Key Points
Question
Is early caffeine citrate administration associated with reduced incidence or severity of acute kidney injury in preterm neonates?
Findings
In this multicenter cohort study of 675 preterm neonates, those who received caffeine in the first 7 days after birth developed acute kidney injury less frequently than neonates who did not (11.2% vs 31.6%).
Meaning
Caffeine administration in the first 7 days after birth may be associated with less frequent acute kidney injury in preterm neonates; further studies on dosage, the timing of administration, and long-term outcomes are needed.
Abstract
Importance
Acute kidney injury (AKI) occurs commonly in preterm neonates and is associated with increased morbidity and mortality.
Objectives
To examine the association between caffeine citrate administration and AKI in preterm neonates in the first 7 days after birth and to test the hypothesis that caffeine administration would be associated with reduced incidence and severity of AKI.
Design, Setting, and Participants
This study was a secondary analysis of the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study, a retrospective observational cohort that enrolled neonates born from January 1 to March 31, 2014. The dates of analysis were October 2016 to December 2017. The setting was an international, multicenter cohort study of neonates admitted to 24 participating level III or IV neonatal intensive care units. Participants met the original inclusion and exclusion criteria of the AWAKEN study. Additional exclusion criteria for this study included participants greater than or equal to 33 weeks’ gestation at birth, admission after age 7 days, use of theophylline in the neonatal intensive care unit, or lack of data to define AKI. There were 675 preterm neonates available for analysis.
Exposure
Administration of caffeine in the first 7 days after birth.
Main Outcomes and Measures
The primary outcome was the incidence of AKI (based on the modified neonatal Kidney Disease: Improving Global Outcomes [KDIGO] definition) in the first 7 days after birth. The hypothesis that caffeine administration would be associated with reduced AKI incidence was formulated before data analysis.
Results
The study cohort (n = 675) was 55.4% (n = 374) male, with a mean (SD) gestational age of 28.9 (2.8) weeks and a mean (SD) birth weight of 1285 (477) g. Acute kidney injury occurred in 122 neonates (18.1%) in the first 7 days after birth. Acute kidney injury occurred less frequently among neonates who received caffeine than among those who did not (50 of 447 [11.2%] vs 72 of 228 [31.6%], P < .01). After multivariable adjustment, administration of caffeine remained associated with reduced odds of developing AKI (adjusted odds ratio, 0.20; 95% CI, 0.11-0.34), indicating that for every 4.3 neonates exposed to caffeine one case of AKI was prevented. Among neonates with early AKI, those receiving caffeine were less likely to develop stage 2 or 3 AKI (adjusted odds ratio, 0.20; 95% CI, 0.12-0.34).
Conclusions and Relevance
Caffeine administration in preterm neonates is associated with reduced incidence and severity of AKI. Further studies should focus on the timing and dosage of caffeine to optimize the prevention of AKI.
This multicenter cohort study examines the association of caffeine administration with acute kidney injury in preterm neonates in the first 7 days after birth.
Introduction
In recent decades, advances in the care of preterm neonates have dramatically lowered mortality rates.1,2,3 As survival has improved, clinicians have been increasingly focused on decreasing the short-term and long-term sequelae of prematurity.4 Acute kidney injury (AKI) occurs frequently in preterm neonates and is associated with increased morbidity and mortality.5,6,7 In 2014, the Neonatal Kidney Collaborative (NKC) initiated an international, multicenter (24 centers in 4 countries) study designed to evaluate the incidence, risk factors, and outcomes associated with neonatal AKI called the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study.8 This study demonstrated that 30% of neonates admitted to a neonatal intensive care unit (NICU) developed AKI and that those with AKI had 4.8 times higher adjusted odds of mortality compared with neonates without AKI.9 However, there are few specific strategies to prevent or ameliorate AKI beyond supportive measures, such as avoidance of nephrotoxins and optimization of blood pressure and fluid balance.10,11,12 Therefore, identifying therapies to prevent or reduce the severity of AKI are of paramount importance.
Methylxanthines are adenosine antagonists that act via A1 and A2A receptors present in the brain, heart, blood vessels, respiratory system, gastrointestinal tract, and kidneys.13 The results of clinical trials using theophylline or aminophylline have suggested that methylxanthines prevent AKI or improve renal function in special populations of high-risk neonates and infants, including those with perinatal hypoxia/ischemia or prematurity and undergoing cardiac surgery.14,15,16,17,18,19,20 However, these medications are no longer widely used in the general neonatal population: a 2014 study21 found that caffeine citrate (another methylxanthine) accounted for 96% of methylxanthine use in 2010. Whether exposure to caffeine might be associated with decreased incidence of AKI was explored by a single-center retrospective study22 of very low-birth-weight (VLBW) neonates (birth weight, <1500 g). Although the results of this study suggested that early caffeine administration might prevent AKI, to date there has been no comprehensive multicenter evaluation of caffeine administration and its association with AKI in preterm neonates.
To address this knowledge gap, we performed a secondary analysis of preterm neonates born at less than 33 weeks’ gestation enrolled in the AWAKEN study. The aim of this analysis was to examine the association of caffeine administration with AKI in the first 7 days after birth. We hypothesized that neonates given caffeine would have reduced incidence and severity of AKI.
Methods
A comprehensive description of the NKC and the methods used to collect the data for the AWAKEN study8 has been published. The University of Alabama at Birmingham Institutional Review Board approved this collaborative study, and each participating center received approval from their respective institutional review boards.
Setting and Participants
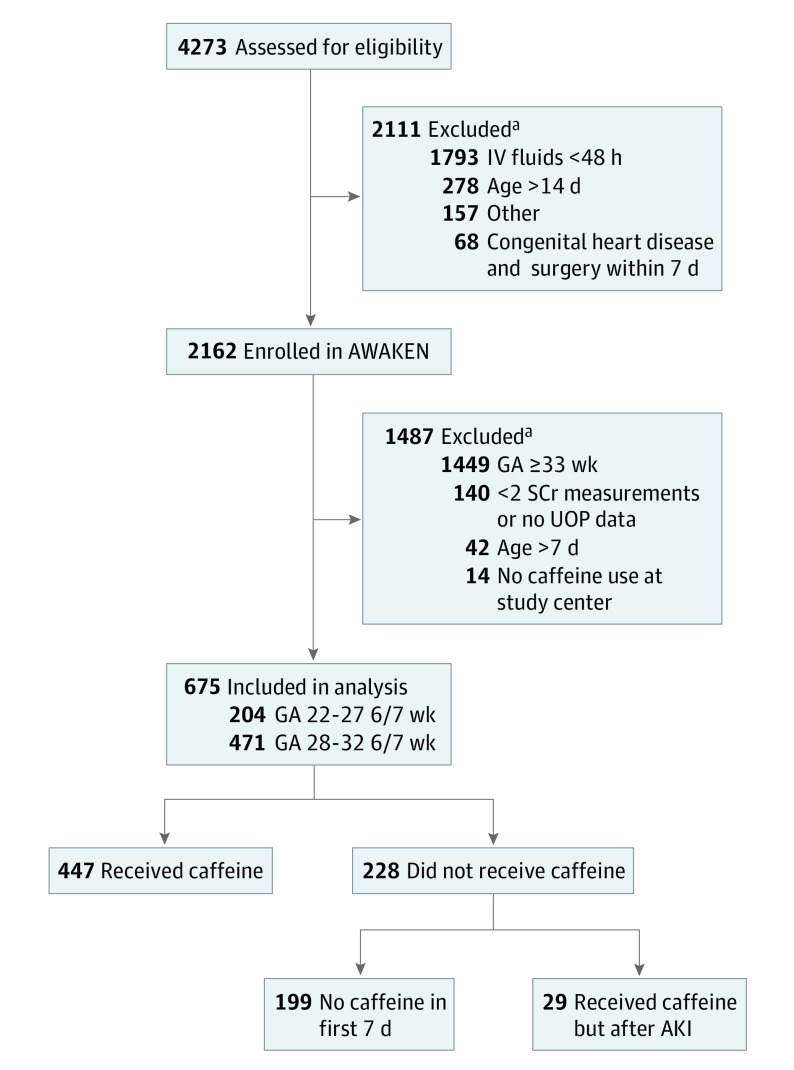
The AWAKEN study was a retrospective observational cohort investigation that enrolled neonates born from January 1 to March 31, 2014. The dates of analysis were October 2016 to December 2017. The setting was an international, multicenter cohort study of neonates admitted to 24 participating level III or IV NICUs. Data were collected from the time of NICU admission until discharge, transfer, death, or 120 days after birth, whichever came first. Inclusion criteria for the AWAKEN study were admission to the NICU and administration of intravenous fluids for at least the first 48 hours after admission. The original exclusion criteria for the AWAKEN study were individuals with admission 14 days or longer after birth, those with congenital heart disease requiring repair less than 7 days after birth, infants with lethal chromosomal anomaly, and neonates with death within 48 hours after birth. Specific to the present study, the following additional exclusion criteria applied: gestational age at least 33 weeks, admission more than 7 days after birth, admission to a NICU that used theophylline rather than caffeine, less than 1 day of measured urine output (UOP) on days 2 to 7 after birth, and fewer than 2 serum creatinine (sCr) measurements (Figure). Analysis was limited to participants younger than 33 weeks’ gestation because few neonates with gestational age at least 33 weeks received caffeine (owing to the low incidence of apnea of prematurity in this group).
Figure. Study Flow Diagram.
Acute kidney injury (AKI) was defined as occurring in the first 7 days after birth, and caffeine citrate administration was defined as occurring before an AKI event. AWAKEN indicates Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates; GA, gestational age; IV, intravenous; SCr, serum creatinine; and UOP, urine output.
aBecause exclusion criteria are not mutually exclusive, some potential participants could have been excluded for multiple reasons and are counted in each exclusion category.
Variables of Outcomes, Exposures, Confounders, and Effect Modifiers
The primary outcome of interest was early AKI occurring in the first 7 days after birth. Neonatal AKI was defined by the modified neonatal Kidney Disease: Improving Global Outcomes (KDIGO) definition (Table 1).23 This is the consensus definition for neonatal AKI based on the recommendation of a recent National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases workshop.24 Secondary outcomes of interest were the severity of AKI, defined by modified neonatal KDIGO staging, and the incidence of AKI within the entire data collection period.
Table 1. Definition of Acute Kidney Injurya.
| Stage | Serum Creatinine (sCr) | UOP During the Past 24 h |
|---|---|---|
| 0 | No change in sCr or rise <0.3 mg/dL |
>1 mL/kg/h |
| 1 | sCr rise ≥0.3 mg/dL within 48 h or sCr rise between ≥1.5 and 1.9 times reference sCr within 7 d |
Between >0.5 and ≤1 mL/kg/h |
| 2 | sCr rise between ≥2 and 2.9 times reference sCr | Between >0.3 and ≤0.5 mL/kg/h |
| 3 | sCr rise ≥3 times reference sCr or sCr ≥2.5 mg/dLb or receipt of dialysis |
≤0.3 mL/kg/h |
Abbreviation: UOP, urine output.
SI conversion factor: To convert serum creatinine value to micromoles per liter, multiply by 88.4.
Reference sCr is the lowest prior sCr value.
This value is lower than the original Kidney Disease: Improving Global Outcomes (KDIGO) definition because an sCr value of 2.5 mg/dL in neonates suggests an estimated glomerular filtration rate less than 10 mL/min/1.73 m2.
The exposure of interest was administration of caffeine before AKI. If administration of caffeine occurred in the first 7 days after birth but after AKI occurred, these neonates were considered not to have received caffeine.
To characterize the cohort and to assess potential confounders, both maternal and neonatal data were collected, including the following: maternal age, mode of delivery, outborn delivery, pregnancy medications, gestational age, birth weight, small for gestational age status, sex, resuscitation efforts, Apgar scores, admission diagnoses, ventilation, patent ductus arteriosus therapies, initial sCr values, type of center, and neonatal medications in the NICU. Similar to the classification of caffeine administration, exposure to other neonatal medications, including antimicrobials, diuretics, and vasopressors, was defined as administration of the medication before AKI. The updated Clinical Risk Index for Babies (CRIB II) score was used to assess the severity of illness on admission.25
Statistical Analysis
Comparisons were made using the χ2 test for categorical variables and the Fisher exact test for continuous variables. All continuous variables were tested for normality using the Shapiro-Wilk test. For normally distributed continuous variables, the means (SDs) were reported and analyzed using the t test. For nonnormally distributed variables, the medians (interquartile ranges) were reported, and groups were compared using the Mann-Whitney test.
To evaluate the association between caffeine administration and AKI—both early (ie, in the first 7 days after birth) and within 120 days—a generalized linear mixed model using a logit link and binary distribution and a random intercept by study site (to account for possible clustering by study site) was used to calculate unadjusted odds ratios (ORs) and associated 95% CIs. Maternal, neonatal, and clinical characteristics with P < .20 in bivariate analysis were considered candidate variables for the final adjusted model. Using a backward selection process, multivariable models were generated that included only variables with P < .05 to create the most parsimonious model to estimate adjusted ORs. To assess possible effect modification by gestational age, an interaction between gestational age and caffeine administration was entered into the final adjusted model to examine whether the observed associations between caffeine administration and AKI differed statistically by gestational age. In secondary analyses, the incidence of AKI was compared between caffeine groups using a generalized linear mixed model with a logit link and binomial distribution, and AKI staging was compared between caffeine groups using a generalized linear mixed model with a cumulative logit link and multinomial distribution for both early AKI and AKI within 120 days. All regression models were adjusted for gestational age, sepsis evaluation, type of center, and antibiotic use.
The number needed to be exposed (NNE), corresponding to the number of patients who would need to be treated with caffeine to prevent one case of AKI after adjustment for confounding variables, was calculated from the adjusted OR obtained in logistic regression using statistical methods described previously.26 For all analyses, 2-sided P < .05 was considered statistically significant. All statistical analyses were performed centrally at the University of Alabama at Birmingham using a software program (SAS, version 9.4; SAS Institute Inc).
Results
Participants
The AWAKEN study enrolled 2162 participants. After the application of the additional exclusion criteria for this study, 675 preterm neonates were eligible for our secondary analysis (Figure). Of the 675 participants, 447 (66.2%) received at least one dose of caffeine in the first 7 days after birth and before AKI. Of the 228 neonates considered to have received no caffeine, 199 received no caffeine at all in the first 7 days after birth, while 29 received caffeine after AKI. For neonates included in the study, 36.9% (249 of 675) received their first dose of caffeine on day 1 after birth, and 53.8% (363 of 675) received their first dose within the first 2 days after birth. Bivariate analyses were conducted to compare the severity of illness between those who received caffeine on day 1 or day 1 and day 2 with those who did not receive caffeine (eTable 1 and eTable 2 in the Supplement). Neonates who did not receive caffeine had older gestational age, higher birth weight, greater Apgar scores at 5 minutes, and lower CRIB II scores, suggesting that these neonates were not sicker than those who received caffeine.
Comparison of Infants by Caffeine Administration
Compared with neonates who did not receive caffeine, neonates who received caffeine had younger gestational age and lower birth weight (Table 2). In addition, these neonates were more likely to be intubated in the delivery room and had lower median Apgar scores at 1 minute and 5 minutes. On admission, those receiving caffeine were more likely to have a diagnosis of respiratory distress, undergo a sepsis evaluation, and receive either invasive or noninvasive respiratory support. Neonates receiving caffeine were more likely to be given a nephrotoxic antimicrobial agent and a nonsteroidal anti-inflammatory drug in the NICU.
Table 2. Characteristics of Patients Stratified by Caffeine Citrate Administration.
| Variable | Whole Cohort (N = 675) |
Caffeine (n = 447) |
No Caffeine (n = 228) |
P Valuea |
|---|---|---|---|---|
| Perinatal Factors | ||||
| HTN gestational disease, No. (%)b | 177 (26.2) | 113 (25.3) | 64 (28.1) | .43 |
| Maternal diabetes, No. (%) | 79 (11.7) | 53 (11.9) | 26 (11.4) | .86 |
| Maternal age, mean (SD), y | 28.9 (6.3) | 28.8 (6.5) | 29.0 (6.0) | .77 |
| Assisted conception, No. (%) | 92 (13.6) | 58 (13.0) | 34 (14.9) | .49 |
| Cesarean delivery, No. (%) | 420 (62.2) | 277 (62.0) | 143 (62.7) | .14 |
| Multiple birth, No. (%) | 216 (32.0) | 153 (34.2) | 63 (27.6) | .08 |
| Outborn delivery, No. (%) | 181 (26.8) | 121 (27.1) | 60 (26.3) | .83 |
| Pregnancy medications | ||||
| Betamethasone, No. (%) | 494 (73.2) | 337 (75.4) | 157 (68.9) | .07 |
| NSAID, No. (%) | 45 (6.7) | 34 (7.6) | 11 (4.8) | .17 |
| Antihypertensive, No. (%)c | 122 (18.1) | 89 (19.9) | 33 (14.5) | .08 |
| Neonatal Factors | ||||
| Gestational age, mean (SD), wk | 28.9 (2.8) | 28.5 (2.6) | 29.6 (2.9) | <.001 |
| Birth weight, mean (SD), g | 1285 (477.0) | 1207 (413.4) | 1436 (552.0) | <.001 |
| SGA status, No. (%) | 97 (14.4) | 61 (13.6) | 36 (15.8) | .47 |
| Male, No. (%) | 374 (55.4) | 249 (55.7) | 125 (54.8) | .75 |
| Ethnicity, No. (%) | ||||
| Hispanic | 87 (12.9) | 61 (13.6) | 26 (11.4) | .67 |
| Non-Hispanic | 502 (74.4) | 331 (74.0) | 171 (75.0) | |
| Unknown | 86 (12.7) | 55 (12.3) | 31 (13.6) | |
| Race, No. (%) | ||||
| White | 367 (54.4) | 238 (53.2) | 129 (56.6) | .70 |
| Black | 149 (22.1) | 102 (22.8) | 47 (20.6) | |
| Other | 159 (23.6) | 107 (23.9) | 52 (22.8) | |
| Delivery room | ||||
| Intubation, No. (%) | 308 (45.6) | 222 (49.7) | 86 (37.7) | .003 |
| Chest compressions, No. (%) | 37 (5.5) | 19 (4.3) | 18 (7.9) | .049 |
| Apgar score at 1 min, median (IQR) | 6 (3, 7) | 5 (3, 7) | 7 (3, 8) | .001 |
| Apgar score at 5 min, median (IQR) | 8 (6, 9) | 8 (6, 8) | 8 (7, 9) | .005 |
| Admission diagnoses, No. (%) | ||||
| Respiratory distress | 594 (88.0) | 415 (92.8) | 179 (78.5) | <.001 |
| Sepsis evaluation | 407 (60.3) | 284 (63.5) | 123 (53.9) | .02 |
| CRIB II score, mean (SD) | 5.5 (4.6) | 5.9 (4.3) | 4.7 (5.1) | .001 |
| Respiratory support, No. (%) | ||||
| Invasive | 387 (57.3) | 278 (62.2) | 109 (47.8) | <.001 |
| Noninvasive | 490 (72.6) | 355 (79.4) | 135 (59.2) | <.001 |
| Treated PDA, No. (%) | 94 (13.9) | 67 (15.0) | 27 (11.8) | .26 |
| Initial sCr value, mean (SD), mg/dL | 0.8 (0.3) | 0.8 (0.2) | 0.8 (0.5) | .13 |
| No. of sCr values measured, mean (SD) | 11.4 (15.3) | 11.6 (15.3) | 11.0 (15.3) | .63 |
| Type of center, No. (%) | ||||
| Children’s hospital | 180 (26.7) | 111 (24.8) | 69 (30.3) | .005 |
| Perinatal center | 115 (17.0) | 62 (13.9) | 53 (23.2) | |
| Surgical perinatal center | 380 (56.3) | 274 (61.3) | 106 (46.5) | |
| Neonatal medications in the NICU, No. (%) | ||||
| Nephrotoxic antimicrobial agentd | 539 (79.7) | 387 (86.6) | 152 (66.7) | <.001 |
| Diuretice | 24 (3.6) | 15 (3.4) | 9 (3.9) | .69 |
| Vasopressorf | 71 (10.5) | 45 (10.1) | 26 (11.4) | .59 |
| NSAIDg | 83 (12.3) | 71 (15.9) | 12 (5.3) | <.001 |
Abbreviations: CRIB, Clinical Risk Index for Babies; HTN, hypertension; IQR, interquartile range; NICU, neonatal intensive care unit; NSAID, nonsteroidal anti-inflammatory drug; PDA, patent ductus arteriosus; SGA, small for gestational age; sCr, serum creatinine.
SI conversion factor: To convert serum creatinine value to micromoles per liter, multiply by 88.4.
Based on χ2 test for categorical variables or t test for continuous variables.
Preeclampsia, eclampsia, or chronic hypertension.
Propranolol, atenolol, carvedilol, metoprolol, esmolol hydrochloride, labetalol hydrochloride, amlodipine, felodipine, isradipine, nicardipine hydrochloride, hydralazine hydrochloride, minoxidil, sodium nitroprusside, or clonidine hydrochloride.
Acyclovir, amphotericin B, aminoglycosides, piperacillin-tazobactam, or vancomycin hydrochloride.
Bumetanide, chlorothiazide, furosemide, or spironolactone.
Dobutamine hydrochloride, dopamine hydrochloride, epinephrine, milrinone lactate, or norepinephrine bitartrate.
Indomethacin or ibuprofen.
Association of Caffeine Administration With Early AKI (Primary Outcome)
Acute kidney injury occurred in the first 7 days after birth in 18.1% (122 of 675) of neonates. Demographics of participants with early AKI compared with those without AKI are listed in eTable 3 in the Supplement. Neonates who received caffeine were less likely to develop early AKI compared with those who did not (11.2% [50 of 447] vs 31.6% [72 of 228], P < .01; unadjusted OR, 0.28; 95% CI, 0.18-0.44) (Table 3). The eFigure in the Supplement shows a Kaplan-Meier AKI-free survival curve for neonates in the first 7 days after birth. After multivariable analysis (adjusting for gestational age, sepsis evaluation, type of center, and nephrotoxic antimicrobial use), neonates who received caffeine remained less likely to have AKI than those who did not receive caffeine (adjusted OR, 0.20; 95% CI, 0.11-0.34; P < .001). The NNE to prevent a single case of AKI was 4.3.
Table 3. Primary Acute Kidney Injury (AKI) Outcomes Stratified by Caffeine Citrate Administrationa.
| Variable | No./Total No. (%) | OR (95% CI) | NNE | ||
|---|---|---|---|---|---|
| Caffeine | No Caffeine | Unadjusted | Adjustedb | ||
| Early AKI, ≤7 dc | |||||
| Overall | 50/447 (11.2) | 72/228 (31.6) | 0.28 (0.18-0.44) | 0.20 (0.11-0.34) | 4.3 |
| Extremely preterm, <27 wk | 30/149 (20.1) | 38/55 (69.1) | 0.07 (0.03-0.16) | 0.13 (0.06-0.31) | 2.2 |
| Very preterm, 28-32 wk | 20/298 (10.1) | 34/173 (19.7) | 0.31 (0.16-0.61) | 0.27 (0.13-0.56) | 8.1 |
| Any AKI, ≤120 dd | |||||
| Overall | 103/447 (23.0) | 83/228 (36.4) | 0.56 (0.38-0.84) | 0.27 (0.16-0.47) | 4.4 |
| Extremely preterm, <27 wk | 69/149 (29.5) | 44/55 (80.0) | 0.12 (0.05-0.30) | 0.24 (0.10-0.58) | 3.1 |
| Very preterm, 28-32 wk | 34/293 (11.6) | 39/170 (22.9) | 0.52 (0.29-0.94) | 0.32 (0.16-0.62) | 8.0 |
Abbreviations: NNE, number needed to be exposed; OR, odds ratio.
Based on generalized linear mixed model with logit link and binary distribution.
Adjusted for gestational age, sepsis evaluation, type of center, and antibiotic use.
P for interaction of gestational age and caffeine administration = .23.
P for interaction of gestational age and caffeine administration = .62.
Association of Caffeine Administration With Secondary AKI Outcomes
Neonates who received caffeine were less likely to develop early AKI based on sCr values alone (adjusted OR, 0.20; 95% CI, 0.11-0.37); however, there was no association of use of caffeine with early AKI as defined by decreased UOP without a rise in creatinine (adjusted OR, 0.40; 95% CI, 0.15-1.06) (Table 4). Among neonates who developed early AKI, those receiving caffeine had an 80% decrease in the odds of stage 2 or 3 AKI (adjusted OR, 0.20; 95% CI, 0.12-0.34). Stage 3 AKI occurred approximately 8 times more frequently among neonates who did not receive caffeine (1.3% [6 of 447] vs 10.5% [24 of 228], P < .001).
Table 4. Secondary Acute Kidney Injury (AKI) Outcomes Stratified by Caffeine Citrate Administration.
| Variable | No. (%) | Adjusted OR (95% CI) | |
|---|---|---|---|
| Caffeine (n = 447) |
No Caffeine (n = 228) |
||
| Early AKI, ≤7 d | |||
| AKI, sCr plus UOP | 50 (11.2) | 72 (31.6) | 0.20 (0.11-0.34)a |
| AKI, sCr | 47 (10.5) | 60 (26.3) | 0.20 (0.11-0.37)a |
| AKI, UOP | 8 (1.8) | 17 (7.5) | 0.40 (0.15-1.06)a |
| Stage 1 | 27 (6.0) | 32 (14.0) | 0.20 (0.12-0.34)b |
| Stage 2 | 17 (3.8) | 16 (7.0) | |
| Stage 3 | 6 (1.3) | 24 (10.5) | |
| Any AKI, ≤120 d | |||
| AKI, sCr | 100 (22.4) | 71 (31.1) | 0.28 (0.16-0.49)a |
| Stage 1 | 54 (12.1) | 40 (17.5) | 0.30 (0.19-0.48)b |
| Stage 2 | 34 (7.6) | 17 (7.5) | |
| Stage 3 | 15 (3.4) | 26 (11.4) | |
Abbreviations: NNE, number needed to be exposed; OR, odds ratio; sCr, serum creatinine; UOP, urine output.
Adjusted for assisted conception, gestational age, birth weight, race, Apgar score at 1 minute, invasive respiratory support, type of center, antibiotic use, and nonsteroidal anti-inflammatory drug use.
Adjusted for assisted conception, birth weight, race, Apgar score at 1 minute, invasive respiratory support, type of center, antibiotic use, and nonsteroidal anti-inflammatory drug use.
Similar associations were observed for AKI that occurred after the initial 7 days (before NICU discharge, transfer, death, or age 120 days). The incidence of AKI through the entire data collection period was 27.6% (186 of 675). Among patients receiving caffeine, the odds of developing AKI were reduced by 44% during the data collection period (crude OR, 0.56; 95% CI, 0.38-0.84), an association that persisted after multivariable adjustment (adjusted OR, 0.27; 95% CI, 0.16-0.47). The NNE to prevent one case of AKI during the participants’ hospitalization (up to 120 days) was 4.4.
Differences in Outcomes by Groups Stratified by Gestational Age
As summarized in eTable 4 and eTable 5 in the Supplement, exposure to caffeine remained associated with decreased frequency of AKI in both gestational age strata. In particular, an 87% decreased odds of early AKI was observed among extremely preterm neonates (adjusted OR, 0.13; 95% CI, 0.06-0.31; NNE, 2.2), and a 73% decreased odds of early AKI was observed for very preterm neonates (adjusted OR, 0.27; 95% CI, 0.13-0.56; NNE, 8.1). There was no statistical interaction between gestational age and caffeine administration, suggesting that there was no difference in the association of caffeine administration with early AKI by gestational age category. Similar, although slightly attenuated, associations were observed for all AKI, with an observed 76% decreased odds of any AKI among extremely preterm neonates (adjusted OR, 0.24; 95% CI, 0.10-0.58; NNE, 3.1) and a 68% decreased odds among very preterm neonates (adjusted OR, 0.32; 95% CI, 0.16-0.62; NNE, 8.0). As with early AKI, no statistical interaction was observed between gestational age and caffeine.
Discussion
In this secondary analysis of the international, multicenter AWAKEN study cohort, preterm neonates who were exposed to caffeine were less likely to develop AKI. This association occurred despite the presence of traditional risk factors for AKI among neonates receiving caffeine (including younger gestational age, lower birth weight, and higher illness severity) and persisted even after multivariable adjustment. Neonates exposed to caffeine had an almost 3-fold reduction in the incidence of AKI and an 8-fold reduction in stage 3 AKI in the first week after birth, supporting the hypothesis that early exposure to caffeine is associated with a reduction in both AKI frequency and severity in preterm infants.
The field of neonatal AKI has undergone a renaissance, with numerous studies5,7,9,27,28,29,30,31 demonstrating that AKI in critically ill neonates is common and consistently associated with adverse outcomes. It is now acknowledged that preterm infants have a higher long-term risk of developing chronic kidney disease (CKD),32,33,34,35,36,37,38,39,40 and the findings of other studies33,41,42,43 have suggested that preterm infants who experience AKI are at particular risk of subsequent diagnosis of CKD. In light of the association of AKI with both short-term and long-term adverse outcomes, it is imperative to develop strategies to prevent or ameliorate AKI.
The results of this multicenter study confirm the association of caffeine administration with AKI in preterm neonates described in smaller studies, with an effect magnitude similar to that found previously in a single-center study22 of caffeine administration and AKI in VLBW neonates (NNE, 2.9 for both cohorts). However, while Carmody et al22 found that most AKI reduction after caffeine administration occurred in neonates with stage 1 AKI (with no association with more severe AKI stages), the findings of the present study suggest that the benefit of caffeine extends to more severe AKI.
In VLBW infants, caffeine use almost doubled between 1997 to 2010, from 40% to 70%.21 This dramatic increase coincides with clinical trials, such as the Caffeine for Apnea of Prematurity Trial,44,45 in which infants randomized to early treatment with caffeine were less likely to develop bronchopulmonary dysplasia. Over the same period, age at initiation of caffeine administration fell from a mean of 10 to 12 days after birth (median, 4-5 days) to a mean of 4 days (median, 1 day).21 In addition, the early use of caffeine has been associated with significant decreases in early-onset and late-onset sepsis, pulmonary interstitial emphysema, intraventricular hemorrhage, retinopathy of prematurity, duration of mechanical ventilation, bronchopulmonary dysplasia, and treatment of patent ductus arteriosus.21 Given these data, it is possible that decreased frequency of AKI among infants receiving caffeine is not mediated by any direct association of caffeine with kidney function but is instead conferred through benefits in neonatal respiratory status or hemodynamic stability.
There are several potential mechanisms of action through which caffeine and possibly other methylxanthines could directly reduce AKI. In investigations involving newborn rabbits exposed to caffeine or theophylline, Gouyon and Guignard46 reported increased renal blood flow, enhanced sodium excretion, and a higher glomerular filtration rate. A subsequent study47 by the same authors demonstrated that theophylline counteracted hypoxemia-induced renal hemodynamic changes by maintaining renal vascular resistance. Another potential mechanism of caffeine-mediated renal protection may involve attenuation of oxidative stress and injury on endoplasmic reticulum.48 Using a rodent hyperoxia model, Teng et al48 demonstrated that caffeine, at clinically appropriate doses, protected against hyperoxia-induced impairment of alveolar formation, improved radial alveolar count and secondary septation, increased vascularity, normalized several signaling pathways, maintained normal endoplasmic reticulum and mitochondrial structure, and decreased apoptosis. Whether any or all of these mechanisms could be relevant in the reduction of AKI seen in the present study requires further evaluation.
Limitations
Although this study included a large, diverse sample enabling control for multiple potential confounders, there are several limitations. First, because this was an observational study, participants were not randomized to receive caffeine, and the indication for caffeine administration could not be ascertained. Second, although we created multivariable models to adjust for potential confounders, patients who received caffeine may nonetheless differ systematically from those who did not in unmeasured ways that could account for differences in AKI occurrence. Third, we were unable to evaluate a dose-dependent effect because medication dosages and systemic levels of caffeine were not collected in the AWAKEN study. Fourth, in this study, data collection was limited to a maximum of 120 days. Therefore, we could only assess short-term associations of caffeine administration with kidney function. Whether exposure to caffeine might confer long-term renal benefits was beyond the scope of this study. Fifth, there is also the possibility of misclassification bias because sCr and UOP values were not recorded every day on every infant. In addition, the definition of neonatal AKI used in this study does not take into account maternal sCr values or the possibility of misquantification of UOP. However, we believe that such misclassification, if present, would occur equally between both groups.
Conclusions
This large multicenter study found that caffeine administration in preterm infants is associated with reduced risk and severity of AKI. Given the established benefits, widespread use, and safety of early caffeine treatment in neonates younger than 28 weeks’ gestational age, it is no longer possible to ethically conduct a randomized clinical trial of caffeine vs placebo for protection against neonatal AKI. While these extremely preterm infants are at the highest risk of neonatal AKI, more mature neonates remain at risk: AKI occurred in almost 20% of the AWAKEN study participants born at 29 to 35 weeks’ gestation in the first week after birth. Because of the benefits and favorable adverse effect profile of caffeine, it may be reasonable to consider routine use of prophylactic caffeine in neonates of 28 to 32 weeks’ gestational age to prevent or reduce AKI, even when apnea of prematurity or the need for positive pressure respiratory support is absent. For extremely preterm neonates, evaluation of the optimal dose, duration, and timing of initiation of caffeine therapy to prevent and reduce the severity of AKI should be explored with a standard protocol for evaluating renal function and injury. Long-term outcomes of renal function and rates of CKD in those treated with caffeine are also needed to inform clinical practice.
eTable 1. Comparison by Caffeine Status on Day 1
eTable 2. Comparison by Caffeine Status on Day 1 and 2
eTable 3. Clinical Characteristics by AKI Diagnosis
eTable 4. Extremely Preterm (<28 Weeks’ Gestation) Demographics
eTable 5. Very Preterm (28-32 6/7 Weeks’ Gestation) Demographics
eFigure. Kaplan-Meier AKI-Free Survival Curve
References
- 1.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manuck TA, Rice MM, Bailit JL, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network . Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103.e1-103.e14.26772790 [Google Scholar]
- 3.Pierrat V, Marchand-Martin L, Arnaud C, et al. ; EPIPAGE-2 Writing Group . Neurodevelopmental outcome at 2 years for preterm children born at 22 to 34 weeks’ gestation in France in 2011: EPIPAGE-2 cohort study. BMJ. 2017;358:j3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel RM. Short- and long-term outcomes for extremely preterm infants. Am J Perinatol. 2016;33(3):318-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan N, Schwartz A, John E, Price R, Amin S. Acute kidney injury impairs postnatal renal adaptation and increases morbidity and mortality in very low-birth-weight infants. Am J Perinatol. 2018;35(1):39-47. [DOI] [PubMed] [Google Scholar]
- 6.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res. 2011;69(4):354-358. [DOI] [PubMed] [Google Scholar]
- 7.Askenazi D, Patil NR, Ambalavanan N, et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr Nephrol. 2015;30(9):1511-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jetton JG, Guillet R, Askenazi DJ, et al. ; Neonatal Kidney Collaborative . Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates: design of a retrospective cohort study. Front Pediatr. 2016;4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jetton JG, Boohaker LJ, Sethi SK, et al. ; Neonatal Kidney Collaborative (NKC) . Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health. 2017;1(3):184-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jetton JG, Sorenson M. Pharmacological management of acute kidney injury and chronic kidney disease in neonates. Semin Fetal Neonatal Med. 2017;22(2):109-115. [DOI] [PubMed] [Google Scholar]
- 11.Coulthard MG. The management of neonatal acute and chronic renal failure: a review. Early Hum Dev. 2016;102:25-29. [DOI] [PubMed] [Google Scholar]
- 12.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2(2):356-365. [DOI] [PubMed] [Google Scholar]
- 13.Dobson NR, Hunt CE. Pharmacology review: caffeine use in neonates: indications, pharmacokinetics, clinical effects, outcomes. Neoreviews. 2013;14(11):e540-e550. doi: 10.1542/neo.14-11-e540 [DOI] [Google Scholar]
- 14.Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia: a study in a developing country. Pediatr Nephrol. 2005;20(9):1249-1252. [DOI] [PubMed] [Google Scholar]
- 15.Bhat MA, Shah ZA, Makhdoomi MS, Mufti MH. Theophylline for renal function in term neonates with perinatal asphyxia: a randomized, placebo-controlled trial. J Pediatr. 2006;149(2):180-184. [DOI] [PubMed] [Google Scholar]
- 16.Jenik AG, Ceriani Cernadas JM, Gorenstein A, et al. A randomized, double-blind, placebo-controlled trial of the effects of prophylactic theophylline on renal function in term neonates with perinatal asphyxia. Pediatrics. 2000;105(4):E45. [DOI] [PubMed] [Google Scholar]
- 17.Raina A, Pandita A, Harish R, Yachha M, Jamwal A. Treating perinatal asphyxia with theophylline at birth helps to reduce the severity of renal dysfunction in term neonates. Acta Paediatr. 2016;105(10):e448-e451. [DOI] [PubMed] [Google Scholar]
- 18.Cho HJ, Jeong IS. The renal effect of prophylactic aminophylline therapy after cardiac surgery in infants. J Crit Care. 2015;30(4):852. [Google Scholar]
- 19.Al-Wassia H, Alshaikh B, Sauve R. Prophylactic theophylline for the prevention of severe renal dysfunction in term and post-term neonates with perinatal asphyxia: a systematic review and meta-analysis of randomized controlled trials. J Perinatol. 2013;33(4):271-277. [DOI] [PubMed] [Google Scholar]
- 20.Cattarelli D, Spandrio M, Gasparoni A, Bottino R, Offer C, Chirico G. A randomised, double blind, placebo controlled trial of the effect of theophylline in prevention of vasomotor nephropathy in very preterm neonates with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed. 2006;91(2):F80-F84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobson NR, Patel RM, Smith PB, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants [published correction appears in J Pediatr. 2014;164(5):1244]. J Pediatr. 2014;164(5):992-998.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmody JB, Harer MW, Denotti AR, Swanson JR, Charlton JR. Caffeine exposure and risk of acute kidney injury in a retrospective cohort of very low birth weight neonates. J Pediatr. 2016;172:63-68.e1. [DOI] [PubMed] [Google Scholar]
- 23.Jetton JG, Askenazi DJ. Acute kidney injury in the neonate. Clin Perinatol. 2014;41(3):487-502. [DOI] [PubMed] [Google Scholar]
- 24.Zappitelli M, Ambalavanan N, Askenazi DJ, et al. Developing a neonatal acute kidney injury research definition: a report from the NIDDK neonatal AKI workshop. Pediatr Res. 2017;82(4):569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parry G, Tucker J, Tarnow-Mordi W; UK Neonatal Staffing Study Collaborative Group . CRIB II: an update of the Clinical Risk Index for Babies score. Lancet. 2003;361(9371):1789-1791. [DOI] [PubMed] [Google Scholar]
- 26.Bender R, Blettner M. Calculating the “number needed to be exposed” with adjustment for confounding variables in epidemiological studies. J Clin Epidemiol. 2002;55(5):525-530. [DOI] [PubMed] [Google Scholar]
- 27.Stoops C, Sims B, Griffin R, Askenazi DJ. Neonatal acute kidney injury and the risk of intraventricular hemorrhage in the very low birth weight infant. Neonatology. 2016;110(4):307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131(6):1168-1179. [DOI] [PubMed] [Google Scholar]
- 29.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017;92(3):751-756. [DOI] [PubMed] [Google Scholar]
- 30.Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney injury. Pediatrics. 2015;136(2):e463-e473. [DOI] [PubMed] [Google Scholar]
- 31.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523-530. [DOI] [PubMed] [Google Scholar]
- 32.Low Birth Weight and Nephron Number Working Group . The impact of kidney development on the life course: a consensus document for action. Nephron. 2017;136(1):3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maqsood S, Fung N, Chowdhary V, Raina R, Mhanna MJ. Outcome of extremely low birth weight infants with a history of neonatal acute kidney injury. Pediatr Nephrol. 2017;32(6):1035-1043. [DOI] [PubMed] [Google Scholar]
- 34.Luyckx VA. Preterm birth and its impact on renal health. Semin Nephrol. 2017;37(4):311-319. [DOI] [PubMed] [Google Scholar]
- 35.Stritzke A, Thomas S, Amin H, Fusch C, Lodha A. Renal consequences of preterm birth. Mol Cell Pediatr. 2017;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starzec K, Klimek M, Grudzień A, Jagła M, Kwinta P. Longitudinal assessment of renal size and function in extremely low birth weight children at 7 and 11 years of age. Pediatr Nephrol. 2016;31(11):2119-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishizaki N, Hirano D, Nishizaki Y, et al. Increased urinary angiotensinogen is an effective marker of chronic renal impairment in very low birth weight children. Clin Exp Nephrol. 2014;18(4):642-648. [DOI] [PubMed] [Google Scholar]
- 38.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. 2012;8(5):265-274. [DOI] [PubMed] [Google Scholar]
- 39.Bonamy AK, Källén K, Norman M. High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics. 2012;129(5):e1199-e1204. [DOI] [PubMed] [Google Scholar]
- 40.Iacobelli S, Loprieno S, Bonsante F, Latorre G, Esposito L, Gouyon JB. Renal function in early childhood in very low birthweight infants. Am J Perinatol. 2007;24(10):587-592. [DOI] [PubMed] [Google Scholar]
- 41.Chaturvedi S, Ng KH, Mammen C. The path to chronic kidney disease following acute kidney injury: a neonatal perspective. Pediatr Nephrol. 2017;32(2):227-241. [DOI] [PubMed] [Google Scholar]
- 42.Abitbol CL, Bauer CR, Montané B, Chandar J, Duara S, Zilleruelo G. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr Nephrol. 2003;18(9):887-893. [DOI] [PubMed] [Google Scholar]
- 43.Harer MW, Pope CF, Conaway MR, Charlton JR. Follow-up of Acute Kidney Injury in Neonates During Childhood Years (FANCY): a prospective cohort study. Pediatr Nephrol. 2017;32(6):1067-1076. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt B, Roberts RS, Davis P, et al. ; Caffeine for Apnea of Prematurity Trial Group . Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112-2121. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt B, Roberts RS, Davis P, et al. ; Caffeine for Apnea of Prematurity Trial Group . Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893-1902. [DOI] [PubMed] [Google Scholar]
- 46.Gouyon JB, Guignard JP. Renal effects of theophylline and caffeine in newborn rabbits. Pediatr Res. 1987;21(6):615-618. [DOI] [PubMed] [Google Scholar]
- 47.Gouyon JB, Guignard JP. Theophylline prevents the hypoxemia-induced renal hemodynamic changes in rabbits. Kidney Int. 1988;33(6):1078-1083. [DOI] [PubMed] [Google Scholar]
- 48.Teng RJ, Jing X, Michalkiewicz T, Afolayan AJ, Wu TJ, Konduri GG. Attenuation of endoplasmic reticulum stress by caffeine ameliorates hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312(5):L586-L598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Comparison by Caffeine Status on Day 1
eTable 2. Comparison by Caffeine Status on Day 1 and 2
eTable 3. Clinical Characteristics by AKI Diagnosis
eTable 4. Extremely Preterm (<28 Weeks’ Gestation) Demographics
eTable 5. Very Preterm (28-32 6/7 Weeks’ Gestation) Demographics
eFigure. Kaplan-Meier AKI-Free Survival Curve