondary outcomes were acute pancreatitis, biliary leak, overall bleeding, operative time, length of hospital stay, total cost, and readmission rate.
Results
The 20 trials comprised 2489 patients (and 2489 procedures). Laparoscopic cholecystectomy plus IntraERCP had the highest probability of being the most successful (SUCRA, 87.2%) and safest (SUCRA, 69.7%) with respect to morbidity. All approaches had similar results regarding overall mortality. Laparoscopic cholecystectomy plus LCBDE was the most successful for avoiding overall bleeding (SUCRA, 83.3%) and for the shortest operative time (SUCRA, 90.2%) and least total cost (SUCRA, 98.9%). Laparoscopic cholecystectomy plus IntraERCP was the best approach for length of hospital stay (SUCRA, 92.7%). Inconsistency was found in operative time (indirect estimate, 19.05; 95% CI, 2.44-35.66; P = .02) and total cost (indirect estimate, 17.06; 95% CI, 3.56-107.21; P = .04). Heterogeneity was observed for success rate (τ, 0.8), operative time (τ, >1), length of stay (τ, >1), and total cost (τ, >1).
Conclusions and Relevance
The combined LC and IntraERCP approach had the greatest odds to be the safest and appears to be the most successful. Laparoscopic cholecystectomy plus LBCDE appears to reduce the risk of acute pancreatitis but may be associated with a higher risk of biliary leak.
This systematic review and meta-analysis compares 4 surgical strategies that combine laparoscopic cholecystectomy and a second technique to assess which strategy is most successful in patients with gallstone disease with common bile duct stones.
Introduction
Gallstone disease with common bile duct (CBD) stones is a common clinical circumstance.1,2 Whereas laparoscopic cholecystectomy (LC) has been established as the criterion standard for symptomatic gallstones,3 the optimal choice for biliary duct calculi remains unaddressed. Four strategies are available: preoperative endoscopic retrograde cholangiopancreatography (PreERCP) plus LC; LC plus laparoscopic CBD exploration (LCDBE); LC plus intraoperative ERCP (IntraERCP), also called rendezvous; and, finally, LC plus postoperative ERCP (PostERCP). The recent update of the British Society of Gastroenterology guidelines for the management of CBD stones states that, despite many randomized clinical trials, there is no evidence of any difference in efficacy, mortality, or morbidity between LBCDE and perioperative ERCP.4 However, it remains unclear whether the equivalence of the approaches available accurately reflects the success of the procedures or whether it is a result of the internal limitations of the studies. We performed a systematic review and meta-analysis of randomized clinical trials by using network meta-analysis as a methodology.5 The primary end point was the safety to efficacy ratio; we used the procedures’ overall mortality and morbidity rates as the main indicators of safety and the success rates as an indicator of efficacy. Success was defined as the clearance of the common bile duct according to the intention-to-treat analysis; success rate was calculated as the ratio of the number of patients in whom the assigned procedure was completed without protocol violations to the number of all randomized patients in each arm. Secondary end points were acute pancreatitis, biliary leak, bleeding, operative time, length of hospital stay (LOS), total cost (US $), and 30-day readmission rate.
Methods
Details of the method are provided in the eAppendix in the Supplement. A systematic review was performed in accordance with a protocol based on the Cochrane Handbook for Systematic Reviews of Interventions6: the manuscript was organized in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) extension statement incorporating network meta-analyses of health care interventions (eFigure 1 in the Supplement).7 The following search terms were used in several logical combinations: LCBDE, LC, preoperative, ERCP, postoperative, period, cholangiopancreatography, endoscopic, retrograde, rendezvous, intraoperative, one-stage, two-stage, single-stage, gallstone, gallstones, calculi, stone, therapy, treatment, therapeutics, surgery, surgical, procedures, clinical trials as topic, random, and allocation in several logical combinations. MEDLINE, Scopus, and the ISI-Web of Science databases were searched from August 12, 2017, to September 14, 2017, for articles published between 1950 and 2017. EndNote version X7 (Thomson Reuters) was used to remove the duplicate studies. The only criterion for eligibility was the randomized study comparing any type of endoscopic or surgical procedure for the management of gallstones and biliary duct calculi. Studies that fulfilled the eligibility criterion were selected for evaluation in full-text form. Studies with all inclusion criteria present and all exclusion criteria were included in the qualitative and quantitative analyses. The data collection was performed by 2 of us (C.A.P. and G.T.) using prefixed data forms. Qualitative evaluation of the studies was performed using the Cochrane Collaboration tool for assessing the risk of bias in randomized trials.8
A frequentist network meta-analysis was carried out to compare all available techniques for treating gallstones and CBD calculi, generating a network for each outcome of interest.9 Treatment effects were reported as the surface under the cumulative ranking curves (SUCRA) and mean ranks. The SUCRA value represented the odds in percentage, without uncertainty, that each technique would be the safest and most successful choice, considering the analyzed outcome represented.10,11,12 The SUCRA values for overall morbidity, mortality, and success rate were used to obtain the safety to efficacy ratio. The reliability of the networks was estimated by evaluating inconsistency.13 Reliability was reported in 2 ways: as the ratio of 2 odds ratios and as the absolute difference between the direct and indirect estimation with 95% CIs. Heterogeneity was evaluated and reported as tau (τ).14 When the τ was greater than 0.5, a multivariate meta-regression analysis was performed to identify factors with a nonnegligible effect (2-sided P < .05). Publication and reporting bias were described with an adjusted funnel plot and was analyzed with the Begg test.15 Statistical significance was set as 2-sided P < .05 for all.
Results
Study Selection
The results of the systematic review of the literature are reported in eFigure 1 in the Supplement. The search identified a total of 1088 records; 271 references were excluded because the title indicated they were duplicate publications. Of the remaining 817 papers, 734 were excluded because, according to the title and abstract, the field of these studies was not pertinent. Eighty-three full-text articles were considered; of these, 63 were removed: 20 were review articles, 20 were meta-analyses, 13 were case reports, 6 reported unextractable data, and 4 were written in a language other than English. Finally, 20 studies16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 were included for quality evaluation and quantitative analysis. On reviewing the data collection, accordance between the 2 reviewers was 100%.
Study Characteristics
Table 1 and eTable 1 in the Supplement show the characteristics of the included studies. Fifteen of the 20 studies (75%) were carried out in Western countries. Fifteen of the studies included only patients with proven CBD calculi, whereas 5 studies also included patients with a strong suspicion of calculi. Data from 2489 patients (representing 2489 operations) were collected and were clustered into 4 arms: 915 (36.7%) in the LC plus LCBDE arm (arm A); 85 (3.5%) in the LC plus PostERCP arm (arm B); 878 (35.3%) in the PreERCP plus LC arm (arm C); and 611 (24.5%) in the LC plus IntraERCP arm (arm D). In the LC plus LCBDE arm, 490 of the 915 (53.5%) procedures included laparoscopic choledochotomy, whereas the transcystic approach was used in 331 cases (36.2%). The data were not extractable for 3 studies and 94 of the 915 procedures (10.3%). One study (6.2%) reported solely on the transcystic approach.
Table 1. Characteristics of the 20 Included Studies.
| Source | Affiliation | Patients | Study Design | Sample Size of Each Arm | Outcomea | |||
|---|---|---|---|---|---|---|---|---|
| LC + LCBDE (n = 915) | LC + PostERCP (n = 85) | PreERCP + LC (n = 878) | LC + IntraERCP (n = 611) |
|||||
| Rhodes et al,16 1998 | Norfolk and Norwich NHS Trust Hospital, United Kingdom | CBD stones confirmed by IOC | LC + LCBDE vs LC + PostERCP | 40 | 40 | NA | NA | A, B, C, D, E, F, G, H |
| Cuschieri et al,17 1999 | Multicenter study, Europe and Australia | CBD stones confirmed or suspected by US, biochemical, or clinical features | LC + LCBDE vs PreERCP + LC | 133b | NA | 133b | NA | A, B, C, D, E, F, H |
| Sgourakis and Karaliotas,18 2002 | Red Cross Hospital Korgialenio-Mpenakio, Athens, Greece | CBD stones confirmed or suspected by US, biochemical, or clinical features | LC + LCBDE vs PreERCP + LC | 36 | NA | 42 | NA | A, B, C, E, F, G |
| Nathanson et al,19 2005 | Royal Brisbane and Princess Alexandra Hospitals, Brisbane, Australia | CBD stones confirmed by IOC and not extractable by transcystic approach | LC + LCBDE vs LC + PostERCP | 41 | 45 | NA | NA | A, B, C, D, E, F |
| Hong et al,20 2006 | Sir Run Run Shaw Hospital, Hangzhou, China | CBD stones confirmed by US, MRCP, or IOC | LC + LCBDE vs LC + IntraERCP | 141 | NA | NA | 93 | A, B, C, D, E, F, G, H, I |
| Lella et al,21 2006 | Policlinico San Marco, Zingonia, Italy | CBD stones confirmed by MRCP in patients with high risk of pancreatitis | PreERCP + LC vs LC + IntraERCP | NA | NA | 60 | 60 | A, B, C, D, E, F, H |
| Morino et al,22 2006 | Molinette Hospital, Turin, Italy | CBD stones confirmed by MRCP | PreERCP + LC vs LC + IntraERCP | NA | NA | 45 | 46 | A, B, C, D, E, F, H |
| Rábago et al,23 2006 | Severo Ochoa’s Hospital, Leganes, Spain | CBD stones suspected by US, biochemical, clinical features | PreERCP + LC vs LC + IntraERCP | NA | NA | 64 | 59 | A, B, C, D, E, F, H, I |
| Noble et al,24 2009 | Southmead Hospital, Bristol, United Kingdom | CBD stones confirmed by imaging or suspected by risk score | LC + LCBDE vs PreERCP + LC | 44 | NA | 47 | NA | A, B, C, D, E, F, H |
| Bansal et al,25 2010 | Multicenter study, New Delhi Hospitals, New Delhi, India | CBD stones confirmed by MRCP, or EUS | LC + LCBDE vs PreERCP + LC | 15 | NA | 15 | NA | A, B, C, D, E, F, H |
| Rogers et al,26 2010 | San Francisco General Hospital, San Francisco, California | CBD stones suspected by US, CT scan, or biochemical or clinical features | LC + LCBDE vs PreERCP + LC | 57 | NA | 54 | NA | A, B, C, D, E, F, G, H, I |
| ElGeidIe et al,27 2011c | Gastroenterology Surgical Center, Mansoura, Egypt | CBD stones confirmed with MRCP | PreERCP + LC vs LC + IntraERCP | NA | NA | 100 | 98 | A, B, C, D, E, F, G, H |
| ElGeidie et al,28 2011c | Gastroenterology Surgical Center, Mansoura, Egypt | CBD stones confirmed with IOC | LC + LCBDE vs LC + IntraERCP | 115 | NA | NA | 111 | A, B, C, D, E, F, G, H |
| Ferulano et al,29 2011 | Federico II Hospital, Naples, Italy | CBD stones confirmed or suspected by US, CT scan, MRCP, or biochemical or clinical features | LC + LCBDE vs PreERCP + LC | 45 | NA | 39 | NA | A, B, C, D, E, F |
| Tzovaras et al,30 2012 | Hospital of Larissa, Larissa, Greece | CBD stones confirmed by MRCP | PreERCP + LC vs LC + IntraERCP | NA | NA | 49 | 50 | A, B, C, D, E, F, H |
| Koc et al,31 2013 | Okmeydani Training and Research Hospital, Istanbul, Turkey | CBD stones confirmed by US, MRCP, or biochemical features | LC + LCBDE vs PreERCP + LC | 57 | NA | 54 | NA | A, B, C, D, E, F, G |
| Ding et al,34 2014 | Tianjin Nankai Hospital, Tianjin, China | CBD stones confirmed by MRCP | LC + LCBDE vs PreERCP + LC | 110 | NA | 111 | NA | A, B, C, D, E, F |
| Sahoo et al,35 2014 | Shrirama Chandra Bhanj Medical College, Cuttack, India | CBD stones confirmed by MRCP | PreERCP + LC vs LC + IntraERCP | NA | NA | 41 | 42 | A, D |
| Lv et al,36 2016 | Beijing Friendship Hospital, Beijing, China | CBD stones confirmed by US, CT scan, or MRCP | LC + LCBDE vs . PreERCP + LC | 29 | NA | 24 | NA | A, B, C, D, E, F, H, I |
| Poh et al,37 2016 | Monash Health Hospital, Victoria, Australia | CBD stones confirmed by IOC | LC + LCBDE vs LC + IntraERCP | 52 | NA | NA | 52 | A, B, C, D, E, F, G, H |
Abbreviations: CBD, common bile duct; CT, computed tomography; EUS, endoscopic ultrasonography; IntraERCP, intraoperative endoscopic retrograde cholangiopancreatography; IOC, intraoperative transcystic cholangiography; LC, laparoscopic cholecystectomy; LCDBE, laparoscopic CBD exploration; MRCP, magnetic resonance cholangiopancreatography; NA, not applicable; PostERCP, postoperative endoscopic retrograde cholangiopancreatography; PreERCP, preoperative endoscopic retrograde cholangiopancreatography; US, transabdominal ultrasound.
A indicates success rate; B, total morbidity; C, total mortality; D, acute pancreatitis ; E, biliary leak ; F, total bleeding; G, total operative time; H, total hospital stay; and I, total costs.
There were 150 randomized patients per arm; however, there were 17 protocol violations per arm.
The quality of the included studies is plotted in eFigure 2 in the Supplement. Eight of the 20 studies (40%) included had an unclear or high risk of selection bias. Twelve of the 20 studies (60%) did not clearly report the allocation of the random sequence (unclear allocation bias). All the studies had a high risk of performance bias owing to the impossibility of double-blinding. All the studies had a low risk of detection bias. Finally, 2 of the 20 studies presented a high risk of attrition (10%), 1 presented a high risk of reporting bias (5%), and 1 (5%) presented a high risk of other biases.
Network Geometry and Risk of Bias Within the Individual Studies
The network geometry and the contribution plots are reported in eFigures 3 and 4 in the Supplement. The network of the success rate (eFigure 3A) included 4 arms, 20 studies, and 2490 patients; the network of overall morbidity included 4 arms (eFigure 3B); overall mortality included 19 studies (eFigure 3C); and acute pancreatitis included 2403 patients (eFigure 3D). The network of biliary leak and overall bleeding rate included 4 arms, 18 studies, and 2320 patients (eFigure 3E and F); the network of overall operative time included 4 arms, 8 studies, and 1142 patients (eFigure 3G); the network of LOS included 4 arms, 14 studies, and 1818 patients (eFigure 3H); and the network of total cost included 3 arms, 4 studies, and 521 patients, but lacked the LCPostERCP arm (eFigure 3I). A network of readmission rate was not generated because these data were reported by a single study.30
A color code was used to define the risk of bias within the individual studies: a green line for comparisons containing 1 or more studies with a low or unclear risk of bias and a red line for comparisons containing 1 or more studies with a high risk of bias. As shown in the contribution plots, the network approach permitted generation of mixed estimates for all end points and 2 main indirect estimates (LCPostERCP vs PreERCPLC, and LCPostERCP vs LCIntraERCP) for all end points (panels A-H) except for total cost (panel I).
Synthesis of Results
Indirect and mixed head-to-head comparisons are summarized in eFigure 5 in the Supplement, and hierarchical rank estimates for each technique are summarized in eTable 2 in the Supplement. The SUCRA and mean rank values for all the procedures available are shown in Table 2.
Table 2. SUCRA Values and Mean Rank for All Outcomesa.
| Outcome of Interest | Surgical Approach | |||||||
|---|---|---|---|---|---|---|---|---|
| LC + LCBDE (Arm A) | L + PostERCP (Arm B) | PreERCP + LC (Arm C) | LC + IntraERCP (Arm D) | |||||
| SUCRA, % | Rank, Mean | SUCRA, % | Rank, Mean | SUCRA, % | Rank, Mean | SUCRA, % | Rank, Mean | |
| Success rate | 55.7 | 2 | 12.8 | 4 | 44.3 | 3 | 87.2 | 1 |
| Overall morbidity | 43.9 | 3 | 62.7 | 2 | 23.8 | 4 | 69.7 | 2 |
| Overall mortality | 54.1 | 2 | 53.9 | 2 | 53.8 | 2 | 38.2 | 3 |
| Acute pancreatitis | 80.3 | 2 | 66.8 | 2 | 1.5 | 4 | 51.3 | 3 |
| Biliary leak | 4.9 | 4 | 86.2 | 1 | 59.6 | 2 | 49.3 | 3 |
| Overall bleeding | 83.3 | 2 | 24.4 | 3 | 52.4 | 2 | 39.9 | 3 |
| Overall operative time | 90.2 | 1 | 29.5 | 3 | 49.6 | 3 | 30.7 | 3 |
| LOS | 68.0 | 2 | 17.2 | 4 | 22.1 | 3 | 92.7 | 1 |
| Total costs | 98.9 | 1 | NA | NA | 11.5 | 3 | 39.6 | 2 |
Abbreviations: IntraERCP, intraoperative endoscopic retrograde cholangiopancreatography; LC, laparoscopic cholecystectomy; LCBDE, laparoscopic common bile duct exploration; LOS, length of postoperative hospital stay; NA, not applicable; PostERCP, postoperative endoscopic retrograde cholangiopancreatography; PreERCP, preoperative endoscopic retrograde cholangiopancreatography; SUCRA, surface under cumulative ranking area curve.
The SUCRA values express the percentage of safety or efficacy of each approach relative to an imaginary approach that was always the best without uncertainty.
Primary End Points
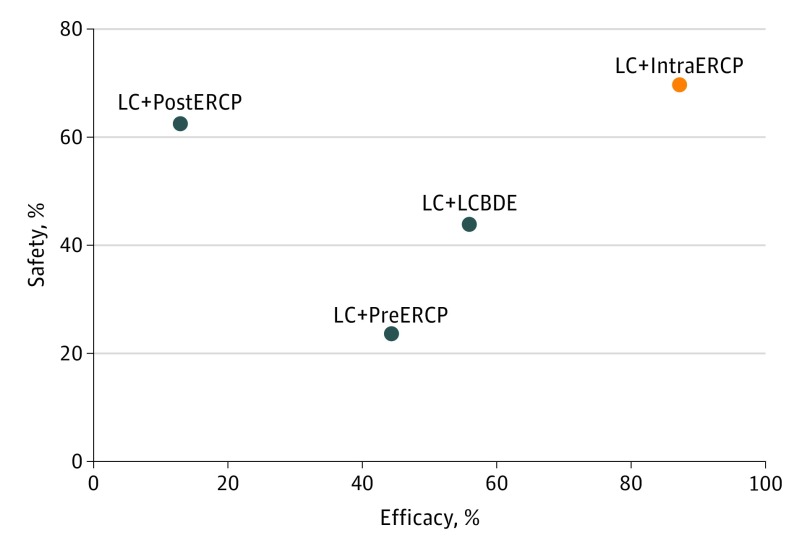
Regarding the efficacy indicator, LC plus IntraERCP was the approach with the greatest probability to be the most successful (SUCRA, 87.2%; mean rank, 1). The remaining 3 approaches had lower probabilities of being the best ranking strategy: the second was LC plus LCBDE (SUCRA, 55.7%), the third was PreERCP plus LC (SUCRA, 44.3%), and the fourth was LC plus PostERCP (SUCRA, 12.8%). Regarding safety indicators, no procedure clearly ranked first without uncertainty. Regarding overall morbidity, the strategies with the highest probability (mean rank, 2) of being the safest were LC plus IntraERCP (SUCRA, 69.7%) and LC plus PostERCP (SUCRA, 62.7%). The approaches LC plus LCBDE, ranking third (SUCRA, 43.9%), and PreERCP plus LC, ranking fourth (SUCRA, 23.8%), had a lower probability of being the best approach. For overall mortality, only the LC plus IntraERCP approach had a slightly worse probability of being the safest (SUCRA, 38.2%; mean rank, 3). The other 3 approaches had similar results, with mean ranks of 2 and SUCRA values of 54.1 %, 53.9 %, and 53.8%. The safety to efficacy ratios are plotted in the Figure and eFigure 6 in the Supplement. Considering overall morbidity as an indicator of safety, the safety to efficacy ratios were as follows: LC plus LCBDE, 0.8; LC plus PostERCP, 4.9; PreERCP plus LC, 0.5; and LC plus IntraERCP, 0.8. As shown in the Figure, the IntraERCP arm performed better than the others, with a safety to efficacy ratio that showed significantly better results in safety and efficacy (cophenetic correlation coefficient c = 0.85; maximum value of clustering gain, 393.54; and optimal number of clusters, 2). On the other hand, when the indicator of safety was overall mortality, the ratios were 0.9 for LC plus LCBDE, 4.2 for LC plus plus PostERCP, 1.2 for PreERCP plus LC, and 0.4 for LC plus IntraERCP. As shown in eFigure 6 in the Supplement, LC plus LCBDE, PreERCP plus LC, and LC plus IntraERCP had similar performance, and LC plus PostERCP had the worst performance (cophenetic correlation coefficient c = 0.88; maximum value of clustering gain, 310.90; and optimal number of clusters, 2) in safety and efficacy.
Figure. Cluster Ranking of the 4 Surgical Strategies.
Cluster rank combined the surface under the cumulative ranking curve (SUCRA) values, success rate, and morbidity rate. The x-axis reports efficacy (success rate). The success of the procedure was defined as the clearance of the common bile duct according to the intention-to-treat analysis. The success rate was calculated as the ratio of patients in whom the assigned procedure was completed without protocol violations to the number of all randomized patients in each arm. The y-axis reports SUCRA values as a percentage of safety, defined as morbidity rate. The different colors represent different clusters. LC + IntraERCP indicates laparoscopic cholecystectomy (LC) plus intraoperative endoscopic retrograde cholangiopancreatography; LC + LCBDE, LC plus laparoscopic common bile duct exploration; LC + PostERCP, LC plus postoperative ERCP; and LC + PreERCP, LC plus preoperative ERCP.
Secondary End Points
When acute pancreatitis was considered, the technique with the least probability to be the best was the PreERCP arm (SUCRA, 1.5%; mean rank, 4). The probability of the other techniques being the safest was as follows: LC plus IntraERCP (51.3%; mean rank, 3); LC plus Post ERCP (66.8%; mean rank, 2); and LC plus LCBDE (80.3%; mean rank, 2). Regarding biliary leak, the worst approach was undoubtedly LCBDE, which had a 4.9% probability of being the safest technique. The PostERCP (86.2%; mean rank, 1), PreERCP (59.6%; mean rank, 2), and IntraERCP (49.3%; mean rank, 3) approaches had a greater probability of being the safest. For overall bleeding, the approach with the highest odds to be the best was LCBDE (SUCRA, 83.3%; mean rank, 2) followed by PreERCP (SUCRA, 52.4%; mean rank, 2). The PostERCP (SUCRA, 24.4%) and the IntraERCP (SUCRA, 39.9%) arms ranked third. Considering overall operative time, the approach with the greatest odds to have lowest operative time was LC plus LCBDE (SUCRA, 90.2%; mean rank, 1). The other techniques had less than 50% odds of being best, with SUCRA results as follows: PostERCP, 29.5%; PreERCP, 49.6%; and IntraERCP, 30.7%. The LOS was probably shorter when the IntraERCP approach was used (SUCRA, 92.7%; mean rank, 1). The probability of a faster discharge was reduced when the LCBDE (SUCRA, 68.1%; mean rank, 2) and PreERCP (SUCRA, 22.1%; mean rank, 3) approaches were used. Finally, considering the total cost in US dollars, the procedure with the highest probability of being the least expensive was LCBDE (SUCRA, 98.9%; mean rank, 1) followed by IntraERCP (SUCRA, 39.6%; mean rank, 2) and PreERCP (SUCRA, 11.5%; mean rank, 3). The readmission rate was not computable.
Inconsistency, Heterogeneity, and Publication Bias
Inconsistency and heterogeneity are shown in Table 3. Success, overall morbidity and mortality, acute pancreatitis, biliary leak, overall bleeding, and LOS did not have significant local inconsistency within the networks. Local inconsistency due to loop A-C-D (see footnote “a” of Table 3 for explanation of these letters) was found in both operative time (indirect estimate, 19.05; 95% CI, 2.44-35.66; P = .02) and total cost (indirect estimate = 17.06; 95% CI, 3.56-107.21; P = .04). Heterogeneity was low (τ, <0.1) for overall mortality, acute pancreatitis, biliary leak, and overall bleeding. On the contrary, heterogeneity was reasonable for overall morbidity (τ, 0.2), higher for the success rate (τ, 0.8) and higher still for overall operative time, LOS, and total cost (τ, >1). No “small-study” effect was present (eFigure 7 in the Supplement) using visual evaluation. The Begg test showed significant P values for the comparisons of LC plus PreERCP vs LC plus IntraERCP regarding mortality (P = .09) and LC plus LCBDE vs LC plus IntraERCP regarding acute pancreatitis (P = .03).
Table 3. Loop Inconsistency and Heterogeneity.
| Outcome of Interest | No. of Studies | No. of Patients | Inconsistency | P Valueb | Heterogeneity, τ | |
|---|---|---|---|---|---|---|
| Loopa | IF or RoR (95% CI) | |||||
| Success rate | 20 | 2490 | A-C-D | 1.30 (1.00-5.70) | .73 | 0.8 |
| Overall morbidity | 19 | 2403 | A-C-D | 1.61 (1.00-3.78) | .27 | 0.2 |
| Overall mortality | 19 | 2403 | A-C-D | 1.21 (1.00-25.34) | .90 | <0.1 |
| Acute Pancreatitis | 19 | 2403 | A-C-D | 4.23 (1.00-24.43) | .11 | <0.1 |
| Biliary leak | 18 | 2320 | A-C-D | 1.91 (1.00-17.85) | .57 | <0.1 |
| Overall bleeding | 18 | 2320 | A-C-D | 4.63 (1.00-36.67) | .15 | <0.1 |
| Overall operative time | 8 | 1142 | A-C-D | 19.05 (2.44-35.65) | .02 | >1 |
| LOS | 14 | 1818 | A-C-D | 1.76 (0.00-4.28) | .64 | >1 |
| Total costs | 4 | 521 | A-C-D | 17.06 (3.56-107.21) | .04 | >1 |
Abbreviations: IF, absolute difference between direct and indirect estimates; LOS, length of postoperative hospital stay; RoR, logarithm of the ratio of 2 odds ratios.
A indicates laparoscopic cholecystectomy plus laparoscopic common bile duct exploration; C, preoperative endoscopic retrograde cholangiopancreatography plus laparoscopic cholecystectomy; and D, laparoscopic cholecystectomy plus intraoperative endoscopic retrograde cholangiopancreatography.
P values were calculated with the χ2 test.
Meta-regression Analysis
Meta-regression was performed for success rate, operative time, and LOS (eTable 3 in the Supplement). Analysis of success rate showed evidence that the success rate of LC plus PostERCP with respect to LC plus LCBDE (β = −0.92; P = .02) decreased in the most recent publications, the IntraERCP approach was more efficacious than LCBDE in Eastern countries (β = 2.8, P = .02), and, when the patients enrolled had only suspected disease, the PreERCP approach could be more efficacious than the LCBDE approach (β = 2.63, P < .001). Meta-regression for operative time and LOS did not show other significant influences.
Discussion
The present systematic review is the first, to our knowledge, to compare the 4 approaches available for the treatment of gallstone disease and biliary duct calculi by using a network meta-analysis. Its sample size of 2490 patients was greater than those of the 2 previous inconclusive meta-analyses,32,33 and it included 4 additional randomized clinical trials34,35,36,37 The first inclusive meta-analysis32 combined 2 very different techniques in addition to LC plus LCBDE and LC plus IntraERCP in the same arm under the category of 1-stage procedures. At the same time, those authors clustered another 2 different endoscopic approaches as well as PreERCP plus LC and LC plus PostERCP in the category of 2-stage procedures, leading to methodological bias. On the other hand, the second meta-analysis37 analyzed all 4 different choices separately but provided no indication as to the best technique. Moreover, in the literature, to date, there are not randomized studies comparing PreERCP vs PostERCP or PostERCP vs IntraERCP or studies with 4 arms. In this setting, the network analysis, with respect to the classic meta-analytic approach, has the important advantage of filling this literature gap, providing indications of comparisons that have never been performed before.
Some information regarding patient distribution was observed in our study. First, the most investigated approach was LCBDE (comprising 36.7% of the patients who provided data), followed by PreERCP plus LC (35.3%), and IntraERCP plus LC (24.5%). Second, the network geometries suggested that 2 main comparisons were lacking: PostERCP vs IntraERCP and PreERCP vs PostERCP. Third, these data suggested the scant appeal of the PostERCP approach (3.5%) to researchers, confirming that this approach was not a priori accepted by physicians and that it has not often been studied, although it is used in clinical practice. Regarding the primary end points, the best approach in terms of the safety to efficacy ratio and expressed as the morbidity to success ratio was the LC plus IntraERCP approach. That approach has the highest probability of being safe (SUCRA, 70%) and also the highest probability of being the most successful (SUCRA, 87%). These results could be explained by the fact that this approach did not require an advanced laparoscopic procedure or a laparoscopic choledochotomy.16 At the same time, it did not expose the patient to complications or even failure related to the difficult endoscopic cannulation of the CBD, which is usually related to use of the PreERCP plus LC approach.38 In addition, our study results showed that LCBDE remains much more effective than LC plus PostERCP because a direct exploration of the CBD during surgery could minimize the risk of incomplete clearance or the failure of ERCP due to the impossibility of CBD cannulation (SUCRA, 56% for LCBDE and 13% for LC plus PostERCP). Also, the PreERCP approach had the lowest probability of being safe (SUCRA, 24%) because the complications of this procedure were added to those of a cholecystectomy, producing an “avalanche” effect on the overall morbidity rate.
After calculating the safety to efficacy ratio as mortality to success, no approach was definitively superior to the others, although the data suggested that the LC plus PostERCP approach performed worst. However, overall mortality was 7 in the entire cohort of 2490 patients (0.003%), and the inferiority of the PostERCP approach depended much more on its low effectiveness than on its related mortality rate. The network approach was without inconsistency for success rate, overall morbidity and mortality, confirming the robustness of mixed estimates for the primary end point. However, a certain degree of heterogeneity was found for the success rate, and many factors could explain this. First, the publication year (ie, date of the study) influenced the effectiveness of the LCBDE approach; this was perhaps a result of the continued improvement in the laparoscopic instruments.39 Second, moving from Western to Eastern countries, the effectiveness of the rendezvous approach was increased with respect to the other 1-stage approach (LCBDE). This was probably owing to the differences in body mass index between the Western and Eastern populations, even if direct extrapolation of these data was rarely possible. It is possible, therefore, that the best results depended on the greater popularity of LCBDE and a greater willingness to use it. Third, patient selection can influence the success rate of the different approaches. In fact, in patients with proven CBD calculi, the success rate of PreERCP was higher than that of LC plus LCBDE, suggesting that laparoscopic exploration of the CBD remains a technical challenge in the case of actual CBD involvement. Regarding the secondary end points, LCBDE has the highest probability of being the best approach for avoiding acute pancreatitis (SUCRA, 80.3%), and PreERCP is indisputably the worst approach (SUCRA, 1.5%). These results can be explained by the fact that laparoscopic exploration of the CBD does not require any invasive procedure on the papilla. The scenario is different with regard to biliary leak: LCBDE was the worst approach (SUCRA, 4.9%). The PostERCP strategy had the highest probability of being the best approach (SUCRA, 86.2%); the remaining 2 approaches were fairly good, with SUCRA values ranging from 50% to 60%. These results can be explained by the fact that choledochotomy can introduce trauma to the CBD, which is not expected during perioperative ERCPs.
The PostERCP approach was the best approach. This is perhaps because ERCP after laparoscopic cholecystectomy could treat biliary leaks resulting from the cholecystectomy itself, resulting in an overall lower rate of fistulas. Potentially, an IntraERCP approach could also reduce a biliary leak due to LC by the routine use of a biliary stent or nasobiliary drainage to protect the cystic stump suture. However, the efficacy of this approach is impossible to establish because it was rarely reported in the included studies. Regarding overall bleeding, none of the approaches was superior, even if LCBDE had the highest SUCRA value, most likely because it does not require a papilla sphincterotomy. The best approach regarding overall operative time was LCBDE (SUCRA, 90.2%; mean rank, 1), reflecting the fact that this procedure is performed at the same time and by the same operator in a single session, saving the time needed to organize the endoscopic step. It should be noted that, even if LC plus IntraERCP is a 1-stage procedure as is LC plus LCBDE, it was not superior to the 2-stage procedures in terms of overall operative time. This could perhaps be explained by the fact that this approach requires 2 operators and different facilities and personnel organization. These data should be interpreted with caution because the network estimates showed conflicting results. On the contrary, LC plus IntraERCP showed a clear advantage in terms of LOS because it was the approach with the highest probability of shortening the LOS (SUCRA, 92.7%; mean rank, 1); it was followed by LCBDE (SUCRA, 68.1%). The PreERCP and PostERCP approaches were clearly the worst, with SUCRA values of approximately 20%. These data were not affected by inconsistency or publication bias; however, they presented high heterogeneity. Regarding total cost, speculation is limited by the presence of inconsistency.
Limitations
This study has some limitations. There was a high selection bias for the absence of a random sequence. Allocation concealment was absent in several included studies. The studies included different types of patients, those with suspected and those with confirmed CBD stones. In addition, there were small differences between the centers in performance of the same procedures.
Conclusions
Despite the aforementioned limitations, the present network meta-analysis is the first, to our knowledge, that attempts to answer the unaddressed question concerning the best operative management for gallstone disease and biliary duct calculi. In terms of morbidity and success rate, the rendezvous approach seemed to perform best. However, the other 3 procedures had specific advantages and disadvantages: LC plus LCBDE helped avoid acute pancreatitis, but it had a risk for biliary leaks; PreERCP plus LC was indisputably the worst choice in terms of acute pancreatitis. Finally, LC plus PostERCP was rarely studied, and it was the one with the lowest success rate.
eAppendix. Methods
eFigure 1. PRISMA Flow Diagram
eFigure 2. Quality Assessment of the Study Based on the Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Trials
eFigure 3. Network Geometry of All Outcomes
eFigure 4. Contribution Plots of All Outcomes
eFigure 5. Forest Plots of All Outcomes
eFigure 6. Cluster Rank Combined the Surface Under the Cumulative Ranking Curve (SUCRA) Values, Success Rate and Mortality Rate
eFigure 7. Funnel Plots of the Network Estimates of All Outcomes
eTable 1. Covariate Potential Source of Heterogeneity in the Studies Included
eTable 2. The Ranking of the Approaches Ranking for All Outcomes
eTable 3. Meta-regression of Confounding Covariates Influencing Heterogeneity
References
- 1.Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004;239(1):-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petelin JB. Laparoscopic common bile duct exploration. Surg Endosc. 2003;17(11):1705-1715. [DOI] [PubMed] [Google Scholar]
- 3.Purkayastha S, Tilney HS, Georgiou P, Athanasiou T, Tekkis PP, Darzi AW. Laparoscopic cholecystectomy versus mini-laparotomy cholecystectomy: a meta-analysis of randomised control trials. Surg Endosc. 2007;21(8):1294-1300. [DOI] [PubMed] [Google Scholar]
- 4.Williams E, Beckingham I, El Sayed G, et al. . Updated guideline on the management of common bile duct stones (CBDS). Gut. 2017;66(5):765-782. [DOI] [PubMed] [Google Scholar]
- 5.Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med. 2013;159(2):130-137. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane Collaboration. http://handbook.cochrane.org. Updated March 2011. Accessed January 3, 2018.
- 7.Hutton B, Salanti G, Caldwell DM, et al. . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta-analysis. JAMA. 2012;308(12):1246-1253. [DOI] [PubMed] [Google Scholar]
- 11.Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163-171. [DOI] [PubMed] [Google Scholar]
- 13.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683-691. [DOI] [PubMed] [Google Scholar]
- 14.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes M, Sussman L, Cohen L, Lewis MP. Randomised trial of laparoscopic exploration of common bile duct versus postoperative endoscopic retrograde cholangiography for common bile duct stones. Lancet. 1998;351(9097):159-161. [DOI] [PubMed] [Google Scholar]
- 17.Cuschieri A, Lezoche E, Morino M, et al. . E.A.E.S. multicenter prospective randomized trial comparing two-stage vs single-stage management of patients with gallstone disease and ductal calculi. Surg Endosc. 1999;13(10):952-957. [DOI] [PubMed] [Google Scholar]
- 18.Sgourakis G, Karaliotas K. Laparoscopic common bile duct exploration and cholecystectomy versus endoscopic stone extraction and laparoscopic cholecystectomy for choledocholithiasis: a prospective randomized study. Minerva Chir. 2002;57(4):467-474. [PubMed] [Google Scholar]
- 19.Nathanson LK, O’Rourke NA, Martin IJ, et al. . Postoperative ERCP versus laparoscopic choledochotomy for clearance of selected bile duct calculi: a randomized trial. Ann Surg. 2005;242(2):188-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong DF, Xin Y, Chen DW. Comparison of laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and laparoscopic exploration of the common bile duct for cholecystocholedocholithiasis. Surg Endosc. 2006;20(3):424-427. [DOI] [PubMed] [Google Scholar]
- 21.Lella F, Bagnolo F, Rebuffat C, Scalambra M, Bonassi U, Colombo E. Use of the laparoscopic-endoscopic approach, the so-called “rendezvous” technique, in cholecystocholedocholithiasis: a valid method in cases with patient-related risk factors for post-ERCP pancreatitis. Surg Endosc. 2006;20(3):419-423. [DOI] [PubMed] [Google Scholar]
- 22.Morino M, Baracchi F, Miglietta C, Furlan N, Ragona R, Garbarini A. Preoperative endoscopic sphincterotomy versus laparoendoscopic rendezvous in patients with gallbladder and bile duct stones. Ann Surg. 2006;244(6):889-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rábago LR, Vicente C, Soler F, et al. . Two-stage treatment with preoperative endoscopic retrograde cholangiopancreatography (ERCP) compared with single-stage treatment with intraoperative ERCP for patients with symptomatic cholelithiasis with possible choledocholithiasis. Endoscopy. 2006;38(8):779-786. [DOI] [PubMed] [Google Scholar]
- 24.Noble H, Tranter S, Chesworth T, Norton S, Thompson M. A randomized, clinical trial to compare endoscopic sphincterotomy and subsequent laparoscopic cholecystectomy with primary laparoscopic bile duct exploration during cholecystectomy in higher risk patients with choledocholithiasis. J Laparoendosc Adv Surg Tech A. 2009;19(6):713-720. [DOI] [PubMed] [Google Scholar]
- 25.Bansal VK, Misra MC, Garg P, Prabhu M. A prospective randomized trial comparing two-stage versus single-stage management of patients with gallstone disease and common bile duct stones. Surg Endosc. 2010;24(8):1986-1989. [DOI] [PubMed] [Google Scholar]
- 26.Rogers SJ, Cello JP, Horn JK, et al. . Prospective randomized trial of LC+LCBDE vs ERCP/S+LC for common bile duct stone disease. Arch Surg. 2010;145(1):28-33. [DOI] [PubMed] [Google Scholar]
- 27.ElGeidie AA, ElEbidy GK, Naeem YM. Preoperative versus intraoperative endoscopic sphincterotomy for management of common bile duct stones. Surg Endosc. 2011;25(4):1230-1237. [DOI] [PubMed] [Google Scholar]
- 28.ElGeidie AA, ElShobary MM, Naeem YM. Laparoscopic exploration versus intraoperative endoscopic sphincterotomy for common bile duct stones: a prospective randomized trial. Dig Surg. 2011;28(5-6):424-431. [DOI] [PubMed] [Google Scholar]
- 29.Ferulano GP, Dilillo S, D’Ambra M, et al. . Laparoscopic one-stage vs endoscopic plus laparoscopic management of common bile ductstones—a prospective randomized study In: Iancu C, ed. Advances in Endoscopic Surgery. London, England: InTech; 2011:291-306. [Google Scholar]
- 30.Tzovaras G, Baloyiannis I, Zachari E, et al. . Laparoendoscopic rendezvous versus preoperative ERCP and laparoscopic cholecystectomy for the management of cholecysto-choledocholithiasis: interim analysis of a controlled randomized trial. Ann Surg. 2012;255(3):435-439. [DOI] [PubMed] [Google Scholar]
- 31.Koc B, Karahan S, Adas G, Tutal F, Guven H, Ozsoy A. Comparison of laparoscopic common bile duct exploration and endoscopic retrograde cholangiopancreatography plus laparoscopic cholecystectomy for choledocholithiasis: a prospective randomized study. Am J Surg. 2013;206(4):457-463. [DOI] [PubMed] [Google Scholar]
- 32.Prasson P, Bai X, Zhang Q, Liang T. One-stage laproendoscopic procedure versus two-stage procedure in the management for gallstone disease and biliary duct calculi: a systemic review and meta-analysis. Surg Endosc. 2016;30(8):3582-3590. [DOI] [PubMed] [Google Scholar]
- 33.Nagaraja V, Eslick GD, Cox MR. Systematic review and meta-analysis of minimally invasive techniques for the management of cholecysto-choledocholithiasis. J Hepatobiliary Pancreat Sci. 2014;21(12):896-901. [DOI] [PubMed] [Google Scholar]
- 34.Ding G, Cai W, Qin M. Single-stage vs. two-stage management for concomitant gallstones and common bile duct stones: a prospective randomized trial with long-term follow-up. J Gastrointest Surg. 2014;18(5):947-951. [DOI] [PubMed] [Google Scholar]
- 35.Sahoo MR, Kumar AT, Patnaik A. Randomised study on single stage laparo-endoscopic rendezvous (intra-operative ERCP) procedure versus two stage approach (pre-operative ERCP followed by laparoscopic cholecystectomy) for the management of cholelithiasis with choledocholithiasis. J Minim Access Surg. 2014;10(3):139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lv F, Zhang S, Ji M, Wang Y, Li P, Han W. Single-stage management with combined tri-endoscopic approach for concomitant cholecystolithiasis and choledocholithiasis. Surg Endosc. 2016;30(12):5615-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poh BR, Ho SP, Sritharan M, et al. . Randomized clinical trial of intraoperative endoscopic retrograde cholangiopancreatography versus laparoscopic bile duct exploration in patients with choledocholithiasis. Br J Surg. 2016;103(9):1117-1124. [DOI] [PubMed] [Google Scholar]
- 38.Löhr JM, Aabakken L, Arnelo U, et al. . How to cannulate? a survey of the Scandinavian Association for Digestive Endoscopy (SADE) in 141 endoscopists [published correction appears in Scand J Gastroenterol. 2014;49(10):1254]. Scand J Gastroenterol. 2012;47(7):861-869. [DOI] [PubMed] [Google Scholar]
- 39.Zhan X, Wang Y, Zhu J, Lin X. Laparoscopic choledocholithotomy with a novel articulating forceps. Surg Innov. 2016;23(2):124-129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eFigure 1. PRISMA Flow Diagram
eFigure 2. Quality Assessment of the Study Based on the Cochrane Collaboration Tool for Assessing Risk of Bias in Randomized Trials
eFigure 3. Network Geometry of All Outcomes
eFigure 4. Contribution Plots of All Outcomes
eFigure 5. Forest Plots of All Outcomes
eFigure 6. Cluster Rank Combined the Surface Under the Cumulative Ranking Curve (SUCRA) Values, Success Rate and Mortality Rate
eFigure 7. Funnel Plots of the Network Estimates of All Outcomes
eTable 1. Covariate Potential Source of Heterogeneity in the Studies Included
eTable 2. The Ranking of the Approaches Ranking for All Outcomes
eTable 3. Meta-regression of Confounding Covariates Influencing Heterogeneity