Key Points
Question
Are early intervention services superior to treatment as usual regarding symptom-related and illness-related treatment outcomes in patients with early-phase psychosis?
Findings
In this meta-analysis of 10 randomized clinical trials (n = 2176 patients), early intervention services were associated with better outcomes than treatment as usual at the end of treatment regarding all meta-analyzable outcomes. These outcomes included all-cause treatment discontinuation from early intervention services or treatment as usual and at least 1 psychiatric hospitalization.
Meaning
In early-phase psychosis, early intervention services were associated with superior outcomes compared with treatment as usual, which supports the need for funding and use of early intervention services in patients with early-phase psychosis.
Abstract
Importance
The value of early intervention in psychosis and allocation of public resources has long been debated because outcomes in people with schizophrenia spectrum disorders have remained suboptimal.
Objective
To compare early intervention services (EIS) with treatment as usual (TAU) for early-phase psychosis.
Data Sources
Systematic literature search of PubMed, PsycINFO, EMBASE, and ClinicalTrials.gov without language restrictions through June 6, 2017.
Study Selection
Randomized trials comparing EIS vs TAU in first-episode psychosis or early-phase schizophrenia spectrum disorders.
Data Extraction and Synthesis
This systematic review was conducted according to PRISMA guidelines. Three independent investigators extracted data for a random-effects meta-analysis and prespecified subgroup and meta-regression analyses.
Main Outcomes and Measures
The coprimary outcomes were all-cause treatment discontinuation and at least 1 psychiatric hospitalization during the treatment period.
Results
Across 10 randomized clinical trials (mean [SD] trial duration, 16.2 [7.4] months; range, 9-24 months) among 2176 patients (mean [SD] age, 27.5 [4.6] years; 1355 [62.3%] male), EIS was associated with better outcomes than TAU at the end of treatment for all 13 meta-analyzable outcomes. These outcomes included the following: all-cause treatment discontinuation (risk ratio [RR], 0.70; 95% CI, 0.61-0.80; P < .001), at least 1 psychiatric hospitalization (RR, 0.74; 95% CI, 0.61-0.90; P = .003), involvement in school or work (RR, 1.13; 95% CI, 1.03-1.24; P = .01), total symptom severity (standardized mean difference [SMD], −0.32; 95% CI, −0.47 to −0.17; P < .001), positive symptom severity (SMD, −0.22; 95% CI, −0.32 to −0.11; P < .001), and negative symptom severity (SMD, −0.28; 95% CI, −0.42 to −0.14; P < .001). Superiority of EIS regarding all outcomes was evident at 6, 9 to 12, and 18 to 24 months of treatment (except for general symptom severity and depressive symptom severity at 18-24 months).
Conclusions and Relevance
In early-phase psychosis, EIS are superior to TAU across all meta-analyzable outcomes. These results support the need for funding and use of EIS in patients with early-phase psychosis.
This meta-analysis compares early intervention services with treatment as usual for patients with early-phase psychosis.
Introduction
Outcomes in people with schizophrenia spectrum disorders have remained suboptimal.1 Schizophrenia is among the 10 most debilitating disorders in the United States,2 being associated with high disability3 and enormous personal and societal cost.1
The results of a 2013 meta-analysis4 suggested that, during the last 5 decades, recovery from schizophrenia remained low (median, 13.5%), without significantly improving. Furthermore, individuals with schizophrenia die on average 15 to 20 years prematurely,5,6,7 with an increasing mortality gap.8 Because people with early-phase schizophrenia spectrum disorders have not endured many years of illness and functional decline and generally respond better to treatment, there has been an increasing focus on early identification and optimized treatment.1,9 Several research programs for patients with early-phase schizophrenia spectrum disorders yielded promising results for early intervention services (EIS) that are specifically designed to meet the needs of patients with early-phase psychosis.10 Early intervention services require a multidisciplinary team of mental health professionals who provide multimodal treatment, including different psychosocial and psychopharmacological interventions that are tailored to the needs of each patient. In EIS programs, these services are provided from one team in a coordinated, integrated fashion instead of referring patients to different health care providers for each service.11,12,13,14,15 These programs aim at decreasing psychosis symptoms, improving functional outcomes, and reducing long-term disability during what has been called a critical illness period.16
In this context, the level of efficacy and effectiveness of EIS for patients with first-episode and early-phase schizophrenia spectrum disorders has been debated. This debate is especially true given necessary societal decisions about resource allocation and funding in times of health care cost cuts and frugality across the world.
To date, only 1 meta-analysis,17 which included 4 randomized clinical trials (RCTs), has compared the effectiveness of EIS vs treatment as usual (TAU) for early-phase psychosis, indicating superiority of EIS approaches. Aside from the limited number of studies included, only published data were used, no subgroup or meta-regression analyses were conducted, and the time course of the treatment effect was not examined.
Because additional RCTs9,18,19,20,21,22,23 of EIS vs TAU have been published, we conducted a comprehensive meta-analysis of all available studies, including additional unpublished data that we received from the authors of all meta-analyzed studies. We hypothesized that EIS would be superior to TAU and expected that a more comprehensive assessment of the treatment effects could inform our understanding of the influence of EIS programs and result in their refinement and implementation across the world.
Methods
This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. A predetermined but unpublished protocol was followed (eMethods 1 in the Supplement).24,25
Literature Search
Three of us (B.G., A.P., and A.K.) independently conducted a systematic literature search in PubMed, PsycINFO, EMBASE, and ClinicalTrials.gov through June 6, 2017, without language restrictions. This search was supplemented by a manual review of reference lists from eligible publications and relevant review articles (eMethods 2 in the Supplement).
Inclusion Criteria
Inclusion criteria were 4-fold. First, a study had to be an RCT to be included. Second, participants had to have a study-defined diagnosis of first-episode psychosis or a study-defined diagnosis of early-phase schizophrenia spectrum disorders (schizophrenia, psychotic disorder not otherwise specified, schizoaffective disorder, schizophreniform disorder, or delusional disorder). Third, the study had to have EIS specifically designed for the needs of people with early-phase psychosis and consisting of a multimodal treatment program, including several psychosocial and psychopharmacological interventions (eg, case management, psychotherapy, supported employment and education, and family support) that are provided from one team in a coordinated, integrated fashion. Fourth, the study had to have a control group consisting of a nonspecialized TAU protocol. Excluded were RCTs randomizing patients to maintenance of EIS vs a step-down or less intense maintenance treatment.
Data Abstraction
Three of us (B.G., A.P., and A.K.) independently identified, checked, and extracted data from eligible trials for all follow-up time points of the treatment phase. Inconsistencies were resolved by involvement of a fourth reviewer (one of us, C.U.C.). Authors were contacted for missing information or unpublished original data.
Outcome and Data Synthesis
The coprimary outcomes were all-cause treatment discontinuation and at least 1 psychiatric hospitalization during the treatment period (excluding a potential initial hospitalization before the initiation of the EIS intervention). Treatment discontinuation is a commonly used outcome in psychiatric research because it is a good indicator of treatment failure for lack of efficacy or tolerability, safety, or acceptability (with nonadherence being a major problem with psychiatric interventions), while hospitalizations are an indicator of marked symptom exacerbation or relapse, as well as of increased health care costs. Therefore, these coprimary outcomes are good indicators of real-life feasibility, acceptability, and effectiveness of an intervention.
Key secondary outcomes were involvement in school or work, total symptom severity improvement, and global functioning (including social and role functioning). These areas represent the illness itself, as well as additional burden of the disease that leads to a poor long-term prognosis.
Additional outcomes included the following: the mean number of psychiatric hospitalizations and bed-days during treatment, study-defined relapse, remission (symptom stability or minimum symptom severity) and recovery (symptom stability or minimum severity plus improved social, educational, and vocational attainment), symptom severity (positive, negative, general, and depressive symptoms26), and subjective quality of life. Details on outcome definitions and scales are provided in eMethods 3 in the Supplement.
All eligible trials were assessed for methodological quality using the Cochrane Collaboration’s tool for assessing risk of bias.27 We extracted data on study design and patient, illness, and treatment components.
Statistical Analysis
We conducted a random-effects meta-analysis28 of outcomes for which at least 2 studies contributed data using Comprehensive Meta-Analysis, version 3 (http://www.meta-analysis.com) (performed by B.G.). Intent-to-treat data were used whenever possible. Continuous outcomes were expressed as the standardized mean difference (SMD), which equals Cohen d, preferring change scores (unless skewed, with the SD exceeding twice the mean) over time point and end point scores, while categorical data were expressed as the pooled risk ratio (RR) using the inverse variance method, each with their 95% CIs. Negative SMD favored EIS when smaller values are better (psychopathology), and positive SMD favored EIS when larger values are better (global functioning and quality of life). Effect sizes of 0.2 were considered small, effect sizes of 0.5 were considered medium, and effect sizes of 0.8 were considered large.29 Risk ratios less than 1 indicate that a specific adverse categorical outcome (all-cause discontinuation, hospitalization, and relapse) occurred less frequently in EIS, and RRs greater than 1 indicate that a desired categorical outcome (remission, recovery, and involvement in school or work) occurred more frequently in EIS. For categorical outcomes, numbers needed to treat (NNTs) were calculated by dividing 1 by the absolute risk difference. Numbers needed to treat of 10 or less were considered clinically relevant.30 We explored study heterogeneity using the χ2 test of homogeneity and I2 statistics, with P < .05 and I2>50%, respectively, indicating significant heterogeneity. All analyses were 2-tailed with an α of .05.
In the primary analyses, EIS and TAU were compared at study end point. We conducted subgroup and exploratory maximum likelihood random-effects meta-regression analyses of the coprimary outcomes and the 3 key secondary outcomes to identify potential moderators or mediators (eMethods 4 in the Supplement). To allow comparability of studies using the Brief Psychiatric Rating Scale (BPRS) and the Positive and Negative Syndrome Scale (PANSS) in the meta-regression, we converted the baseline BPRS scores to PANSS scores using an established method.31 Treatment intensity was defined as the number of therapeutic interventions per month.
The following post hoc sensitivity analyses were added. First, meta-regression analyses were performed to investigate the potential influence of the overall attrition rate and between-group attrition difference on the coprimary outcomes and the 3 key secondary outcomes (eMethods 5 in the Supplement). Second, a subgroup analysis was performed that excluded 2 studies20,22 from Mexico because their effect sizes were particularly large.
Finally, we inspected funnel plots. The regression test by Egger et al32 and the trim-and-fill method by Duval and Tweedie33 were used to examine the presence of publication bias.
Results
Search
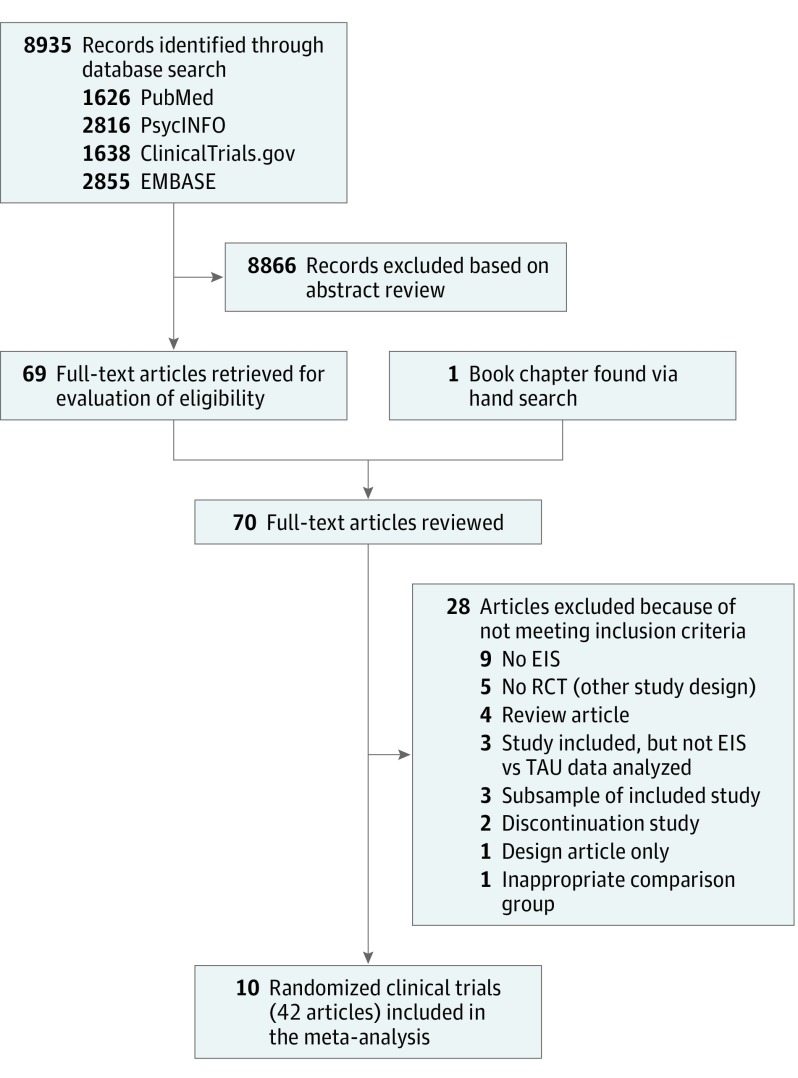
The initial search identified 8935 records, and study selection procedures yielded 41 articles and 1 book chapter reporting on 10 meta-analyzable EIS trials (Figure 1 and eTable 1 in the Supplement). All studies were published, but unpublished data were obtained from all 10 studies to be included in the meta-analysis. Study authors either shared their original data set or reanalyzed the data as needed. Across the 10 RCTs, the mean (SD) trial duration was 16.2 (7.4) months (range, 9-24 months). Among 2176 total patients, the mean (SD) age was 27.5 (4.6) years, and 1355 (62.3%) were male.
Figure 1. PRISMA Diagram of Included and Excluded Studies.
EIS indicates early intervention services; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized clinical trial; and TAU, treatment as usual.
Study, Patient, and Treatment Characteristics
Altogether, 10 studies9,18,19,20,21,22,34,35,36,37 (n = 2176 patients) were included. Patients had a mean (SD) baseline PANSS-converted BPRS score of 72.8 (11.7) (9 studies), a mean (SD) total illness duration (defined as the interval between the onset of positive psychotic symptoms and the study entry) of 159.8 (125.4) weeks (6 studies), and a mean (SD) duration of untreated psychosis (DUP) (defined as the interval between the onset of positive psychotic symptoms and the first antipsychotic treatment) of 79.9 (71.1) weeks (5 studies). All EIS programs were team-based, multicomponent interventions, which included a mean (SD) of 4.8 (0.9) components (range, 4-6 components). All EIS interventions included psychopharmacological treatment by a licensed and qualified prescriber (with steady medication review and monitoring) and family psychoeducation and counseling. Other common components were cognitive behavior therapy (7 studies), family therapy (7 studies), vocational and educational counseling (5 studies), social skills training (5 studies), and crisis response team and crisis management (4 studies). Fidelity of EIS intervention was assessed in all studies via treatment and team supervision. Rating scales to measure fidelity were used in 6 studies, confirming medium to high fidelity in the 3 studies that reported measurement-based outcomes on the fidelity testing (more details are listed in Table 1, eTable 2, and eTable 3 and in the Supplement).
Table 1. Study, Patient and Treatment Characteristics.
| Characteristic | Study Name | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| COAST34 | JCEP23 | LEO35 | OPUS36 | OTP37 | PIANO18 | RAISE-ETP9 | STEP19 | Valencia 12 Months20 | Valencia 6 Months22 | |
| Study Characteristics | ||||||||||
| Duration, mo | 9 | 24 | 18 | 24 | 24 | 9 | 24 | 12 | 12 | 6 |
| No. of sites, location | 1, South London/UK | 1, Hong Kong | 1, London/UK | 5, Denmark | 1, Norway | 117, Italy | 34, US | 1, US | 1, Mexico | 1, Mexico |
| No. at baseline (EIS/TAU) | 59 (32/27) |
200 (100/100) |
144 (71/73) |
547 (275/272) |
50 (30/20) |
444 (272/172) |
404 (223/181) |
117 (60/57) |
88 (44/44) |
120 (60/60) |
| Inclusion criteria | 1st Episode of any functional psychosis in past 5 y of contact | FEP according to DSM IV | ≤2 Episodes of nonaffective PSY; SCZ; SzT; DEL | 1st Episode SCZ spectrum d/o including DEL and SzT; ≤12 wk AP medication | Recent-onset SCZ (≤2 y since FEP), with more than 1 acute episode | 1st Lifetime contact with the center for any functional PSY | FEP (SCZ-spectrum disorder; psychosis NOS, brief psychosis), ≤6 mo AP medication | FEP (nonaffective); ≤5 y ago; ≤12 wk AP medication | FEP (SCZ); stable after first AP medication (≥15 d); no substance abuse | FEP (SCZ); stable after first AP medication (≥15 d); no substance abuse |
| Early intervention services components | Medication review; vocational/ educational counseling; CBT; family PE/counseling; family therapy; crisis response team/crisis management | Medication review; vocational/ educational counseling; CBT; family PE/counseling; crisis response team/crisis management; SST | Medication review; vocational/ educational counseling; CBT; family PE/counseling | Medication review; family PE/counseling; family therapy; crisis response team/crisis management; SST | Medication review; CBT; family PE/counseling; family therapy; crisis response team/crisis management; SST | Medication review; CBT; family PE/counseling; family therapy | Medication review; vocational/ educational counseling; CBT; family PE/counseling | Medication review; vocational/ educational counseling; CBT; family PE/counseling; family therapy | Medication review; family PE/counseling; family therapy; SST | Medication review; family PE/counseling; family therapy; SST |
| Patient Characteristics | ||||||||||
| Age, mean (SD) [range] | 28 (8) [8-65] |
36.6 (8.7) [26-55] |
26.3 (6.2) [16-40] |
26.6 (6.4) [18-45] |
25.4 (4.6) [18-35] |
30.2 (9.6) [18-54] |
23.1 (5.1) [16-45] |
22.5 (4.9) [16-45] |
24.3 (3.1) [16-50] |
26.8 (5.0) [16-50] |
| Male, % | 75 | 43 | 65 | 59 | 62 | 59 | 73 | 98 | 75 | 66 |
| Illness Characteristics | ||||||||||
| Diagnosis, % | SCZ/SzA: 83.1; BP: 12.5; substance-induced PSY: 1.0 | SCZ: 44.0; SzF: 17.0; brief PSY: 12.0; PSY NOS: 6.0; SzA: 1.0 | SCZ: 69.4 | SCZ: 66.2; SzT: 14.4; brief PSY: 8.2; SzA: 4.6; DEL: 4.6; PSY NOS: 2.0 | SCZ: 80; SzA: 12; SzF: 8 | SCZ: 27; brief PSY: 18; DEL: 16; mania with PSY: 13; MDD with PSY: 9; SzA: 9; PSY NOS: 6; SzT: 2 | SCZ: 52.9; SzF: 16.6; SzA DEP: 14.1; PSY NOS: 9.9; SzA BP: 5.9; brief PSY: 0.5 | SCZ/SzA: 29.0 | SCZ: 100.0 | SCZ: 100.0 |
| DUP, mean (median), wk | NR | 73.6 (13.3) | 9.1 (16.0) | NR | NR | 45.2 (8.0) | 193.5 (74.0) | 43.9 (12.0) | NR | NR |
Abbreviations: AP, antipsychotic; BP, bipolar; CBT, cognitive behavioral therapy; COAST, Croydon Outreach and Assertive Support Team; DEL, delusional disorder; DUP, duration of untreated psychosis; EIS, early intervention services; FEP, first episode of psychosis; JCEP, Jockey Club Early Psychosis; LEO, Lambeth Early Onset; MDD, major depressive disorder; NOS, not otherwise specified; NR, not reported; OPUS, specialized assertive intervention; OTP, Optimal Treatment Project; PE, psychoeducation; PIANO, Psychosis: Early Intervention and Assessment of Needs and Outcome; PSY, psychosis; RAISE-ETP, Recovery After an Initial Schizophrenia Episode–Early Treatment Program; SCZ, schizophrenia; SST, social skills training; STEP, Specialized Treatment Early in Psychosis; SzA, schizoaffective; SzA BP, schizoaffective bipolar; SzA DEP, SzA depressive; SzF, schizophreniform disorder; SzT, schizotypal disorder; TAU, treatment as usual.
There was a 2-fold higher treatment intensity in EIS vs TAU (4 studies; ratio of EIS to TAU, 2.14; range, 1.36-3.00). The mean (SD) number of low-risk ratings across the 7 domains of the Cochrane Collaboration’s tool for assessing risk of bias (higher numbers are better) was 5.2 (0.9) (range, 4-7), indicating an overall low risk of bias. Masking of participants and personnel was rated as high risk in all but 3 studies because knowledge of the treatment received is almost inevitable with these types of setting- and service-based interventions (eTable 4 and eTable 5 in the Supplement).
All-Cause Treatment Discontinuation
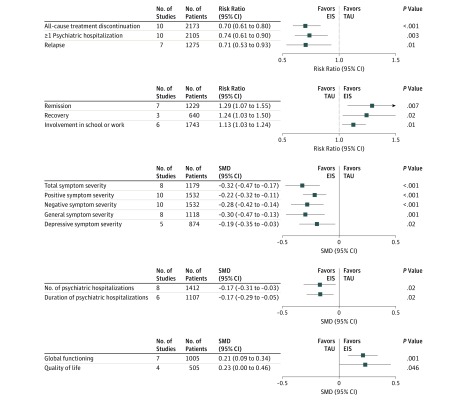
All-cause treatment discontinuation was significantly lower with EIS than with TAU (21.3% vs 31.3%) in 10 studies among 2173 patients (RR, 0.70; 95% CI, 0.61-0.80; P < .001; NNT, 12.4; 95% CI, 7.3-40.5; P = .005). The regression test by Egger et al32 did not indicate publication bias (Figure 2 and eFigure 1 and eFigure 2 in the Supplement).
Figure 2. Summary of Pooled Results.
EIS indicates early intervention services; SMD, standardized mean difference; and TAU, treatment as usual.
Effect sizes did not differ statistically between any analyzed subgroups (eTable 6 in the Supplement). Higher baseline PANSS negative scores were associated with even less treatment discontinuation in EIS (coefficient, −0.08; 95% CI, −0.15 to −0.01; P = .03).
At Least 1 Psychiatric Hospitalization
Risk of at least 1 psychiatric hospitalization in 10 studies among 2105 patients was significantly lower with EIS than TAU (32.3% vs 42.4%) (RR, 0.74; 95% CI, 0.61-0.90; P = .003; NNT, 10.1; 95% CI, 6.4-23.9; P = .001). The regression test by Egger et al32 indicated potential publication bias. After statistical adjustment for 5 potentially missing studies using the trim-and-fill method by Duval and Tweedie,33 the RR increased to 0.87 (95% CI, 0.71-1.07) (Figure 2 and eFigure 3 and eFigure 4 in the Supplement).
In subgroup analyses, a significant between-subgroup difference was only found regarding presence or absence of fidelity monitoring. Studies that included fidelity monitoring had comparatively fewer hospitalizations with EIS than with TAU (RR, 0.88 vs 0.50; P = .001). In meta-regression analyses, larger study sample size was associated with lower hospitalization risk (coefficient, 0.001; 95% CI, 0.000-0.002; P = .002) (eTable 6 in the Supplement).
The number of psychiatric hospitalizations (mean [SD], 0.41 [0.30] for EIS and 0.59 [1.11] for TAU) and the number of bed-days during treatment (mean [SD], 21.20 [48.94] for EIS and 30.41 [61.05] for TAU) were significantly lower in EIS than in TAU. The SMD for hospitalizations was −0.17 (95% CI, −0.31 to −0.03; P = .02), and the SMD for bed-days was −0.17 (95% CI, −0.29 to −0.05; P = .006). These results are shown in eFigure 5 and eFigure 6 in the Supplement.
Total and Specific Symptom Severity
Total symptom severity improvement in 8 studies among 1179 patients was significantly greater in EIS than in TAU (SMD, −0.32; 95% CI, −0.47 to −0.17; P < .001). Effect sizes did not differ statistically between any analyzed subgroups. However, in meta-regression analyses of continuous variables, younger age, male sex, higher baseline symptom severity (PANSS total, PANSS negative, and PANSS positive), and percentage of patients with schizophrenia were each associated with larger advantages for EIS (Figure 2 and eTable 7 and eFigure 7 in the Supplement).
Superiority of EIS was supported by analysis of positive symptom severity (SMD, −0.22; 95% CI, −0.32 to −0.11; P < .001), negative symptom severity (SMD, −0.28; 95% CI, −0.42 to −0.14; P < .001), general symptom severity (SMD, −0.30; 95% CI, −0.47 to −0.13; P = .001), and depressive symptom severity (SMD, −0.19; 95% CI, −0.35 to −0.03; P = .02). These results are shown in Figure 2 and eFigures 8, 9, 10, and 11 in the Supplement.
Relapse, Remission, and Recovery
Relapse rates in 7 studies among 1275 patients were significantly lower in EIS than in TAU (19.6% [141 of 719] vs 29.1% [162 of 556]) (RR, 0.71; 95% CI, 0.53-0.93; P = .01; NNT, 10.0; 95% CI, 5.5-54.0; P = .02). Patients in EIS more often achieved study-defined remission in 7 studies among 1229 patients (57.3% vs 50.7%) (RR, 1.29; 95% CI, 1.07-1.55; P = .007; NNT, 5.7; 95% CI, 3.3-20.4; P = .006) and recovery in 3 studies among 640 patients (30.3% vs 27.6%) (RR, 1.24; 95% CI, 1.03-1.50; P = .02; NNT, 13.9; 95% CI, 5.6-27.5; P = .19) (eFigures 12, 13, and 14 in the Supplement).
Global Functioning and Involvement in School or Work
Global functioning in 7 studies among 1005 patients improved significantly more in EIS than in TAU (SMD, 0.21; 95% CI, 0.09-0.34; P = .001). The proportion of patients in school or employed in 6 studies among 1743 patients was significantly higher with EIS than with TAU (52.5% vs 45.3%) (RR, 1.13; 95% CI, 1.03-1.24; P = .01; NNT, 17.8; 95% CI, 9.8-100.0; P = .02) (Figure 2 and eTable 7, eFigure 15, and eFigure 16 in the Supplement). Effect sizes did not statistically differ in any subgroup analysis. In the meta-regression analyses, no significant moderators were identified (eTable 7 in the Supplement).
Quality of Life
Quality of life in 4 studies among 505 patients was significantly higher with EIS than with TAU (SMD, 0.23; 95% CI, 0.00-0.46; P = .046). Detailed results are shown in eFigure 17 in the Supplement.
Time Point Analyses
Superiority of EIS was consistent across almost all time points (6, 9-12, and 18-24 months of treatment). The exceptions were at 18 to 24 months for general symptom severity in 3 studies among 489 patients (SMD, −0.14; 95% CI, −0.32 to 0.04; P = .12) and for depressive symptom severity in 3 studies among 474 patients (SMD, −0.21; 95% CI, −0.51 to 0.08; P = .16) (Table 2).
Table 2. Outcomes at End Point and at Different Time Pointsa.
| Variable | Baseline to End Point | Short-term (6 mo) | Medium-term (9-12 mo) | Long-term (18-24 mo) | Difference End Point vs 18-24 mo, P Value | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies (No. of Patients) | SMD (95% CI) | Result P Value | Heterogeneity | No. of Studies (No. of Patients) | SMD (95% CI) | Result P Value | Heterogeneity | No. of Patients (No. of Studies) | SMD (95% CI) | Result P Value | Heterogeneity | No. of Studies (No. of Patients) | SMD (95% CI) | Result P Value | Heterogeneity | ||||||
| P Value | I2 | P Value | I2 | P Value | I2 | P Value | I2 | ||||||||||||||
| Total symptom severity | 8 (1179) | −0.322 (−0.474 to −0.170) |
<.001 | .18 | 31.7 | 4 (671) | −0.447 (−0.672 to −0.223) |
<.001 | .14 | 44.8 | 8 (1179) | −0.322 (−0.474 to −0.170) |
<.001 | .18 | 31.7 | 4 (559) | −0.210 (−0.390 to −0.031) |
.02 | .35 | 8.1 | .42 |
| Positive symptom severity | 10 (1532) | −0.215 (−0.318 to −0.113) |
<.001 | .43 | 0.5 | 5 (695) | −0.306 (−0.497 to −0.116) |
.002 | .25 | 25.6 | 10 (1532) | −0.215 (−0.318 to −0.113) |
<.001 | .43 | 0.5 | 5 (900) | −0.148 (−0.281 to −0.015) |
.03 | .90 | 0.0 | .43 |
| Negative symptom severity | 10 (1532) | −0.280 (−0.424 to −0.137) |
<.001 | .10 | 38.4 | 5 (695) | −0.333 (−0.527 to −0.139) |
.001 | .24 | 27.4 | 10 (1532) | −0.280 (−0.424 to −0.137) |
<.001 | .10 | 38.4 | 5 (899) | −0.245 (−0.385 to −0.105) |
.001 | .37 | 6.7 | .73 |
| General symptom severity | 8 (1118) | −0.297 (−0.468 to −0.127) |
.001 | .11 | 40.2 | 5 (695) | −0.340 (−0.501 to −0.178) |
<.001 | .37 | 7.1 | 8 (1118) | −0.297 (−0.468 to −0.127) |
.001 | .11 | 40.2 | 3 (489) | −0.143 (−0.324 to 0.037) |
.12 | .43 | 0.0 | .30 |
| Depressive symptom severity | 5 (874) | −0.193 (−0.351 to −0.034) |
.02 | .30 | 17.9 | 3 (511) | −0.276 (−0.451 to −0.101) |
.002 | .58 | 0.0 | 5 (874) | −0.193 (−0.351 to −0.034) |
.02 | .30 | 17.9 | 3 (474) | −0.212 (−0.509 to 0.084) |
.16 | .09 | 58.5 | .10 |
| Global functioningb | 7 (1005) | 0.210 (0.085 to 0.336) |
.001 | .59 | 0 | 3 (286) | 0.307 (0.026 to 0.589) |
.03 | .30 | 16.8 | 7 (1005) | 0.210 (0.085 to 0.336) |
.001 | .59 | 0 | 4 (708) | 0.244 (0.095 to 0.393) |
.001 | .65 | 0 | .73 |
| Quality of life | 4 (505) | 0.230 (0.004 to 0.456) |
.046 | .21 | 34.1 | 3 (544) | 0.193 (0.024 to 0.363) |
.02 | .51 | 0 | 2 (295) | 0.282 (0.050 to 0.515) |
.02 | .73 | 0 | 3 (437) | 0.269 (0.497 to 0.041) |
.02 | .26 | 26.9 | .97 |
Abbreviation: SMD, standardized mean difference.
Negative SMD favored early intervention services when smaller values are better (psychopathology), and positive SMD favored early intervention services when larger values are better (global functioning and quality of life).
Sensitivity Analyses
In the post hoc meta-regression analysis, the overall attrition rate and between-group attrition difference did not mediate any of the outcomes (eTable 6 and eTable 7 in the Supplement). The results of the post hoc subgroup analyses that excluded the 2 studies from Mexico essentially confirmed the findings of the entire available data set (eTable 8 in the Supplement).
Discussion
In this comprehensive meta-analysis of 10 RCTs (n = 2176), 6 to 24 months of EIS that consisted of 4 to 6 evidence-based38 coordinated and integrated treatment components was associated with superior outcomes compared with TAU regarding all meta-analyzable outcomes. Our meta-analysis demonstrates that EIS programs, all of which comprised antipsychotic treatment and various psychosocial interventions, are associated with significant superiority to TAU across a wide range of clinically relevant outcomes, including hospitalization risk, bed-days, symptoms, and global functioning. The mean effect sizes were small for continuous outcomes, ranging from an SMD of −0.19 for depression symptom severity (for which patients were not selected) and an SMD of 0.21 for global functioning to an SMD of −0.32 for total symptom severity. Effect sizes were small to medium for categorical outcomes. For example, compared with TAU, participants in EIS had a 12.6% greater likelihood of being in school or employed (NNT, 17.8) and improved by 24% to 30% more than with TAU on other outcomes, such as remission (NNT, 5.7), relapse prevention (NNT, 10.0), hospitalization (NNT, 10.1), treatment engagement (NNT, 12.4), and recovery (NNT, 13.9).
The I2 statistics describe the percentage of variation across studies that is due to heterogeneity rather than chance, all of which were less than 50% except for remission, for which I2 was 68.9%, suggesting low outcome heterogeneity across studies. However, the respective 95% CIs imply some relevant heterogeneity of the treatment effect across the study populations, indicating that sources for this heterogeneity need to be identified that could help detect patient subgroups requiring a dynamic adaptation of EIS in terms of the intensity and duration of individual or combined EIS components.
Subgroup and meta-regression analyses were exploratory owing to the small number of studies. However, the results indicated robust findings across various potential sources of heterogeneity, such as study quality, observed case analyses, and lack of masking.
In the Recovery After an Initial Schizophrenia Episode–Early Treatment Program (RAISE-ETP) study,9 DUP less than 74 weeks (ie, the median) significantly increased effect sizes for the primary outcome of subjective quality of life from 0.31 to 0.54 and for total symptoms from −0.29 to −0.42. In contrast, in our meta-regression analysis, DUP did not significantly moderate the effectiveness of EIS. However, DUP data (provided by only 5 trials) varied greatly (mean, 9-194 weeks; median, 8-74 weeks). Only patient-level analyses can shed more light on the effect of DUP on the efficacy of EIS.
Superior involvement in school or work and global functioning were associated only with provision of vocational intervention and family therapy, respectively. These findings suggest that family involvement might independently improve symptomatic and functional outcomes, whereas educational or vocational rehabilitation succeeded in improving involvement in school and work. Both of these results should be investigated further.
The consistent significant advantage of EIS at the end of the intervention period in RCTs raises the question of generalizability of the findings to patients not captured in RCTs and of durability of the effects. Consistent with the treatment results in the meta-analyzed RCTs, the findings of a naturalistic, 10-year follow-up study39 in Hong Kong using a matched historical control (n = 296) suggested that 2 years of EIS significantly reduced suicides and suicide attempts and resulted in fewer admissions (odds ratio [OR], 1.56; P < .001), shorter hospitalizations (OR, 1.29; P = .04), and longer employment tenure (OR, −0.28; P < .001). However, no differences emerged in psychotic symptoms, symptomatic remission, and functional recovery. In the German, naturalistic Integrated Care in Early Psychosis study40 of EIS that included an early detection program, EIS (n = 120) was associated with better outcomes than a historical control (n = 105) at 1 year regarding remission, psychotic psychopathology, and global functioning. Although these superior findings may be driven by the addition of an early detection program, within the treated cohort, DUP did not appear to be predictive of the superior psychopathological and functional outcomes. However, as mentioned above, DUP was only reported in half of the studies and had a very heterogeneous distribution (weighted mean [SD], 79.9 [71.1] weeks), limiting the informative value of meta-regression analyses. Nevertheless, the addition of early detection elements as part of EIS that aim at reducing DUP could be considered in future studies. Moreover, studies should also include more minors to better represent the clinical sample of patients with early-phase psychosis.
Targeting the question of whether an extended duration of EIS would be superior to shorter intervention periods, an uncontrolled EIS study41 reported that gains made at year 2 could be sustained or increased with EIS at a lower level for another 3 years. Two studies that randomized patients after 2 years of EIS to either extended EIS by 3 years vs TAU found beneficial effects at 5 years. The first study42 found significant superiority of extended EIS for adherence, work alliance, and patient satisfaction, while the treatment effects remained stable in both groups. The second study43 found a significant effect of EIS on the treatment duration and both positive and negative symptom remission. The treatment duration and treatment intensity had an independent effect on positive and negative symptoms and total symptoms, respectively. Another RCT44 (n = 160) comparing extended EIS vs step-down treatment (even more intense than TAU) after 2 years of EIS indicated significant superiority of the 12-month extended EIS for several outcomes, such as negative and depressive symptoms, general psychopathology, global functioning, independent living skills, and work productivity. However, the fact that, in this study,44 only 20% of patients achieved functional recovery (which included competitive employment) at year 3, as well as that supported employment and educational intervention outside of the context of EIS was successful in increasing vocational or educational attainment in first-episode psychosis,45,46 suggests the need for greater focus on functional reintegration and employment or education.
Future research should focus on a better understanding of the sources of heterogeneity in treatment response. The research should seek to identify patient characteristics that determine the magnitude of gains overall and from individual EIS components, as well as their respective intensity and duration to achieve the targeted outcomes in the short term and in the long term. To allow comparability of the effects, EIS research studies and real-world programs should adhere to a set of treatment standards, although adaptations of EIS based on country and health care systems might be needed.19 To allow meaningful comparisons of EIS across implementation sites and systems that will likely vary in their ability to improve different outcomes (probably reflecting their focus, priorities, and resources), EIS initiatives should also be oriented to a standard set of stakeholder-relevant outcomes (similar to the National Institute of Mental Health Early Psychosis Intervention Network47). In addition, information on the cost-effectiveness of specific EIS packages across variable treatment settings is needed.45,46
Given that schizophrenia is one of the disorders most associated with personal distress and societal cost,1 sustaining gains achieved by EIS could be cost-effective.48 Therefore, additional trials are needed that study different EIS extension vs step-down procedures for patient subgroups that can move between these options based on identified needs. Such research is especially relevant because data from 3 programs suggest that 2 to 3 years,49,50 5 years,49 7 to 8 years,51 or 10 years52 after the discontinuation of EIS, the prior gains may largely be lost.
Limitations
Several limitations of this meta-analysis need to considered. Although we included 10 studies and 2176 patients (6 trials and 1554 patients more than the prior meta-analysis10), the numbers of trials and participants were modest, limiting the informative value of analyses regarding possible publication bias as well as subgroup and meta-regression analyses. Moreover, patient and treatment characteristics were heterogeneous in several dimensions, complicating the interpretation of independent effects of specific moderators and mediators, including the important variable of DUP, which is associated with the prognosis of psychosis in general and which also significantly moderated EIS outcomes.9,53 In addition, the results for relapse, symptomatic remission, and recovery could be influenced by heterogeneous outcome definitions, although the same definitions were used for EIS and TAU in each individual study.
Furthermore, although 3 studies did not use masked assessors, this variable did not significantly moderate EIS superiority. Moreover, the only outcome potentially related to the variable of fidelity monitoring was a lower number of hospitalizations in EIS vs TAU in studies that reported fidelity monitoring outcomes.
Conversely, the TAU condition delivered by centers involved in an RCT may have consisted of care that is better and more comprehensive than real-world TAU, as indicated by the number of treatment elements and treatment intensity in TAU. If correct, this factor would have lowered effect sizes. Because the active treatment duration ranged from 6 to 24 months, we cannot comment on the efficacy of longer-term EIS interventions. Furthermore, the observed effect sizes were small to medium, and meta-analyzable cost-effectiveness data across variable settings and health care systems were lacking. Although each EIS program studied used 4 to 6 similar, evidence-based intervention components, differences in the choice and delivery of each component and in country-specific and setting-specific TAU conditions could be relevant. In addition, because each EIS program used 4 to 6 components, it was impossible to tease apart the effect of individual combinations of EIS elements. Finally, adherence to each of the treatments may have differed but was insufficiently reported.
Conclusions
Based on the results from this comprehensive meta-analysis, EIS was associated with better outcomes than TAU across many sources of variability. These findings should provide further impetus for the widespread implementation and funding of EIS in the United States and across the world, as has already begun.54,55,56,57,58,59,60
eMethods 1. Study Protocol
eMethods 2. Search Terms
eMethods 3. Overview Outcome Definitions and Scales
eMethods 4. Subgroup and Meta-Regression Analyses
eMethods 5. Post Hoc Sensitivity Analyses
eTable 1. Included Studies and Papers
eTable 2. Study, Patient, and Treatment Characteristics
eTable 3. Detailed Information on EIS Interventions and Standard Treatment Comparator
eTable 4. Risk of Bias Summary Table
eTable 5. Risk of Bias for Single Studies
eTable 6. Co-Primary Outcomes: Overall Results, Subgroup Analyses, and Meta-Regression
eTable 7. Key Secondary: Overall Results, Subgroup Analyses, and Meta-Regression
eTable 8. Sensitivity Subgroup Analysis (Excluding Two Studies From Mexico)
eFigure 1. Forest Plot: All-Cause Treatment Discontinuation
eFigure 2. Funnel Plot: All-Cause Treatment Discontinuation
eFigure 3. Forest Plot: Hospitalization
eFigure 4. Funnel Plot: Hospitalization
eFigure 5. Forest Plot: Mean Number of Hospital Admissions
eFigure 6. Forest Plot: Hospital Bed Days
eFigure 7. Forest Plot: Total Symptom Improvement
eFigure 8. Forest Plot: Positive Symptom Improvement
eFigure 9. Forest Plot: Negative Symptom Improvement
eFigure 10. Forest Plot: General Symptom Improvement
eFigure 11. Forest Plot: Depressive Symptom Improvement
eFigure 12. Forest Plot: Relapse
eFigure 13. Forest Plot: Remission
eFigure 14. Forest Plot: Recovery
eFigure 15. Forest Plot: Functioning
eFigure 16. Forest Plot: Involvement in School or Work
eFigure 17. Forest Plot: Quality of Life
References
References
- 1.Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon JA, Vos T, Hogan DR, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2129-2143. doi: 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jääskeläinen E, Juola P, Hirvonen N, et al. A systematic review and meta-analysis of recovery in schizophrenia. Schizophr Bull. 2013;39(6):1296-1306. doi: 10.1093/schbul/sbs130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 6.Nordentoft M, Wahlbeck K, Hällgren J, et al. Excess mortality, causes of death and life expectancy in 270,770 patients with recent onset of mental disorders in Denmark, Finland and Sweden. PLoS One. 2013;8(1):e55176. doi: 10.1371/journal.pone.0055176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hjorthøj C, Stürup AE, McGrath JJ, Nordentoft M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry. 2017;4(4):295-301. doi: 10.1016/S2215-0366(17)30078-0 [DOI] [PubMed] [Google Scholar]
- 8.Nielsen RE, Uggerby AS, Jensen SO, McGrath JJ. Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades: a Danish nationwide study from 1980 to 2010. Schizophr Res. 2013;146(1-3):22-27. doi: 10.1016/j.schres.2013.02.025 [DOI] [PubMed] [Google Scholar]
- 9.Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordentoft M, Rasmussen JØ, Melau M, Hjorthøj CR, Thorup AA. How successful are first episode programs? a review of the evidence for specialized assertive early intervention. Curr Opin Psychiatry. 2014;27(3):167-172. doi: 10.1097/YCO.0000000000000052 [DOI] [PubMed] [Google Scholar]
- 11.Chang WC, Chan GH, Jim OT, et al. Optimal duration of an early intervention programme for first-episode psychosis: randomised controlled trial. Br J Psychiatry. 2015;206(6):492-500. [DOI] [PubMed] [Google Scholar]
- 12.Birchwood M, Lester H, McCarthy L, et al. The UK national evaluation of the development and impact of Early Intervention Services (the National EDEN studies): study rationale, design and baseline characteristics. Early Interv Psychiatry. 2014;8(1):59-67. doi: 10.1111/eip.12007 [DOI] [PubMed] [Google Scholar]
- 13.Verma S, Poon LY, Subramaniam M, Abdin E, Chong SA. The Singapore Early Psychosis Intervention Programme (EPIP): a programme evaluation. Asian J Psychiatr. 2012;5(1):63-67. doi: 10.1016/j.ajp.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen P, Nordentoft M, Abel MB, Gouliaev G, Jeppesen P, Kassow P. Early detection and assertive community treatment of young psychotics: the OPUS study rationale and design of the trial. Soc Psychiatry Psychiatr Epidemiol. 2000;35(7):283-287. [DOI] [PubMed] [Google Scholar]
- 15.Lambert M, Schöttle D, Sengutta M, et al. Early detection and integrated care in adolescents and young adults with severe psychotic illnesses [in German]. Psychiatr Prax. 2015;42(suppl 1):S49-S53. doi: 10.1055/s-0034-1387652 [DOI] [PubMed] [Google Scholar]
- 16.Birchwood M, Todd P, Jackson C. Early intervention in psychosis: the critical period hypothesis. Br J Psychiatry Suppl. 1998;172(33):53-59. [PubMed] [Google Scholar]
- 17.Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. Early intervention services, cognitive-behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry. 2010;197(5):350-356. doi: 10.1192/bjp.bp.109.074526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggeri M, Bonetto C, Lasalvia A, et al. ; GET UP Group . Feasibility and effectiveness of a multi-element psychosocial intervention for first-episode psychosis: results from the cluster-randomized controlled GET UP PIANO trial in a catchment area of 10 million inhabitants. Schizophr Bull. 2015;41(5):1192-1203. doi: 10.1093/schbul/sbv058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srihari VH, Tek C, Kucukgoncu S, et al. First-episode services for psychotic disorders in the U.S. public sector: a pragmatic randomized controlled trial. Psychiatr Serv. 2015;66(7):705-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valencia M, Juarez F, Ortega H. Integrated treatment to achieve functional recovery for first-episode psychosis. Schizophr Res Treatment. 2012;2012:962371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui CL, Lau WW, Leung CM, et al. Clinical and social correlates of duration of untreated psychosis among adult-onset psychosis in Hong Kong Chinese: the JCEP study. Early Interv Psychiatry. 2015;9(2):118-125. doi: 10.1111/eip.12094 [DOI] [PubMed] [Google Scholar]
- 22.Valencia M, Juarez F, Delgado M, Díaz A Early intervention to improve clinical and functional outcome in patients with first episode-psychosis. https://www.iconceptpress.com/book/mental-disorder/11000123/.../1305000979.pdf. Accessed June 6, 2017.
- 23.Hui CL, Chang WC, Chan SK, et al. Early intervention and evaluation for adult-onset psychosis: the JCEP study rationale and design. Early Interv Psychiatry. 2014;8(3):261-268. doi: 10.1111/eip.12034 [DOI] [PubMed] [Google Scholar]
- 24.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008-2012. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. A power primer. Psychol Bull. 1992;112(1):155-159. [DOI] [PubMed] [Google Scholar]
- 30.Citrome L. Compelling or irrelevant? using number needed to treat can help decide. Acta Psychiatr Scand. 2008;117(6):412-419. doi: 10.1111/j.1600-0447.2008.01194.x [DOI] [PubMed] [Google Scholar]
- 31.Leucht S, Rothe P, Davis JM, Engel RR. Equipercentile linking of the BPRS and the PANSS. Eur Neuropsychopharmacol. 2013;23(8):956-959. doi: 10.1016/j.euroneuro.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89-98. doi: 10.2307/2669529 [DOI] [Google Scholar]
- 34.Kuipers E, Holloway F, Rabe-Hesketh S, Tennakoon L; Croydon Outreach and Assertive Support Team (COAST) . An RCT of early intervention in psychosis: Croydon Outreach and Assertive Support Team (COAST). Soc Psychiatry Psychiatr Epidemiol. 2004;39(5):358-363. doi: 10.1007/s00127-004-0754-4 [DOI] [PubMed] [Google Scholar]
- 35.Craig TK, Garety P, Power P, et al. The Lambeth Early Onset (LEO) Team: randomised controlled trial of the effectiveness of specialised care for early psychosis. BMJ. 2004;329(7474):1067. doi: 10.1136/bmj.38246.594873.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen L, Jeppesen P, Thorup A, et al. A randomised multicentre trial of integrated versus standard treatment for patients with a first episode of psychotic illness [published correction appears in BMJ. 2005;331(7524):1065]. BMJ. 2005;331(7517):602. doi: 10.1136/bmj.38565.415000.E01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grawe RW, Falloon IR, Widen JH, Skogvoll E. Two years of continued early treatment for recent-onset schizophrenia: a randomised controlled study. Acta Psychiatr Scand. 2006;114(5):328-336. doi: 10.1111/j.1600-0447.2006.00799.x [DOI] [PubMed] [Google Scholar]
- 38.Addington DE, McKenzie E, Norman R, Wang J, Bond GR. Essential evidence-based components of first-episode psychosis services. Psychiatr Serv. 2013;64(5):452-457. doi: 10.1176/appi.ps.201200156 [DOI] [PubMed] [Google Scholar]
- 39.Chan SK, So HC, Hui CL, et al. 10-Year outcome study of an early intervention program for psychosis compared with standard care service. Psychol Med. 2015;45(6):1181-1193. doi: 10.1017/S0033291714002220 [DOI] [PubMed] [Google Scholar]
- 40.Lambert M, Schöttle D, Ruppelt F, et al. Early detection and integrated care for adolescents and young adults with psychotic disorders: the ACCESS III study. Acta Psychiatr Scand. 2017;136(2):188-200. [DOI] [PubMed] [Google Scholar]
- 41.Norman RM, Manchanda R, Malla AK, Windell D, Harricharan R, Northcott S. Symptom and functional outcomes for a 5 year early intervention program for psychoses. Schizophr Res. 2011;129(2-3):111-115. doi: 10.1016/j.schres.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 42.Albert N, Melau M, Jensen H, et al. Five years of specialised early intervention versus two years of specialised early intervention followed by three years of standard treatment for patients with a first episode psychosis: randomised, superiority, parallel group trial in Denmark (OPUS II). BMJ. 2017;356:i6681. doi: 10.1136/bmj.i6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malla A, Joober R, Iyer S, et al. Comparing three-year extension of early intervention service to regular care following two years of early intervention service in first-episode psychosis: a randomized single blind clinical trial. World Psychiatry. 2017;16(3):278-286. doi: 10.1002/wps.20456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang WC, Kwong VW, Chan GH, et al. Prediction of functional remission in first-episode psychosis: 12-month follow-up of the randomized-controlled trial on extended early intervention in Hong Kong. Schizophr Res. 2016;173(1-2):79-83. doi: 10.1016/j.schres.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 45.Nuechterlein KH, Subotnik KL, Turner LR, Ventura J, Becker DR, Drake RE. Individual placement and support for individuals with recent-onset schizophrenia: integrating supported education and supported employment. Psychiatr Rehabil J. 2008;31(4):340-349. doi: 10.2975/31.4.2008.340.349 [DOI] [PubMed] [Google Scholar]
- 46.Killackey E, Jackson HJ, McGorry PD. Vocational intervention in first-episode psychosis: individual placement and support v. treatment as usual. Br J Psychiatry. 2008;193(2):114-120. [DOI] [PubMed] [Google Scholar]
- 47.National Institute of Mental Health. Early Psychosis Intervention Network (EPINET): a learning healthcare system for early serious mental illness. https://www.nimh.nih.gov/funding/grant-writing-and-application-process/concept-clearances/2015/early-psychosis-intervention-network-epinet-a-learning-healthcare-system-for-early-serious-mental-illness.shtml. Accessed July 15, 2017.
- 48.Rosenheck R, Leslie D, Sint K, et al. Cost-effectiveness of comprehensive, integrated care for first episode psychosis in the NIMH RAISE Early Treatment Program. Schizophr Bull. 2016;42(4):896-906. doi: 10.1093/schbul/sbv224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gafoor R, Nitsch D, McCrone P, et al. Effect of early intervention on 5-year outcome in non-affective psychosis. Br J Psychiatry. 2010;196(5):372-376. doi: 10.1192/bjp.bp.109.066050 [DOI] [PubMed] [Google Scholar]
- 50.Bertelsen M, Jeppesen P, Petersen L, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry. 2008;65(7):762-771. doi: 10.1001/archpsyc.65.7.762 [DOI] [PubMed] [Google Scholar]
- 51.Secher RG, Hjorthøj CR, Austin SF, et al. Ten-year follow-up of the OPUS specialized early intervention trial for patients with a first episode of psychosis. Schizophr Bull. 2015;41(3):617-626. doi: 10.1093/schbul/sbu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sigrúnarson V, Gråwe RW, Morken G. Integrated treatment vs. treatment-as-usual for recent onset schizophrenia; 12 year follow-up on a randomized controlled trial. BMC Psychiatry. 2013;13:200. doi: 10.1186/1471-244X-13-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hegelstad WT, Larsen TK, Auestad B, et al. Long-term follow-up of the TIPS early detection in psychosis study: effects on 10-year outcome. Am J Psychiatry. 2012;169(4):374-380. doi: 10.1176/appi.ajp.2011.11030459 [DOI] [PubMed] [Google Scholar]
- 54.Consolidated Appropriations Act of 2014, HR 3547, 113th Cong. https://www.congress.gov/bill/113th-congress/house-bill/3547. Updated january 17, 2014. Accessed May 28, 2017.
- 55.Nordentoft M, Melau M, Iversen T, et al. From research to practice: how OPUS treatment was accepted and implemented throughout Denmark. Early Interv Psychiatry. 2015;9(2):156-162. doi: 10.1111/eip.12108 [DOI] [PubMed] [Google Scholar]
- 56.Department of Health The NHS Plan: a plan for investment, a plan for reform. http://webarchive.nationalarchives.gov.uk/content/+/http://www.dh.gov.uk/en/publicationsandstatistics/publications/publicationspolicyandguidance/dh_4002960. Published July 1, 2000. Accessed May 28, 2017.
- 57.National Health Service A national service framework for mental health. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/198051/National_Service_Framework_for_Mental_Health.pdf. Published September 1999. Accessed June 21, 2017.
- 58.Department of Health and Social Care. No health without mental health: a cross-government mental health outcomes strategy for people of all ages. https://www.gov.uk/government/publications/no-health-without-mental-health-a-cross-government-mental-health-outcomes-strategy-for-people-of-all-ages-a-call-to-action. Published February 2, 2011. Accessed May 28, 2017.
- 59.Azrin ST, Goldstein AB, Heinssen RK Expansion of coordinated specialty care for first-episode psychosis in the US. https://www.pathwaysrtc.pdx.edu/focal-point-S1603. Accessed June 21, 2017.
- 60.National Institute of Mental Health. Evidence-based treatments for first episode psychosis: components of coordinated specialty care. https://www.nimh.nih.gov/health/topics/schizophrenia/raise/evidence-based-treatments-for-first-episode-psychosis-components-of-coordinated-specialty-care.shtml. Published April 14, 2014. Accessed July 15, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Study Protocol
eMethods 2. Search Terms
eMethods 3. Overview Outcome Definitions and Scales
eMethods 4. Subgroup and Meta-Regression Analyses
eMethods 5. Post Hoc Sensitivity Analyses
eTable 1. Included Studies and Papers
eTable 2. Study, Patient, and Treatment Characteristics
eTable 3. Detailed Information on EIS Interventions and Standard Treatment Comparator
eTable 4. Risk of Bias Summary Table
eTable 5. Risk of Bias for Single Studies
eTable 6. Co-Primary Outcomes: Overall Results, Subgroup Analyses, and Meta-Regression
eTable 7. Key Secondary: Overall Results, Subgroup Analyses, and Meta-Regression
eTable 8. Sensitivity Subgroup Analysis (Excluding Two Studies From Mexico)
eFigure 1. Forest Plot: All-Cause Treatment Discontinuation
eFigure 2. Funnel Plot: All-Cause Treatment Discontinuation
eFigure 3. Forest Plot: Hospitalization
eFigure 4. Funnel Plot: Hospitalization
eFigure 5. Forest Plot: Mean Number of Hospital Admissions
eFigure 6. Forest Plot: Hospital Bed Days
eFigure 7. Forest Plot: Total Symptom Improvement
eFigure 8. Forest Plot: Positive Symptom Improvement
eFigure 9. Forest Plot: Negative Symptom Improvement
eFigure 10. Forest Plot: General Symptom Improvement
eFigure 11. Forest Plot: Depressive Symptom Improvement
eFigure 12. Forest Plot: Relapse
eFigure 13. Forest Plot: Remission
eFigure 14. Forest Plot: Recovery
eFigure 15. Forest Plot: Functioning
eFigure 16. Forest Plot: Involvement in School or Work
eFigure 17. Forest Plot: Quality of Life
References