This multicenter cohort study investigates the association of histologic chorioamnionitis with intraventricular hemorrhage and punctate white matter injury among preterm neonates and with their early childhood neurodevelopmental outcomes.
Key Points
Question
Is histologic chorioamnionitis associated with perinatal brain injury and adverse neurodevelopmental outcomes among children born preterm?
Findings
In this multicenter cohort study of 350 children, histologic chorioamnionitis was not associated with perinatal intraventricular hemorrhage or punctate white matter injury nor with lower motor or cognitive scores at 18 to 24 months’ corrected age.
Meaning
Histologic chorioamnionitis is not associated with adverse early childhood neurodevelopmental outcomes among preterm neonates.
Abstract
Importance
Understanding the role of chorioamnionitis, a major factor leading to preterm birth, in the pathogenesis of neonatal brain injury and adverse neurodevelopmental outcomes may help in identifying potentially modifiable perinatal variables affecting brain health and outcomes among children born preterm.
Objective
To evaluate whether histologic chorioamnionitis among neonates born very preterm is associated with intraventricular hemorrhage (IVH) and punctate white matter injury (WMI) or with adverse neurodevelopmental outcomes during early childhood.
Design, Setting, and Participants
Prospective cohort study conducted across 3 academic centers (from April 2006 to September 2013 in Canada, from March 2007 to March 2013 in the Netherlands, and from January 2004 to August 2011 in the United States). Children who were born preterm (24-32 weeks’ gestation) and who had undergone a placental pathologic evaluation, magnetic resonance imaging as soon as clinically stable, and Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) assessments between 18 and 24 months’ corrected age (CA) were included. Magnetic resonance imaging scans were assessed for grade of IVH and volume of punctate WMI. Data analysis occurred between December 2016 and January 2018. Final multivariable analyses examining the association of chorioamnionitis with motor and cognitive outcomes accounted for academic center and perinatal and postnatal factors.
Main Outcomes and Measures
Punctate WMI volume and IVH detected on neonatal magnetic resonance imaging scans; motor and cognitive outcomes defined using Bayley-III assessments conducted among these children between 18 and 24 months’ CA.
Results
Of 350 neonates (182 male) in the final cohort, 145 (41.4%) had histologic chorioamnionitis. Gestational age was significantly lower among those with chorioamnionitis (median, 26.4 weeks; interquartile range [IQR], 25.6-27.7 weeks) than among those without chorioamnionitis (median, 28.0 weeks; IQR, 27.0-29.7 weeks). Chorioamnionitis was not associated with IVH or WMI, nor was it associated with worse motor outcomes in univariable or multivariable analyses (adjusted Bayley-III motor score, −2.2; 95% CI, −5.6 to 1.3). Cognitive scores were marginally yet statistically significantly lower among children with chorioamnionitis (median, 105; IQR, 95-110) than among those without chorioamnionitis (median, 105; IQR, 100-115) in the univariable model. This difference was attenuated in the multivariable model (adjusted Bayley-III cognitive score, −3.0; 95% CI, −6.4 to 0.4).
Conclusions and Relevance
Histologic chorioamnionitis was not associated with IVH or WMI near birth or with worse cognitive or motor outcomes from 18 to 24 months’ CA after accounting for perinatal factors. Postnatal factors attenuated the association between chorioamnionitis and neurodevelopmental outcomes, highlighting the importance of preventing postnatal illness, such as infection, to promote optimal outcomes among children born preterm.
Introduction
Acute chorioamnionitis (hereinafter called chorioamnionitis) refers to the neutrophilic inflammation of the placental tissues thought to result from an ascending bacterial infection.1 It is considered a major factor associated with preterm birth and has been estimated to occur in 40% to 80% of preterm deliveries.1,2 Chorioamnionitis is associated with several adverse neonatal outcomes, including respiratory distress syndrome, sepsis, bronchopulmonary dysplasia (BPD), and death.3 In both animal and clinical studies, chorioamnionitis has been associated with perinatal brain injury.4 Brain injury is thought to result either through placental inflammation, causing local vasoconstriction and ischemic injury in the fetal brain, or through contributions of circulating proinflammatory cytokines and reactive oxygen and nitrogen species to vulnerable brain cell populations.4,5,6,7
Clinical studies examining brain injury and neurodevelopmental outcomes among infants with chorioamnionitis have yielded inconsistent results. Most of these studies have focused on intraventricular hemorrhage (IVH) and cystic periventricular leukomalacia. Although individual studies have not consistently detected associations between chorioamnionitis and IVH or cystic periventricular leukomalacia (for review, see Chau et al4 and Ylijoki et al8), when pooled in a meta-analysis, chorioamnionitis was an independent risk factor for cystic periventricular leukomalacia.9 Because the incidence of cystic periventricular leukomalacia has greatly decreased during the past decades concurrent with improvements in neonatal intensive care, punctate white matter injury (WMI) is increasingly recognized as the most prevalent pattern of brain injury among preterm neonates.10,11,12,13 One prospective cohort study found no difference in the severity of WMI, brain metabolism, or white matter microstructure between neonates with or without histologic chorioamnionitis.11 The results of studies examining neurodevelopmental outcomes among infants with chorioamnionitis have been inconsistent. Chorioamnionitis has been associated with cerebral palsy in 2 meta-analyses.14,15 Mild neurocognitive impairments have been reported among certain studies, albeit with very small effect sizes,3,16,17 whereas other groups have not found significant differences.18,19
Some of the heterogeneity reflected by previous studies has been attributed to discrepancies in study criteria, such as the definition of chorioamnionitis (ie, histologic vs clinical), the gestational age of the infants studied, and differences among clinical practices that could contribute to outcomes in single-center studies.9,17 These prior studies have also suggested the need to account for other pathways to preterm birth that may contribute to adverse outcomes in the nonchorioamnionitis-exposed groups, such as preeclampsia.
The primary objective of the present prospective multicenter study was to examine the association of histopathologic chorioamnionitis with IVH, WMI, and neurodevelopmental outcomes in a cohort of very preterm infants. We hypothesized that chorioamnionitis would be associated with adverse motor and cognitive outcomes when accounting for prenatal factors, including preeclampsia. A secondary objective was to compare findings across academic centers to identify center-specific factors that might mediate differences in the association between chorioamnionitis and outcome.
Methods
Study Population
Individual prospective cohorts were drawn from 3 academic centers: BC Children’s and Women’s Hospital and the University of British Columbia (UBC) in Vancouver, British Columbia, Canada from April 2006 to September 2013; the University Medical Center Utrecht (UMCU) in the Netherlands from March 2007 to March 2013; and the UCSF Benioff Children’s Hospital in the United States from January 2004 to August 2011. The study was approved by the research ethics board or institutional review board at each center, and written informed consent was obtained from the parent or legal guardian of each infant. Further details on study enrollment are provided in the eMethods in the Supplement.
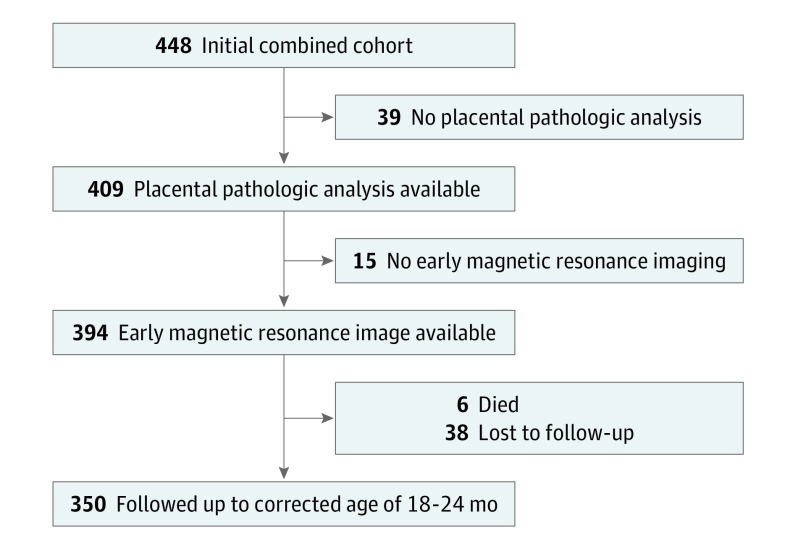
There were 448 preterm infants (24-32 weeks’ gestation) in the total cohort: 232 from UBC, 70 from UCSF Benioff Children’s Hospital, and 146 from UMCU. Infants were included in the analysis (Figure 1) if they had undergone placental pathologic assessments, early brain magnetic resonance imaging (MRI), and 18 to 24 months of follow-up. Among the cohorts from the 3 academic centers, infants with evidence of a congenital infection, genetic syndrome, or large parenchymal hemorrhagic infarction (>2 cm) were excluded.
Figure 1. Participant Flowchart.
Placental Histologic Assessment
Each placenta was sent fresh for macroscopic and microscopic analyses, which were conducted using the same clinical protocols at each center. Histologic chorioamnionitis was defined by clinical pathologists (including J.T. and C.C.) using criteria defined by Redline et al,20 and the degree of placental inflammation was scored as outlined as the eMethods in the Supplement.
Clinical Factors
Systematic medical record reviews were conducted to collect information about pregnancy, delivery, and perinatal course. Definite episodes of necrotizing enterocolitis stage 2 and higher were recorded using the criteria of Bell et al.21 Postnatal positive-culture infection was noted.22 In the absence of a universally accepted definition, newborns were considered to have hypotension if they were treated with saline boluses or vasopressors for low blood pressure.22 Data on maternal educational level were collected as an important mediator of neurodevelopmental outcomes among preterm-born children.23,24
Magnetic Resonance Imaging
Newborns underwent MRI as soon as they were clinically stable at a median age of 3.4 weeks (interquartile range [IQR], 2.0-5.1 weeks) using well-established protocols at each center.11,12,13,25 Early postnatal MRI has been shown to provide the best representation of focal noncystic WMI occurring as a result of in utero exposures.11,12,13,25,26 In addition, WMI detected on early postnatal MRI scans has been shown to be associated with adverse neurodevelopmental outcomes at 18 months of corrected age (CA).26
Experienced assessors (including A.J.B., L.S.d.V., M.B., and S.P.M.) at each center noted IVH and punctate WMI. Good interobserver agreement for punctate WMI has been shown in a previous study between radiologists at UBC and UCSF Benioff Children’s Hospital.27 Further details on labeling of WMI are available in the eMethods in the Supplement. Punctate WMI volume was calculated through manual segmentation according to the method described in Guo et al26 and was normalized as a percentage of total brain volume.
Although there were slight differences in the T1-weighted MRI acquisition protocols used among the centers, the acquisition resolution was similar. Furthermore, we accounted for the center as a potential confounder in the regression models examining WMI volume.
Developmental Follow-up
Neurodevelopmental outcome was assessed using the US version of the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III)28 in established neonatal follow-up programs between 18 and 24 months’ CA by experienced clinical assessors. Clinical assessors were blinded to imaging data and placental pathologic findings. Composite scores were obtained for motor and cognitive domains, with a normative mean of 100 and standard deviation of 15; higher composite scores reflect better function. Infants who were too impaired to undergo formal neurodevelopmental testing were imputed a score of 49.
Statistical Analysis
Statistical analysis was performed using Stata, version 14.2 (StataCorp) and occurred between December 2016 and January 2018. Demographic and clinical characteristics were compared using the Fisher exact and Kruskal-Wallis tests for categorical and continuous variables, respectively. Multivariable linear regression was used to examine the association between chorioamnionitis and WMI volume accounting for gestational age at birth and postmenstrual age at MRI. Multivariable models were constructed to examine the association between histologic chorioamnionitis and Bayley-III motor and cognitive scores, first controlling for center, maternal educational level, and gestational age, then also controlling for preeclampsia, and finally controlling for postnatal variables that have been associated with neurodevelopmental outcomes and might be on the causal pathway between chorioamnionitis and outcome (ie, BPD, postnatal sepsis, and brain injury).22,29,30 In a fourth model, we accounted for the interaction between histologic chorioamnionitis and center to examine whether any association with chorioamnionitis was modified by center.
Results
Cohort Demographic and Clinical Characteristics
As shown in Figure 1, of the 448 infants in the total initial cohort, 39 did not undergo placental pathologic assessment, 15 did not undergo early brain MRI, 38 were lost to follow-up, and 6 died. Thus, a total of 350 infants were included in the analysis: 183 infants from UBC, 106 from UMCU, and 61 from UCSF Benioff Children’s Hospital.
There were significant differences among the clinical characteristics of the infants across the 3 centers (Table 1). In particular, the UMCU cohort had the lowest median gestational age at birth, the highest incidence of grades 3 and 4 IVH, and the highest incidence of histologic chorioamnionitis, yet this cohort also had the lowest WMI volume and the highest motor scores. Cognitive scores among the infants did not differ across the 3 centers.
Table 1. Demographic and Clinical Characteristics of Newborns by Academic Center.
| Characteristic | No. Available for Analysis | Newborns, No. (%) | P Value | ||
|---|---|---|---|---|---|
| UBC (n = 183) | UMCU (n = 106) | UCSF (n = 61) | |||
| Prenatal factor | |||||
| Male | 350 | 94 (51) | 56 (53) | 32 (52) | .98 |
| Preeclampsia | 349 | 46 (25) | 15 (14) | 10 (17) | .06 |
| Gestational diabetes | 349 | 15 (8) | 5 (5) | 6 (10) | .36 |
| Antenatal antibiotics | 348 | 112 (61) | 58 (55) | 38 (63) | .52 |
| Antenatal steroids | 348 | 165 (90) | 103 (97) | 58 (98) | .02 |
| Antenatal magnesium sulfate | 346 | 40 (22) | 16 (15) | 33 (57) | <.001 |
| University educational level | 315 | 82 (47) | 38 (37) | 30 (77) | <.001 |
| Histologic chorioamnionitis | 350 | 68 (37) | 62 (58) | 15 (24) | <.001 |
| Clinical chorioamnionitis | 331 | 6 (3) | 5 (5) | 7 (12) | .07 |
| Postnatal factor | |||||
| GA at birth, median (IQR), wk | 350 | 28.0 (26.1-29.9) | 26.7 (26.0-27.6) | 28.4 (27.1-29.6) | <.001 |
| Birth weight, median (IQR), g | 350 | 1060 (813-1285) | 915 (800-1030) | 1030 (840-1200) | <.001 |
| Definite NEC | 350 | 8 (4) | 6 (6) | 2 (3) | .83 |
| Postnatal sepsis | 349 | 88 (48) | 36 (34) | 17 (28) | .005 |
| Neonatal hypotension | 350 | 67 (37) | 58 (55) | 25 (41) | .01 |
| BPD | 350 | 33 (18) | 26 (24) | 13 (21) | .41 |
| Brain injury | |||||
| IVH, grades 1 and 2 | 347 | 72 (40) | 34 (32) | 14 (23) | .05 |
| IVH, grades 3 and 4 | 347 | 5 (3) | 13 (12) | 6 (10) | .004 |
| WMI volume, median (IQR), % TCV | 350 | 0.03 (0.01-0.2) | 0.01 (0.002-0.07) | 0.03 (0.02-0.03) | .04 |
| Outcome | |||||
| Motor score, median (IQR) | 348 | 100 (88-107) | 107 (100-115) | 98.5 (91-107) | <.001 |
| Cognitive score, median (IQR) | 350 | 105 (100-115) | 105 (95-110) | 105 (95-115) | .21 |
| Cerebral palsy | 341 | 14 (8) | 4 (4) | 4 (7) | .40 |
Abbreviations: BPD, bronchopulmonary dysplasia; GA, gestational age; IQR, interquartile range; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; TCV, total cerebral volume; UBC, British Columbia Children’s and Women’s Hospital, University of British Columbia; UCSF, University of California, San Francisco Benioff Children’s Hospital; UMCU, the University Medical Center Utrecht; WMI, white matter injury.
Chorioamnionitis
Histopathologic chorioamnionitis was identified in 145 of the 350 infants (41.4%) in the total cohort. In 74 of these 145 infants (51.0%), a severe maternal inflammatory response was identified, and in 14 infants (9.7%), a severe fetal inflammatory response was identified. Only 18 infants (5.1%) were born to mothers who received a diagnosis of clinical chorioamnionitis.
The clinical characteristics of infants born to mothers with or without chorioamnionitis are summarized in Table 2. Infants in the chorioamnionitis group were less likely to be born to mothers with preeclampsia, were born at a lower gestational age, and had a higher incidence of postnatal sepsis.
Table 2. Demographic and Clinical Characteristics of Newborns With or Without Histologic Chorioamnionitis in the Combined Cohort.
| Characteristic | No. Analyzed | Newborns, No. (%) | P Value | |
|---|---|---|---|---|
| Without Chorioamnionitis (n = 205) | With Chorioamnionitis (n = 145) | |||
| Prenatal factor | ||||
| Male | 350 | 113 (55.1) | 69 (47.6) | .19 |
| Preeclampsia | 349 | 64 (31.4) | 7 (4.8) | <.001 |
| Gestational diabetes | 349 | 18 (8.8) | 8 (5.5) | .30 |
| Antenatal antibiotics | 348 | 98 (48.0) | 110 (76.4) | <.001 |
| Antenatal steroids | 348 | 186 (91.6) | 140 (96.6) | .08 |
| Antenatal magnesium sulfate | 346 | 64 (31.8) | 25 (17.2) | .003 |
| Postnatal factor | ||||
| GA at birth, median (IQR), wk | 350 | 28.0 (27.0-29.7) | 26.4 (25.6-27.7) | <.001 |
| Birth weight, median (IQR), g | 350 | 1055 (840-1251) | 943 (790-1090) | .002 |
| University educational level | 315 | 88 (48.4) | 62 (46.6) | .82 |
| Definite NEC | 350 | 7 (3.4) | 9 (6.2) | .30 |
| Postnatal sepsis | 349 | 68 (33.3) | 73 (50.3) | .002 |
| Neonatal hypotension | 350 | 84 (41.0) | 66 (45.5) | .44 |
| Chronic lung disease | 350 | 39 (19.0) | 33 (22.8) | .42 |
| Brain injury | ||||
| IVH, grades 1 and 2 | 347 | 67 (32.8) | 53 (37.1) | .42 |
| IVH, grades 3 and 4 | 347 | 9 (4.4) | 15 (10.5) | .03 |
| WMI volume, median (IQR), % TCV | 350 | 0.02 (0.01-0.09) | 0.03 (0.01-0.15) | .90 |
| Outcome | ||||
| Motor score, median (IQR) | 348 | 100 (94-110) | 100 (91-110) | .32 |
| Cognitive score, median (IQR) | 350 | 105 (100-115) | 105 (95-110) | .003 |
| Cerebral palsy | 341 | 13 (6.60) | 9 (6.2) | .99 |
Abbreviations: GA, gestational age; IQR, interquartile range; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; TCV, total cerebral volume; WMI, white matter injury.
Chorioamnionitis and Perinatal Brain Injury
The incidence of moderate to severe IVH (grades 3-4) did not differ significantly among neonates exposed to chorioamnionitis (15 of 143 [10.5%]) compared with those who were unexposed (9 of 205 [4.4%]). White matter lesion volumes, expressed as a percentage of total cerebral volume, did not differ between infants who were exposed to chorioamnionitis (median, 0.03%; IQR, 0.01%-0.15%) and those who were unexposed to chorioamnionitis (median, 0.02%; IQR, 0.01%-0.09%) in the univariable analysis. There was likewise no association between chorioamnionitis and WMI volume as a percent of total cerebral volume when accounting for birth gestational age, postmenstrual age at MRI, and center (mean, 0.02%; 95% CI, −0.02% to 0.08%).
Chorioamnionitis and Neurodevelopmental Outcomes
A univariable analysis indicated no significant difference in motor scores between infants of 18 to 24 months CA with (median, 100; IQR, 91-110) or without chorioamnionitis (median, 100; IQR, 94-110) (Figure 2). There was likewise no association using multivariable analysis accounting for perinatal factors, BPD, postnatal sepsis, and brain injury (Bayley-III motor score, −2.2; 95% CI, −5.6 to 1.3) (Table 3, eTable 1 in the Supplement).
Figure 2. Motor and Cognitive Outcomes at 18 to 24 Months’ Corrected Age Among Newborns With or Without Histologic Chorioamnionitis.
Box and whisker plots showing median score (horizontal line in the box), interquartile ranges (top and bottom borders of the box, which mark the 75th and 25th percentiles, respectively), and upper and lower adjacent lines (whiskers, which mark the 90th and 10th percentiles). The points beyond the whiskers are outliers beyond the 90th and 10th percentiles.
Table 3. Motor and Cognitive Outcomes at 18 to 24 Months’ Corrected Age Using Multivariable Analysis of Newborns With or Without Histologic Chorioamnionitis.
| Outcome | Newborns Without Chorioamnionitis | Newborns With Chorioamnionitis | Adjusted Differences in Bayley-III Score (95% CI) | |||
|---|---|---|---|---|---|---|
| Mean (SD) Score | No. | Mean (SD) Score | No. | Adjusting Only for Perinatal Factors | Adjusting for Perinatal and Postnatal Factors and Brain Injury | |
| Motor score | 100.8 (13.8) | 203 | 99.0 (15.4) | 145 | −3.6 (−7.1 to −0.1) | −2.2 (−5.6 to 1.3) |
| Cognition | 106.3 (12.9) | 205 | 101.5 (14.1) | 145 | −4.2 (−7.6 to −0.9) | −3.0 (−6.4 to 0.4) |
Abbreviation: Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition.
Cognitive scores were marginally lower among children with chorioamnionitis (median, 105; IQR, 95-110) than among those without chorioamnionitis (median, 105; IQR, 100-115) in the univariable model (Figure 2). This association persisted when adjusting for center, maternal educational level, and gestational age (Bayley-III cognitive score, −3.5; 95% CI, −6.6 to −0.3), with an increase in effect size when preeclampsia was added to the model (Bayley-III cognitive score, −4.2; 95% CI, −7.6 to −0.9) (Table 3, eTable 2 in the Supplement). The association was attenuated when BPD, sepsis, and brain injury were incorporated into the model (Bayley-III cognitive score, −3.0; 95% CI, −6.4 to 0.4) (Table 3; eTable 2 in the Supplement).
Differences in cognitive outcome were not associated with center-specific factors (eTables 1 and 2 in the Supplement). In addition, there were no significant center by chorioamnionitis interactions for either motor or cognitive scores in the full models. The severities of the maternal and fetal inflammatory responses were not associated with cognitive outcomes in multivariable models (details reported in eTables 3 and 4, eResults, eFigure, and eDiscussion in the Supplement).
Discussion
Chorioamnionitis and Perinatal Brain Injury
In this multicenter cohort of preterm infants, we found that histopathologic chorioamnionitis was not associated with IVH or punctate WMI among preterm neonates. Although some studies have found an association between IVH and chorioamnionitis, our data are consistent with most studies reporting no association (for review, see Ylijoki et al8). Our finding that histopathologic chorioamnionitis was not associated with WMI is consistent with a previous analysis of the Vancouver subset of our study cohort, which showed no association with punctate WMI among preterm newborns.11 In that prior analysis, WMI was assessed categorically rather than quantitatively with volumes.25 Our present study extends those findings in a larger and heterogeneous multicenter cohort with WMI volumes and uniform outcome assessments. Use of white matter lesion volume enables a more robust assessment of WMI severity.26 Consistent with prior work,26 WMI volume normalized to total cerebral volume was associated with motor and cognitive outcomes.
Chorioamnionitis and Neurodevelopmental Outcomes
Histologic chorioamnionitis was not associated with motor outcomes among infants at 18 to 24 months’ CA. Although chorioamnionitis was associated with a small decrease in cognitive scores even when accounting for perinatal factors, the association was attenuated when sepsis, BPD, IVH, and WMI were included in the model. Sepsis, BPD, and brain injury are postnatal factors occurring distal to chorioamnionitis and may lie on the causal pathway between chorioamnionitis and neurodevelopmental outcome. The results of our multivariable model indicated that postnatal sepsis and WMI were each predictive of cognitive outcome (eTable 2 in the Supplement). Chorioamnionitis is a known risk factor for postnatal sepsis.31 For the preterm neonate, postnatal infection, even in the absence of positive cultures, has been associated with WMI, brain dysmaturation,22 and adverse cognitive and motor functions, including cerebral palsy.29,30 Our findings therefore underscore the pressing need to prevent postnatal sepsis, BPD, and WMI, with the goal of improving the neurodevelopmental outcomes of preterm neonates.
The lack of an association between histologic chorioamnionitis and cognitive outcome in our cohort is consistent with another, larger prospective study of 1375 preterm infants, among whom the combination of histologic and clinical chorioamnionitis was associated with low 18-month cognitive scores.17 In another preterm cohort, histologic chorioamnionitis was not associated with worse neurocognitive performance at 2 years of age but was associated with poorer cognitive development and learning and memory functions at 5 years.32 Our findings differ from those of another prospective study of 628 preterm infants, which found a 4-point decrease in cognitive scores among children with histologic chorioamnionitis, even when accounting for perinatal factors.3 Although these previous studies accounted for perinatal variables, none of them accounted for postnatal variables, which, in our analysis, further attenuated the association between chorioamnionitis and outcome.
A notable outcome of our analysis was the increased effect size of the association between chorioamnionitis and cognitive outcome when accounting for preeclampsia. In a previous preterm cohort study in which infants were grouped into different categories reflecting the most likely cause of preterm birth, infants with maternal preeclampsia had worse cognitive scores at 2 years of age than infants with chorioamnionitis or infants with neither condition.19 A similar study that specifically examined placental histologic features as the cause for preterm birth likewise found that infants with placental underperfusion had worse cognitive scores at 2 years of age than infants with histologic chorioamnionitis.33 In our analysis, we compared infants with chorioamnionitis with those born preterm owing to any other cause. Because our comparison group included infants born preterm owing to preeclampsia and were therefore themselves at risk of adverse neurodevelopmental outcomes, including preeclampsia in the model likely enhanced the association of chorioamnionitis with cognitive outcome. Preeclampsia has not been consistently accounted for in previous cohort studies of chorioamnionitis,3,17,32 although the results of our analysis suggest that it is important to consider the etiology of preterm birth when studying chorioamnionitis.
Limitations
A limitation of our study is that infants were assessed only at 18 to 24 months’ CA; thus, we cannot exclude a link between chorioamnionitis and more subtle neuropsychologic deficits apparent only at school age, as in the study by Ylijoki et al.32 Because neonates with large periventricular hemorrhagic infarctions were excluded, the link between chorioamnionitis and this severe form of IVH could not be examined. Likewise, because unstable neonates did not undergo an early MRI and thus were not included in the analysis, it is possible that some neonates with more extensive brain injury were missed. Another limitation of our study is that we did not assess measures of brain development, such as those obtained though diffusion tensor imaging, given variations in MRI acquisition across centers. Although a recent study found an association between altered fractional anisotropy and histologic chorioamnionitis,2 in an earlier subset of our UBC cohort with very similar characteristics, histologic chorioamnionitis was not associated with diffusion measures of brain maturation.11 We used uniform assessments of brain injury and functional outcomes across 3 centers and did not detect an association between histologic chorioamnionitis and these measures.
Conclusions
The main strength of our study was the use of a heterogeneous multicenter cohort to assess center-specific factors mediating the association between chorioamnionitis and outcome that could underlie the heterogeneity found among previous studies. Consistent with other reports,11,22,23,24,29,30 in our multivariable analysis, motor outcomes were associated with moderate to severe IVH and WMI, whereas cognitive outcomes were most robustly associated with maternal educational level, postnatal sepsis, and WMI. Yet in our multicenter cohort, histologic chorioamnionitis was not associated with punctate WMI volume or with worse motor or cognitive outcomes at 18 to 24 months’ CA when accounting for clinical factors and brain injury. Preventing postnatal illness, such as infection, remains an important opportunity to improve the brain health of children born preterm.
eMethods. Study Enrollment, Placental, and White Matter Injury Analyses, and Clinical Measures of Chorioamnionitis
eTable 1. Motor Outcomes at 18 to 24 months’ Corrected Age From Multivariable Analysis of Newborns With and Without Chorioamnionitis
eTable 2. Cognitive Outcomes at 18 to 24 Months’ Corrected Age From Multivariable Analysis of Newborns With and Without Chorioamnionitis
eTable 3. Cognitive Outcomes at 18 to 24 Months’ Corrected Age From Multivariable Analysis of Newborns With Mild and Severe Inflammation of Maternal Placental Tissues
eTable 4. Cognitive Outcomes at 18 to 24 Months’ Corrected Age From Multivariable Analysis of Newborns With Mild and Severe Inflammation of Fetal Placental Tissues
eResults. Associations of Maternal and Fetal Inflammatory Responses, and of Clinical Features of Chorioamnionitis With Outcome
eFigure. Associations Between Cognitive Outcome and Severity of Maternal and Fetal Inflammatory Response
eDiscussion. Associations of Maternal and Fetal Inflammatory Responses, and of Clinical Features of Chorioamnionitis With Outcome
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anblagan D, Pataky R, Evans MJ, et al. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep. 2016;6:37932. doi: 10.1038/srep37932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendson L, Russell L, Robertson CMT, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr. 2011;158(3):397-402. doi: 10.1016/j.jpeds.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 4.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41(1):83-103. doi: 10.1016/j.clp.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 5.Garnier Y, Coumans ABC, Jensen A, Hasaart THM, Berger R. Infection-related perinatal brain injury: the pathogenic role of impaired fetal cardiovascular control. J Soc Gynecol Investig. 2003;10(8):450-459. doi: 10.1016/S1071-5576(03)00150-3 [DOI] [PubMed] [Google Scholar]
- 6.Leviton A, Dammann O, Durum SK. The adaptive immune response in neonatal cerebral white matter damage. Ann Neurol. 2005;58(6):821-828. doi: 10.1002/ana.20662 [DOI] [PubMed] [Google Scholar]
- 7.Dammann O, O’Shea TM. Cytokines and perinatal brain damage. Clin Perinatol. 2008;35(4):643-663, v. doi: 10.1016/j.clp.2008.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ylijoki M, Ekholm E, Haataja L, Lehtonen L; PIPARI study group . Is chorioamnionitis harmful for the brain of preterm infants? a clinical overview. Acta Obstet Gynecol Scand. 2012;91(4):403-419. doi: 10.1111/j.1600-0412.2012.01349.x [DOI] [PubMed] [Google Scholar]
- 9.Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284(11):1417-1424. doi: 10.1001/jama.284.11.1417 [DOI] [PubMed] [Google Scholar]
- 10.Hamrick SEG, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. J Pediatr. 2004;145(5):593-599. doi: 10.1016/j.jpeds.2004.05.042 [DOI] [PubMed] [Google Scholar]
- 11.Chau V, Poskitt KJ, McFadden DE, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66(2):155-164. doi: 10.1002/ana.21713 [DOI] [PubMed] [Google Scholar]
- 12.Wagenaar N, Chau V, Groenendaal F, et al. Clinical risk factors for punctate white matter lesions on early magnetic resonance imaging in preterm newborns. J Pediatr. 2017;182:34-40.e1. doi: 10.1016/j.jpeds.2016.11.073 [DOI] [PubMed] [Google Scholar]
- 13.Kersbergen KJ, Benders MJNL, Groenendaal F, et al. Different patterns of punctate white matter lesions in serially scanned preterm infants. PLoS One. 2014;9(10):e108904. doi: 10.1371/journal.pone.0108904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8(1):25-29. doi: 10.1002/mrdd.10003 [DOI] [PubMed] [Google Scholar]
- 15.Shatrov JG, Birch SCM, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116(2, pt 1):387-392. doi: 10.1097/AOG.0b013e3181e90046 [DOI] [PubMed] [Google Scholar]
- 16.Hardt NS, Kostenbauder M, Ogburn M, Behnke M, Resnick M, Cruz A. Influence of chorioamnionitis on long-term prognosis in low birth weight infants. Obstet Gynecol. 1985;65(1):5-10. [PubMed] [Google Scholar]
- 17.Pappas A, Kendrick DE, Shankaran S, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168(2):137-147. doi: 10.1001/jamapediatrics.2013.4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Källén K, Serenius F, Westgren M, Maršál K; EXPRESS Group . Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS). Acta Obstet Gynecol Scand. 2015;94(11):1203-1214. doi: 10.1111/aogs.12726 [DOI] [PubMed] [Google Scholar]
- 19.Schlapbach LJ, Ersch J, Adams M, Bernet V, Bucher HU, Latal B. Impact of chorioamnionitis and preeclampsia on neurodevelopmental outcome in preterm infants below 32 weeks gestational age. Acta Paediatr. 2010;99(10):1504-1509. doi: 10.1111/j.1651-2227.2010.01861.x [DOI] [PubMed] [Google Scholar]
- 20.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C; Society for Pediatric Pathology, Perinatal Section, Amniotic Fluid Infection Nosology Committee . Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435-448. doi: 10.1007/s10024-003-7070-y [DOI] [PubMed] [Google Scholar]
- 21.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1-7. doi: 10.1097/00000658-197801000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chau V, Brant R, Poskitt KJ, Tam EWY, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71(3):274-279. doi: 10.1038/pr.2011.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potijk MR, Kerstjens JM, Bos AF, Reijneveld SA, de Winter AF. Developmental delay in moderately preterm-born children with low socioeconomic status: risks multiply. J Pediatr. 2013;163(5):1289-1295. doi: 10.1016/j.jpeds.2013.07.001 [DOI] [PubMed] [Google Scholar]
- 24.Johnson S, Evans TA, Draper ES, et al. Neurodevelopmental outcomes following late and moderate prematurity: a population-based cohort study. Arch Dis Child Fetal Neonatal Ed. 2015;100(4):F301-F308. doi: 10.1136/archdischild-2014-307684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SP, Ferriero DM, Leonard C, et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147(5):609-616. doi: 10.1016/j.jpeds.2005.06.033 [DOI] [PubMed] [Google Scholar]
- 26.Guo T, Duerden EG, Adams E, et al. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurology. 2017;88(7):614-622. doi: 10.1212/WNL.0000000000003606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glass HC, Bonifacio SL, Chau V, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122(2):299-305. doi: 10.1542/peds.2007-2184 [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. Manual for the Bayley Scales of Infant and Toddler Development. 3rd ed. San Antonio, TX: Harcourt Assessment, 2006. [Google Scholar]
- 29.Synnes A, Luu TM, Moddemann D, et al. ; Canadian Neonatal Network and the Canadian Neonatal Follow-Up Network . Determinants of developmental outcomes in a very preterm Canadian cohort. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F235-F234. doi: 10.1136/archdischild-2016-311228 [DOI] [PubMed] [Google Scholar]
- 30.Stoll BJ, Hansen NI, Adams-Chapman I, et al. ; National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357-2365. doi: 10.1001/jama.292.19.2357 [DOI] [PubMed] [Google Scholar]
- 31.Pugni L, Pietrasanta C, Acaia B, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonatal Med. 2016;29(9):1525-1529. doi: 10.3109/14767058.2015.1053862 [DOI] [PubMed] [Google Scholar]
- 32.Ylijoki M, Lehtonen L, Lind A, et al. ; PIPARI Study Group . Chorioamnionitis and five-year neurodevelopmental outcome in preterm infants. Neonatology. 2016;110(4):286-295. doi: 10.1159/000446236 [DOI] [PubMed] [Google Scholar]
- 33.van Vliet EOG, de Kieviet JF, van der Voorn JP, Been JV, Oosterlaan J, van Elburg RM. Placental pathology and long-term neurodevelopment of very preterm infants. Am J Obstet Gynecol. 2012;206(6):489.e1-489.e7. doi: 10.1016/j.ajog.2012.03.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Study Enrollment, Placental, and White Matter Injury Analyses, and Clinical Measures of Chorioamnionitis
eTable 1. Motor Outcomes at 18 to 24 months’ Corrected Age From Multivariable Analysis of Newborns With and Without Chorioamnionitis
eTable 2. Cognitive Outcomes at 18 to 24 Months’ Corrected Age From Multivariable Analysis of Newborns With and Without Chorioamnionitis
eTable 3. Cognitive Outcomes at 18 to 24 Months’ Corrected Age From Multivariable Analysis of Newborns With Mild and Severe Inflammation of Maternal Placental Tissues
eTable 4. Cognitive Outcomes at 18 to 24 Months’ Corrected Age From Multivariable Analysis of Newborns With Mild and Severe Inflammation of Fetal Placental Tissues
eResults. Associations of Maternal and Fetal Inflammatory Responses, and of Clinical Features of Chorioamnionitis With Outcome
eFigure. Associations Between Cognitive Outcome and Severity of Maternal and Fetal Inflammatory Response
eDiscussion. Associations of Maternal and Fetal Inflammatory Responses, and of Clinical Features of Chorioamnionitis With Outcome