Key Points
Question
Is frequent or heavy cannabis use associated with cognitive dysfunction in adolescents and young adults?
Findings
This systematic review and meta-analysis of 69 cross-sectional studies of 2152 cannabis users and 6575 comparison participants showed a small but significant overall effect size for reduced cognitive functioning in adolescents and young adults who reported frequent cannabis use. However, studies requiring abstinence from cannabis for longer than 72 hours had a very small, nonsignificant effect size.
Meaning
Although continued cannabis use may be associated with small reductions in cognitive functioning, results suggest that cognitive deficits are substantially diminished with abstinence.
This systematic review and meta-analysis investigates the association of cannabis with cognitive functioning in adolescents and young adults.
Abstract
Importance
Substantial shifts in perception and policy regarding cannabis have recently occurred, with use of cannabis increasing while its perceived harm decreases. One possible risk of increased cannabis use is poorer cognitive functioning, especially in youth.
Objective
To provide the first quantitative synthesis of the literature examining cannabis and cognitive functioning in adolescents and young adults (with a mean age of 26 years and younger).
Data Sources
PubMed, PsycInfo, Academic Search Premier, Scopus, and bibliographies of relevant reviews were searched for peer-reviewed, English-language studies from the date the databases began through May 2017.
Study Selection
Consensus criteria were used to determine study inclusion through abstract and manuscript review.
Data Extraction and Synthesis
This study followed Meta-analysis of Observational Studies in Epidemiology guidelines. Effect size estimates were calculated using multivariate mixed-effects models for cognitive functioning outcomes classified into 10 domains.
Main Outcomes and Measures
Results from neurocognitive tests administered in cross-sectional studies were primary outcomes, and we examined the influence of a priori explanatory variables on variability in effect size.
Results
Sixty-nine studies of 2152 cannabis users (mean [SD] age, 20.6 [2.8] years; 1472 [68.4%] male) and 6575 comparison participants with minimal cannabis exposure were included (mean [SD] age, 20.8 [3.4]; 3669 [55.8%] male). Results indicated a small overall effect size (presented as mean d) for reduced cognitive functioning associated with frequent or heavy cannabis use (d, −0.25; 95% CI, −0.32 to −0.17; P < .001). The magnitude of effect sizes did not vary by sample age or age at cannabis use onset. However, studies requiring an abstinence period longer than 72 hours (15 studies; n = 928) had an overall effect size (d, −0.08; 95% CI, −0.22 to 0.07) that was not significantly different from 0 and smaller than studies with less stringent abstinence criteria (54 studies; n = 7799; d, −0.30; 95% CI, −0.37 to −0.22; P = .01).
Conclusions and Relevance
Associations between cannabis use and cognitive functioning in cross-sectional studies of adolescents and young adults are small and may be of questionable clinical importance for most individuals. Furthermore, abstinence of longer than 72 hours diminishes cognitive deficits associated with cannabis use. Although other outcomes (eg, psychosis) were not examined in the included studies, results indicate that previous studies of cannabis in youth may have overstated the magnitude and persistence of cognitive deficits associated with use. Reported deficits may reflect residual effects from acute use or withdrawal. Future studies should examine individual differences in susceptibility to cannabis-associated cognitive dysfunction.
Introduction
Substantial shifts in the legality and public perceptions of cannabis have recently occurred in the United States. Cannabis use has increased, while the perception of its harms has decreased.1,2 In view of these trends, it is of considerable public health importance to delineate potential risks of cannabis use. However, scientific debates about physical and mental health consequences of cannabis remain unresolved. A critical question concerns potential cognitive dysfunction associated with cannabis use during adolescence and early adulthood, when use typically begins and substantial neurodevelopment continues to occur. To address this question, we conducted a meta-analysis specifically examining studies of cognitive functioning in adolescent and young adult cannabis users.
Adolescence is a period of dynamic neurobiological and behavioral changes. Substantial increases in cognitive capacities, particularly in executive functioning,3 occur alongside marked neurodevelopmental changes (eg, maturation of prefrontal networks) that continue into the mid-20s.4,5 Because of this prolonged neurodevelopmental period and the potential involvement of the endocannabinoid system in such changes,6,7 concerns have increased regarding use of cannabis during this putative critical period of brain development.8,9
While there is consensus that acute cannabis intoxication results in cognitive deficits, residual cognitive effects from cannabis (ie, ones that persist after acute intoxication) are still debated, particularly after a period of abstinence. Numerous studies in adolescents and young adults have reported associations between frequent or early-onset cannabis use and poorer cognitive performance in tasks requiring executive functioning, attention, and episodic memory.10,11,12,13,14 However, findings are somewhat inconsistent,15,16 with several explanatory and confounding variables contributing to variability; these include psychiatric and substance use comorbidities, frequency of cannabis use, and length of abstinence.17,18,19,20
Qualitative reviews of this literature have provided valuable insights, and most have concluded that adolescents and young adults are at heightened risk of cannabis-associated cognitive deficits, especially with early cannabis use.8,15,21,22 However, qualitative reviews can be selective; they rely primarily on statistical significance, typically do not conduct analyses of potential bias, and cannot provide accurate estimates of the magnitude of associations or influence of important variables that might contribute to variability in findings. Meta-analysis is a powerful method for synthesizing results across existing literature and examining whether explanatory variables affect variability in outcomes. Meta-analysis also addresses inconsistences by standardizing outcomes and diminishing the effects of varying statistical power. To date, 3 meta-analyses of adult cannabis users exist,23,24,25 reporting small negative associations between attention, learning, memory, and executive functioning and frequent or heavy cannabis use. Yet effects were almost undetectable in studies that require users to maintain a few days to weeks of abstinence prior to assessment.23,24 However, a meta-analysis has not been conducted specifically in adolescents or young adults. In this study, we extend prior qualitative reviews by providing quantitative estimates of potential associations between heavy/frequent cannabis use and cognitive functioning in adolescents and young adults. We also examined potential associations between variability in effect sizes and a predetermined set of explanatory variables, including study design and subject characteristics proposed to influence cognition in cannabis-using youth.15,21,22
Methods
Study Eligibility
We followed Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines.26 We began by defining a priori meta-analysis study inclusion criteria as any study that (1) assessed human adolescents and/or young adults (with a mean age of 26 years or younger, to include potentially sensitive neurodevelopmental periods22); (2) identified heavy, frequent, and/or problematic cannabis use as the primary variable of interest; (3) did not solely identify cannabis as a comorbidity to another substance use or mental health disorder; (4) did not focus on acute effects; (5) included an appropriate comparison group; (6) reported at least 1 standardized neurocognitive test; (7) was written in English; and (8) provided sufficient data to calculate effect sizes. These criteria were intentionally designed to provide a comprehensive representation of existing research while also allowing the empirical examination of relationships between variability in study methods or study samples and effect sizes. (Details are presented in the eMethods in the Supplement.)
Only observational, cross-sectional studies were included. Reliable estimates for longitudinal studies were indeterminable; there were few such studies, with heterogeneity in length of follow-up and methods of reporting cognitive data, and we believed that inference would be imprecise and unreliable with this small number of heterogeneous studies. However, baseline data from longitudinal studies were used where available.
Search Strategies and Study Selection
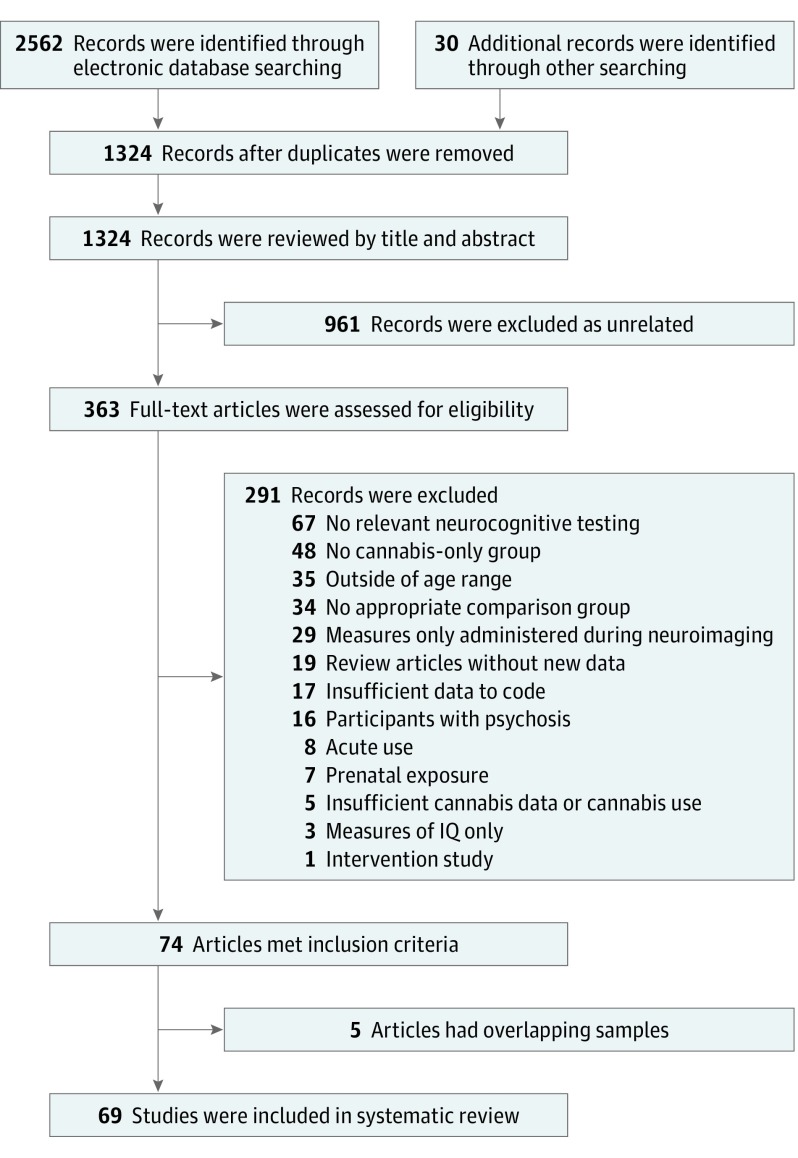
Systematic literature searches were independently conducted by 2 of us (J.C.S. and S.T.S.) in PubMed, PsycINFO, Academic Search Premier, and Scopus, beginning on December 10, 2016, and continuing until final searches were completed on May 12, 2017. The publication date range for included studies was from the database start date to May 12, 2017. The eMethods in the Supplement include an example full electronic search for PubMed. All identified articles were independently reviewed by the same 2 authors and supplemented by searches of qualitative reviews.8,15,21,22 Of the 2592 records initially retrieved, 363 full-text articles were assessed and 74 met inclusion criteria. After 5 studies with overlapping samples were removed, 69 studies were found to be eligible (Figure 1).
Figure 1. Flowchart of Searches for Studies Included in the Meta-analysis.
Data Extraction
Study information was independently extracted by 2 researchers (S.T.S. and J.D.J.), with discrepancies in coding resolved with by a third researcher (J.C.S.). Because certain cognitive domains may have different sensitivities to cannabis-associated effects,15 raters classified tests into domains based on evidence of construct validity. These domains were attention, learning, delayed memory, speed of information processing, verbal/language, visuospatial, motor functioning, and executive functioning (eMethods in the Supplement). To examine specific subcomponents of executive functioning, this domain was separated into abstraction/shifting, updating/working memory, and inhibition subdomains based on a well-supported model of executive functioning.27,28 See eTable 1 in the Supplement for tests in each cognitive domain.
Effect Size Calculation
We used the standardized mean difference statistic (d) as the measure of effect size, applied Hedges and Olkin correction for small sample bias,29 and used the variance for each d to determine a weighting factor for the unbiased effect size. Measures where low scores indicated better performance were adjusted so that a negative d indicated worse performance in the cannabis group.
Funnel plot tests and exploratory analyses were conducted to examine potential small study bias including the method of Egger et al30 to test for small study effects. Since no trim-and-fill method exists for multivariate mixed-effects meta-analysis, the Duval and Tweedie trim-and-fill method31 for random-effects analyses provided an estimate of potentially missing effect sizes.
Statistical Analyses
Analyses were conducted using a mixed-effects multivariate model (eMethods in the Supplement).32,33 Since most studies reported multiple cognitive measures, this method was chosen to allow for multiple outcomes per study. A multivariate model allows for multiple correlated within-study effect sizes, takes the hierarchical (clustered) data structure into account, and permits different cluster sizes (ie, effect sizes per study). A framework for such analyses is provided by Generalized Linear Latent and Mixed Models (GLLAMM) implemented in Stata version 13 (StataCorp),34 which we have applied in prior meta-analyses.35,36
We defined a 2-level mixed-effects model; level 1 is represented by effect sizes within studies, and level 2 is represented by different studies. This model examines variability of effect sizes between studies (random factor) and associations between various explanatory variables (fixed factors) and effect sizes. Fixed-effects and random-effects parameters and their variances and covariance are estimated via adaptive quadrature, a robust and flexible numeric integration approach allowing heteroscedastic level 1 variances.37
Results
Preliminary Analyses
There were 69 eligible studies (Figure 1; Table) with 8727 participants, including 2152 cannabis users and 6575 comparison participants who had minimal cannabis use. Studies were published between 1973 and 2017. We coded 384 effect sizes from 69 studies (mean (SD), 9.46 [5.32]; range, 1-17). Cannabis users in the studies had a mean (SD) age of 20.6 (2.8) years and were 68.1% male. Comparison participants had a mean (SD) age of 20.8 (3.4) years and were 55.8% male. Studies included were predominantly conducted in the United States, United Kingdom, Europe, and Australia. Cannabis users had a mean (SD) age at cannabis use initiation of 15.2 (1.5) years. The mean (SD) time of abstinence required by the studies was 152.7 (335.2) hours. Twenty-two studies (32%) reported either 0 hours of abstinence or no specificity in abstinence criteria, 32 studies (46%) reported between 1 and 72 hours of abstinence, and 15 studies (22%) reported greater than 72 hours of abstinence.
Table. Overview of the 69 Studies Included in the Meta-analysisa.
| Source | No. of Participants | Cognitive Domains Assessed | Age Range of Participants, y | Minimum Required Abstinence Period, h | |
|---|---|---|---|---|---|
| Cannabis User Group | Comparison Group | ||||
| Ashtari et al,38 2011 | 14 | 14 | Learning, delayed memory | 18-20 | 720 |
| Becker et al,39 2014 | 35 | 35 | Attention, learning, delayed memory, SIP, EF-U/WM, EF-A/S, V/L, motor | 18-20 | 12 |
| Brown et al,40 2010 | 32 | 33 | Learning, delayed memory | ≥18 | 48 |
| Churchwell et al,41 2010 | 18 | 18 | V/L | 16-19 | 0 |
| Cousijn et al,42 2013 | 17 | 26 | EF-I | 18-30 | 0 |
| Cousijn et al,42 2013 | 10 | 26 | EF-I | 18-30 | 0 |
| Croft et al,43 2001 | 18 | 31 | Learning, delayed memory, SIP, EF-I, EF-U/WM, V/L, motor | ≥18 | 48 |
| Cuttler et al,44 2012 | 48 | 48 | Learning, EF-U/WM | 17-33 | 0 |
| Cuyàs et al,45 2011 | 110 | 93 | Delayed memory, learning, visuospatial, SIP, V/L | ≥18 | 72 |
| Dougherty et al,10 2013 | 45 | 48 | Attention, EF-U/WM, EF-A/S, EF-I, learning | 14-17 | 18 |
| Ehrenreich et al,14 1999 | 48 | 49 | Attention, EF-U/WM, EF-A/S | ≥18 | 24 |
| Ehrenreich et al,14 1999 | 51 | 49 | Attention, EF-U/WM, EF-A/S | ≥18 | 24 |
| Epstein & Kumra,46 2014 | 29 | 53 | Attention, EF-I | 10-23 | 0 |
| Filbey et al,47 2015 | 36 | 16 | Learning, delayed memory | 18-50 | 72 |
| Filbey et al,47 2015 | 19 | 16 | Learning, delayed memory | 18-50 | 72 |
| Flavel et al,48 2013 | 10 | 10 | Motor | ≥18 | 12 |
| Fried et al,17 2005 | 35 | 59 | Attention, learning, delayed memory, SIP, EF-U/WM, EF-A/S | 17-21 | 2160 |
| Fried et al,17 2005 | 35 | 59 | Attention, learning, delayed memory, SIP, EF-U/WM, EF-A/S | 17-21 | 0 |
| Gonzalez et al,49 2012 | 65 | 65 | EF-I, learning | 17-24 | 24 |
| Gouzoulis-Mayfrank et al,50 2000 | 28 | 28 | Attention, learning, delayed memory, EF-U/WM, EF-A/S, EF-I, V/L | 18-31 | 24 |
| Grant et al,51 2012 | 16 | 214 | Attention, EF-U/WM, EF-A/S, EF-I | 18-29 | 0 |
| Grant et al,52 1973 | 29 | 29 | Learning, EF-A/S, SIP | ≥18 | 0 |
| Gruber et al,13 2012 | 19 | 28 | Attention, learning, delayed memory, SIP, EF-U/WM, EF-A/S, EF-I, visuospatial, V/L | ≥18 | 12 |
| Gruber et al,13 2012 | 15 | 28 | Attention, learning, delayed memory, SIP, EF-U/WM, EF-A/S, EF-I, visuospatial, V/L | ≥18 | 12 |
| Hadjiefthyvoulou et al,53 2011 | 12 | 18 | Learning, delayed memory | ≥18 | 24 |
| Hanson et al,54 2010 | 19 | 21 | Attention, EF-U/WM | 15-19 | 504 |
| Hanson et al,55 2014 | 24 | 34 | Attention, EF-U/WM, EF-A/S, SIP, V/L | 17-20 | 336 |
| Harvey et al,56 2007 | 34 | 36 | Attention, learning, delayed memory, EF-U/WM, EF-A/S, SIP | 13-18 | 12 |
| Hermann et al,57 2007 | 13 | 13 | Attention, learning, delayed memory, EF-U/WM, EF-A/S | ≥18 | 0 |
| Herzig et al,58 2014 | 35 | 48 | Delayed memory, EF-U/WM, EF-A/S | ≥18 | 2 |
| Hooper et al,18 2014 | 33 | 43 | Attention, learning, delayed memory, EF-I, EF-A/S, EF-U/WM | 12-17 | 720 |
| Houck et al,59 2013 | 36 | 33 | EF-U/WM | 14-18 | 0 |
| Jacobsen et al,60 2004 | 20 | 25 | Attention | 13-18 | 720 |
| Jacobus et al,61 2014 | 24 | 30 | Attention, learning, delayed memory, EF-I, EF-A/S, EF-U/WM, V/L, visuospatial, SIP, motor | 15-18 | 672 |
| Jacobus et al,62 2015 | 49 | 59 | Attention, learning, delayed memory, EF-I, EF-A/S, EF-U/WM, V/L, visuospatial, SIP, motor | 15-18 | 672 |
| Lamers et al,63 2006 | 15 | 15 | EF-I, EF-A/S, learning, delayed memory, SIP, visuospatial | 21-42 | 0 |
| Lane et al,64 2007 | 22 | 31 | EF-A/S | 14-18 | 0 |
| Lisdahl & Price,65 2012 | 23 | 36 | Attention, learning, delayed memory, EF-I, EF-A/S, V/L | 18-28 | 168 |
| de Sola Llopis66 et al, 2008 | 23 | 34 | Attention, EF-I, EF-A/S, learning, delayed memory, V/L, SIP | ≥18 | 72 |
| Mahmood et al,67 2010 | 65 | 65 | Learning, delayed memory, visuospatial | 15-19 | 552 |
| Medina et al,11 2007 | 31 | 34 | Attention, learning, delayed memory, EF-A/S, EF-U/WM, SIP, visuospatial, V/L | 16-18 | 552 |
| Messinis et al,68 2006 | 20 | 24 | Attention, learning, delayed memory, EF-A/S, SIP, V/L | 17-49 | 24 |
| Morgan et al,69 2012 | 29 | 30 | Attention, learning, delayed memory, V/L | 18-50 | 0 |
| Murphy et al,70 2011 | 13 | 12 | EF-I | 18-30 | 168 |
| Nestor et al,71 2008 | 35 | 38 | Learning, delayed memory | ≥18 | 0 |
| Price et al,72 2015 | 27 | 32 | EF-I, EF-U/WM | 18-25 | 168 |
| Pujol et al,73 2014 | 28 | 29 | Attention, learning, delayed memory | 18-30 | 12 |
| Quednow et al,74 2006 | 19 | 19 | Attention, learning, delayed memory | ≥18 | 72 |
| Rochford et al,75 1977 | 26 | 25 | Learning, visuospatial | ≥18 | 0 |
| Schwartz et al,76 1989 | 10 | 8 | Learning, delayed memory | 14-16 | 0 |
| Schweinsburg et al,77 2005 | 15 | 19 | Learning, delayed memory, EF-U/WM, EF-A/S, SIP, visuospatial | 15-17 | 48 |
| Schweinsburg et al,78 2010 | 13 | 18 | EF-U/WM | 15-18 | 48 |
| Schweinsburg et al,78 2010 | 13 | 18 | EF-U/WM | 15-18 | 648 |
| Scott et al,16 2017 | 227 | 3401 | Attention, EF-U/WM, EF-A/S, learning, visuospatial | 14-21 | 0 |
| Skosnik et al,79 2008 | 14 | 10 | EF-U/WM, SIP | 18-35 | 24 |
| Smith et al,80 2014 | 10 | 44 | EF-U/WM | ≥18 | 0 |
| Smith et al,81 2015 | 10 | 44 | Delayed memory | ≥18 | 0 |
| Solowij et al,12 2011 | 52 | 62 | Attention, learning, delayed memory | 16-20 | 12 |
| Tait et al,20 2011 | 60 | 420 | Learning, delayed memory, SIP, EF-U/WM | 20-24 | 0 |
| Tait et al,20 2011 | 60 | 420 | Learning, delayed memory, SIP, EF-U/WM | 20-24 | 0 |
| Takagi et al,82 2011b | 19 | 19 | EF-I | 13-24 | 24 |
| Takagi et al,83 2011a | 21 | 21 | Attention, learning, delayed memory | 13-24 | 24 |
| Takagi et al,84 2014 | 19 | 19 | EF-I | 13-24 | 24 |
| Tamm et al,85 2013 | 20 | 21 | Learning, delayed memory, EF-I, EF-U/WM, EF-A/S | ≥18 | 36 |
| Varma et al,86 1988 | 26 | 26 | SIP, visuospatial, learning, delayed memory | 15-35 | 12 |
| Verdejo-García et al,87 2013 | 86 | 58 | EF-U/WM, EF-A/S, SIP | 18-30 | 72 |
| Vilar-López et al,88 2013 | 19 | 18 | Attention, EF-I | 12-25 | 24 |
| Whitehurst et al,89 2015 | 17 | 13 | EF-I, learning, delayed memory, SIP | ≥18 | 0 |
| Winward et al,90 2014 | 20 | 55 | Learning, delayed memory, EF-U/WM, EF-A/S, SIP, visuospatial | 16-18 | 672 |
Abbreviations: EF-A/S, Executive functioning–abstraction/shifting; EF-I, Executive functioning–inhibition; EF-U/WM, Executive functioning–updating/working memory; SIP, speed of information processing; V/L, Verbal/Language.
eTable 2 in the Supplement contains a more complete overview of each study.
We first tested a model without explanatory variables,35,36 revealing that the overall mean neurocognitive effect size was d was −0.247 (SE, 0.038; 95% CI, −0.32 to −0.17), and the between-study variance estimate was 0.070 (SE, 0.018; P < .001), indicating that variance between studies was significantly more than that explained by sampling error alone.
eFigure 1A in the Supplement displays a funnel plot of effect size estimates against their standard error. Visual inspection of this funnel plot revealed asymmetry, and the test of Egger et al30 for small study effects revealed significant bias (t = 4.70; P < .001).
The Duval and Tweedie trim-and-fill method filled an additional 44 effect sizes and reduced the effect size by approximately 37.9% in random-effects analyses (from d = −.206; 95% CI, −0.24 to −0.16 to d = −0.128; 95% CI, −0.17 to −0.09; P < .001), although a significant effect size remained (eResults in the Supplement). However, the exact reduction in magnitude should be interpreted with caution.
Neurocognitive Domains
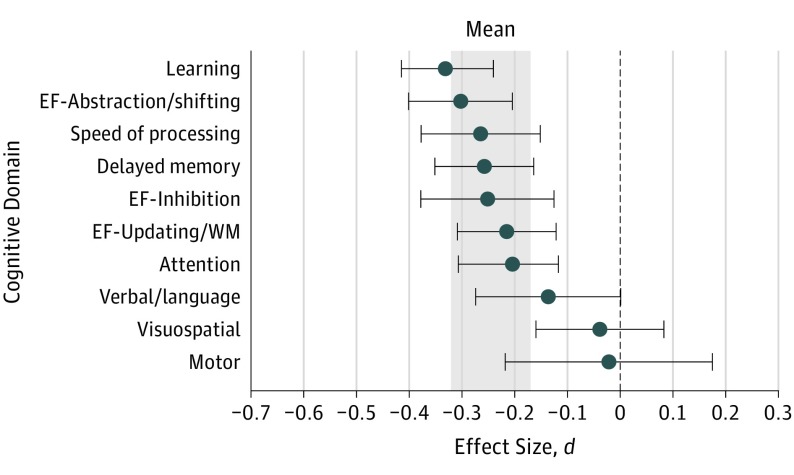
Figure 2 displays effect sizes by neurocognitive domain, which ranged from d = −0.33 to −0.02 (eResults in the Supplement). Effect sizes were significant in the domains of learning (d = −0.33; 95% CI, −0.42 to −0.24; P < .001), executive functioning-abstraction/shifting (d = −0.30; 95% CI, −0.40 to −0.20; P < .001), speed of information processing (d = −0.26; 95% CI, −0.38 to −0.15; P < .001), delayed memory (d = −0.26; 95% CI, −0.35 to −0.16; P < .001), executive functioning-inhibition (d = −0.25; 95% CI, −0.38 to −0.13; P < .001), executive functioning-updating/working memory (d = −.22; 95% CI, −0.31 to −0.12; P < .001), and attention (d = −0.21; 95% CI, −0.31 to −0.12; P < .001). Nonsignificant effect sizes were found in the domains of verbal/language (d = −0.14; 95% CI, −0.27 to 0.001; P = .05), visuospatial (d = −0.04; 95% CI, −0.16 to 0.08; P = .53), and motor functioning (d = −0.02; 95% CI, −0.22 to 0.18; P = .83). Significant differences in mean effect size estimates were found across neurocognitive domains (χ29 = 41.14; P < .001). However, there were no significant differences in effect size estimates between learning, delayed memory, attention, speed of information processing, or executive functioning domains after applying Bonferroni corrections.
Figure 2. Mean Weighted Effect Sizes for Each Neurocognitive Test Domain.
The mean value shown is the grand mean effect size of 69 included studies; d is the standardized mean difference. The shaded area indicates the 95% CI around the mean, −0.247. EF indicates executive functioning; SIP, speed of information processing; WM, working memory. Blue circles indicate the domain effect size d; gray bands, the overall means; error bars, 95% CIs.
Follow-up Analyses
Follow-up analyses were performed with several predetermined explanatory variables, including age at first cannabis use, sample sociodemographic characteristics, clinical characteristics (eg, depression), publication year, mean hours of abstinence, and length of required abstinence (longer than 72 hours vs 72 hours or less), given prior literature hypothesizing such moderating effects (hereafter, k indicates the number of studies corresponding to each variable).15,21,22
Subgroup analyses revealed no significant differences in effect sizes by the age category (adolescents or adults) of the sample population (eFigure 2 in the Supplement), early vs late cannabis use onset (ranging from 15 to 18 years old as defined by each individual study; studies were inconsistent in what age was considered early onset), whether studies matched groups by alcohol use, or period of publication (eFigure 3 in the Supplement). Further, mean age, mean age at first use, and between-groups difference in depression were not associated with variability in effect size estimates (eResults in the Supplement). However, studies with treatment-seeking samples (k = 12; n = 581; d, −0.43; 95% CI, −0.62 to −0.24) showed larger magnitude effect sizes (χ21 = 4.32; uncorrected P = .04) compared with non–treatment-seeking samples (k = 56; n = 8146; d, −0.22; 95% CI, −0.29 to −0.14) in a test for subgroup differences.
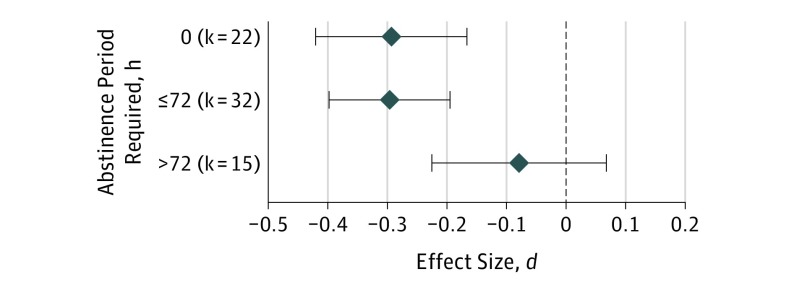
At an uncorrected threshold for multiple comparisons, mean hours of reported abstinence (which were available for k = 28; n = 1661; β = 0.059; P = .04) in each study was associated with variability in effect sizes, such that longer abstinence periods were associated with reductions in effect size magnitude. Furthermore, as shown in Figure 3, studies requiring an abstinence period longer than 72 hours (k = 15; n = 928) had an overall effect size that was not significantly different from 0 (d = −0.08, 95% CI, −0.22 to 0.07; P = .29) and was significantly smaller than studies with less stringent abstinence criteria (k = 54; n = 7799; χ21 = 6.36; P = .01).
Figure 3. Mean Weighted Effect Sizes for Varying Abstinence Criteria.
Subgroup analyses compared effect sizes (standardized mean difference d) from studies with abstinence periods longer than 72 hours to effect sizes from studies with abstinence lengths equal to or less than 72 hours. Data from all 3 groups are presented here to show that the subgroup of studies with unknown or 0 abstinence are not the primary contributor to reported subgroup differences. Blue diamonds indicate the domain effect size d; error bars, 95% CIs.
Discussion
Prior reviews have concluded that frequent use of cannabis impairs cognitive functioning in several domains, with greater deficits associated with adolescent vs adult onset of use.8,21,22,25 Our quantitative synthesis of data from 69 studies of adolescents and young adults revealed statistically significant but small cognitive effects associated with heavy/frequent cannabis use. These effects did not vary systematically by the age range studied or the age at which cannabis was initiated, although help-seeking samples in treatment evidenced slightly larger effects. Importantly, increasing abstinence was associated with smaller effect sizes, and studies that required an abstinence period from cannabis of longer than 72 hours had a very small, nonsignificant effect size. The magnitude of these deficits and their reduction by abstinence are consistent with prior meta-analyses conducted in adults with more chronic use patterns.23,24 Taken together, our analyses suggest a detectable but limited association between cannabis use and cognitive functioning in adolescents and young adults; for a majority of individuals, such effects may be of questionable clinical significance, especially after sustained abstinence. These findings converge with a recent report from the National Academies of Sciences, Engineering, and Medicine,91 which highlighted the multitude of confounders present in many studies and concluded that there is significant uncertainty about the presence of cannabis-associated cognitive deficits after sustained abstinence.
Findings Across Cognitive Domains
We found variability in effect sizes across cognitive domains, with the largest effects in learning and delayed memory, executive functioning, speed of processing, and attention. However, effect sizes in these domains were similar and within a relatively constricted range (mean d, −0.33 to −0.21). It is important to consider the practical implications of these effect size magnitudes. Although traditional conceptualizations of effect size magnitude do not necessarily correspond to clinical significance, all effect sizes in this study were below one-third of a standard deviation. Thus our results do not support the conclusion that frequent cannabis use is associated with large or even medium magnitude deficits in memory, attention, or other aspects of cognitive functioning. Although it could be argued that neurocognitive testing lacks sensitivity to detect cognitive abnormalities in cannabis abusers, prior meta-analyses in substances such as alcohol,92 methamphetamine,35 benzodiazepines,93,94 and cocaine95 have shown medium to large effect sizes, arguing against a lack of sensitivity. Moreover, recent large-scale structural neuroimaging studies also report conflicting data on cannabis-associated alterations in adolescents and adults.96,97,98,99,100
Length of Abstinence and Reduction of Effect Sizes
A notable finding in this meta-analysis was that the length of abstinence was associated with variance in effect sizes across studies, albeit at thresholds uncorrected for multiple comparisons. Although accurate measurement of abstinence is challenging because only 14 studies reported monitored abstinence, a longer required length of abstinence was associated with smaller magnitude effect sizes. Similarly, increasing the reported (as opposed to required) length of abstinence was associated with decreased magnitude of effect sizes. Moreover, studies with abstinence periods longer than 72 hours had small, nonsignificant effect sizes that were significantly less than studies with shorter abstinence periods, suggesting that some effects observed in studies associating cannabis use with cognitive dysfunction may be due to residual effects of recent use or withdrawal, rather than persistent changes associated with chronic use.23,101 Thus, small negative associations between continued cannabis use and cognitive functioning may diminish after sustained abstinence. However, these findings contrast with those from a large longitudinal study102 and a recent systematic review in adolescents and adults.25 Discrepancies with the latter may reflect differences in the age range covered, study selection, and methods of analysis.
Association of Age With Effect Sizes
Age did not influence cognitive effect size estimates. In fact, older samples had slightly larger (nonsignificant) effect sizes overall. Additionally, studies of early-onset cannabis users did not have significantly larger effects than studies examining late-onset users. Perhaps studies not specifically focusing on early-onset users nonetheless included substantial numbers of these individuals, because heavy cannabis users are more likely to initiate use at an early age. Taken together, these results do not support a heightened risk for poor cognitive outcomes in cannabis-using adolescents compared with adults, although such differences may emerge with adolescent onset and long-term frequent use, as previously reported.102,103 Only longitudinal data can delineate whether initiation of cannabis use during adolescence vs adulthood results in greater risks for brain-behavior functioning.
Considerations for Interpretation
The magnitude of these effect sizes and potential implications of findings should be considered in the context of additional relevant factors. First, it is critical to highlight that all psychoactive substances are associated with risks of use, and cannabis is no exception. Importantly, the data reported here do not address associations between cannabis use and other significant physical and mental health outcomes, such as negative lung functioning outcomes, deleterious outcomes on motivation, or risk for psychosis, which have been reported as heightened with chronic or early use.104,105,106
A second consideration is that functional outcomes may ultimately be more important than measures of cognitive functioning, and some studies suggest particular risks of early, heavy use for academic and occupational outcomes.107,108 However, findings regarding academic functioning have been inconsistent and may depend on other substance use or familial factors.109,110 These associations are obviously complex and will require more specific prospective modeling.
Third, there is likely heterogeneity in who is at greatest risk of brain-behavior problems associated with frequent cannabis use. Studies show interparticipant variability in behavioral and brain response to cannabis,111,112 which could contribute to individual differences in cognitive outcomes. Moreover, for certain individuals, small effects could be clinically meaningful because of individual differences influencing cognitive functioning (eg, socioeconomic status). On the other hand, most of the studies that were included predominantly enrolled frequent cannabis users or those with cannabis use disorders, and findings may not generalize to more occasional users or to those administered cannabinoids in medical settings.
Fourth, reported effect sizes may actually be overestimates, considering results from measures of bias. Smaller published studies often show larger effects than large studies, which can bias meta-analyses.30 Several factors can lead to these effects,113 including methodological differences or publication bias, in which statistically significant findings are more likely to be published.114 We found potential small study effects in this literature, and a data augmentation method that imputes missing studies (accounting for potential bias) suggested that effect sizes might be inflated. Furthermore, though some studies used normative neurocognitive data that adjust for influential demographic factors, our analyses primarily used raw scores to calculate effect sizes. As such, results do not account for sociodemographic, psychiatric, or substance use confounders, which are common in case-control studies of cannabis115,116; effects may be further attenuated once such factors are accounted for.
Finally, we cannot make conclusions about the causal contribution of cannabis to alterations in cognitive functioning since results do not account for cognitive deficits that may have existed prior to cannabis use initiation. Adolescents at risk of substance use problems may display cognitive vulnerabilities,17,117,118,119,120 which could partially contribute to cognitive findings described here, although they do not exclude the possibility of additional deficits. Furthermore, our data do not address associations between cannabis use and cognitive functioning over longer periods, although some included studies did examine chronic cannabis users and outcomes after protracted abstinence. Consideration of results from longitudinal studies offers conflicting evidence regarding long-term trajectories of cognitive functioning in cannabis users, especially after abstinence. Strong evidence for cognitive dysfunction associated with adolescent-onset, long-term frequent cannabis use comes from a longitudinal study of the Dunedin cohort,102 showing that individuals with adolescent-onset daily cannabis use who continued heavy use throughout adulthood showed declines in IQ and poorer cognitive functioning at age 38 years, even after adjusting for multiple relevant covariates. However, the sample size of this specific subgroup was small, which raises questions about generalizability. Further, other longitudinal studies argue against the strength or persistence of deficits over shorter periods,17,20 especially in studies where abstinence was carefully monitored.101 Two recent, large-scale studies also question the specificity of cannabis as a causal factor in predicting cognitive change after adjusting for confounding variables and familial factors.118,121 The landmark Adolescent Brain Cognitive Development study (https://abcdstudy.org) will hopefully help resolve discrepancies and answer critical questions about consequences of cannabis use with longitudinal data on 10 000 children aged 9 to 10 years in the United States. Additionally, once the quantity of longitudinal research increases, additional research syntheses should be conducted to ascertain the long-term effects of cannabis.
Future Directions
Studies of the therapeutic potential of cannabinoids continue to progress, with evidence of efficacy for several conditions (eg, nausea with cancer treatment).91 However, optimizing the risk/benefit profile of cannabinoids will require focused research into variables affecting outcomes. Studies would benefit from detailed characterization of cannabinoid content, as there may be divergent behavioral effects that depend on cannabinoid concentrations/ratios.122,123,124 Optimizing cannabis therapeutics will also require a comprehensive understanding of patient factors that affect risks to facilitate patient selection. There is likely substantial variability in risk for cognitive and mental health problems associated with cannabis use, and research into such factors (eg, genomic profiles) will be crucial to avoid unnecessary cannabinoid-related adverse effects.
Limitations
A substantial limitation in this literature is the heterogeneity in measurement of cannabis use (eTable 2 in the Supplement). There is little consensus regarding what level of cannabis use is hazardous for cognitive or mental health outcomes. A continuous measure of cannabis use could be useful to this end, but it is likely to be unreliable except in studies of consistent, frequent users or studies with detailed microlongitudinal data collection, which is often unfeasible. Research is also limited by variation in how cannabis use data are collected. For example, studies report data that vary substantially across time (eg, past year, past month), frequency levels (eg, per week, per day), measurements of quantity (eg, grams, joints), and, potentially, across cannabinoid content. To advance knowledge and provide valuable public health information, the field needs to converge on standardized cannabis use metrics125 or devise innovative ways of measuring cannabinoid levels126 to examine cumulative and frequency effects.
Neuropsychological meta-analyses are hindered by variability in tests administered across a body of literature, creating challenges in assigning outcomes into specific cognitive domains. Although tests purport to measure specific cognitive functions, most tests involve multiple cognitive processes. Thus, cautious interpretation of effect size differences between domains is warranted. However, this limitation is diminished in our meta-analysis given the substantial overlap in effect size magnitudes. Furthermore, studies often report multiple neurocognitive outcomes and focus primarily on significant between-group differences as evidence of cognitive deficits. We attempted to mitigate this outcome selection bias by selecting measures based on construct validity and not statistical significance, and avoided selecting multiple indices from individual tests that measure similar constructs. Moreover, we used sophisticated analytic models to account for within-study correlations. Together, these methods reduce the problems of multiple comparisons evident in this literature.
Conclusions
In light of the changing perceptions of cannabis use and an evolving policy landscape surrounding cannabis, understanding the potential risks of cannabis use for mental health and brain functioning is of paramount importance. In the first quantitative synthesis of 69 studies examining frequent or heavy use of cannabis by adolescents and young adults, we found statistically significant but small negative effect sizes in cognitive functioning associated with cannabis. Inconsistent with conclusions from previous reviews, we found little evidence for more severe effects with cannabis use at earlier ages or specifically in adolescence. Moreover, the data suggest associations between length of abstinence and restored cognitive functioning, with greater abstinence associated with smaller group differences. Furthermore, we found very small, nonsignificant effect sizes in studies that required more than 72 hours of cannabis abstinence. Large-scale longitudinal studies are needed to examine the effects of sustained, heavy cannabis use and identify genetic factors, individual differences, and cannabis use parameters that may affect risk for brain-behavior dysfunction in individuals who use cannabis.
eMethods. Supplementary Methods.
eResults. Results.
eTable 1. Overview of 69 Studies Included in the Meta-Analysis.
eTable 2. Neurocognitive Tests Analyzed in the Meta-Analysis, by Cognitive Domain.
eFigure 1. (A) Unadjusted and (B) trim-and-fill funnel plots with standardized mean difference effect sizes (d).
eFigure 2. Mean weighted effect sizes and 95% confidence intervals for age groups.
eFigure 3. Mean weighted effect sizes and 95% confidence intervals for year of publication.
References
- 1.Substance Abuse and Mental Health Services Administration Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 2.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future National Survey Results on Drug Use, 1975-2016: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2017. [Google Scholar]
- 3.Gur RC, Richard J, Calkins ME, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology. 2012;26(2):251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861-863. [DOI] [PubMed] [Google Scholar]
- 5.Satterthwaite TD, Wolf DH, Erus G, et al. Functional maturation of the executive system during adolescence. J Neurosci. 2013;33(41):16249-16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galve-Roperh I, Palazuelos J, Aguado T, Guzmán M. The endocannabinoid system and the regulation of neural development: potential implications in psychiatric disorders. Eur Arch Psychiatry Clin Neurosci. 2009;259(7):371-382. [DOI] [PubMed] [Google Scholar]
- 7.Ellgren M, Artmann A, Tkalych O, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol. 2008;18(11):826-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobus J, Tapert SF. Effects of cannabis on the adolescent brain. Curr Pharm Des. 2014;20(13):2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13(2):253-263. [DOI] [PubMed] [Google Scholar]
- 10.Dougherty DM, Mathias CW, Dawes MA, et al. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology (Berl). 2013;226(2):307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13(5):807-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solowij N, Jones KA, Rozman ME, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl). 2011;216(1):131-144. [DOI] [PubMed] [Google Scholar]
- 13.Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav. 2012;26(3):496-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenreich H, Rinn T, Kunert HJ, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology (Berl). 1999;142(3):295-301. [DOI] [PubMed] [Google Scholar]
- 15.Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev. 2013;23(2):117-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott JC, Wolf DH, Calkins ME, et al. Cognitive functioning of adolescent and young adult cannabis users in the Philadelphia Neurodevelopmental Cohort. Psychol Addict Behav. 2017;31(4):423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—a comparison with pre-drug performance. Neurotoxicol Teratol. 2005;27(2):231-239. [DOI] [PubMed] [Google Scholar]
- 18.Hooper SR, Woolley D, De Bellis MD. Intellectual, neurocognitive, and academic achievement in abstinent adolescents with cannabis use disorder. Psychopharmacology (Berl). 2014;231(8):1467-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardini D, White HR, Xiong S, et al. Unfazed or dazed and confused: does early adolescent marijuana use cause sustained impairments in attention and academic functioning? J Abnorm Child Psychol. 2015;43(7):1203-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106(12):2195-2203. [DOI] [PubMed] [Google Scholar]
- 21.Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. Considering cannabis: the effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addict Rep. 2014;1(2):144-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lubman DI, Cheetham A, Yücel M. Cannabis and adolescent brain development. Pharmacol Ther. 2015;148:1-16. [DOI] [PubMed] [Google Scholar]
- 23.Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9(5):679-689. [DOI] [PubMed] [Google Scholar]
- 24.Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp Clin Psychopharmacol. 2012;20(5):420-429. [DOI] [PubMed] [Google Scholar]
- 25.Ganzer F, Bröning S, Kraft S, Sack P-M, Thomasius R. Weighing the evidence: a systematic review on long-term neurocognitive effects of cannabis use in abstinent adolescents and adults. Neuropsychol Rev. 2016;26(2):186-222. [DOI] [PubMed] [Google Scholar]
- 26.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting; Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 27.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49-100. [DOI] [PubMed] [Google Scholar]
- 28.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. 2015;6:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedges LV, Olkin LI. Statistical Methods for Meta-Analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. [DOI] [PubMed] [Google Scholar]
- 32.Arends LR, Vokó Z, Stijnen T. Combining multiple outcome measures in a meta-analysis: an application. Stat Med. 2003;22(8):1335-1353. [DOI] [PubMed] [Google Scholar]
- 33.Kalaian HA, Raudenbush SW. A multivariate mixed linear model for meta-analysis. Psychol Methods. 1996;1(3):227-235. [Google Scholar]
- 34.Rabe-Hesketh S, Skrondal A, Pickles A.. GLLAMM manual; UC Berkeley Division of Biostatistics working paper series, paper 160. http://biostat.jhsph.edu/~fdominic/teaching/bio656/software/gllamm.manual.pdf. Published 2004. Accessed February 14, 2018.
- 35.Scott JC, Woods SP, Matt GE, et al. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17(3):275-297. [DOI] [PubMed] [Google Scholar]
- 36.Scott JC, Matt GE, Wrocklage KM, et al. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull. 2015;141(1):105-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rabe-Hesketh S, Skrondal A, Pickles A. Maximum likelihood estimation of limited and discrete dependent variable models with nested random effects. J Econom. 2005;128(2):301-323. [Google Scholar]
- 38.Ashtari M, Avants B, Cyckowski L, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45(8):1055-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Becker MP, Collins PF, Luciana M. Neurocognition in college-aged daily marijuana users. J Clin Exp Neuropsychol. 2014;36(4):379-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown J, McKone E, Ward J. Deficits of long-term memory in ecstasy users are related to cognitive complexity of the task. Psychopharmacology (Berl). 2010;209(1):51-67. [DOI] [PubMed] [Google Scholar]
- 41.Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 2010;1:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cousijn J, Watson P, Koenders L, Vingerhoets WA, Goudriaan AE, Wiers RW. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. 2013;38(12):2825-2832. [DOI] [PubMed] [Google Scholar]
- 43.Croft RJ, Mackay AJ, Mills AT, Gruzelier JG. The relative contributions of ecstasy and cannabis to cognitive impairment. Psychopharmacology (Berl). 2001;153(3):373-379. [DOI] [PubMed] [Google Scholar]
- 44.Cuttler C, McLaughlin RJ, Graf P. Mechanisms underlying the link between cannabis use and prospective memory. PLoS One. 2012;7(5):e36820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuyàs E, Verdejo-García A, Fagundo AB, et al. The influence of genetic and environmental factors among MDMA users in cognitive performance. PLoS One. 2011;6(11):e27206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Epstein KA, Kumra S. Executive attention impairment in adolescents with schizophrenia who have used cannabis. Schizophr Res. 2014;157(1-3):48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filbey FM, McQueeny T, Kadamangudi S, Bice C, Ketcherside A. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res. 2015;293:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flavel SC, White JM, Todd G. Abnormal maximal finger tapping in abstinent cannabis users. Hum Psychopharmacol. 2013;28(6):612-614. [DOI] [PubMed] [Google Scholar]
- 49.Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J Clin Exp Neuropsychol. 2012;34(9):962-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gouzoulis-Mayfrank E, Daumann J, Tuchtenhagen F, et al. Impaired cognitive performance in drug free users of recreational ecstasy (MDMA). J Neurol Neurosurg Psychiatry. 2000;68(6):719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug Alcohol Depend. 2012;121(1-2):159-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grant I, Rochford J, Fleming T, Stunkard A. A neuropsychological assessment of the effects of moderate marihuana use. J Nerv Ment Dis. 1973;156(4):278-280. [DOI] [PubMed] [Google Scholar]
- 53.Hadjiefthyvoulou F, Fisk JE, Montgomery C, Bridges N. Everyday and prospective memory deficits in ecstasy/polydrug users. J Psychopharmacol. 2011;25(4):453-464. [DOI] [PubMed] [Google Scholar]
- 54.Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanson KL, Thayer RE, Tapert SF. Adolescent marijuana users have elevated risk-taking on the balloon analog risk task. J Psychopharmacol. 2014;28(11):1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26(3):309-319. [DOI] [PubMed] [Google Scholar]
- 57.Hermann D, Sartorius A, Welzel H, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry. 2007;61(11):1281-1289. [DOI] [PubMed] [Google Scholar]
- 58.Herzig DA, Nutt DJ, Mohr C. Alcohol and relatively pure cannabis use, but not schizotypy, are associated with cognitive attenuations. Front Psychiatry. 2014;5:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abuse. 2013;39(6):414-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. 2004;1021:384-390. [DOI] [PubMed] [Google Scholar]
- 61.Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75(5):729-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobus J, Squeglia LM, Infante MA, et al. Neuropsychological performance in adolescent marijuana users with co-occurring alcohol use: a three-year longitudinal study. Neuropsychology. 2015;29(6):829-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamers CTJ, Bechara A, Rizzo M, Ramaekers JG. Cognitive function and mood in MDMA/THC users, THC users and non-drug using controls. J Psychopharmacol. 2006;20(2):302-311. [DOI] [PubMed] [Google Scholar]
- 64.Lane SD, Cherek DR, Tcheremissine OV, Steinberg JL, Sharon JL. Response perseveration and adaptation in heavy marijuana-smoking adolescents. Addict Behav. 2007;32(5):977-990. [DOI] [PubMed] [Google Scholar]
- 65.Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. J Int Neuropsychol Soc. 2012;18(4):678-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Sola Llopis S, Miguelez-Pan M, Peña-Casanova J, et al. Cognitive performance in recreational ecstasy polydrug users: a two-year follow-up study. J Psychopharmacol. 2008;22(5):498-510. [DOI] [PubMed] [Google Scholar]
- 67.Mahmood OM, Jacobus J, Bava S, Scarlett A, Tapert SF. Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. J Stud Alcohol Drugs. 2010;71(6):885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Messinis L, Kyprianidou A, Malefaki S, Papathanasopoulos P. Neuropsychological deficits in long-term frequent cannabis users. Neurology. 2006;66(5):737-739. [DOI] [PubMed] [Google Scholar]
- 69.Morgan CJA, Duffin S, Hunt S, Monaghan L, Mason O, Curran HV. Neurocognitive function and schizophrenia-proneness in individuals dependent on ketamine, on high potency cannabis (‘skunk’) or on cocaine. Pharmacopsychiatry. 2012;45(7):269-274. [DOI] [PubMed] [Google Scholar]
- 70.Murphy PN, Erwin PG, Maciver L, et al. The relationships of ‘ecstasy’ (MDMA) and cannabis use to impaired executive inhibition and access to semantic long-term memory. Hum Psychopharmacol. 2011;26(7):460-469. [DOI] [PubMed] [Google Scholar]
- 71.Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40(3):1328-1339. [DOI] [PubMed] [Google Scholar]
- 72.Price JS, McQueeny T, Shollenbarger S, Browning EL, Wieser J, Lisdahl KM. Effects of marijuana use on prefrontal and parietal volumes and cognition in emerging adults. Psychopharmacology (Berl). 2015;232(16):2939-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pujol J, Blanco-Hinojo L, Batalla A, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51:68-78. [DOI] [PubMed] [Google Scholar]
- 74.Quednow BB, Jessen F, Kuhn K-U, Maier W, Daum I, Wagner M. Memory deficits in abstinent MDMA (ecstasy) users: neuropsychological evidence of frontal dysfunction. J Psychopharmacol. 2006;20(3):373-384. [DOI] [PubMed] [Google Scholar]
- 75.Rochford J, Grant I, LaVigne G. Medical students and drugs: further neuropsychological and use pattern considerations. Int J Addict. 1977;12(8):1057-1065. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child. 1989;143(10):1214-1219. [DOI] [PubMed] [Google Scholar]
- 77.Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79(2):201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J Psychoactive Drugs. 2010;42(3):401-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Skosnik PD, Edwards CR, O’Donnell BF, Steffen A, Steinmetz JE, Hetrick WP. Cannabis use disrupts eyeblink conditioning: evidence for cannabinoid modulation of cerebellar-dependent learning. Neuropsychopharmacology. 2008;33(6):1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith MJ, Cobia DJ, Wang L, et al. Cannabis-related working memory deficits and associated subcortical morphological differences in healthy individuals and schizophrenia subjects. Schizophr Bull. 2014;40(2):287-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith MJ, Cobia DJ, Reilly JL, et al. Cannabis-related episodic memory deficits and hippocampal morphological differences in healthy individuals and schizophrenia subjects. Hippocampus. 2015;25(9):1042-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takagi M, Yücel M, Cotton SM, et al. Verbal memory, learning, and executive functioning among adolescent inhalant and cannabis users. J Stud Alcohol Drugs. 2011b;72(1):96-105. [DOI] [PubMed] [Google Scholar]
- 83.Takagi M, Lubman DI, Cotton S, et al. Executive control among adolescent inhalant and cannabis users. Drug Alcohol Rev. 2011a;30(6):629-637. [DOI] [PubMed] [Google Scholar]
- 84.Takagi MJ, Lubman DI, Cotton SM, Verdejo-García A, Vilar-López R, Yücel M. A signal detection analysis of executive control performance among adolescent inhalant and cannabis users. Subst Use Misuse. 2014;49(14):1920-1927. [DOI] [PubMed] [Google Scholar]
- 85.Tamm L, Epstein JN, Lisdahl KM, et al. ; MTA Neuroimaging Group . Impact of ADHD and cannabis use on executive functioning in young adults. Drug Alcohol Depend. 2013;133(2):607-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varma VK, Malhotra AK, Dang R, Das K, Nehra R. Cannabis and cognitive functions: a prospective study. Drug Alcohol Depend. 1988;21(2):147-152. [DOI] [PubMed] [Google Scholar]
- 87.Verdejo-García A, Fagundo AB, Cuenca A, et al. COMT val158met and 5-HTTLPR genetic polymorphisms moderate executive control in cannabis users. Neuropsychopharmacology. 2013;38(8):1598-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vilar-López R, Takagi M, Lubman DI, et al. The effects of inhalant misuse on attentional networks. Dev Neuropsychol. 2013;38(2):126-136. [DOI] [PubMed] [Google Scholar]
- 89.Whitehurst LN, Fogler K, Hall K, Hartmann M, Dyche J. The effects of chronic marijuana use on circadian entrainment. Chronobiol Int. 2015;32(4):561-567. [DOI] [PubMed] [Google Scholar]
- 90.Winward JL, Hanson KL, Tapert SF, Brown SA. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J Int Neuropsychol Soc. 2014;20(8):784-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.National Academies of Sciences, Engineering, and Medicine The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 92.Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18(2):203-213. [DOI] [PubMed] [Google Scholar]
- 93.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long-term benzodiazepine use: a meta-analysis. CNS Drugs. 2004;18(1):37-48. [DOI] [PubMed] [Google Scholar]
- 94.Barker MJ, Greenwood KM, Jackson M, Crowe SF. Persistence of cognitive effects after withdrawal from long-term benzodiazepine use: a meta-analysis. Arch Clin Neuropsychol. 2004;19(3):437-454. [DOI] [PubMed] [Google Scholar]
- 95.Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8(5):368-376. [DOI] [PubMed] [Google Scholar]
- 96.Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34(16):5529-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orr JM, Paschall CJ, Banich MT. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. Neuroimage Clin. 2016;12:47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pagliaccio D, Barch DM, Bogdan R, et al. Shared predisposition in the association between cannabis use and subcortical brain structure. JAMA Psychiatry. 2015;72(10):994-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thayer RE, YorkWilliams S, Karoly HC, et al. Structural neuroimaging correlates of alcohol and cannabis use in adolescents and adults. Addiction. 2017;112(12):2144-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. 2015;35(4):1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pope HG Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58(10):909-915. [DOI] [PubMed] [Google Scholar]
- 102.Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657-E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pope HG Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69(3):303-310. [DOI] [PubMed] [Google Scholar]
- 104.Jones JD, Calkins ME, Scott JC, Bach EC, Gur RE. Cannabis use, polysubstance use, and psychosis spectrum symptoms in a community-based sample of U.S. youth. J Adolesc Health. 2017;60(6):653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the association between the level of cannabis use and risk of psychosis. Schizophr Bull. 2016;42(5):1262-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167(3):221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Silins E, Horwood LJ, Patton GC, et al. ; Cannabis Cohorts Research Consortium . Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286-293. [DOI] [PubMed] [Google Scholar]
- 108.Castellanos-Ryan N, Pingault J-B, Parent S, Vitaro F, Tremblay RE, Séguin JR. Adolescent cannabis use, change in neurocognitive function, and high-school graduation: a longitudinal study from early adolescence to young adulthood. Dev Psychopathol. 2017;29(4):1253-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meier MH, Hill ML, Small PJ, Luthar SS. Associations of adolescent cannabis use with academic performance and mental health: a longitudinal study of upper middle class youth. Drug Alcohol Depend. 2015;156(suppl C):207-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Verweij KJH, Huizink AC, Agrawal A, Martin NG, Lynskey MT. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug Alcohol Depend. 2013;133(2):580-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Atakan Z, Bhattacharyya S, Allen P, et al. Cannabis affects people differently: inter-subject variation in the psychotogenic effects of Δ9-tetrahydrocannabinol: a functional magnetic resonance imaging study with healthy volunteers. Psychol Med. 2013;43(6):1255-1267. [DOI] [PubMed] [Google Scholar]
- 112.Batalla A, Crippa JA, Busatto GF, et al. Neuroimaging studies of acute effects of THC and CBD in humans and animals: a systematic review. Curr Pharm Des. 2014;20(13):2168-2185. [DOI] [PubMed] [Google Scholar]
- 113.Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119-1129. [DOI] [PubMed] [Google Scholar]
- 114.Dwan K, Altman DG, Arnaiz JA, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLoS One. 2008;3(8):e3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Daly M. Personality may explain the association between cannabis use and neuropsychological impairment. Proc Natl Acad Sci U S A. 2013;110(11):E979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rogeberg O. Correlations between cannabis use and IQ change in the Dunedin cohort are consistent with confounding from socioeconomic status. Proc Natl Acad Sci U S A. 2013;110(11):4251-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giancola PR, Tarter RE. Executive cognitive functioning and risk for substance abuse. Psychol Sci. 1999;10(3):203-205. [Google Scholar]
- 118.Meier MH, Caspi A, Danese A, et al. Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction. 2018;113(2):257-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sloboda Z, Glantz MD, Tarter RE. Revisiting the concepts of risk and protective factors for understanding the etiology and development of substance use and substance use disorders: implications for prevention. Subst Use Misuse. 2012;47(8-9):944-962. [DOI] [PubMed] [Google Scholar]
- 120.Tarter RE, Kirisci L, Mezzich A, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160(6):1078-1085. [DOI] [PubMed] [Google Scholar]
- 121.Jackson NJ, Isen JD, Khoddam R, et al. Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc Natl Acad Sci U S A. 2016;113(5):E500-E508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rømer Thomsen K, Callesen MB, Feldstein Ewing SW. Recommendation to reconsider examining cannabis subtypes together due to opposing effects on brain, cognition and behavior. Neurosci Biobehav Rev. 2017;80:156-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Morgan CJA, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study: naturalistic study [published correction appears in Br J Psychiatry. 2010;197(5):416]. Br J Psychiatry. 2010;197(4):285-290. [DOI] [PubMed] [Google Scholar]
- 124.Gruber SA, Sagar KA, Dahlgren MK, Racine MT, Smith RT, Lukas SE. Splendor in the grass? a pilot study assessing the impact of medical marijuana on executive function. Front Pharmacol. 2016;7:355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Solowij N, Lorenzetti V, Yücel M. Effects of cannabis use on human behavior: a call for standardization of cannabis use metrics. JAMA Psychiatry. 2016;73(9):995-996. [DOI] [PubMed] [Google Scholar]
- 126.Kevin RC, Allsop DJ, Lintzeris N, Dunlop AJ, Booth J, McGregor IS. Urinary cannabinoid levels during nabiximols (Sativex®)-medicated inpatient cannabis withdrawal. Forensic Toxicol. 2017;35(1):33-44.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29367861&dopt=Abstract29367861 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplementary Methods.
eResults. Results.
eTable 1. Overview of 69 Studies Included in the Meta-Analysis.
eTable 2. Neurocognitive Tests Analyzed in the Meta-Analysis, by Cognitive Domain.
eFigure 1. (A) Unadjusted and (B) trim-and-fill funnel plots with standardized mean difference effect sizes (d).
eFigure 2. Mean weighted effect sizes and 95% confidence intervals for age groups.
eFigure 3. Mean weighted effect sizes and 95% confidence intervals for year of publication.