Abstract
Importance
The physical benefits of resistance exercise training (RET) are well documented, but less is known regarding the association of RET with mental health outcomes. To date, no quantitative synthesis of the antidepressant effects of RET has been conducted.
Objectives
To estimate the association of efficacy of RET with depressive symptoms and determine the extent to which logical, theoretical, and/or prior empirical variables are associated with depressive symptoms and whether the association of efficacy of RET with depressive symptoms accounts for variability in the overall effect size.
Data Sources
Articles published before August 2017, located using Google Scholar, MEDLINE, PsycINFO, PubMed, and Web of Science.
Study Selection
Randomized clinical trials included randomization to RET (n = 947) or a nonactive control condition (n = 930).
Data Extraction and Synthesis
Hedges d effect sizes were computed and random-effects models were used for all analyses. Meta-regression was conducted to quantify the potential moderating influence of participant and trial characteristics.
Main Outcomes and Measures
Randomized clinical trials used validated measures of depressive symptoms assessed at baseline and midintervention and/or postintervention. Four primary moderators were selected a priori to provide focused research hypotheses about variation in effect size: total volume of prescribed RET, whether participants were healthy or physically or mentally ill, whether or not allocation and/or assessment were blinded, and whether or not the RET intervention resulted in a significant improvement in strength.
Results
Fifty-four effects were derived from 33 randomized clinical trials involving 1877 participants. Resistance exercise training was associated with a significant reduction in depressive symptoms with a moderate-sized mean effect ∆ of 0.66 (95% CI, 0.48-0.83; z = 7.35; P < .001). Significant heterogeneity was indicated (total Q = 216.92, df = 53; P < .001; I2 = 76.0% [95% CI, 72.7%-79.0%]), and sampling error accounted for 32.9% of observed variance. The number needed to treat was 4. Total volume of prescribed RET, participant health status, and strength improvements were not significantly associated with the antidepressant effect of RET. However, smaller reductions in depressive symptoms were derived from randomized clinical trials with blinded allocation and/or assessment.
Conclusions and Relevance
Resistance exercise training significantly reduced depressive symptoms among adults regardless of health status, total prescribed volume of RET, or significant improvements in strength. Better-quality randomized clinical trials blinding both allocation and assessment and comparing RET with other empirically supported treatments for depressive symptoms are needed.
This meta-analysis examines the association of resistance exercise with depressive symptoms and the extent to which variables of logical, theoretical, and/or prior empirical relation to depressive symptoms and/or associations of resistance exercise with depressive symptoms account for variability in the outcome.
Key Points
Question
What is the overall association of efficacy of resistance exercise training with depressive symptoms, and which logical, theoretical, and/or prior empirical variables are associated with depressive symptoms?
Findings
In this meta-analysis of 33 clinical trials including 1877 participants, resistance exercise training was associated with a significant reduction in depressive symptoms, with a moderate-sized mean effect. Total volume of resistance exercise training, health status, and strength improvements were not associated with the antidepressant effect; however, smaller reductions in depressive symptoms were derived from trials with blinded allocation and/or assessment.
Meaning
The available empirical evidence supports resistance exercise training as an alternative and/or adjuvant therapy for depressive symptoms.
Introduction
Depression is a highly prevalent global burden, affecting more than 300 million people worldwide1; is a significant source of absenteeism and disability in the work force2; has an economic burden of approximately $118 billion annually3; and is the most costly mental health disorder in Europe, accounting for 1% of the total gross domestic product.4 Depressive symptoms are highly comorbid and significantly associated with poor health,5 including an increased risk of cardiovascular diseases,6,7 Alzheimer disease,8 type 2 diabetes,9 mortality,10 and noncompliance with medical treatment.11
Current frontline treatments for depression include medication and psychotherapy. However, for individuals with mild to moderate or severe depression, medication can be expensive, with limited efficacy (d < 0.20).12,13 Psychotherapy can be expensive and inaccessible, and previously reported effects may be overestimated owing to publication bias.14 Moreover, among individuals with depression who are seeking treatment, depressive symptoms persist for approximately 67% after first-line treatment of up to 14 weeks, and at least 30% remain depressed after 4 rounds of distinct 12-week treatments.15 Thus, there is continued interest in alternative treatments for depression and continued need to compare potential alternative treatments with established treatments.
Exercise interventions are promising treatments for depressive symptoms, and these interventions are free from the adverse effects and high costs associated with antidepressant medications and psychotherapy.16,17 Exercise interventions also have established benefits for cardiovascular diseases, the leading cause of death among individuals with major depressive disorder.6 Exercise training improves depressive symptoms among otherwise healthy adults,18 chronically ill adults,19 and adults with a depressive disorder.17 However, the magnitude of the effect remains unclear, as publication bias and flawed inclusion criteria may have resulted in underestimations of the magnitude of exercise effects.17,20 The benefits of acute aerobic exercise and aerobic exercise training (AET) for depressive symptoms among otherwise healthy adults and chronically ill adults are well established,18,19,21,22 but less is known regarding the associations of resistance exercise training (RET) with depressive symptoms. In addition, few trials have included both an RET and an AET arm in the same investigation, limiting direct comparisons between the modalities.
Resistance exercise training interventions are generally designed to increase strength, skeletal muscle mass, endurance, and/or power.23 Evidence has supported significant anxiolytic effects of RET among adults, regardless of their health status,24 and a previous narrative review supported the antidepressant effects of RET.25 However, no quantitative synthesis of randomized clinical trials (RCTs) of the antidepressant effect of RET has been conducted. Furthermore, there is a need to identify potential sources of variability in the antidepressant effect of RET, particularly modifiable participant and trial characteristics, to better inform the prescription of RET and future RET interventions.
The key objectives of this meta-analysis and meta-regression analysis were to estimate the overall association of efficacy of RET with depressive symptoms; determine the extent to which the overall effect varies based on variables of logical, theoretical, and/or prior empirical variables associated with depressive symptoms; and compare the effect of different exercise modes derived from RCTs in which participants were randomized to RET, AET, or a nonactive control condition.
Methods
Data Sources and Searches
This systematic review was conducted in accordance with the PRISMA guidelines.26 Articles published before August 2017 were identified using Google Scholar, MEDLINE, PsycINFO, PubMed, and Web of Science. Key words used included combinations of strength training, resistance training, and weight training, along with depress*. Supplementary searches of relevant systematic reviews17,18,24,25,27 and references within included articles were performed manually.
Study Selection and Inclusion Criteria
Inclusion criteria were peer-reviewed publication, clinical trials, randomized allocation to either an RET intervention or a nonactive control condition, and a validated self-report or clinician-rated measure of depressive symptoms assessed at baseline and at midintervention and/or postintervention. Investigations were excluded that included exercise as part of a multicomponent intervention but did not include the additional component in comparison conditions, and/or compared RET only with an active treatment for depression, including cognitive therapy, pharmacotherapy, relaxation or meditation, and flexibility training. One article28 was excluded because the depressive outcomes were reported in an earlier included article.29 eFigure 1 in the Supplement provides a flowchart of article inclusion and exclusion.
Data Extraction
Data were extracted from the included RCTs into an SPSS (SPSS Inc) file by 3 of us (B.R.G., C.P.M., and M.P.H.). The data extracted included the characteristics of the participants and the trials and the associations of exercise with outcomes of logical, theoretical, and/or prior empirical relation to depressive symptoms and/or the associations of RET with depressive symptoms; these included age, sex, physical and mental health status, type of control condition, whether allocation and/or assessment were blinded, duration of exercise program, frequency, session duration, RET intensity, whether or not RET sessions were supervised, whether or not the primary outcome of the trial was depressive symptoms, depressive symptom measure used, and whether or not there was a significant improvement in strength. To calculate total volume of RET prescribed, intervention duration (weeks), weekly frequency (days), and session duration (minutes) were multiplied together.
Study Quality Assessment
Two of us (B.R.G. and M.P.H.) independently assessed trial quality (scored 0-13) using the Detsky scale.30 This scale was amended to include research design, control condition, randomization and blinding methods, outcome measures, adherence, and characteristics of the exercise intervention. Higher scores indicated better study quality. The individual scores of each included RCT are presented in eTable 1 in the Supplement.
Effect Size Calculation
To calculate Hedges d effect sizes, the mean change for the control was subtracted from the mean change for RET, and the difference was divided by the pooled baseline SD.31 Larger reductions in depressive symptoms for RET resulted in positive effect sizes. eTable 2 in the Supplement presents the values used to calculate Hedges d and primary moderator values. Interrater reliability for effect size calculations was examined by calculating 2-way (effects × raters) intraclass correlation coefficients for absolute agreement. The initial intraclass correlation coefficients were greater than 0.90. When means and SDs were not reported, the authors were contacted. When these values could not be provided (k = 5), they were estimated from exact P values reported in the trial,32 included graphs,33,34 or from the largest other study of the same population sample that used the same measure of depressive symptoms,35,36 in accordance with common meta-analytic protocols.37 Discrepancies (eg, values of SDs estimated from included graphs) were resolved by consensus among the investigators involved in the data extraction (B.R.G., C.P.M., and M.P.H.).
Data Synthesis and Analysis
Meta-regression was used for moderator analyses because it reduces the probability of type I error by computing concurrent estimates of independent effects by multiple moderators on the variation in effect size across trials. Random-effects models were used with macros (MeanES; MetaReg)38 to aggregate the mean effect size delta (Δ) and test the variation in effects according to moderator variables.31,38 Heterogeneity was evaluated with Cochrane Q, and consistency was evaluated with I2.37 If sampling error accounted for less than 75% of the observed variance, heterogeneity was indicated.31 The mean reduction in depressive symptoms among participants engaging in RET, expressed as a function of absolute risk reduction, was calculated to determine the number needed to treat.39 The number of unretrieved or unpublished studies of null effect that would diminish the significance of observed effects of P > .05 was estimated as fail-safe N+.40
As a sensitivity analysis, the mean effect was recalculated, extracting single effects from the included RCTs determined by the effect with the maximum dose of RET, and the effect in which the Beck Depression Inventory was used,41 for homogeneity of results. There were 3 exceptions in which 2 effects remained extracted from single RCTs because these RCTs each contained 2 treatment groups and 2 control groups.33,42,43
To examine publication bias, funnel plot symmetry was examined, Egger regression44 and Begg rank correlation tests were calculated,45 and trim and fill analysis adjusting to the left of the mean was performed.46 Potential outliers, effects substantially larger than most, were also removed, and the mean effect size ∆ was recalculated for additional sensitivity analysis.
Primary Moderators
Four primary moderators were selected a priori to provide focused research hypotheses about variation in effect size: total volume of prescribed RET, participant’s health status, whether or not allocation and/or assessment was blinded, and whether or not the RET intervention resulted in a significant improvement in strength. Definitions for each primary and secondary moderator and associated levels are presented in eTable 3 in the Supplement.
Primary Moderator Analysis
Each of the 4 primary moderators were coded according to the planned contrasts (P ≤ .05) among its levels.47 Primary moderators were included in the mixed-effects multiple linear regression analyses with maximum likelihood estimation, adjusting for nonindependence of multiple effects contributed by single studies, baseline depressive symptoms, and the depressive symptom measure.31,38 Tests of the regression model (QR) and its residual error (QE) are reported.
Univariate Meta-regression Analyses
Secondary moderators were selected for exploratory univariate analyses. Random-effects models were used to calculate the mean effect sizes (Δ) and 95% CIs for moderator variables.38 Each secondary moderator was included in random-effects univariate meta-regression analysis with maximum likelihood estimation.31,38
Results
Study Characteristics
Fifty-four effects were derived from 33 RCTs of 1877 participants (RET group, 947 participants; control group, 930 participants). Table 1 presents the relevant characteristics for each of the included RCTs.28,32,33,34,35,36,42,43,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72 Depressive symptoms were the primary outcome in 18 RCTs (k = 37). The mean (SD) sample age was 52 (18) years, and 67% of participants were female. The mean prescribed RET program duration was 16 weeks (range, 6-52 weeks). The frequency of RET sessions ranged from 2 to 7 days per week; the most common frequency was 3 days per week (20 RCTs; k = 30). Twenty-five RCTs (k = 39) evaluated participants with a physical or mental illness. Twenty-five RET interventions (k = 44) were fully supervised by various health care professionals. Seven RET interventions (k = 9) included a combination of supervised and unsupervised sessions, and 1 RET intervention was unsupervised. Adherence or compliance was reported in 15 of the 33 RCTs; the mean (SD) adherence rate was 78% (18%). Of the 18 remaining RCTs that did not report adherence or compliance, 2 reported attendance rates, which ranged from 87.5%53 to 94%.71 The Beck Depression Inventory41 was the most frequently used measure of depressive symptoms (k = 21).
Table 1. Characteristics of Included Randomized Clinical Trials.
| Source | Measure | Intensity | Intervention Length, wk | Age, Mean (SD), y | Control | Sex | Participant Characteristics |
|---|---|---|---|---|---|---|---|
| Abrahao et al,48 2016 | BDI | Low to moderate | 12 | 39 (14) | Wait list | Mixed | Systemic lupus erythematosus |
| Aidar et al,49 2014 | BDI | Low to moderate | 12 | 53 (8) | No treatment | Mixed | Survivors of ischemic stroke |
| Alves et al,42 2013 | GDS | Low to moderate | 24 | 64 (4) | No treatment + placebo supplement | Female | Elderly |
| Ansai et al,50 2015 | GDS | Low to moderate | 16 | >80 | No treatment | Mixed | Elderly |
| Courneya et al,51 2007 | CESD | Low to moderate | Duration of treatment | Range, 25-76 | Wait list | Female | Breast cancer |
| Dalgas et al,52 2010 | MDI | Low to moderate | 12 | 48 (10) | Wait list | Mixed | Multiple sclerosis |
| Damush et al,53 1999 | MHFI | Low to moderate | 8 | 68 (6) | Wait list | Female | Elderly |
| Doyne et al,54 1987 | BDI, DACL, HRSD | Low to moderate | 8 | 28 (5) | Wait list | Female | Major or minor depressive disorder |
| Geliebter et al,55 1997 | BDI | Low to moderate | 8 | 35 (6) | No training | Mixed | Obesity |
| Goldfield et al,56 2015 | BRUMS-D | Low to moderate | 22 | 16 (2) | Wait list | Mixed | Obesity |
| Häkkinen et al,57 2001 | BDI | Low to moderate | 21 | 36 (6) | No treatment | Female | Fibromyalgia |
| Herring et al,58 2011 | BDI | Low to moderate | 6 | 24 (6) | Wait list | Female | Generalized anxiety disorder |
| Herring et al,35 2014 | HADS | Low to moderate | 6 | Range, 24-68 | Patient education | Mixed | Obesity |
| Karahan et al,59 2017 | BDI | Low to moderate | 8 | 40 (8) | Patient education | Mixed | Failed back surgery syndrome |
| Lau et al,36 2004 | HADS | Vigorous | 6 | Range, 10-17 | No treatment | Mixed | Obesity |
| Levinger et al,33 2011 | CDS | Low to moderate | 10 | 51 (7) | No treatment | Mixed | Type 2 diabetes |
| Lincoln et al,60 2011 | GDS | Low to moderate | 16 | 66 (8) | No treatment | Mixed | Type 2 diabetes |
| Martins et al,61 2011 | POMS-D | Low to moderate | 16 | 76 (8) | No treatment | Mixed | Elderly |
| Norvell et al,72 1993 | SCL-90-D | Low to moderate | 16 | 33 (8) | Wait list | Male | Law enforcement personnel |
| Nyberg et al,62 2015 | HADS | Low to moderate | 8 | 69 (5) | Patient education | Mixed | Chronic obstructive pulmonary disorder |
| O’Reilly et al,63 1999 | HADS | Low to moderate | 24 | 62 (10) | No treatment | Mixed | Knee osteoarthritis |
| Penninx et al,32 2002 | CESD | Low to moderate | 12 | 69 (6) | Patient education | Mixed | Knee osteoarthritis |
| Pilu et al,64 2007 | HRSD | Not Reported | 32 | Range, 40-60 | Usual care | Female | Major depressive disorder |
| Putiri et al,65 2012 | BDI | Not Reported | 12 | 58 (7) | Usual care | Mixed | Type 2 diabetes |
| Sarsan et al,66 2006 | BDI | Low to moderate | 12 | 43 (10) | No treatment | Female | Obesity |
| Sims et al,67 2009 | CESD | Vigorous | 10 | 68 (15) | Wait list | Mixed | Chronic poststroke patients |
| Singh et al,29 1997 | BDI, DSM, GDS, HRSD | Vigorous | 10 | 71 (7) | Patient education | Mixed | Major or minor depression |
| Singh et al,68 2001 | BDI, GDS, HRSD | Vigorous | 6 | 71 (7) | Patient education | Mixed | Major or minor depression |
| Sparrow et al,34 2011 | BDI | Low to moderate | 24 | 70 (8) | Patient education | Mixed | Elderly |
| Tapps et al,69 2013 | BDI | Low to moderate | 12 | 75 (3) | No treatment | Mixed | Elderly |
| Van der Kooi et al,43 2007 | BDI | Low to moderate | 52 | 38 (10) | No treatment | Mixed | Facioscapulohumeral muscular dystrophy |
| Vizza et al,70 2016 | DASS-21 | Low to moderate | 12 | 26 (7) | Usual care | Female | Polycystic ovary syndrome |
| Zanuso et al,71 2012 | POMS-D | Low to moderate | 12 | 74 (4) | Wait list | Mixed | Elderly |
Abbreviations: BDI, Beck Depression Inventory; BRUMS-D, Brunel Mood Scale Questionnaire–Depression; CDS, Cardiac Depression Scale; CESD, Center for Epidemiologic Studies Depression Scale; DACL, Depression Adjective Checklist; DASS-21, Depression, Anxiety and Stress Scale; GDS, Geriatric Depression Scale; HADS, Hospital Anxiety and Depression Scale; HRSD, Hamilton Rating Scale for Depression; MDI, Major Depression Inventory; MHFI, Mental Health Functioning Index–Depression; POMS-D, Profile of Mood States–Depression; SCL-90-D, Hopkins Symptom Checklist–Depression.
Mean Effect ∆, Heterogeneity, and Publication Bias
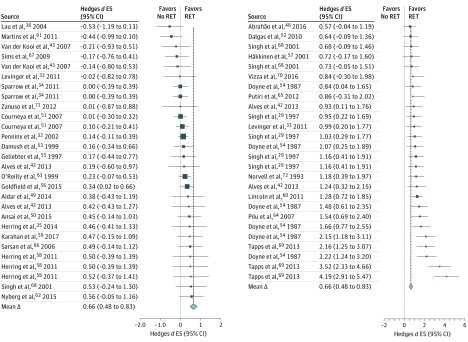
A forest plot of the distribution of effects is presented in the Figure. Forty-eight of the 54 effects (89%) were larger than zero, indicating a reduction in depressive symptoms favoring RET. Twenty effects significantly favored RET. The mean effect size ∆ was 0.66 (95% CI, 0.48-0.83; z = 7.35; P < .001). The effect was heterogeneous (total Q = 216.92, df = 53; P < .001; I2 = 76.0% [95% CI, 72.7%-79.0%]), and sampling error accounted for 32.9% of observed variance. The mean quality score was 10.5 (range, 7-13). The fail-safe number of effects was 1358, indicating that 1358 null effects would be needed to diminish the overall effect to P > .05. Significant Begg rank correlation (Kendall τ = 0.45; P < .001) and Egger regression tests (intercept = –1.34; SE = 0.52; P = .01) indicated significant funnel plot asymmetry (eFigure 2 in the Supplement). Trim and fill analyses did not change the overall effect (∆ = 0.66; 95% CI, 0.48-0.83; 0 RCTs trimmed). The mean reduction in depressive symptoms among participants engaging in RET resulted in a number needed to treat of 4.
Figure. Forest Plot of Distribution of Hedges d Effect Sizes (ES).
Individual effects and overall effect of resistance exercise training on depressive symptoms. The different sizes of the data markers indicate the respective weight of the individual effects in the overall analysis. Studies are cited multiple times because multiple effects were derived from individual trials. Each citation represents a unique effect. The dashed vertical lines show the difference between the overall effect and each individual effect.
Three effects substantially larger than most were derived from 1 RCT.69 The magnitude of these effects appeared to be due partly to greater depressive symptoms among participants who were randomized to the intervention group compared with controls. The mean effect was recalculated with this RCT removed, and the effect remained moderate and significant (∆ = 0.53; 95% CI, 0.38-0.68; z = 7.00; P < .001). Similarly, a nonsignificant reduction in the overall effect was observed when calculated with single effects derived from each study (∆ = 0.48; 95% CI, 0.30-0.67; z = 5.08; P < .001).
Primary Moderator Analyses
The overall meta-regression model was significant (QR = 17.97, df = 7; P = .01; R2 = 0.30; QE = 42.57, df = 31; P = .08; I2 = 38.88% [95% CI, 25.63%-49.77%]). Blinded allocation and/or assessment of outcomes accounted for significant variation in the antidepressant effects of RET (β = –0.39; z = –2.50; P = .01). Effects were significantly smaller when outcome allocation and/or assessment was blinded (∆ = 0.56; 95% CI, 0.40-0.71) compared with when outcome allocation and/or assessment was not blinded (∆ = 1.07; 95% CI, 0.36-1.78). Total volume of prescribed exercise (β = –0.28; P = .09), significant improvements in strength (β = 0.32; P = .09), and participant’s health status (β = –0.23; P = .17) were not significantly related to effect size (Table 2).
Table 2. Summary of Primary Moderator Analysis.
| Primary Moderator | β | P Value | B (SE) | Adjusted 95% CIa |
|---|---|---|---|---|
| Blinded allocation and/or assessment | −0.39 | .01 | −0.036 (0.14) | −0.63 to −0.08 |
| Significant improvement in strength | −0.32 | .09 | 0.35 (0.21) | −0.76 to 0.06 |
| Total volume of RET prescribed | −0.28 | .09 | −0.0002 (0.0001) | −0.0004 to 0 |
| Participant health status | −0.23 | .17 | −0.19 (0.14) | −0.46 to 0.08 |
Abbreviation: RET, resistance exercise training.
Adjusted for nonindependence of multiple effects contributed by single studies, baseline depressive symptoms, and the depressive symptom measure.
Univariate Meta-regression Analyses
The results of univariate moderator analyses for the primary and secondary moderators are presented in Table 3.
Table 3. Summary of Univariate Analyses.
| Effect Moderator | Contrast Weights | Effects (k) | Δ (95% CI) | P Valuea | |
|---|---|---|---|---|---|
| Moderator | Contrast | ||||
| Sex | |||||
| Female | 1 | 20 | 0.81 (0.51 to 1.10) | <.001 | .28 |
| Mixed | −1 | 34 | 0.58 (0.36 to 0.80) | <.001 | |
| Age, y | |||||
| <25 | −0.5 | 2 | −0.04 (−0.89 to 0.80) | .92 | .63 |
| 25-54 | −0.5 | 26 | 0.67 (0.43 to 0.91) | <.001 | |
| ≥55 | 1 | 26 | 0.72 (0.45 to 1.00) | <.001 | |
| Health | |||||
| Healthy | 1 | 15 | 0.81 (0.33 to 1.29) | <.001 | .63 |
| Physical illness | −0.5 | 20 | 0.34 (0.17 to 0.52) | <.001 | |
| Mental illness (MDD, GAD) | −0.5 | 18 | 1.00 (0.69 to 1.31) | <.001 | |
| Baseline depression | |||||
| Indicative of mild to moderate depression | 1 | 25 | 0.90 (0.68 to 1.11) | <.001 | .02 |
| Not indicative | −1 | 29 | 0.45 (0.23 to 0.67) | <.001 | |
| Control condition | |||||
| Attention placebo control | 1 | 15 | 0.98 (0.56 to 1.41) | <.001 | .09 |
| No attention placebo control | −1 | 39 | 0.54 (0.36 to 0.73) | <.001 | |
| Comparison type | |||||
| Wait list | NA | 17 | 0.71 (0.39 to 1.02) | <.001 | NA |
| Patient education | NA | 13 | 0.51 (0.27 to 0.75) | <.001 | |
| No treatment | NA | 11 | 0.33 (0.02 to 0.64) | .04 | |
| Usual care | NA | 5 | 2.30 (1.05 to 3.55) | <.001 | |
| Placebo or second treatment | NA | 8 | 0.48 (0.07 to 0.88) | .02 | |
| Program length, wk | |||||
| <12 | −1 | 26 | 0.88 (0.58 to 1.18) | <.001 | .70 |
| ≥12 | 1 | 26 | 0.51 (0.28 to 0.73) | <.001 | |
| Session, min | |||||
| <45 | −1 | 12 | 1.10 (0.49 to 1.70) | <.001 | .049 |
| ≥45 | 1 | 28 | 0.48 (0.29 to 0.68) | <.001 | |
| Frequency, d/wk | |||||
| 2 | −0.5 | 12 | 0.53 (0.25 to 0.81) | <.001 | .19 |
| 3 | −0.5 | 32 | 0.60 (0.37 to 0.84) | <.001 | |
| ≥4 | 1 | 10 | 1.00 (0.55 to 1.46) | <.001 | |
| Intensity | |||||
| Low to moderate | −1 | 45 | 0.67 (0.49 to 0.87) | <.001 | .72 |
| Vigorous | 1 | 9 | 0.59 (0.17 to 1.01) | .006 | |
| Blinded assessment | |||||
| Yes | 1 | 42 | 0.56 (0.40 to 0.71) | <.001 | .15 |
| No | −1 | 12 | 1.07 (0.36 to 1.78) | .003 | |
| Supervision | |||||
| Combination of supervised and unsupervised | −1 | 9 | 0.14 (0 to 0.29) | .05 | .02 |
| Fully supervised | 1 | 44 | 0.79 (0.57 to 1.02) | <.001 | |
| Primary outcome depression | |||||
| Yes | 1 | 38 | 0.88 (0.63 to 1.13) | <.001 | .002 |
| No | −1 | 16 | 0.19 (0.06 to 0.32) | .006 | |
| Significant improvement in strength | |||||
| Yes | 1 | 19 | 0.50 (0.32 to 0.68) | <.001 | .45 |
| No | −0.5 | 7 | 0.09 (−0.08 to 0.27) | .30 | |
| Not reported | −0.5 | 28 | 0.94 (0.62 to 1.26) | <.001 | |
Abbreviations: GAD, generalized anxiety disorder; MDD, major or minor depressive disorder.
The moderator P value indicates the P value for the mean effect of the individual moderator. The contrast P value indicates the P value of the comparison between the moderator levels.
Subanalysis Between RET and AET
To facilitate subanalyses between RET and AET, data were extracted from 9 RCTs (k = 17) in which participants were randomized to RET, AET, or a nonactive control condition.32,35,48,51,54,55,56,58,61,66 Effects were not significantly different for the RET interventions (∆ = 0.64; 95% CI, 0.34-0.93) than for the AET interventions (∆ = 0.46; 95% CI, 0.22-0.70) compared with the control groups (P = .48). When directly comparing the effects of RET with AET (positive effects favoring RET), a small, nonsignificant mean effect ∆ favoring RET was found (∆ = 0.15; 95% CI, –0.004 to 0.30; z = 1.91; P = .06).
Discussion
To our knowledge, this is the first meta-analysis to examine RCTs to assess the efficacy of RET on depressive symptoms. Across 33 RCTs, RET was associated with a significant reduction in depressive symptoms regardless of the participants’ characteristics (ie, age, sex, and health status) or the features of the RET stimulus (ie, program duration, session duration, intensity, frequency, or total prescribed volume). However, while simultaneously considering the potential variation associated with baseline depressive scores, multiple effects from single RCTs, whether or not strength was significantly improved, total prescribed RET volume, and participant’s health status, blinded allocation and/or assessment was significantly associated with the overall effect of RET, such that significantly smaller reductions in depressive symptoms were found when investigators were blinded to allocation and/or assessment.
Univariate analyses showed that significantly larger reductions in depressive symptoms were derived from RCTs of participants with scores indicative of mild to moderate depression compared with RCTs of participants without scores indicating mild to moderate depression, and from RCTs of shorter RET sessions (<45 minutes) compared with RCTs featuring longer session durations. In addition, significantly larger reductions were found in fully supervised RCTs compared with RCTs that used combinations of supervised and unsupervised RET, and in RCTs in which the primary outcome was depressive symptoms (Table 3).
The magnitude of the overall mean effect (Δ = 0.66; 95% CI, 0.48-0.83) is consistent with the association of diverse types of exercise training with depression (pooled standardized mean difference, –0.62; 95% CI, –0.81 to 0.42, with negative scores favoring exercise)18 and is larger than the recently reported association of RET with anxiety (∆ = 0.31).24 In addition, the magnitude of the overall mean effect and the magnitude of the effects among important subsamples are consistent with previously reported effects. Specifically, the mean effect for individuals with a physical illness (∆ = 0.34; 95% CI, 0.17-0.52) is consistent with previous evidence of the associations of all types of exercise training with depressive symptoms among adults with a chronic illness (∆ = 0.30; 95% CI, 0.25-0.36)19 and adults with neurologic disorders (∆ = 0.28; 95% CI, 0.15-0.41).73
The large effect of RET found among adults with depressive symptoms indicative of mild to moderate depression (∆ = 0.90; 95% CI, 0.68-1.11) is consistent with previously reported effects of all exercise modes among people with major depressive disorder (standardized mean difference, 1.11; 95% CI, 0.79-1.43).17 Twelve RCTs (k = 25) included samples that reported clinically significant elevations in depressive symptoms, based on cutoff scores commonly used for clinical screening.74,75,76,77 The mean scores for 10 of the 25 effects (40%) suggested potential remission based on a frequently used response threshold of a 50% or greater reduction in baseline scores.78 The mean percentage reduction from baseline scores for all 25 of these effects was 45%. Moreover, the mean effect for RCTs in which baseline scores were indicative of mild to moderate depression (Δ = 0.90; 95% CI, 0.68-1.12; z = 8.12; P < .001) was significantly larger than effects from RCTs in which baseline scores were below suggested clinical cutoff scores (Δ = 0.45; 95% CI, 0.23-0.67; z = 4.02; P = .03) (Table 3). The larger percentage reduction found from RCTs of participants with elevated depressive symptoms, coupled with the significant difference based on initial severity of depressive symptoms, suggests that RET may be particularly helpful for reducing depressive symptoms in people with greater depressive symptoms. These findings support potentially different mechanisms of action and/or unique interactions in participants with clinical depression that may not be present in participants with subclinical depressive symptoms.
Primary Moderators of the Effect
Blinded allocation and/or assessment was independently and significantly associated with reductions in depressive symptoms; smaller reductions occurred in RCTs with blinded allocation and/or assessment (∆ = 0.56; 95% CI, 0.40-0.71). Blinded allocation and assessment of outcomes can limit biases associated with self-reported measures in exercise interventions.79,80,81 Previous reports have demonstrated a reduction in the overall effect of exercise on depression after exclusion of trials that do not adequately blind allocation and/or assessment.18
Blinded allocation and/or assessment is also an indication of intervention quality.30,82 Based on the study quality assessment used here, the overall quality of RCTs was high, with a mean score of 10.5 (range, 7-13) on a 13-point scale. When blinding was removed from the overall quality score, such that the maximum total score was 11, RCTs that reported blinded allocation and/or assessment had significantly higher mean (SD) quality scores (10.0 [1.0]) compared with those without blinded allocation and/or assessment (8.0 [0.9]) (t = 5.82, df = 31; P < .001). Blinded allocation and/or assessment may indicate a higher-quality research design, which may have resulted in smaller effects by providing a more rigorous estimation of the “true” effect of RET on depressive symptoms.
Participant’s health status, volume of prescribed RET, and whether or not strength was significantly improved were not independently associated with the overall mean reduction in depressive symptoms. These findings are consistent with previous evidence showing that the antidepressant effects of exercise training were not dependent on a significant improvement in fitness.19 These findings are also consistent with recently reported associations of RET with anxiety.24
Although RET significantly reduced depressive symptoms independent of total prescribed volume of RET, this measure of total volume (intervention length × frequency × session duration) could not be extracted for all RCTs because 8 RCTs (k = 14) did not report the duration of RET sessions. In addition, this measure of total volume did not include the intensity of prescribed RET. Heterogeneous reporting of prescribed intensity did not allow differentiation between low-intensity RET and moderate-intensity RET, necessitating their merger and comparison with vigorous-intensity RET. Only 4 interventions (k = 9)28,36,70,71 were of vigorous intensity. The relationship between RET intensity and strength gains is moderated by participant training status, as moderate-intensity RET improves strength most in untrained participants, and vigorous-intensity RET improves strength most in trained participants.83 There is a paucity of within-study comparisons of RET dose, multiarm RCTs comparing RET and other strictly matched exercise modalities, and investigations of the influence of exercise volume, exercise intensity, and their interaction. For example, more frequently completed vigorous RET may afford the possibility of shorter exercise sessions while meeting recommended guidelines,84 potentially increasing feasibility while maintaining positive mental health benefits.
There is continued interest in the comparative effects of different exercise modes on mental health outcomes. However, with one notable exception,85,86 few RCTs have directly compared the antidepressant effects of different exercise modes in a single study sample. Nine RCTs included here directly compared RET with AET and a nonactive control condition.32,35,48,51,54,55,56,58,61,66 Although the magnitude of improvement for AET and RET did not differ significantly, consistent with recent results of the comparative associations of AET and RET with anxiety symptoms,24 only 2 RCTs attempted to match AET and RET interventions in any capacity. One trial matched AET and RET based on energy expenditure,55 and 1 trial more thoroughly matched AET and RET based on body region, positive work, time actively engaged in exercise, and load progression.58 Future trials, matching different exercise modes on relevant features of the exercise stimulus, will allow more rigorous and controlled comparisons between exercise modalities, and the examination of interactions between factors such as frequency, intensity, duration, and exercise modality.
Future Research
In addition, authors should report the mean session duration, the numbers of sets performed, the numbers of repetitions, the lengths of rest periods between sets, and the intensity (eg, the percentages of 1-repetition maximum and the rate of perceived exertion), to more thoroughly assess the total volume of exercise prescribed. Authors should report whether interventions were performed in groups or individually. When exercise sessions are supervised, the efforts made to control for social interaction during sessions should be reported. Future trials should blind allocation, blind assessors from group assignment, explicitly report this process, and state how missing data and dropouts were handled, including explicitly stating if intention-to-treat analyses were conducted.
Six RCTs assessed the effects of RET on depressive symptoms in participants with a clinical diagnosis of depression or anxiety, and 8 RCTs assessed depressive symptoms in participants who had scores indicative of moderate depression without an actual diagnosis. More important, individuals who display elevated subclinical depressive or anxiety symptoms are at increased risk of developing clinically significant psychopathologic features.87 Because participants with baseline scores indicative of mild to moderate depression had significantly larger improvements than those who did not, investigating RET interventions among individuals at different points on the severity spectrum may be particularly interesting.
Limitations
There was a notable lack of clear and complete reporting of intervention design, protocol, data analyses, participant information, medication use, adherence, and compliance, which should be emphasized in future trial reporting. Medication use was insufficiently reported to allow comparisons between RCTs; 12 of the 33 RCTs (36%) did not report information regarding medication use. Twenty-one of 33 RCTs (64%) did not report adherence or compliance with the interventions. Prescribed antidepressant medication use is associated with poor adherence to exercise programs among patients,88 making this omission particularly problematic.
Conclusions
The available empirical evidence supports RET as an alternative or adjuvant therapy for depressive symptoms. Future trials should include thorough reporting of trial and RET design, specifically blinded allocation, assessment, and adherence. In addition, future trials should compare RET with other empirically supported therapies for depressive symptoms.
eFigure 1. Flowchart of Study Selection
eFigure 2. Funnel Plot of Hedges d Effect Sizes vs Study Standard Error
eTable 1. Individual Scores on Amended Detsky Quality Assessment
eTable 2. Values Used to Calculate Hedges d Effect Size and Primary Moderator Values
eTable 3. Definitions for Each Moderator and Associated Levels
eReferences
References
- 1.World Health Organization Depression fact sheet. http://www.who.int/mediacentre/factsheets/fs369/en/. Updated February 2017. Accessed March 1, 2017.
- 2.Munce SEP, Stansfeld SA, Blackmore ER, Stewart DE. The role of depression and chronic pain conditions in absenteeism: results from a national epidemiologic survey. J Occup Environ Med. 2007;49(11):566-576. [DOI] [PubMed] [Google Scholar]
- 3.Mrazek DA, Hornberger JC, Altar CA, Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996-2013. Psychiatr Serv. 2014;65(8):977-987. [DOI] [PubMed] [Google Scholar]
- 4.Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. J Ment Health Policy Econ. 2006;9(2):87-98. [PubMed] [Google Scholar]
- 5.Penninx BWJH, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lett HS, Blumenthal JA, Babyak MA, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med. 2004;66(3):305-315. [DOI] [PubMed] [Google Scholar]
- 8.Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753-759. [DOI] [PubMed] [Google Scholar]
- 9.Knol MJ, Twisk JWR, Beekman ATF, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus: a meta-analysis. Diabetologia. 2006;49(5):837-845. [DOI] [PubMed] [Google Scholar]
- 10.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. [DOI] [PubMed] [Google Scholar]
- 12.Barrett B, Byford S, Knapp M. Evidence of cost-effective treatments for depression: a systematic review. J Affect Disord. 2005;84(1):1-13. [DOI] [PubMed] [Google Scholar]
- 13.Fournier JC, DeRubeis RJ, Hollon SD, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA. 2010;303(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cuijpers P, Smit F, Bohlmeijer E, Hollon SD, Andersson G. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178. [DOI] [PubMed] [Google Scholar]
- 15.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57-66. [DOI] [PubMed] [Google Scholar]
- 16.Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder third edition. Am J Psychiatry. 2010;167(10):1. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf. Accessed March 1, 2017.20068118 [Google Scholar]
- 17.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42-51. [DOI] [PubMed] [Google Scholar]
- 18.Cooney GM, Dwan K, Greig CA, et al. Exercise for depression. Cochrane Database Syst Rev. 2013;12(9):CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herring MP, Puetz TW, O’Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101-111. [DOI] [PubMed] [Google Scholar]
- 20.Ekkekakis P. Honey, I shrunk the pooled SMD! guide to critical appraisal of systematic reviews and meta-analyses using the Cochrane review on exercise for depression as example. Ment Health Phys Act. 2015;8:21-36. doi: 10.1016/j.mhpa.2014.12.001 [DOI] [Google Scholar]
- 21.McDowell CP, Campbell MJ, Herring MP. Sex-related differences in mood responses to acute aerobic exercise. Med Sci Sports Exerc. 2016;48(9):1798-1802. [DOI] [PubMed] [Google Scholar]
- 22.Meyer JD, Koltyn KF, Stegner AJ, Kim JS, Cook DB. Influence of exercise intensity for improving depressed mood in depression: a dose-response study. Behav Ther. 2016;47(4):527-537. [DOI] [PubMed] [Google Scholar]
- 23.Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008:A1-H14. [Google Scholar]
- 24.Gordon BR, McDowell CP, Lyons M, Herring MP. The effects of resistance exercise training on anxiety: a meta-analysis and meta-regression analysis of randomized controlled trials. Sports Med. 2017;47(12):2521-2532. [DOI] [PubMed] [Google Scholar]
- 25.O’Connor PJ, Herring MP, Caravalho A. Mental health benefits of strength training in adults. Am J Lifestyle Med. 2010;4(5):377-396. doi: 10.1177/1559827610368771 [DOI] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubbs B, Rosenbaum S, Vancampfort D, Ward PB, Schuch FB. Exercise improves cardiorespiratory fitness in people with depression: a meta-analysis of randomized control trials. J Affect Disord. 2016;190:249-253. [DOI] [PubMed] [Google Scholar]
- 28.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20(2):95-101. [DOI] [PubMed] [Google Scholar]
- 29.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52(1):M27-M35. [DOI] [PubMed] [Google Scholar]
- 30.Detsky AS, Naylor CD, O’Rourke K, McGeer AJ, L’Abbé KA. Incorporating variations in the quality of individual randomized trials into meta-analysis. J Clin Epidemiol. 1992;45(3):255-265. [DOI] [PubMed] [Google Scholar]
- 31.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. San Diego, CA: Academic Press; 1985. [Google Scholar]
- 32.Penninx BWJH, Rejeski WJ, Pandya J, et al. Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):124-132. [DOI] [PubMed] [Google Scholar]
- 33.Levinger I, Selig S, Goodman C, Jerums G, Stewart A, Hare DL. Resistance training improves depressive symptoms in individuals at high risk for type 2 diabetes. J Strength Cond Res. 2011;25(8):2328-2333. [DOI] [PubMed] [Google Scholar]
- 34.Sparrow D, Gottlieb DJ, Demolles D, Fielding RA. Increases in muscle strength and balance using a resistance training program administered via a telecommunications system in older adults. J Gerontol A Biol Sci Med Sci. 2011;66(11):1251-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herring LY, Wagstaff C, Scott A. The efficacy of 12 weeks supervised exercise in obesity management. Clin Obes. 2014;4(4):220-227. [DOI] [PubMed] [Google Scholar]
- 36.Lau PWC, Yu CW, Lee A, Sung RYT. The physiological and psychological effects of resistance training on Chinese obese adolescents. J Exerc Sci Fit. 2004;2(2):115-120. [Google Scholar]
- 37.Green S, Higgins JPT, Alderson P, Clarke M, Mulrow CD, Oxman AD. Cochrane Handbook for Systematic Reviews of Interventions. Vol 4. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 38.Lipsey MW, Wilson DB. Practical Meta-Analysis. Vol 49 Thousand Oaks, CA: Sage publications; 2001. [Google Scholar]
- 39.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310(6977):452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59(2):464-468. [PubMed] [Google Scholar]
- 41.Beck AT, Steer RA, Brown GK. Beck Depression Inventory–II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 42.Alves CRR, Merege Filho CA, Benatti FB, et al. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: a randomized double-blind study. PLoS One. 2013;8(10):e76301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Kooi EL, Kalkman JS, Lindeman E, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol. 2007;254(7):931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. [PubMed] [Google Scholar]
- 46.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. [DOI] [PubMed] [Google Scholar]
- 47.Rosenthal R. Meta-Analytic Procedures for Social Research. Vol 6 Thousand Oaks, CA: Sage publications; 1991. [Google Scholar]
- 48.Abrahão MI, Gomiero AB, Peccin MS, Grande AJ, Trevisani VF. Cardiovascular training vs. resistance training for improving quality of life and physical function in patients with systemic lupus erythematosus: a randomized controlled trial. Scand J Rheumatol. 2016;45(3):197-201. [DOI] [PubMed] [Google Scholar]
- 49.Aidar FJ, de Matos DG, de Oliveira RJ, et al. Relationship between depression and strength training in survivors of the ischemic stroke. J Hum Kinet. 2014;43(1):7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ansai JH, Rebelatto JR. Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: A randomized controlled trial. Geriatr Gerontol Int. 2015;15(9):1127-1134. [DOI] [PubMed] [Google Scholar]
- 51.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396-4404. [DOI] [PubMed] [Google Scholar]
- 52.Dalgas U, Stenager E, Jakobsen J, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler. 2010;16(4):480-490. [DOI] [PubMed] [Google Scholar]
- 53.Damush TM, Damush JG Jr. The effects of strength training on strength and health-related quality of life in older adult women. Gerontologist. 1999;39(6):705-710. [DOI] [PubMed] [Google Scholar]
- 54.Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, McDougall-Wilson IB, Neimeyer RA. Running versus weight lifting in the treatment of depression. J Consult Clin Psychol. 1987;55(5):748-754. [DOI] [PubMed] [Google Scholar]
- 55.Geliebter A, Maher MM, Gerace L, Gutin B, Heymsfield SB, Hashim SA. Effects of strength or aerobic training on body composition, resting metabolic rate, and peak oxygen consumption in obese dieting subjects. Am J Clin Nutr. 1997;66(3):557-563. [DOI] [PubMed] [Google Scholar]
- 56.Goldfield GS, Kenny GP, Alberga AS, et al. Effects of aerobic training, resistance training, or both on psychological health in adolescents with obesity: The HEARTY randomized controlled trial. J Consult Clin Psychol. 2015;83(6):1123-1135. [DOI] [PubMed] [Google Scholar]
- 57.Häkkinen A, Häkkinen K, Hannonen P, Alen M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: comparison with healthy women. Ann Rheum Dis. 2001;60(1):21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Herring MP, Jacob ML, Suveg C, O’Connor PJ. Effects of short-term exercise training on signs and symptoms of generalized anxiety disorder. Ment Health Phys Act. 2011;4(2):71-77. doi: 10.1016/j.mhpa.2011.07.002 [DOI] [Google Scholar]
- 59.Karahan AY, Sahin N, Baskent A. Comparison of effectiveness of different exercise programs in treatment of failed back surgery syndrome: a randomized controlled trial. J Back Musculoskeletal Rehabil. 2017;30(1):109-120. doi: 10.3233/BMR-160722 [DOI] [PubMed] [Google Scholar]
- 60.Lincoln AK, Shepherd A, Johnson PL, Castaneda-Sceppa C. The impact of resistance exercise training on the mental health of older Puerto Rican adults with type 2 diabetes. J Gerontol B Psychol Sci Soc Sci. 2011;66(5):567-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martins R, Coelho E Silva M, Pindus D, Cumming S, Teixeira A, Veríssimo M. Effects of strength and aerobic-based training on functional fitness, mood and the relationship between fatness and mood in older adults. J Sports Med Phys Fitness. 2011;51(3):489-496. [PubMed] [Google Scholar]
- 62.Nyberg A, Lindström B, Rickenlund A, Wadell K. Low-load/high-repetition elastic band resistance training in patients with COPD: a randomized, controlled, multicenter trial. Clin Respir J. 2015;9(3):278-288. [DOI] [PubMed] [Google Scholar]
- 63.O’Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised controlled trial. Ann Rheum Dis. 1999;58(1):15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pilu A, Sorba M, Hardoy MC, et al. Efficacy of physical activity in the adjunctive treatment of major depressive disorders: preliminary results. Clin Pract Epidemiol Ment Health. 2007;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Putiri AL, Lovejoy JC, Gillham S, Sasagawa M, Bradley R, Sun GC. Psychological effects of Yi Ren Medical Qigong and progressive resistance training in adults with type 2 diabetes mellitus: a randomized controlled pilot study. Altern Ther Health Med. 2012;18(1):30-34. [PubMed] [Google Scholar]
- 66.Sarsan A, Ardiç F, Özgen M, Topuz O, Sermez Y. The effects of aerobic and resistance exercises in obese women. Clin Rehabil. 2006;20(9):773-782. [DOI] [PubMed] [Google Scholar]
- 67.Sims J, Galea M, Taylor N, et al. Regenerate: assessing the feasibility of a strength-training program to enhance the physical and mental health of chronic post stroke patients with depression. Int J Geriatr Psychiatry. 2009;24(1):76-83. [DOI] [PubMed] [Google Scholar]
- 68.Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci. 2001;56(8):M497-M504. [DOI] [PubMed] [Google Scholar]
- 69.Tapps T, Passmore T, Lindenmeier D, Bishop A. An investigation into the effects of resistance based physical activity participation on depression of older adults in a long-term care facility. Annu Ther Recreation. 2013;21:63-72. [Google Scholar]
- 70.Vizza L, Smith CA, Swaraj S, Agho K, Cheema BS. The feasibility of progressive resistance training in women with polycystic ovary syndrome: a pilot randomized controlled trial. BMC Sports Sci Med Rehabil. 2016;8(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zanuso S, Sieverdes JC, Smith N, Carraro A, Bergamin M. The effect of a strength training program on affect, mood, anxiety, and strength performance in older individuals. Int J Sport Psychol. 2012;43(1):53-66. [Google Scholar]
- 72.Norvell N, Belles D. Psychological and physical benefits of circuit weight training in law enforcement personnel. J Consult Clin Psychol. 1993;61(3):520-527. [DOI] [PubMed] [Google Scholar]
- 73.Adamson BC, Ensari I, Motl RW. Effect of exercise on depressive symptoms in adults with neurologic disorders: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(7):1329-1338. [DOI] [PubMed] [Google Scholar]
- 74.Kendall PC, Hollon SD, Beck AT, Hammen CL, Ingram RE. Issues and recommendations regarding use of the Beck Depression Inventory. Cognit Ther Res. 1987;11(3):289-299. doi: 10.1007/BF01186280 [DOI] [Google Scholar]
- 75.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203-214. [DOI] [PubMed] [Google Scholar]
- 76.Crawford JR, Henry JD, Crombie C, Taylor EP. Normative data for the HADS from a large non-clinical sample. Br J Clin Psychol. 2001;40(pt 4):429-434. [DOI] [PubMed] [Google Scholar]
- 77.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982-1983;17(1):37-49. [DOI] [PubMed] [Google Scholar]
- 78.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. [DOI] [PubMed] [Google Scholar]
- 79.Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ. 2008;336(7644):601-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non-blinded outcome assessors. BMJ. 2012;344:e1119. [DOI] [PubMed] [Google Scholar]
- 81.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408-412. [DOI] [PubMed] [Google Scholar]
- 82.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rhea MR, Alvar BA, Burkett LN, Ball SD. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc. 2003;35(3):456-464. [DOI] [PubMed] [Google Scholar]
- 84.World Health Organization Global strategy on diet, physical activity and health: physical activity and adults. http://www.who.int/dietphysicalactivity/factsheet_adults/en/. Accessed March 1, 2017.
- 85.Hallgren M, Helgadóttir B, Herring MP, et al. Exercise and internet-based cognitive-behavioural therapy for depression: multicentre randomised controlled trial with 12-month follow-up. Br J Psychiatry. 2016;209(5):414-420. [DOI] [PubMed] [Google Scholar]
- 86.Hallgren M, Kraepelien M, Öjehagen A, et al. Physical exercise and internet-based cognitive-behavioural therapy in the treatment of depression: randomised controlled trial. Br J Psychiatry. 2015;207(3):227-234. [DOI] [PubMed] [Google Scholar]
- 87.Wolitzky-Taylor K, Dour H, Zinbarg R, et al. Experiencing core symptoms of anxiety and unipolar mood disorders in late adolescence predicts disorder onset in early adulthood. Depress Anxiety. 2014;31(3):207-213. [DOI] [PubMed] [Google Scholar]
- 88.Laustsen S, Hjortdal VE, Petersen AK. Predictors for not completing exercise-based rehabilitation following cardiac surgery. Scand Cardiovasc J. 2013;47(6):344-351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Flowchart of Study Selection
eFigure 2. Funnel Plot of Hedges d Effect Sizes vs Study Standard Error
eTable 1. Individual Scores on Amended Detsky Quality Assessment
eTable 2. Values Used to Calculate Hedges d Effect Size and Primary Moderator Values
eTable 3. Definitions for Each Moderator and Associated Levels
eReferences