Key Points
Question
Do patients at high risk of psychosis exhibit disorganized gyrification network properties, and is it possible to predict transition to a first episode of psychosis on the basis of gyrification?
Findings
In this cross-sectional magnetic resonance imaging study among 161 individuals in 4 study groups, patients who later develop psychosis exhibit disorganized gyrification network properties even at a clinically high-risk state. Gyrification network measures predict the future outcome of transition with more than 80% accuracy.
Meaning
Constructing gyrification-based networks from structural magnetic resonance imaging may facilitate individual prediction of future psychosis in those at clinical high risk for psychosis.
Abstract
Importance
There is urgent need to improve the limited prognostic accuracy of clinical instruments to predict psychosis onset in individuals at clinical high risk (CHR) for psychosis. As yet, no reliable biological marker has been established to delineate CHR individuals who will develop psychosis from those who will not.
Objectives
To investigate abnormalities in a graph-based gyrification connectome in the early stages of psychosis and to test the accuracy of this systems-based approach to predict a transition to psychosis among CHR individuals.
Design, Setting, and Participants
This investigation was a cross-sectional magnetic resonance imaging (MRI) study with follow-up assessment to determine the transition status of CHR individuals. Participants were recruited from a specialized clinic for the early detection of psychosis at the Department of Psychiatry (Universitäre Psychiatrische Kliniken [UPK]), University of Basel, Basel, Switzerland. Participants included individuals in the following 4 study groups: 44 healthy controls (HC group), 63 at-risk mental state (ARMS) individuals without later transition to psychosis (ARMS-NT group), 16 ARMS individuals with later transition to psychosis (ARMS-T group), and 38 antipsychotic-free patients with first-episode psychosis (FEP group). The study dates were November 2008 to November 2014. The dates of analysis were March to November 2017.
Main Outcomes and Measures
Gyrification-based structural covariance networks (connectomes) were constructed to quantify global integration, segregation, and small-worldness. Group differences in network measures were assessed using functional data analysis across a range of network densities. The extremely randomized trees algorithm with repeated 5-fold cross-validation was used to delineate ARMS-T individuals from ARMS-NT individuals. Permutation tests were conducted to assess the significance of classification performance measures.
Results
The 4 study groups comprised 161 participants with mean (SD) ages ranging from 24.0 (4.7) to 25.9 (5.7) years. Small-worldness was reduced in the ARMS-T and FEP groups and was associated with decreased integration and increased segregation in both groups (Hedges g range, 0.666-1.050). Using the connectome properties as features, a good classification performance was obtained (accuracy, 90.49%; balanced accuracy, 81.34%; positive predictive value, 84.47%; negative predictive value, 92.18%; sensitivity, 66.11%; specificity, 96.58%; and area under the curve, 88.30%).
Conclusions and Relevance
These findings suggest that there is poor integration in the coordinated development of cortical folding in patients who develop psychosis. These results further suggest that gyrification-based connectomes might be a promising means to generate systems-based measures from anatomical data to improve individual prediction of a transition to psychosis in CHR individuals.
This cross-sectional magnetic resonance imaging study investigates abnormalities in a graph-based gyrification connectome in the early stages of psychosis and tests the accuracy of this systems-based approach to predict a transition to psychosis among individuals at clinical high risk for psychosis.
Introduction
Predicting psychosis onset in individuals at clinical high risk (CHR) for psychosis1 is essential to administer preventive interventions. However, it is not yet possible to make any personalized prediction of psychosis onset relying only on the initial clinical assessment.2 Therefore, research is striving for reliable brain markers to improve the prediction of psychosis onset in these individuals.3,4
To date, most studies have used structural magnetic resonance imaging (MRI) to investigate the alterations in regional gray matter volume (GMV) in CHR individuals.5 However, most of the available evidence shows that GMV reductions seen in patients occur before the transition to psychosis, during the transition, or in the immediate postonset phase, which may indicate that morphometric methods, such as the measurement of gyrification, are perhaps more sensitive to detect the pathophysiology in the prodromal phase.6 Indeed, altered local gyrification indexes (LGIs) have been reported not only in patients with schizophrenia7 but also in patients with first-episode psychosis (FEP),8 those at genetic risk for schizophrenia,9,10 and CHR individuals,11 as well as in those who later developed an overt psychotic disorder.12,13,14 However, these localized regional changes fail to quantify the association between concomitant changes in different brain areas.15 The gross morphology of the developing brain undergoes several well-coordinated maturational events to establish neural networks,16 and developmental disturbances in the topological organization of these networks can result in various psychiatric disorders, such as schizophrenia.17,18 Maldevelopment of the structural connectome can be inferred by studying the covariance of morphology using graph theory.15 Such graph-based network studies capture an important aspect of developmental maturation that is crucial for understanding the pathophysiology of psychotic disorders.19,20 Previous studies reported reduced small-worldness of structural brain networks in patients with schizophrenia,21,22,23 CHR individuals,24 people at increased familial risk for schizophrenia,25,26,27 and those with subclinical psychotic experiences,28 characterized by increased segregation and decreased interaction of anatomical covariance (see the published reviews18,19,29,30,31 of network analyses in schizophrenia). Of various regional morphometric properties that can be assessed for structural covariance, gyrification is a compelling marker of early neurodevelopment. The overall pattern of cortical gyrification is established by year 2 of human life and remains consistent for most of the adult life.32 Perinatal complications that affect brain development result in aberrant gyrification.33,34 Notably, experimental introduction of white matter lesions in early life produces localized changes in cortical folding in distant regions that are axonally connected.35 Therefore, aberrations in structural covariance of gyrification can provide an index of the integrity of cortical connectivity in early life. It has previously been shown that the gyrification-based structural connectome is indeed not normal in patients with schizophrenia, with an abnormally segregated pattern of cortical folding in distributed brain regions, especially in those with more severe illness36 and in nonresponders to antipsychotics.37
This study explored the topological organization of gyrification networks in the following 4 study groups: 44 healthy controls (HC group), 63 at-risk mental state (ARMS) individuals without later transition to psychosis (ARMS-NT group), 16 ARMS individuals with later transition to psychosis (ARMS-T group), and 38 antipsychotic-free patients with FEP (FEP group). The study dates were November 2008 to November 2014. The dates of analysis were March to November 2017. Our aims were to investigate whether the network properties of the gyrification-based connectome differed at baseline among the 4 study groups and to test if this information was sufficiently discriminatory to delineate ARMS individuals transitioning vs nontransitioning to psychosis.
Methods
Patients
We recruited 44 HCs, 79 ARMS individuals, and 38 antipsychotic-free patients with FEP in our specialized clinic for the early detection of psychosis at the Department of Psychiatry (Universitäre Psychiatrische Kliniken [UPK]), University of Basel, Basel, Switzerland. All participants provided written informed consent, and the study was approved by the local ethics committee (Ethikkommission Nordwest-und Zentralschweiz).
The ARMS and FEP statuses were assessed using the Basel Screening Instrument for Psychosis (BSIP),38 the Brief Psychiatric Rating Scale (BPRS),39 the Scale for the Assessment of Negative Symptoms (SANS),40 and the Global Assessment of Functioning (GAF).41 In addition, we recorded current and previous psychotropic medication, as well as nicotine and illegal drug consumption, by using a semistructured interview. The following exclusion criteria were applied to all study groups: history of a psychotic disorder; psychotic symptoms secondary to an organic disorder; substance abuse according to International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) research criteria; psychotic symptoms associated with a bipolar disorder, major depression, or a borderline personality disorder; age younger than 18 years; inadequate knowledge of the German language; and IQ less than 70, measured with the Mehrfachwahl-Wortschatz-Test Form B.42
The ARMS status required 1 or more of the following: (1) attenuated psychoticlike symptoms, (2) brief limited intermittent psychotic symptoms (BLIPS), or (3) a first-degree or second-degree relative with a psychotic disorder plus at least 2 additional risk factors for or indicators of beginning psychosis according to the BSIP screening instrument. Inclusion because of attenuated psychotic symptoms required that change in mental state had to be present at least several times a week and for more than 1 week (a score of 2 or 3 on the BPRS hallucination item or a score of 3 or 4 on BPRS items for unusual thought content or suspiciousness). Inclusion because of BLIPS required a score of 4 or higher on the BPRS hallucination item or a score of 5 or higher on BPRS items for unusual thought content, suspiciousness, or conceptual disorganization, with each symptom lasting less than 1 week before resolving spontaneously. After the baseline assessment, ARMS individuals were followed up clinically and received standard psychiatric case management (mean [SD] follow-up, 3.8 [3.2] years). All ARMS individuals were antipsychotic naive, while 29 were taking low-dose antidepressants at the time of imaging (see the eMethods in the Supplement). Sixteen ARMS individuals have transitioned to psychosis (transition rate, 20%; mean [SD] time of transition after MRI, 19.85 [19.60] months).
Patients with FEP were those who met the criteria for a transition to psychosis according to the classification by Yung et al.43 These patients already fulfilled criteria for acute psychotic disorder according to the ICD-10 or the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) but not yet for schizophrenia. Inclusion required a score of 4 or higher on the BPRS hallucination item or a score of 5 or higher on BPRS items for unusual thought content, suspiciousness, or conceptual disorganization. The symptoms must have occurred at least several times a week and persisted for more than 1 week. All patients with FEP were antipsychotic free at the time of imaging. Six patients with FEP were taking antidepressants (see the eMethods in the Supplement).
Healthy controls were recruited from the same geographic area as the other groups. They had no current psychiatric disorder; no history of a psychiatric illness, head trauma, neurologic illness, serious medical or surgical illness, or substance abuse; and no family history of any psychiatric disorder as assessed by an experienced psychiatrist in a detailed clinical interview.
MRI Data Acquisition
Magnetic resonance imaging data were obtained in all participants. Details of the data acquisition protocol can be found in the eMethods in the Supplement.
Computation of LGIs
Local gyrification indexes were obtained using the method by Schaer et al,44 with images reconstructed via a software program (FreeSurfer, version 5.3.0; http://surfer.nmr.mgh.harvard.edu/).45 The LGIs were computed for 68 parcellated brain regions according to the atlas by Desikan et al46 (see eFigure 1 in the Supplement). More details can be found in the eMethods in the Supplement.
Group Comparison on LGIs
Multiple analysis of covariance (MANCOVA) was performed. The comparison used LGIs as dependent variables, group as a fixed factor, and age, sex, and intracranial volume as covariates.
Gyrification Network Construction
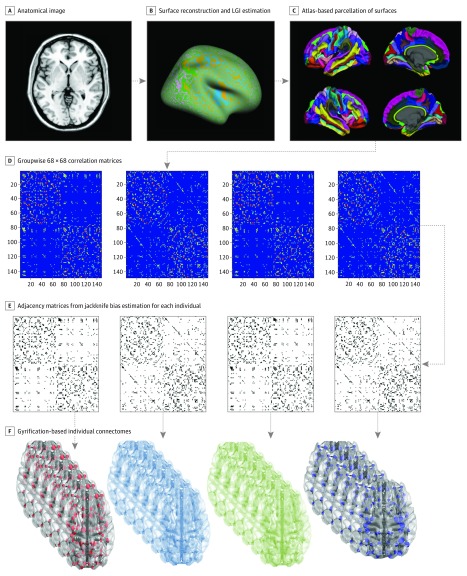
We first generated a 68 × 68 correlation matrix based on LGIs for each of the 4 study groups, adjusted for the effect of age, sex, and intracranial volume. We then used a jackknife bias estimation procedure to determine each individual’s contribution to the overall group-level covariance structure, providing an individual-wise 68 × 68 distance matrix for the 4 study groups (see the eMethods in the Supplement for more information).47,48 A graph analysis toolbox49 was used for studying various topological properties. For the weighted connectivity matrices obtained from each individual, a range of network thresholds based on connection density (ie, 0.05-0.25, with interval steps of 0.01) was applied to generate binary undirected adjacency matrices. This choice of range enabled between-group comparisons in topological measures across graphs with comparable number of edges but without inducing disconnection or losing small-worldness. Topological measures were normalized to equivalent values derived from 20 random (“null”) networks with the same degree of distribution. Figure 1 shows a flow diagram of the graph analysis.
Figure 1. Steps in Processing the Gyrification-Based Connectome.
A, Acquisition of anatomical image. B, Surface reconstruction was carried out using a software program (FreeSurfer, version 5.3.0; http://surfer.nmr.mgh.harvard.edu/). Local gyrification index (LGI) estimation was performed using the method by Schaer et al.44 C, The atlas by Desikan et al46 was used for parcellating the cortical surface to 68 regions (34 on each hemisphere). D, Association matrices were obtained by calculating the correlations between regional gyrification across individuals within each group separately. E, Binary adjacency matrices were derived from bias estimates using the jackknife procedure for each individual within the 4 study groups. F, Binarization used the selected range of cost densities whereby the resulting graphs were always fully connected and had small-world properties.
Topological Properties of Gyrification Networks
In a gyrification-based connectome, the simplest measure of the overall network strength is provided by the average degree of individual nodes. Modular development and regionally selective functional dependency within clusters of high covariance result in a high degree of segregation. In contrast, high integration indicates a coordinated maturational process affecting the entire brain; this effect may result from the presence of certain “central” hub regions whose structure covaries with a large number of other brain regions, leading to widely distributed structural coupling. Such hub regions often show a preferential covariance among each other, making the connectome resilient to pathological processes affecting a single hub region. Assortativity is an index of such resilience. The presence of high segregation in the context of optimum integration gives rise to small-worldness, measured using the small-world index (σ). The σ is the ratio between the normalized clustering coefficient (ϒ) and the normalized characteristic path length (λ). The ϒ is normalized for each node and averaged across the network; hence, it is likely to be influenced disproportionately by low-degree (or weakly connected) nodes. Transitivity, another topological measure, is free of this bias because it is normalized collectively, providing more intuitive information regarding segregated structural covariance. See the eMethods in the Supplement for formal descriptions of these properties.
Group Comparisons on Network Measures
To perform statistical group comparisons across the range of densities between 0.05 and 0.25, we first constructed curves showing the change in the measures of interest as a function of the density. Functional data analysis was performed with topological measures treated as a function of y = f(x), allowing summation across densities and obviating the need for multiple testing. A 1-way analysis of variance was used, followed by post hoc t tests to compare the density function as obtained using functional data analysis among the 4 study groups. Given that we examined 6 topological measures, Bonferroni-corrected 2-tailed P = .05 divided by 6 (ie, .008) was chosen as the threshold of statistical significance. The unbiased effect size between-group comparisons were estimated using Hedges g.50
Association Between Topological Measures and Clinical Variables
Spearman rank correlations were used to test the association between topological measures and clinical variables (BPRS, SANS, and GAF total scores). These correlations were tested for each patient group separately.
Individual Prediction of a Transition to Psychosis
The extremely randomized trees algorithm51 (see the eMethods in the Supplement) with all 6 network measures as features was used to delineate ARMS-NT individuals from AMRS-T individuals. We were specifically interested in whether we could improve the positive predictive value (PPV) because clinical instruments have a limited ability to rule in heightened risk of subsequent psychosis in CHR individuals.2 While increasing PPV is important to make clinically viable predictions, reducing the number of false-positives to a minimum is essential, especially because treatments to prevent transition are of uncertain efficacy at present. In line with prior work,52 we thus calculated 2 diagnostic indexes in addition to pretest and posttest probabilities and the positive likelihood ratio. These factors included the predictive summary index (PSI) to quantify the total amount of uncertainty reduced by the gyrification test (PSI = [PPV + NPV] − 1) (where NPV indicates the negative predictive value) and the number needed to predict (NNP) (NNP = 1 / PSI),53 an estimate of the number of patients that need to be examined to correctly predict diagnosis in one person.
Results
Demographic and Clinical Features
The 4 study groups comprised 161 participants with mean (SD) ages ranging from 24.0 (4.7) to 25.9 (5.7) years. The 4 study groups were well matched on age, handedness, cannabis consumption, and antidepressant treatment, but they differed on sex, years of education and premorbid IQ, intracranial volume, cigarettes per day and alcohol consumption, and global functioning. The 3 clinical groups differed on the BPRS and GAF total scores but not on the SANS total score (Table 1). Because premorbid IQ differed between study groups as a feature of the illness, it was not included as a covariate in all group comparisons (see eTable 1 in the Supplement for correlations between IQ and graph variables).
Table 1. Clinical and Demographic Characteristics of the Study Samplea.
| Variable | HC (n = 44) |
ARMS-NT (n = 63) |
ARMS-T (n = 16) |
FEP (n = 38) |
Group Statistic | P Value |
|---|---|---|---|---|---|---|
| Age, mean (SD), yb | 25.52 (4.27) | 24.03 (4.67) | 25.63 (7.08) | 25.87 (5.67) | F3,160 = 1.36 | .26 |
| Sex, No. (%)c | ||||||
| Men | 17 (39) | 49 (78) | 8 (50) | 34 (89) | χ23 = 30.13 | <.001 |
| Women | 27 (61) | 14 (22) | 8 (50) | 4 (11) | ||
| Right-handedness, No. (%)c | 40 (91) | 59 (94) | 16 (100) | 36 (95) | χ26 = 3.61 | .73 |
| Years of education, mean (SD)b | 15.45 (2.65) | 13.65 (2.58) | 13.81 (3.00) | 13.11 (3.04) | F3,160 = 5.78 | .001 |
| HC>ARMS-NT | .006 | |||||
| HC>FEP | .001 | |||||
| Premorbid IQ on the MWT-B, mean (SD)b | 118.48 (12.13) | 114.19 (14.88) | 105.36 (11.84) | 106.61 (15.40) | F3,111 = 4.36 | .006 |
| HC>ARMS-T | .04 | |||||
| HC>FEP | .02 | |||||
| Intracranial volume, mean (SD), cm3b | 1613.78 (154.73) | 1687.59 (153.70) | 1585.14 (133.96) | 1644.44 (143.67) | F3,160 = 3.17 | .03 |
| Cigarettes per day, mean (SD), No.b | 2.45 (5.74) | 7.35 (8.91) | 6.81 (8.42) | 11.11 (10.89) | F3,160 = 6.90 | <.001 |
| HC<ARMS-NT | .03 | |||||
| HC<FEP | .001 | |||||
| Alcohol consumption, No. (%)c | ||||||
| None | 5 (11) | 6 (9) | 7 (44) | 14 (37) | χ26 = 23.00 | .001 |
| Moderate | 37 (84) | 47 (75) | 8 (50) | 22 (58) | ||
| Uncontrolled | 2 (5) | 10 (16) | 1 (6) | 2 (5) | ||
| Cannabis consumption, No.c | ||||||
| Yes | 5 (11) | 20 (32) | 4 (25) | 11 (29) | χ23 = 6.23 | .10 |
| No | 39 (89) | 43 (68) | 12 (75) | 27 (71) | ||
| BPRS total score, mean (SD)d | NA | 36.90 (8.33) | 38.00 (6.64) | 50.03 (13.42) | F2,116 = 20.99 | <.001 |
| ARMS-NT<FEP | <.001 | |||||
| ARMS-T < FEP | <.001 | |||||
| SANS total score, mean (SD)d | NA | 13.87 (11.97) | 18.06 (11.94) | 18.26 (13.57) | F2,116 = 1.74 | .18 |
| GAF total score, mean (SD)b | 90.34 (4.68) | 70.98 (12.54) | 63.38 (13.84) | 54.74 (17.21) | F3,160 = 58.41 | <.001 |
| HC>ARMS-NT | <.001 | |||||
| HC>ARMS-T | <.001 | |||||
| HC>FEP | <.001 | |||||
| Antidepressant treatment, No. (%)e | ||||||
| Yes | NA | 23 (37) | 6 (38) | 7 (18) | χ22 = 4.03 | .133 |
| No | NA | 40 (63) | 10 (63) | 31 (82) |
Abbreviations: ARMS-NT, at-risk mental state nontransition; ARMS-T, at-risk mental state transition; BPRS, Brief Psychiatric Rating Scale; FEP, first-episode psychosis; GAF, Global Assessment of Functioning; HC, healthy control; MWT-B, Mehrfachwahl-Wortschatz-Test Form B; NA, not applicable; SANS, Scale for the Assessment of Negative Symptoms.
Controlling for group effect, there is a significant negative association between years of education and cigarettes per day (r = −0.277, P = .003) and a significant positive association between years of education and premorbid IQ (r = 0.262, P = .005). Controlling for group effect, cannabis consumption is positively associated with cigarettes per day (F = 12.822, P = .001) but not years of education (F = 0.231, P = .63) or premorbid IQ (F = 0.193, P = .66). Controlling for group effect, alcohol consumption is not associated with years of education (F = 0.385, P = .68), premorbid IQ (F = 1.991, P = .14), or cigarettes per day (F = 0.811, P = .45).
Analysis of variance between all 4 study groups.
χ2 Test between all 4 study groups.
Analysis of variance between ARMS-NT, ARMS-T, and FEP.
χ2 Test between ARMS-NT, ARMS-T, and FEP.
Group Differences in Raw LGIs
MANCOVA revealed no group effect on regional LGIs (F = 0.9, P = .666). eTable 2 in the Supplement lists the raw LGI values.
Gyrification Network Properties
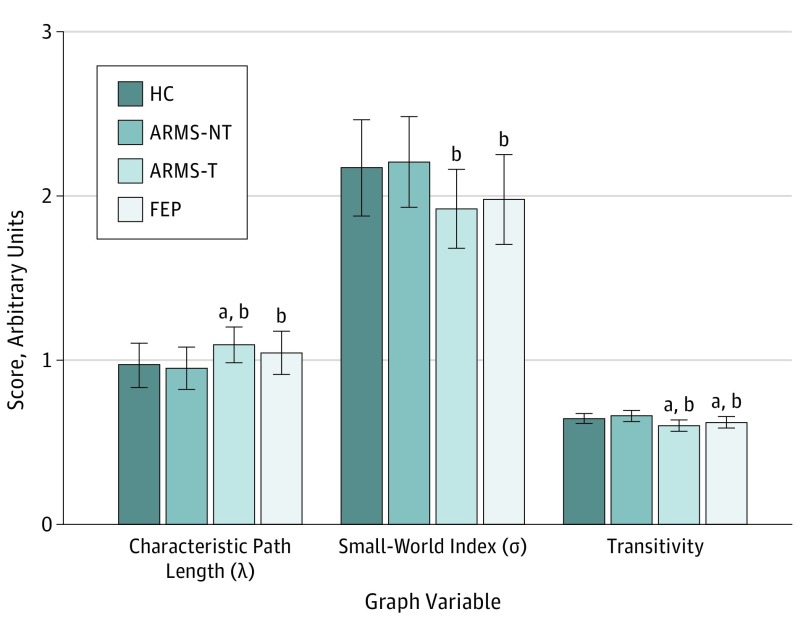
We noted statistically significant group differences in the topological properties of λ, σ, and transitivity but not in the overall network strength, assortativity, or ϒ (Table 2 and Figure 2). Post hoc comparisons revealed that small-worldness σ was reduced in the ARMS-T and FEP groups compared with the HC and ARMS-NT groups, with differences between the ARMS-NT and ARMS-T groups showing large effect sizes (Hedges g, >1). Characteristic path length λ was significantly higher in the ARMS-T and FEP groups, indicating reduced integration compared with the HC and ARMS-NT groups. In both cases (for small-worldness σ and characteristic path length λ), there was no difference between the ARMS-T and FEP groups, indicating that these parameters are observable before the transition to psychosis and remain unaltered in the presence of FEP. In addition, we found that transitivity (segregation) was significantly higher in the ARMS-T and FEP groups compared with the HC and ARMS-NT groups. Transitivity was higher in the FEP group compared with the ARMS-T group.
Table 2. Graph Variables and Their Effect Sizes in the HC, ARMS-NT, ARMS-T, and Antipsychotic-Free FEP Groups.
| Graph Variable | Mean (SD) | F Score | P Value | HC vs ARMS-NT | HC vs ARMS-T | HC vs FEP | ARMS-NT vs FEP | ARMS-T vs FEP | ARMS-NT vs ARMS-T | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC (n = 44) |
ARMS-NT (n = 63) |
ARMS-T (n = 16) |
FEP (n = 38) |
Hedges g | P Value | Hedges g | P Value | Hedges g | P Value | Hedges g | P Value | Hedges g | P Value | Hedges g | P Value | |||
| λ | 0.969 (0.135) |
0.953 (0.128) |
1.095 (0.107) |
1.046 (0.132) |
7.871 | <.001 | 0.121 | .535 | −0.969 | .001 | −0.571 | .011 | −0.713 | <.001 | 0.385 | .195 | −1.132 | <.001 |
| ϒ | 1.960 (0.086) |
1.961 (0.104) |
1.958 (0.066) |
1.914 (0.107) |
2.162 | .095 | −0.010 | .958 | 0.024 | .933 | 0.473 | .034 | 0.444 | .032 | 0.447 | .134 | 0.030 | .913 |
| σ | 2.170 (0.293) |
2.207 (0.276) |
1.921 (0.242) |
1.979 (0.273) |
8.581 | <.001 | −0.130 | .507 | 0.876 | .004 | 0.666 | .003 | 0.823 | <.001 | −0.216 | .465 | 1.050 | <.001 |
| Transitivity | 0.647 (0.031) |
0.662 (0.034) |
0.602 (0.033) |
0.623 (0.034) |
20.518 | <.001 | −0.454 | .022 | 1.409 | <.001 | 0.733 | .001 | 1.138 | <.001 | −0.614 | .042 | 1.757 | <.001 |
| Average degree | 10.126 (0.007) |
10.123 (0.009) |
10.123 (0.002) |
10.122 (0.006) |
2.229 | .087 | 0.361 | .067 | 0.484 | .098 | 0.604 | .007 | 0.124 | .545 | 0.190 | .520 | 0.011 | .996 |
| Assortativity | −0.395 (0.114) |
−0.377 (0.108) |
−0.403 (0.083) |
−0.409 (0.107) |
0.829 | .480 | 0.162 | .409 | −0.073 | .798 | −0.125 | .570 | −0.295 | .151 | −0.059 | .842 | −0.249 | .373 |
Abbreviations: ARMS-NT, at-risk mental state nontransition; ARMS-T, at-risk mental state transition; FEP, first-episode psychosis; HC, healthy control; .λ, characteristic path length; ϒ, clustering coefficient; σ, small-world index.
Figure 2. Graph Variable Comparisons for the HC, ARMS-NT, ARMS-T, and FEP Groups.
The mean (SD) scores of each graph variable were estimated from functional data analysis with the selected density range of 0.05 to 0.25. ARMS-NT indicates at-risk mental state nontransition; ARMS-T, at-risk mental state transition; FEP, first-episode psychosis; and HC, healthy control.
aP < .001 compared with HC.
bP < .001 compared with ARMS-NT.
Association Between Graph Metrics and Clinical Symptoms
Associations between BPRS, SANS, and GAF total scores and the 6 network measures were tested for each patient group (ARMS-NT, ARMS-T, and FEP) separately. There were no significant correlations between clinical variables and network metrics.
Classification Analysis
Using network measures as features (see the eResults and eTable 3 in the Supplement for classification performance on the raw LGIs), we obtained a good classification performance (Table 3 and eFigure 2 in the Supplement). With 5000 permutations, both balanced accuracy (classification value, 0.813; permutation mean, 0.499; permutation SD, 0.029) and area under the curve (classification value, 0.883; permutation mean, 0.499; permutation SD, 0.094) reached statistical significance (P < .001) (see eFigure 3 in the Supplement for supporting data on permutation tests). eTable 4 in the Supplement lists details for all permutation tests. Based on this test performance, we estimated a range of diagnostic indexes (Table 3). Notably, the posttest probability of a transition to psychosis increased by more than 60%, up to 83% and 87% compared with the pretest probability, based on standard clinical assessment both in our sample and compared with meta-analytic validity of psychometric CHR interviews.2 We estimated the PSI to be 0.76 and the NNP to be 1.30. These estimates indicate that the use of gyrification connectomes can reduce the uncertainty in predicting a transition to psychosis by 76%, a large gain that is likely to be clinically significant. The NNP of 1.30 also indicates that the overall testing burden is likely to be tolerable to gain the improved precision as observed in this study.
Table 3. Classification Performance Measures and Diagnostic Indexes of Clinical Utility.
| Performance Measure | Value |
|---|---|
| Accuracy, % | 90.49 |
| Balanced accuracy, % | 81.34 |
| Positive predictive value, % | 84.47 |
| Negative predictive value, % | 92.18 |
| Sensitivity, % | 66.11 |
| Specificity, % | 96.58 |
| Area under the curve, % | 88.30 |
| Positive likelihood ratio | 19.31 |
| Pretest odds (own sample) | 0.25 |
| Posttest odds (own sample) | 4.90 |
| Pretest probability (own sample) | 0.20 |
| Posttest probability (own sample) | 0.83 |
| Pretest probability (meta-analytic2) | 0.26 |
| Posttest probability (meta-analytic2) | 0.87 |
| Predictive summary index | 0.76 |
| Number needed to predict | 1.30 |
Discussion
To our knowledge, this is the first gyrification-based connectomic study to predict the transition to psychosis from a CHR state. We had 3 major findings. First, we found that ARMS individuals who later transition to FEP already show abnormalities in the gyrification connectome, indicating subtle neurodevelopmental aberrations in this group. In particular, those who later transition to psychosis demonstrate a highly segregated but poorly integrated pattern in structural covariance of cortical folding compared with nonconverters and healthy individuals. Second, the alterations in the gyrification connectome seen in the ARMS-T group were also present in antipsychotic-free patients with FEP. Third, by using topological measures of the gyrification connectome, we were able to predict the future outcome of a transition to psychosis with 81.34% balanced accuracy.
In search of the neural mechanisms that contribute to emerging psychosis, developmental processes are increasingly being recognized to be crucial for a transition to psychosis.54 Structural covariance based on morphometric measures, especially gyrification, represents synchronized developmental changes.55,56,57 When developmental lesions are experimentally induced in the white matter, covarying proximal and distal changes occur in gyrification patterns,35 indicating the influence of axonal integrity during early life. Because most cortical folding is complete in fetal life,32 it is likely that the topological abnormalities associated with a transition to psychosis that we report herein had been already present in early childhood. Various perinatal insults and defenses against these insults could result in a pattern of segregated cortical development.33,34 Nevertheless, the lack of regional changes in the degree of cortical folding among the 4 study groups indicates that either these insults were widespread (not localized) but brief or were partially compensated by other factors controlling the morphogenesis.58
As argued by Van Os and Delespaul,59 the number of CHR individuals who need to be treated to prevent one case of full-blown psychotic disorder relates directly to both the PPV of the prognostic test used and the success rate of the preventive treatment. Assuming the latter to be 50%,59 we would need a PPV greater than 50% to obtain a number needed to treat of 4. Given that only 4% to 5% of patients who eventually develop FEP seek help for CHR features even in specialized settings,60,61 it is important that false-negatives are kept to a minimum. To date, approaches like the North American Prodrome Longitudinal Study risk calculator62 and the polyenviromic risk score risk prediction tool63 have not provided a high PPV alongside a high sensitivity. The gyrification connectome offers predictors that appear to achieve both high PPV and moderate sensitivity.
Our results are comparable with the findings of GMV-based transition prediction biomarkers.64,65,66 While we achieved comparable balanced accuracy and higher PPV, NPV, and specificity, our sensitivity was lower. Low sensitivity may relate to sampling imbalance (63 ARMS-NT vs 16 ARMS-T), although it reflects the true base rates in this population. Furthermore, pathophysiological (ie, GMV vs gyrification67) and methodological differences (support vector machine vs extremely randomized trees algorithm, as well as different cross-validation strategies) may also explain the divergent results. Gyrification connectomes may be well suited as part of sequential testing approaches to improve psychosis prediction.3 Our results need to be validated in larger, enriched samples to ensure their real-world utility.
Limitations
Several limitations of our study merit comment. It is not yet clear what is the best way of defining individual nodes when constructing graph networks.68 We used neuroanatomically defined boundaries of cortical folding to generate intuitively meaningful values of gyrification that can be applied invariably across different groups being compared and be reliably replicated in future studies. This approach is also likely to provide greater convergence with developmental changes.57 We did not assess perinatal complications and thus could not explore whether developmental problems have driven the topological abnormalities seen in those transitioning to psychosis. Also, we did not consider other developmentally influenced brain measures, such as surface area, that might facilitate improve psychosis prediction.69 Furthermore, we had approximately a 20% transition rate in our sample during almost 4 years of follow-up. Although the transition to psychosis generally plateaus the first 2 years after the initial clinical assessment and the transition rate after this period is likely to be small,70 the duration of the follow-up period should be considered when testing the predictive accuracy of gyrification networks. The lack of a significant association between topological alterations and symptom expression should be considered with caution; disorganized gyrification could also be a marker of poor cognitive or functional outcomes not assessed in this study.
Conclusions
In summary, we provide the first report to date that a transition to psychosis is associated with developmental disruptions in the morphogenesis of cortical folding. This observation makes perturbed neurodevelopment directly relevant to the neurobiology of psychosis onset in a sample with clinically defined ARMS. Gyrification connectomes can potentially be used to provide sample enrichment among CHR individuals to promote prevention of psychosis.
eMethods. Supplemental Methods
eFigure 1. Parcellated Brain Regions Based on Desikan’s Atlas
eFigure 2. Receiver Operating Characteristic Curve for the Graph-Based Classification Performance
eFigure 3. Permutation Results (Balanced Accuracy and Area Under the Curve)
eTable 1. Pearson’s Correlations Between Graph Variables and IQ (Premorbid) Within Each Group
eTable 2. Raw Local Gyrification Indices (LGI) Across Groups
eTable 3. Classification Performance Measures With Raw LGIs
eTable 4. P Values of Permutation Tests
eResults. Supplemental Results
References
- 1.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fusar-Poli P, Cappucciati M, Rutigliano G, et al. At risk or not at risk? a meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry. 2015;14(3):322-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt A, Cappucciati M, Radua J, et al. Improving prognostic accuracy in subjects at clinical high risk for psychosis: systematic review of predictive models and meta-analytical sequential testing simulation. Schizophr Bull. 2017;43(2):375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borgwardt S, Schmidt A. Is neuroimaging clinically useful in subjects at high risk for psychosis? World Psychiatry. 2016;15(2):178-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38(6):1297-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palaniyappan L. Progressive cortical reorganisation: a framework for investigating structural changes in schizophrenia. Neurosci Biobehav Rev. 2017;79:1-13. [DOI] [PubMed] [Google Scholar]
- 7.Palaniyappan L, Liddle PF. Aberrant cortical gyrification in schizophrenia: a surface-based morphometry study. J Psychiatry Neurosci. 2012;37(6):399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris JM, Yates S, Miller P, Best JJ, Johnstone EC, Lawrie SM. Gyrification in first-episode schizophrenia: a morphometric study. Biol Psychiatry. 2004;55(2):141-147. [DOI] [PubMed] [Google Scholar]
- 9.Falkai P, Honer WG, Kamer T, et al. Disturbed frontal gyrification within families affected with schizophrenia. J Psychiatr Res. 2007;41(10):805-813. [DOI] [PubMed] [Google Scholar]
- 10.Nanda P, Tandon N, Mathew IT, et al. Local gyrification index in probands with psychotic disorders and their first-degree relatives [published correction appears in Biol Psychiatry. 2015;77(9):841]. Biol Psychiatry. 2014;76(6):447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tepest R, Schwarzbach CJ, Krug B, Klosterkötter J, Ruhrmann S, Vogeley K. Morphometry of structural disconnectivity indicators in subjects at risk and in age-matched patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2013;263(1):15-24. [DOI] [PubMed] [Google Scholar]
- 12.Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol Psychiatry. 2004;56(3):182-189. [DOI] [PubMed] [Google Scholar]
- 13.Harris JM, Moorhead TW, Miller P, et al. Increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry. 2007;62(7):722-729. [DOI] [PubMed] [Google Scholar]
- 14.Sasabayashi D, Takayanagi Y, Takahashi T, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol Psychiatry. 2017;82(10):737-745. [DOI] [PubMed] [Google Scholar]
- 15.Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186-198. [DOI] [PubMed] [Google Scholar]
- 16.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20(4):327-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crossley NA, Mechelli A, Ginestet C, Rubinov M, Bullmore ET, McGuire P. Altered hub functioning and compensatory activations in the connectome: a meta-analysis of functional neuroimaging studies in schizophrenia. Schizophr Bull. 2016;42(2):434-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62(4):2296-2314. [DOI] [PubMed] [Google Scholar]
- 19.Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14(5):322-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans AC. Networks of anatomical covariance. Neuroimage. 2013;80:489-504. [DOI] [PubMed] [Google Scholar]
- 21.Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28(37):9239-9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Lin L, Lin CP, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141(2-3):109-118. [DOI] [PubMed] [Google Scholar]
- 23.van den Heuvel MP, Sporns O, Collin G, et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70(8):783-792. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt A, Crossley NA, Harrisberger F, et al. Structural network disorganization in subjects at clinical high risk for psychosis. Schizophr Bull. 2016;43(3):583-591.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27481826&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tijms BM, Sprooten E, Job D, et al. Grey matter networks in people at increased familial risk for schizophrenia. Schizophr Res. 2015;168(1-2):1-8. [DOI] [PubMed] [Google Scholar]
- 26.Shi F, Yap PT, Gao W, Lin W, Gilmore JH, Shen D. Altered structural connectivity in neonates at genetic risk for schizophrenia: a combined study using morphological and white matter networks. Neuroimage. 2012;62(3):1622-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan H, Tian L, Wang Q, et al. Compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents. Neurosci Bull. 2015;31(3):275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drakesmith M, Caeyenberghs K, Dutt A, et al. Schizophrenia-like topological changes in the structural connectome of individuals with subclinical psychotic experiences. Hum Brain Mapp. 2015;36(7):2629-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornito A, Bullmore ET. Reconciling abnormalities of brain network structure and function in schizophrenia. Curr Opin Neurobiol. 2015;30:44-50. [DOI] [PubMed] [Google Scholar]
- 30.van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24(1):32-48. [DOI] [PubMed] [Google Scholar]
- 31.Rubinov M, Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci. 2013;15(3):339-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31(19):7174-7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haukvik UK, Schaer M, Nesvåg R, et al. Cortical folding in Broca’s area relates to obstetric complications in schizophrenia patients and healthy controls. Psychol Med. 2012;42(6):1329-1337. [DOI] [PubMed] [Google Scholar]
- 34.Dubois J, Benders M, Cachia A, et al. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008;18(6):1444-1454. [DOI] [PubMed] [Google Scholar]
- 35.Goldman-Rakic PS, Rakic P. Experimental modification of gyral patterns In: Geschwind N, Galaburda A, eds. Cerebral Dominance. Cambridge, MA: Harvard University Press; 1984:179-192. [Google Scholar]
- 36.Palaniyappan L, Park B, Balain V, Dangi R, Liddle P. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct. 2015;220(4):2059-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palaniyappan L, Marques TR, Taylor H, et al. Globally efficient brain organization and treatment response in psychosis: a connectomic study of gyrification. Schizophr Bull. 2016;42(6):1446-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riecher-Rössler A, Aston J, Ventura J, et al. The Basel Screening Instrument for Psychosis (BSIP): development, structure, reliability and validity [in German]. Fortschr Neurol Psychiatr. 2008;76(4):207-216. [DOI] [PubMed] [Google Scholar]
- 39.Lukoff D, Liberman RP, Nuechterlein KH. Symptom monitoring in the rehabilitation of schizophrenic patients. Schizophr Bull. 1986;12(4):578-602. [DOI] [PubMed] [Google Scholar]
- 40.Andreasen NC. The scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. 1989;(7):49-58. [PubMed] [Google Scholar]
- 41.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 42.Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91(5):335-345. [DOI] [PubMed] [Google Scholar]
- 43.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis: a step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172(33):14-20. [PubMed] [Google Scholar]
- 44.Schaer M, Cuadra MB, Tamarit L, Lazeyras F, Eliez S, Thiran JP. A surface-based approach to quantify local cortical gyrification. IEEE Trans Med Imaging. 2008;27(2):161-170. [DOI] [PubMed] [Google Scholar]
- 45.Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran JP, Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J Vis Exp. 2012;(59):e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. [DOI] [PubMed] [Google Scholar]
- 47.Miller RG. The jackknife: a review. Biometrika. 1974;61(1):1-15. [Google Scholar]
- 48.Richter CG, Thompson WH, Bosman CA, Fries P. A jackknife approach to quantifying single-trial correlation between covariance-based metrics undefined on a single-trial basis. Neuroimage. 2015;114:57-70. [DOI] [PubMed] [Google Scholar]
- 49.Hosseini SM, Hoeft F, Kesler SR Sr. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012;7(7):e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedges LV. Distribution theory for Glass’s estimator of effect size and related estimators. J Educ Stat. 1981;6(2):107-128. [Google Scholar]
- 51.Geurts P, Ernst D, Wehenkel L. Extremely randomized trees. Mach Learn. 2006;63(1):3-42. [Google Scholar]
- 52.Iwabuchi SJ, Liddle PF, Palaniyappan L. Clinical utility of machine-learning approaches in schizophrenia: improving diagnostic confidence for translational neuroimaging. Front Psychiatry. 2013;4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Linn S, Grunau PD. New patient-oriented summary measure of net total gain in certainty for dichotomous diagnostic tests. Epidemiol Perspect Innov. 2006;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray RM, Bhavsar V, Tripoli G, Howes O. 30 Years on: how the neurodevelopmental hypothesis of schizophrenia morphed into the developmental risk factor model of psychosis. Schizophr Bull. 2017;43(6):1190-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zielinski BA, Gennatas ED, Zhou J, Seeley WW. Network-level structural covariance in the developing brain. Proc Natl Acad Sci U S A. 2010;107(42):18191-18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khundrakpam BS, Reid A, Brauer J, et al. ; Brain Development Cooperative Group . Developmental changes in organization of structural brain networks. Cereb Cortex. 2013;23(9):2072-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 2013;33(7):2889-2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36(5):275-284. [DOI] [PubMed] [Google Scholar]
- 59.Van Os J, Delespaul P. Toward a world consensus on prevention of schizophrenia. Dialogues Clin Neurosci. 2005;7(1):53-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fusar-Poli P, Rutigliano G, Stahl D, et al. Development and validation of a clinically based risk calculator for the transdiagnostic prediction of psychosis. JAMA Psychiatry. 2017;74(5):493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ajnakina O, Morgan C, Gayer-Anderson C, et al. Only a small proportion of patients with first episode psychosis come via prodromal services: a retrospective survey of a large UK mental health programme. BMC Psychiatry. 2017;17(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cannon TD, Yu C, Addington J, et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173(10):980-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Padmanabhan JL, Shah JL, Tandon N, Keshavan MS. The “polyenviromic risk score”: aggregating environmental risk factors predicts conversion to psychosis in familial high-risk subjects. Schizophr Res. 2017;181:17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koutsouleris N, Riecher-Rössler A, Meisenzahl EM, et al. Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophr Bull. 2015;41(2):471-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koutsouleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern classification to identify subjects in at-risk mental states of psychosis and predict disease transition. Arch Gen Psychiatry. 2009;66(7):700-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koutsouleris N, Borgwardt S, Meisenzahl EM, Bottlender R, Möller HJ, Riecher-Rössler A. Disease prediction in the at-risk mental state for psychosis using neuroanatomical biomarkers: results from the FePsy study. Schizophr Bull. 2012;38(6):1234-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palaniyappan L, Liddle PF. Differential effects of surface area, gyrification and cortical thickness on voxel based morphometric deficits in schizophrenia. Neuroimage. 2012;60(1):693-699. [DOI] [PubMed] [Google Scholar]
- 68.Zalesky A, Fornito A, Harding IH, et al. Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage. 2010;50(3):970-983. [DOI] [PubMed] [Google Scholar]
- 69.Bois C, Ronan L, Levita L, et al. Cortical surface area differentiates familial high risk individuals who go on to develop schizophrenia. Biol Psychiatry. 2015;78(6):413-420. [DOI] [PubMed] [Google Scholar]
- 70.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220-229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Parcellated Brain Regions Based on Desikan’s Atlas
eFigure 2. Receiver Operating Characteristic Curve for the Graph-Based Classification Performance
eFigure 3. Permutation Results (Balanced Accuracy and Area Under the Curve)
eTable 1. Pearson’s Correlations Between Graph Variables and IQ (Premorbid) Within Each Group
eTable 2. Raw Local Gyrification Indices (LGI) Across Groups
eTable 3. Classification Performance Measures With Raw LGIs
eTable 4. P Values of Permutation Tests
eResults. Supplemental Results