Key Points
Question
Can latent variable statistical methods differentiate neural mechanisms of co-occurring symptom dimensions in youth?
Findings
In this magnetic resonance imaging study of 197 youth aged 8 to 18 years, bifactor analysis decomposed the unique and shared variances of irritability and anxiety symptoms. On a functional neuroimaging task assessing threat orienting, these phenotypes showed a double dissociation: irritability was associated with widespread perturbed neural activation, whereas anxiety was associated with perturbed amygdala connectivity.
Meaning
A bifactor approach to modeling pediatric psychopathology revealed a novel double dissociation in which phenotype-specific mechanisms were found to underlie clinically relevant threat orienting.
Abstract
Importance
Comorbidity is ubiquitous in psychiatry, but it is unclear how to differentiate neural mechanisms of co-occurring symptoms. Pediatric irritability and anxiety symptoms are prevalent and frequently co-occur. Threat orienting is pertinent to both phenotypes and is an ideal context in which to examine their unique and common neural mechanisms.
Objectives
To decompose the unique and shared variances of pediatric irritability and anxiety symptoms and to determine neural correlates of these differentiated phenotypes during threat orienting.
Design, Setting, and Participants
This investigation was a cross-sectional functional magnetic resonance imaging study. The setting was a research clinic at the National Institute of Mental Health. Participants were youth aged 8 to 18 years spanning multiple diagnostic categories (141 youth with disruptive mood dysregulation disorder, anxiety disorder, and/or attention-deficit/hyperactivity disorder and 56 healthy youth). This combination provided wide variation in levels of irritability and anxiety symptoms. Data were acquired between June 30, 2012, and June 28, 2016.
Main Outcomes and Measures
Participants and parents rated youth’s irritability on the Affective Reactivity Index and anxiety on the Screen for Child Anxiety Related Emotional Disorders. Bifactor analysis decomposed the unique and shared variances. A functional magnetic resonance imaging dot-probe task assessed attention orienting to angry (ie, threat) vs neutral faces. Whole-brain analyses examined associations between the bifactor-derived phenotypes and both neural activity and amygdala functional connectivity.
Results
Among 197 participants included in the final analysis, the mean (SD) age was 13.1 (2.7) years, and 91 (46.2%) were female. The best-fit bifactor model (Comparative Fit Index, 0.959; Root Mean Square Error of Approximation, 0.066) included unique factors of parent-reported irritability, youth-reported irritability, and anxiety, as well as a common factor of negative affectivity. When the task required attention away from threat, higher parent-reported irritability was associated with increased activity in the insula, caudate, dorsolateral and ventrolateral prefrontal cortex, and inferior parietal lobule (t189≥4.15 for all, P < .001 for all). In contrast, higher anxiety was associated with decreased amygdala connectivity to the cingulate, thalamus, and precentral gyrus (t189≤−4.19 for all, P < .001 for all). These distinctive neural correlates did not emerge using a diagnostic approach.
Conclusions and Relevance
A latent variable approach to parsing co-occurring symptom dimensions revealed a novel double dissociation. During orientation away from threat, only irritability was associated with neural activity, whereas only anxiety was associated with amygdala connectivity. Despite the challenges of symptom co-occurrence for clinical neuroscience, data-driven phenotyping may facilitate a path forward.
This cross-sectional functional magnetic resonance imaging study decomposes the unique and shared variances of pediatric irritability and anxiety symptoms and determines neural correlates of these differentiated phenotypes during threat orienting.
Introduction
One goal of precision psychiatry is to identify clear brain-behavior associations. However, a major challenge is co-occurrence among clinical phenotypes, which raises questions about specific vs shared pathophysiology.1,2,3 To date, most relevant studies have focused on diagnostic categories, but these do not track closely with biology. As a result, the field is moving toward alternative phenotyping strategies, such as transdiagnostic dimensionally assessed symptoms,3,4,5,6 hierarchical clustering of symptoms,7 and symptom networks.8 How to parse neural mechanisms of distinct but correlated symptom dimensions remains an open question. In this study, we used latent variable methods to differentiate mechanisms of co-occurring symptom dimensions in a transdiagnostic sample of youth.
Children seen for psychiatric care typically exhibit multiple co-occurring symptoms, complicating treatment.9 In particular, individual differences in chronic irritability and anxiety are correlated in both clinical10,11 and community12,13,14,15 pediatric samples. Irritability refers to an increased proneness to anger relative to peers.16,17 Levels of both irritability18 and anxiety19,20 are distributed continuously in youth. Clinically significant irritability or anxiety in early life predicts elevated risk for negative outcomes, including depression and functional impairment in adulthood.21,22,23,24 Parsing the unique and common neural mechanisms of irritability and anxiety in early life could reveal precise targets for treatment and prevention.
Both irritability and anxiety are characterized by high-arousal negative affect states (ie, negative affectivity).25,26 In addition, both irritability and anxiety have been associated with biased attention orienting toward social threats, such as angry faces.27,28,29 However, the phenotypes differ in their behavioral output. Whereas irritability is associated with approach behavior in response to threat (eg, reactive aggression),16,17 anxiety is associated with avoidant behavior.16,20 Therefore, threat orienting is an ideal domain in which to examine the unique and common neural mechanisms of these phenotypes.
In the present study, we used bifactor analysis30 to examine the unique and common variances of dimensionally assessed irritability and anxiety in relation to neural mechanisms of threat orienting. Bifactor analysis is one type of latent variable analysis that uses observed data to estimate underlying constructs.31,32 It specifically handles correlated data, such as symptom reports of irritability and anxiety, that are posited to reflect an overarching or common construct (ie, negative affectivity), as well as unique constructs. In this study, we estimated a common latent factor (negative affectivity) reflecting associations between irritability and anxiety symptoms, thereby accounting for their co-occurrence, and unique latent factors reflecting only irritability or only anxiety symptoms, thereby accounting for their specificity. We hypothesized that these differentiated phenotypes would show distinct associations with neural activity and amygdala connectivity during threat orienting, which would not be found using a traditional diagnostic approach.
Methods
Participants
At the National Institute of Mental Health, neuroimaging data were acquired from youth aged 8 to 18 years. To obtain full, distributed ranges of irritability and anxiety symptoms, the transdiagnostic sample included youth with clinically significant irritability and/or anxiety, youth with subthreshold symptoms, and healthy youth. Specifically, participants had no psychiatric diagnosis (n = 56) or had a presenting diagnosis of disruptive mood dysregulation disorder (DMDD), characterized by severe, chronic irritability (n = 54); an anxiety disorder (generalized, social, or separation anxiety disorder) (n = 50); or attention-deficit/hyperactivity disorder (ADHD) (n = 37). Primary ADHD was included because it is associated strongly with chronic irritability in this age group and thus is a common comorbidity of DMDD.33 Participants were assessed on levels of irritability using the Affective Reactivity Index (ARI) parent-report and youth-report forms34 and on levels of anxiety using the Screen for Child Anxiety Related Emotional Disorders (SCARED) parent-report and youth-report forms.35 These assessments were conducted within 3 months of the imaging with the exception of 6 participants having no psychiatric diagnosis whose data were data collected outside of this window. Exclusion criteria were IQ below 70 or presence of a pervasive developmental disorder, posttraumatic stress disorder, schizophrenia, substance use within the preceding 3 months, neurological disorder, or unstable medical illness (eMethods in the Supplement). Participants were recruited through advertisements in the community. Parents gave written informed consent, and youth gave written assent. Data were acquired between June 30, 2012, and June 28, 2016. Youth received monetary compensation for participation. Study procedures were approved by the National Institute of Mental Health Institutional Review Board.
Functional Magnetic Resonance Imaging Paradigm
Participants completed an event-related dot-probe task (eFigure 1 in the Supplement).36,37 Each trial began with a fixation cross (500 milliseconds), followed by a pair of faces of an identical actor (angry-neutral or neutral-neutral, 500 milliseconds). After this display, a probe (< or >) appeared; participants identified the direction of the probe as quickly and accurately as possible. The task conditions were threat congruent (the probe appeared in the location of the angry face after angry-neutral pairs), threat incongruent (the probe appeared in the location of the neutral face after angry-neutral pairs), and neutral (the probe followed neutral-neutral face pairs). The location of the angry face and the location and direction of the probe were counterbalanced. Trials were administered randomly in 2 runs, with a total of 80 congruent, 80 incongruent, and 80 neutral trials. Eighty fixation-only trials provided an additional baseline.
Imaging Procedures
Functional magnetic resonance imaging data were acquired on a 3-T imaging system (HDx; General Electric) with an 8-channel head coil. Functional image volumes were collected with an in-plane resolution of 2.5 × 2.5 mm using a T2-weighted gradient-echo pulse sequence (repetition time/echo time of 2300/25 milliseconds, flip angle of 50°, field of view of 24 cm, 96 × 96–pixel matrix, and 41 contiguous 3-mm interleaved axial sections). Total acquisition time was 14 minutes. A high-resolution 3-dimensional MPRAGE spin-echo sequence (NEX of 1, echo time/inversion time of minimum full echo time/725 milliseconds, field of view of 22 cm, 256 × 192–pixel matrix, and bandwidth of 31.25 Hz per 256 voxels) was acquired for use in coregistration and normalization procedures.
Data were processed and analyzed using Analysis of Functional NeuroImages (AFNI).38 A general linear model estimated blood oxygenation level–dependent signal change and voxelwise functional connectivity of the bilateral amygdala using generalized psychophysiological interaction39 methods (eMethods in the Supplement).
Statistical Analysis
Bifactor Model of Irritability and Anxiety
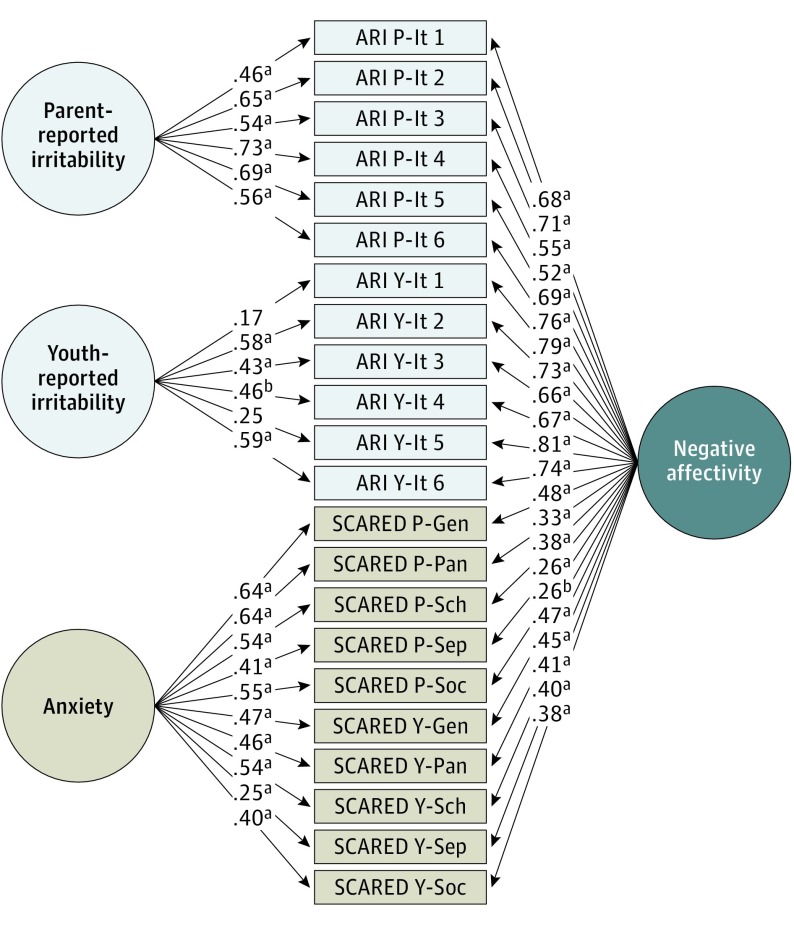
Bifactor analysis quantified the unique and shared variances of irritability and anxiety symptoms (eMethods in the Supplement). The best-fit model included the following 4 factors: unique factors of parent-reported irritability, youth-reported irritability, and anxiety (parent-reported and youth-reported), as well as a common factor that we termed negative affectivity (Comparative Fit Index, 0.959; Non-Normed Fit Index, 0.950; and Root Mean Square Error of Approximation, 0.066 [90% CI, 0.055-0.077]) (Figure 1). Participants’ scores on the factors were extracted for use as predictors of neural activation and connectivity. Consistent with a dimensional, transdiagnostic approach, factor scores showed variability within and across diagnostic groups (eFigure 2 in the Supplement). Repeated 5-fold cross-validation (10 repeats)40 supported model robustness (eMethods, eFigure 3, and eFigure 4 in the Supplement).
Figure 1. Bifactor Model of Pediatric Irritability and Anxiety.
Shown is the best-fit model, including unique factors of parent-reported irritability, youth-reported irritability, and anxiety (parent reported and youth reported), as well as a common factor of negative affectivity. Data represent the series of factor loadings of each measure on each latent factor. ARI indicates Affective Reactivity Index34; Gen, generalized anxiety disorder subscale; It, item; P, parent report; Pan, panic disorder subscale; SCARED, Screen for Child Anxiety Related Emotional Disorders35; Sch, school avoidance subscale; Sep, separation anxiety disorder subscale; Soc, social anxiety disorder subscale; and Y, youth report.
aP ≤ .001.
bP < .005.
Imaging Analyses
Analyses were conducted between September 2016 and November 2017 using AFNI. Group analyses used AFNI’s 3dMVM program. The between-subject independent variables were the continuous factor scores (grand-mean centered) of parent-reported irritability, youth-reported irritability, anxiety, and negative affectivity. These variables were tested together in the 3dMVM model. The within-subject independent variable was the task condition (threat congruent, threat incongruent, and neutral). General linear t tests modeled the a priori task condition contrasts of attention orienting to threat (threat-incongruent vs threat-congruent trials) and general viewing of threat (threat-incongruent/threat-congruent vs neutral trials) together within the model, as a function of the unique and common phenotype variables. Age, sex, and motion (grand-mean centered) were used as covariates in the model because of their associations with selected between-subject variables (P < .05 for all). All variance inflation factor indexes were less than 1.54.
Whole-brain analyses were conducted using a gray matter mask with the cerebellum removed. This mask was intersected with a group mask that included only those voxels in which data existed for at least 90% of participants. The initial voxelwise threshold was set at 2-sided P < .005. Multiple-testing correction was set to α = .05 for activation and to α = .025 for functional connectivity (based on 2 seeds) via Monte Carlo cluster-size simulation with a gaussian plus exponential spatial autocorrelation function to estimate smoothness (AFNI’s 3dClustSim program) (a = 0.49478, b = 4.14409, and c = 11.13640).41 These parameters resulted in a cluster-size threshold of k>69 (1078 mm3) for activation and k>84 (1313 mm3) for connectivity. Left and right amygdala regions of interest (ROIs) were anatomically defined using the Talairach Daemon atlas,42 resampled, and intersected with a whole-brain mask (α = .025 based on 2 ROIs).
To characterize whole-brain and ROI associations, the mean activity and connectivity values for significant clusters were extracted using AFNI’s 3dROIstat program. Using statistical software (SPSS, version 23.0; SPSS Inc),43 contrasts were calculated for attention orienting to threat (threat-incongruent vs threat-congruent trials) and general viewing of threat (threat-incongruent/threat-congruent vs neutral trials). Multivariate linear regression models used the same variables as in the group analyses. All reported associations (t statistics and P values) are derived from these exploratory post hoc correlations (2-sided tests with no additional correction). Data were assessed for influential cases (standardized residual, >3). When excluding influential cases, all results remained significant; therefore, these cases were retained.
Last, a parallel diagnostic group analysis was conducted for comparison purposes. Details are given in the eMethods in the Supplement.
Behavioral Analyses
Reaction times (RTs) were calculated. Incorrect trials and trials in which RTs were less than 150 milliseconds, greater than 2000 milliseconds, or exceeding 2.5 SDs from the participant’s mean RT for the task condition were removed.36,37,44 Behavioral measures of attention orienting to threat, attention distraction by threat,36,37,44 and attention orienting variability45 were calculated.
Results
In total, 197 participants were included in the final analysis. Their mean (SD) age was 13.1 (2.7) years, and 91 (46.2%) were female (Table 1).
Table 1. Sample Characteristics Among 197 Youths.
| Characteristic | Value |
|---|---|
| Age, mean (SD), y | 13.06 (2.68) |
| Male, No. (%) | 106 (53.8) |
| IQ, mean (SD)a | 112.46 (13.09) |
| Socioeconomic status, mean (SD)b | 35.21 (18.96) |
| Dimensional measures total scores, mean (SD)c | |
| ARI parent report | 3.19 (3.35) |
| ARI youth report | 2.56 (2.74) |
| SCARED parent report | 16.35 (14.16) |
| SCARED youth report | 16.73 (13.90) |
| Presenting diagnosis, No. (%) | |
| None | 56 (28.4) |
| Disruptive mood dysregulation disorder | 54 (27.4) |
| Anxiety disorder | 50 (25.4) |
| Attention-deficit/hyperactivity disorder | 37 (18.8) |
| Medications, No. (%) | |
| Selective serotonin reuptake inhibitor | 28 (14.2) |
| Stimulant | 56 (28.4) |
| Second-generation antipsychotic | 16 (8.1) |
| Antiepileptic drug | 13 (6.6) |
| Image quality, mean (SD) | |
| Average motion after censoring | 0.07 (0.04) |
Abbreviations: ARI, Affective Reactivity Index34; SCARED, Screen for Child Anxiety Related Emotional Disorders.35
IQ assessed using Wechsler Abbreviated Scale of Intelligence.46 Data were missing for 2 participants.
Socioeconomic status assessed using Two Factor Index of Social Position by Hollingshead.47 Data were missing for 14 participants.
The ARI parent-report and youth-report forms and SCARED youth-report form each were missing total scores for 1 participant.
Behavior
Attention orienting to threat and attention distraction by threat did not vary significantly by any phenotype. Greater attention orienting variability was associated with higher parent-reported irritability (r = 0.19, P < .01) and higher negative affectivity (r = 0.18, P = .01) (eTable 1 in the Supplement).
Activation
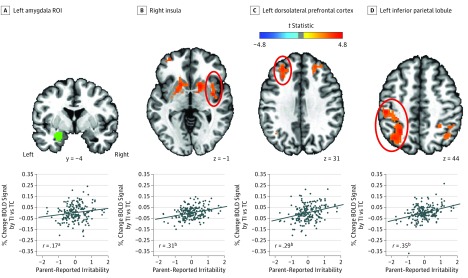
Table 2 lists all significant results for activation. On threat-incongruent vs threat-congruent trials, higher levels of parent-reported irritability were associated with increased activity in multiple regions mediating attentional and motor responses to negatively valenced stimuli.48,49 These regions included the left amygdala (ROI) (t189 = 2.30, P = .02) (Figure 2A), right insula (t189 = 4.47, P < .001) (Figure 2B), bilateral dorsolateral prefrontal cortex (t189 = 4.15, P < .001 for the left; t189 = 4.32, P < .001 for the right) (Figure 2C), left ventrolateral prefrontal cortex (vlPFC) (t189 = 4.50, P < .001), bilateral inferior parietal lobule (t189 = 5.19, P < .001 for the left; t189 = 4.53, P < .001 for the right) (Figure 2D), and bilateral caudate (t189 = 4.74, P < .001 for the left; t189 = 4.66, P < .001 for the right) (eFigure 5A in the Supplement). There was a positive association between bilateral caudate activity and RT to the probe on threat-incongruent vs threat-congruent trials (r = 0.17, P = .02 for the left caudate; r = 0.16, P = .03 for the right caudate) (eFigure 5B in the Supplement) and an indirect association of higher parent-reported irritability with this increased RT via increased caudate activity (β = 1.343, P < .05 for the left caudate; β = 1.346, P < .05 for the right caudate) (eFigure 5B in the Supplement).
Table 2. Significant Associations of Neural Activity and Amygdala Functional Connectivity With the Unique and Common Phenotypes.
| Contrast | Peak Talairach Coordinates (LPI) | Size, mm3 | t Statistica | P Valuea | Locationb | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Whole-Brain BOLD Activityc | |||||||
| Parent-reported irritability by TI vs TC | −46 | −26 | 44 | 9125 | 5.19 | <.001 | Left inferior parietal lobule/left postcentral gyrus/left superior parietal lobule |
| −9 | 6 | 11 | 4625 | 4.74 | <.001 | Left caudate/left lentiform nucleus | |
| −26 | 39 | 31 | 2438 | 4.15 | <.001 | Left dorsolateral prefrontal cortex | |
| 54 | −59 | 36 | 2172 | 4.53 | <.001 | Right inferior parietal lobule | |
| 36 | −4 | −1 | 1563 | 4.47 | <.001 | Right insula | |
| 21 | 41 | 34 | 1563 | 4.32 | <.001 | Right dorsolateral prefrontal cortex | |
| 16 | 9 | −1 | 1453 | 4.66 | <.001 | Right caudate/right lentiform nucleus | |
| −34 | 51 | 6 | 1359 | 4.50 | <.001 | Left ventrolateral prefrontal cortex | |
| Negative affectivity by T vs N | 4 | −11 | 9 | 1234 | 4.10 | <.001 | Right dorsomedial nucleus of thalamus/left dorsomedial nucleus of thalamus |
| Amygdala ROI BOLD Activityd | |||||||
| Parent-reported irritability by TI vs TC | −21 | −4 | −21 | 1063 | 2.30 | .02 | Left amygdala |
| Right Amygdala Seed Functional Connectivitye | |||||||
| Anxiety by TI vs TC | −11 | −21 | 14 | 2969 | −4.27 | <.001 | Left thalamus/right thalamus |
| 4 | −1 | 39 | 2375 | −4.24 | <.001 | Right cingulate/left cingulate | |
| −49 | −6 | 34 | 1484 | −4.19 | <.001 | Left precentral gyrus | |
Abbreviations: BOLD, blood oxygenation level–dependent; LPI, left-posterior-inferior; N, neutral trials; ROI, region of interest; T, threat trials; TC, threat-congruent trials; TI, threat-incongruent trials.
Value from post hoc multivariate linear regression on the mean BOLD signal for extracted cluster.
Location represents anatomical overlap of cluster with region.
No significant effects for parent-reported irritability by T vs N, youth-reported irritability or anxiety by TI vs TC or T vs N, or negative affectivity by TI vs TC.
No significant effects for parent-reported irritability by T vs N or for youth-reported irritability, anxiety, or negative affectivity by TI vs TC or T vs N.
No significant effects for parent-reported irritability, youth-reported irritability, or negative affectivity by TI vs TC or T vs N.
Figure 2. Association of Irritability With Widespread Neural Activation as a Function of Attention Orienting to Threat.
Shown are selected significant clusters and associated partial regression plots and partial correlation coefficients from post hoc multivariate linear regression on the mean blood oxygenation level–dependent (BOLD) signal. A, Left amygdala ROI. B, Right insula. C, Left dorsolateral prefrontal cortex. D, Left inferior parietal lobule. ROI, region of interest; TI, threat-incongruent trials; and TC, threat-congruent trials. The ovals delineate the regions listed in B, C, and D. The diagonal lines indicate the fitted regression lines.
aP = .02.
bP < .001.
On threat vs neutral trials, higher levels of negative affectivity were associated with increased activity in the right dorsomedial nucleus of the thalamus (t189 = 4.10, P < .001) (eFigure 6 in the Supplement).
Functional Connectivity
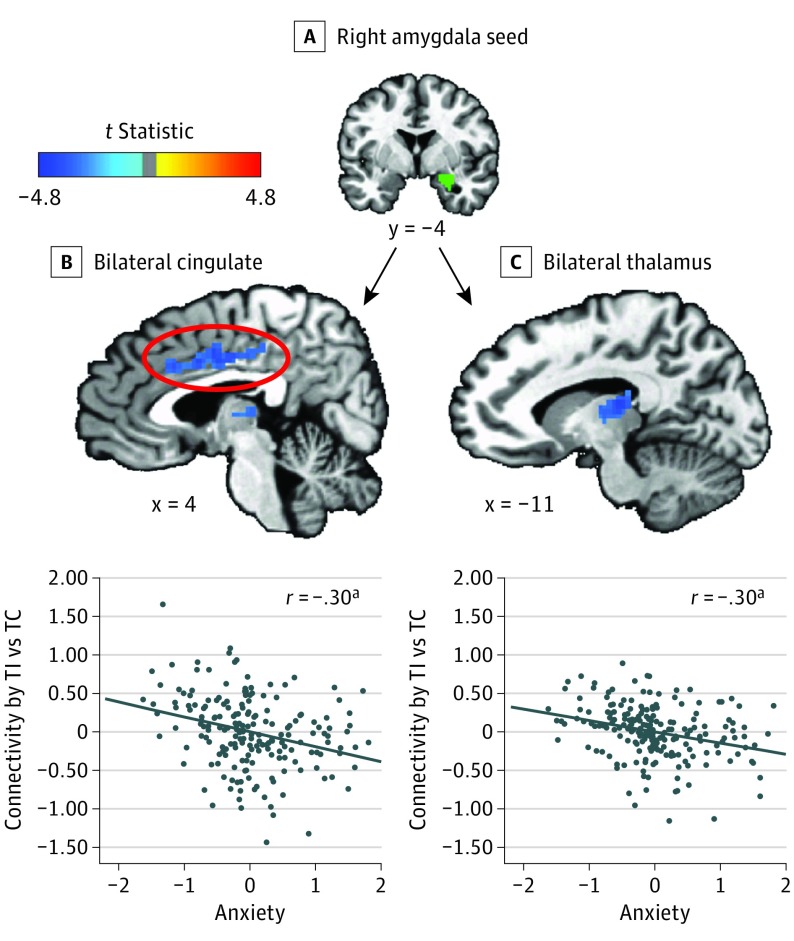
Table 2 lists all significant results for amygdala connectivity. On threat-incongruent vs threat-congruent trials, higher levels of anxiety were associated with decreased right amygdala connectivity (Figure 3A) to the bilateral cingulate (t189 = −4.24, P < .001) (Figure 3B), bilateral thalamus (t189 = −4.27, P < .001) (Figure 3C), and left precentral gyrus (t189 = −4.19) (P < .001).
Figure 3. Association of Anxiety With Amygdala Connectivity as a Function of Attention Orienting to Threat.
Shown are selected significant clusters and associated partial regression plots and partial correlation coefficients from post hoc multivariate linear regression on the mean right amygdala–based connectivity value. A, Right amygdala seed. B, Bilateral cingulate. C, Bilateral thalamus. TI indicates threat-incongruent trials; TC, threat-congruent trials. The oval delineates the region listed in B. The diagonal lines indicate the fitted regression lines.
aP < .001.
Diagnostic Approach
These distinctive neural correlates of irritability and anxiety did not emerge from an analysis that examined phenotypes diagnostically. Participants whose presenting diagnosis reflected the clinical threshold for severe irritability (DMDD) or anxiety (anxiety disorder) largely did not differ from participants with no psychiatric diagnosis. The one significant finding indicated that, on threat-incongruent vs threat-congruent trials, participants with an anxiety disorder (without DMDD) exhibited decreased connectivity between the right amygdala and posterior cingulate/precuneus relative to participants with no psychiatric diagnosis (eTable 2 and eFigure 7 in the Supplement). This cluster did not overlap with that found in the dimensional analysis of anxiety.
Supplementary Analyses
Supplementary post hoc analyses of the dimensional phenotypes controlled for levels of ADHD symptoms (n = 161) (eTable 3 in the Supplement) and depressive symptoms (n = 175) (eTable 4 in the Supplement) assessed within 3 months of the imaging. For both sets of analyses, all whole-brain findings remained significant, but the left amygdala ROI finding was not significant. Post hoc analyses also examined results by medication status within 30 days before imaging (eTable 5 in the Supplement). In the subsample excluding participants taking stimulants (n = 141) or selective serotonin reuptake inhibitors (n = 169), all whole-brain findings remained significant, but the left amygdala ROI finding was not significant. In the subsample excluding participants taking second-generation antipsychotics (n = 181) or antiepileptic drugs (n = 184), all findings remained significant.
Supplementary analyses estimated functional connectivity of the bilateral vlPFC (eMethods in the Supplement). On threat-incongruent vs threat-congruent trials, higher levels of anxiety were associated with decreased right vlPFC connectivity to the right caudate/putamen (eFigure 8 in the Supplement).
Last, we reanalyzed the whole-brain data using threshold-free cluster enhancement50 with familywise error rate correction to 0.05 via permutation testing (eMethods and eFigure 9 in the Supplement). Results were largely consistent with the original analyses. The primary differences were that more extensive regions were associated with parent-reported irritability and that the region associated with negative affectivity was not significant. In the diagnostic group analysis, no regions were significant.
Discussion
A latent variable approach to parsing co-occurring symptom dimensions revealed a double dissociation between irritability and anxiety. On a threat-orienting task, only irritability was associated with increased neural activity, including activity in the insula, caudate, dorsolateral and ventrolateral prefrontal cortex, and inferior parietal lobule. Only anxiety was associated with decreased amygdala connectivity, including to the cingulate, thalamus, and precentral gyrus. In supplementary analyses, anxiety was also associated with decreased vlPFC connectivity. Therefore, while pediatric irritability and anxiety often co-occur, phenotype-specific brain mechanisms are involved in threat orienting.
The widespread, increased activity associated with higher levels of parent-reported irritability may reflect that greater neural engagement is required to maintain attentional and motor control during threat-incongruent trials, when the task requires attending away from threat. Using a threat imminence framework,51 the increased neural activity specific to irritability may reflect heightened arousal that, in specific contexts, can contribute to maladaptive approach behavior toward nonimminent threats. In contrast, when the task required attending away from threat, anxiety was uniquely related to decreased connectivity between the amygdala and hubs of cortico-limbic networks. This pattern may reflect subtle aberrations in higher-order processes that mediate maladaptive avoidant behavior.52 For example, functional connectivity of the amygdala has been found to vary based on whether threat stimuli are presented subliminally vs supraliminally.53 The associations that we found between anxiety and amygdala connectivity may reflect differential levels of awareness of, or attention to, task-irrelevant threats. Indeed, a prior study37 also found associations between anxiety and functional connectivity of the amygdala during threat orienting.
To our knowledge, this is the first study to examine neural mechanisms of threat orienting in irritability. These results extend prior work on threat orienting in anxiety.37,44,54,55,56 In fact, previous findings relating anxiety to increased prefrontal activity on threat-orienting tasks54,55,56 may have been driven, in part, by co-occurring irritability that was not examined. We also found that negative affectivity was associated with increased activity in the dorsomedial nucleus of the thalamus during threat viewing. This association may reflect a general increase in motivation-driven visual processing of threat shared by irritability and anxiety.57 However, it should be noted that this region was not significant in the supplementary threshold-free cluster enhancement analysis. In addition, it is notable that parent-reported irritability and youth-reported irritability formed distinct factors in the bifactor analysis. This outcome is consistent with well-known informant discrepancies in developmental psychopathology58 and suggests that informant effects are important to consider in irritability. In this study, youth-reported irritability was not associated significantly with any brain data. Based on the distribution of diagnostic groups across youth-reported irritability scores, it appears that some youth with psychopathology (eg, DMDD) may underreport levels of irritability relative to parents. This possibility may have influenced our ability to detect neural correlates with youth-reported irritability.
Given these results, treatment prediction algorithms for irritability vs anxiety will likely be fundamentally distinct.59,60 The widespread pattern of perturbed neural activity in irritability is likely to require clinical interventions targeting extensive distributed dysfunction in regions mediating attentional and motor control in the context of threat. Widespread dysfunction is also seen in other phenotypes (eg, bipolar disorder and schizophrenia)61,62; this pattern is in contrast to pediatric anxiety, in which the dysfunction and treatment may be more targeted. Specifically, promising treatments for anxiety may target perturbed amygdala connectivity, which we have extended herein to perturbed vlPFC connectivity. Recent data suggest that amygdala connectivity may be engaged by attention bias modification therapy.37 The relevance of these distinct neural mechanisms for behavior and treatment response should be a focus of further work. In future pediatric intervention trials, participants could be phenotyped using this bifactor model of irritability and anxiety and stratified by their scores on the respective factors. New treatments may also be developed to target these neural alterations. For instance, noninvasive stimulation of the lateral prefrontal cortex has been shown to enhance the effects of attention bias modification63,64; such an approach could be tested for target regions in irritability and anxiety.
Limitations
This study had several limitations. First, the sample did not include all diagnoses that may involve irritability and/or anxiety. In particular, youth with primary unipolar or bipolar depression were not included, although mood disorder episodes can involve irritability.65 Future studies should recruit additional diagnostic groups (eg, major depressive disorder, bipolar disorder, posttraumatic stress disorder, and schizophrenia). A fully transdiagnostic approach to unique and common neural correlates would include all diagnoses feasible for imaging. Furthermore, given an appropriate sample, depressive symptoms could be incorporated in a future bifactor model that includes both negative and positive affectivity. Multisite investigations with larger samples and a broader array of symptom measures will help advance latent variable approaches to neuroimaging data. Second, the design of our study was cross-sectional. Follow-up studies should examine mechanisms of irritability and anxiety that may unfold across development. Third, some participants were taking psychotropic medication. Although post hoc analyses supported the robustness of the findings to medication, it is possible that results would be different in unmedicated individuals. Fourth, it will be important to replicate these findings, including in community samples.
Conclusions
The ubiquity of symptom co-occurrence and imprecision of diagnostic categories complicate research on pathophysiology and treatment of mental illness.1,2,3,4 Latent variable approaches may facilitate a path forward. For example, a bifactor approach may be useful in parsing symptom dimensions within syndromes and investigating neural substrates across a wide range of co-occurring symptoms. The identification of discrete early-expressed biomarkers of psychiatric disease may inform more effective, targeted treatments in youth.
eMethods. Supplemental Methods
eFigure 1. Dot-Probe Task Schematic
eFigure 2. Histograms of Factor Scores by Diagnostic Group
eFigure 3. Results of Repeated Five-Fold Cross Validation (10 Repeats) on Loadings of Observed Variables on the Common Factor
eFigure 4. Results of Repeated Five-Fold Cross Validation (10 Repeats) on Loadings of Observed Variables on the Unique Factors
eFigure 5. Indirect Effect of Parent-Reported Irritability on Reaction Time (RT) Index of Biased Attention Orienting Toward Threat as Mediated by Caudate Activity
eFigure 6. Negative Affectivity Is Associated With Increased Activity in Right Dorsomedial Nucleus of the Thalamus on Threat Vs Neutral Trials
eFigure 7. Anxiety Disorder Diagnosis Is Associated With Decreased Amygdala Connectivity to Posterior Cingulate/Precuneus as a Function of Threat Orienting
eFigure 8. Anxiety Is Associated With Decreased Ventrolateral Prefrontal Cortex Connectivity to Striatum as a Function of Attention Orienting to Threat
eFigure 9. Results of Threshold-Free Cluster Enhancement (TFCE) Analysis
eTable 1. Task Behavior as a Function of the Unique and Common Phenotypes
eTable 2. Significant Association of Neural Connectivity With Presenting Diagnosis
eTable 3. Associations of Neural Activity and Connectivity With the Unique and Common Phenotypes, Covarying ADHD Symptoms Post Hoc (N = 161)
eTable 4. Associations of Neural Activity and Connectivity With the Unique and Common Phenotypes, Covarying Depressive Symptoms Post Hoc (N = 175)
eTable 5. Associations of Neural Activity and Connectivity With the Unique and Common Phenotypes, Excluding Medication Classes Post Hoc Using a Leave-Out Approach
References
- 1.Hyman SE. Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007;8(9):725-732. [DOI] [PubMed] [Google Scholar]
- 2.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14(1):29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zald DH, Lahey BB. Implications of the hierarchical structure of psychopathology for psychiatric neuroimaging. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(4):310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Insel T, Cuthbert B, Garvey M, et al. Research Domain Criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751. [DOI] [PubMed] [Google Scholar]
- 5.Castellanos-Ryan N, Struve M, Whelan R, et al. ; IMAGEN Consortium . Neural and cognitive correlates of the common and specific variance across externalizing problems in young adolescence. Am J Psychiatry. 2014;171(12):1310-1319. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugan S, Wolf DH, Calkins ME, et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry. 2016;173(5):517-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chekroud AM, Gueorguieva R, Krumholz HM, Trivedi MH, Krystal JH, McCarthy G. Reevaluating the efficacy and predictability of antidepressant treatments: a symptom clustering approach. JAMA Psychiatry. 2017;74(4):370-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fried EI, van Borkulo CD, Cramer AO, Boschloo L, Schoevers RA, Borsboom D. Mental disorders as networks of problems: a review of recent insights. Soc Psychiatry Psychiatr Epidemiol. 2017;52(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57-87. [PubMed] [Google Scholar]
- 10.Stoddard J, Stringaris A, Brotman MA, Montville D, Pine DS, Leibenluft E. Irritability in child and adolescent anxiety disorders. Depress Anxiety. 2014;31(7):566-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornacchio D, Crum KI, Coxe S, Pincus DB, Comer JS. Irritability and anxiety severity among youth with anxiety. J Am Acad Child Adolesc Psychiatry. 2016;55(1):54-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brotman MA, Schmajuk M, Rich BA, et al. Prevalence, clinical correlates, and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60(9):991-997. [DOI] [PubMed] [Google Scholar]
- 13.Stringaris A, Goodman R. Three dimensions of oppositionality in youth. J Child Psychol Psychiatry. 2009;50(3):216-223. [DOI] [PubMed] [Google Scholar]
- 14.Leadbeater BJ, Homel J. Irritable and defiant sub-dimensions of ODD: their stability and prediction of internalizing symptoms and conduct problems from adolescence to young adulthood. J Abnorm Child Psychol. 2015;43(3):407-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savage J, Verhulst B, Copeland W, Althoff RR, Lichtenstein P, Roberson-Nay R. A genetically informed study of the longitudinal relation between irritability and anxious/depressed symptoms. J Am Acad Child Adolesc Psychiatry. 2015;54(5):377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brotman MA, Kircanski K, Stringaris A, Pine DS, Leibenluft E. Irritability in youths: a translational model. Am J Psychiatry. 2017;174(6):520-532. [DOI] [PubMed] [Google Scholar]
- 17.Leibenluft E. Pediatric irritability: a systems neuroscience approach. Trends Cogn Sci. 2017;21(4):277-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copeland WE, Brotman MA, Costello EJ. Normative irritability in youth: developmental findings from the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry. 2015;54(8):635-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox NA, Pine DS. Temperament and the emergence of anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2012;51(2):125-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry. 2007;48(7):631-648. [DOI] [PubMed] [Google Scholar]
- 21.Stringaris A, Cohen P, Pine DS, Leibenluft E. Adult outcomes of youth irritability: a 20-year prospective community-based study. Am J Psychiatry. 2009;166(9):1048-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copeland WE, Shanahan L, Egger H, Angold A, Costello EJ. Adult diagnostic and functional outcomes of DSM-5 disruptive mood dysregulation disorder. Am J Psychiatry. 2014;171(6):668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidal-Ribas P, Brotman MA, Valdivieso I, Leibenluft E, Stringaris A. The status of irritability in psychiatry: a conceptual and quantitative review. J Am Acad Child Adolesc Psychiatry. 2016;55(7):556-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56-64. [DOI] [PubMed] [Google Scholar]
- 25.Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96(3):465-490. [PubMed] [Google Scholar]
- 26.Rothbart MK. Temperament, development, and personality. Curr Dir Psychol Sci. 2007;16(4):207-212. [Google Scholar]
- 27.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1-24. [DOI] [PubMed] [Google Scholar]
- 28.Hommer RE, Meyer A, Stoddard J, et al. Attention bias to threat faces in severe mood dysregulation. Depress Anxiety. 2014;31(7):559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salum GA, Mogg K, Bradley BP, et al. Association between irritability and bias in attention orienting to threat in children and adolescents. J Child Psychol Psychiatry. 2017;58(5):595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holzinger KJ, Swineford F. The bi-factor method. Psychometrika. 1937;2(1):41-54. [Google Scholar]
- 31.Friston KJ, Redish AD, Gordon JA. Computational nosology and precision psychiatry. Comput Psychiatr. 2017;1:2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flagel SB, Pine DS, Ahmari SE, et al. A novel framework for improving psychiatric diagnostic nosology In: Computational Psychiatry: New Perspectives on Mental Illness. Cambridge, MA: MIT Press; 2016:168-199. [Google Scholar]
- 33.Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry. 2014;171(3):276-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stringaris A, Goodman R, Ferdinando S, et al. The Affective Reactivity Index: a concise irritability scale for clinical and research settings. J Child Psychol Psychiatry. 2012;53(11):1109-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230-1236. [DOI] [PubMed] [Google Scholar]
- 36.White LK, Britton JC, Sequeira S, et al. Behavioral and neural stability of attention bias to threat in healthy adolescents. Neuroimage. 2016;136:84-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White LK, Sequeira S, Britton JC, et al. Complementary features of attention bias modification therapy and cognitive behavioral therapy in pediatric anxiety disorders. Am J Psychiatry. 2017;174(8):775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173. [DOI] [PubMed] [Google Scholar]
- 39.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grimm KJ, Mazza GL, Davoudzadeh P. Model selection in finite mixture models: a k-fold cross-validation approach. Struct Equ Modeling. 2017;24(2):246-256. [Google Scholar]
- 41.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. AFNI and clustering: false-positive rates redux. Brain Connect. 2017;7(3):152-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.IBM SPSS Statistics for Windows [computer program]. Version 23.0. Armonk, NY: IBM Corp; 2015.
- 44.Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y. Neural changes with attention bias modification for anxiety: a randomized trial. Soc Cogn Affect Neurosci. 2015;10(7):913-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naim R, Abend R, Wald I, et al. Threat-related attention bias variability and posttraumatic stress. Am J Psychiatry. 2015;172(12):1242-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wechsler D. WASI Manual. San Antonio, TX: Psychological Corp; 1999. [Google Scholar]
- 47.Hollingshead AB. Two Factor Index of Social Position. New Haven, CT: Yale University; 1957. [Google Scholar]
- 48.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception, I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504-514. [DOI] [PubMed] [Google Scholar]
- 49.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4(3):165-178. [DOI] [PubMed] [Google Scholar]
- 50.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83-98. [DOI] [PubMed] [Google Scholar]
- 51.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1(4):429-438. [DOI] [PubMed] [Google Scholar]
- 52.Price RB, Allen KB, Silk JS, et al. Vigilance in the laboratory predicts avoidance in the real world: a dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Dev Cogn Neurosci. 2016;19:128-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26(36):9264-9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu X, Taber-Thomas BC, Pérez-Edgar K. Frontolimbic functioning during threat-related attention: relations to early behavioral inhibition and anxiety in children. Biol Psychol. 2017;122:98-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091-1097. [DOI] [PubMed] [Google Scholar]
- 56.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carretié L, Albert J, López-Martín S, Tapia M. Negative brain: an integrative review on the neural processes activated by unpleasant stimuli. Int J Psychophysiol. 2009;71(1):57-63. [DOI] [PubMed] [Google Scholar]
- 58.De Los Reyes A, Kazdin AE. Informant discrepancies in the assessment of childhood psychopathology: a critical review, theoretical framework, and recommendations for further study. Psychol Bull. 2005;131(4):483-509. [DOI] [PubMed] [Google Scholar]
- 59.Fonzo GA, Goodkind MS, Oathes DJ, et al. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am J Psychiatry. 2017;174(12):1163-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fonzo GA, Goodkind MS, Oathes DJ, et al. Selective effects of psychotherapy on frontopolar cortical function in PTSD. Am J Psychiatry. 2017;174(12):1175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hibar DP, Westlye LT, Doan NT, et al. Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group [published online May 2, 2017]. Mol Psychiatry. doi: 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Erp TG, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21(4):547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol Psychiatry. 2010;67(10):919-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clarke PJ, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biol Psychiatry. 2014;76(12):946-952. [DOI] [PubMed] [Google Scholar]
- 65.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 66.clinicaltrials.gov. Studies of Brain Function and Course of Illness in Pediatric Bipolar Disorder. NCT00025935. https://clinicaltrials.gov/ct2/results?cond=&term=NCT00025935. Accessed February 22, 2018.
- 67.clinicaltrials.gov. Clinical Trial of Fluoxetine in Anxiety and Depression in Children, and Associated Brain Changes. NCT00018057. https://clinicaltrials.gov/ct2/results?cond=&term=NCT00018057. Accessed February 22, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Dot-Probe Task Schematic
eFigure 2. Histograms of Factor Scores by Diagnostic Group
eFigure 3. Results of Repeated Five-Fold Cross Validation (10 Repeats) on Loadings of Observed Variables on the Common Factor
eFigure 4. Results of Repeated Five-Fold Cross Validation (10 Repeats) on Loadings of Observed Variables on the Unique Factors
eFigure 5. Indirect Effect of Parent-Reported Irritability on Reaction Time (RT) Index of Biased Attention Orienting Toward Threat as Mediated by Caudate Activity
eFigure 6. Negative Affectivity Is Associated With Increased Activity in Right Dorsomedial Nucleus of the Thalamus on Threat Vs Neutral Trials
eFigure 7. Anxiety Disorder Diagnosis Is Associated With Decreased Amygdala Connectivity to Posterior Cingulate/Precuneus as a Function of Threat Orienting
eFigure 8. Anxiety Is Associated With Decreased Ventrolateral Prefrontal Cortex Connectivity to Striatum as a Function of Attention Orienting to Threat
eFigure 9. Results of Threshold-Free Cluster Enhancement (TFCE) Analysis
eTable 1. Task Behavior as a Function of the Unique and Common Phenotypes
eTable 2. Significant Association of Neural Connectivity With Presenting Diagnosis
eTable 3. Associations of Neural Activity and Connectivity With the Unique and Common Phenotypes, Covarying ADHD Symptoms Post Hoc (N = 161)
eTable 4. Associations of Neural Activity and Connectivity With the Unique and Common Phenotypes, Covarying Depressive Symptoms Post Hoc (N = 175)
eTable 5. Associations of Neural Activity and Connectivity With the Unique and Common Phenotypes, Excluding Medication Classes Post Hoc Using a Leave-Out Approach