Key Points
Question
Is prenatal exposure to selective serotonin reuptake inhibitors associated with fetal brain development?
Findings
In this cohort study including 98 infants, significant gray matter volume expansion was noted in the amygdala and insula, as well as an increase in white matter structural connectivity between these same regions in selective serotonin reuptake inhibitor–exposed infants, compared with infants exposed to untreated prenatal maternal depression and healthy controls.
Meaning
In line with prior animal studies, these multimodal brain imaging findings suggest that prenatal selective serotonin reuptake inhibitor exposure has a significant association with fetal brain development.
Abstract
Importance
Selective serotonin reuptake inhibitor (SSRI) use among pregnant women is increasing, yet the association between prenatal SSRI exposure and fetal neurodevelopment is poorly understood. Animal studies show that perinatal SSRI exposure alters limbic circuitry and produces anxiety and depressive-like behaviors after adolescence, but literature on prenatal SSRI exposure in humans is limited and mixed.
Objective
To examine associations between prenatal SSRI exposure and brain development using structural and diffusion magnetic resonance imaging (MRI).
Design, Setting, and Participants
A cohort study conducted at Columbia University Medical Center and New York State Psychiatric Institute included 98 infants: 16 with in utero SSRI exposure, 21 with in utero untreated maternal depression exposure, and 61 healthy controls. Data were collected between January 6, 2011, and October 25, 2016.
Exposures
Selective serotonin reuptake inhibitors and untreated maternal depression.
Main Outcomes and Measures
Gray matter volume estimates using structural MRI with voxel-based morphometry and white matter structural connectivity (connectome) using diffusion MRI with probabilistic tractography.
Results
The sample included 98 mother (31 [32%] white, 26 [27%] Hispanic/Latina, 26 [27%] black/African American, 15 [15%] other) and infant (46 [47%] boys, 52 [53%] girls) dyads. Mean (SD) age of the infants at the time of the scan was 3.43 (1.50) weeks. Voxel-based morphometry showed significant gray matter volume expansion in the right amygdala (Cohen d = 0.65; 95% CI, 0.06-1.23) and right insula (Cohen d = 0.86; 95% CI, 0.26-1.14) in SSRI-exposed infants compared with both healthy controls and infants exposed to untreated maternal depression (P < .05; whole-brain correction). In connectome-level analysis of white matter structural connectivity, the SSRI group showed a significant increase in connectivity between the right amygdala and the right insula with a large effect size (Cohen d = 0.99; 95% CI, 0.40-1.57) compared with healthy controls and untreated depression (P < .05; whole connectome correction).
Conclusions and Relevance
Our findings suggest that prenatal SSRI exposure has an association with fetal brain development, particularly in brain regions critical to emotional processing. The study highlights the need for further research on the potential long-term behavioral and psychological outcomes of these neurodevelopmental changes.
This cohort study examines the association between prenatal exposure to selective serotonin reuptake inhibitors and fetal brain development.
Introduction
The prescription of selective serotonin reuptake inhibitor (SSRI) medications for pregnant women has accelerated over the past 30 years.1 To some extent, this rise may be attributable to increased awareness of the detrimental effects of untreated prenatal maternal depression (PMD) on women and children,2 along with early studies failing to document immediate effects of SSRI exposure in offspring (although later rodent studies document postpubertal alterations3). However, little is known about the association between prenatal SSRI exposure and human fetal neurodevelopment.
Serotonin (5-hydroxytryptamine [5-HT]) plays a vital role in neurodevelopment. In the fetal brain, 5-HT signaling affects cell proliferation, differentiation, neuronal migration, network formation, and synaptogenesis.4 The 5-HT transporter is widely expressed in the fetal brain in both serotonergic and nonserotonergic neurons,5 thus providing a developmentally transient target for SSRIs. Atypical serotonergic signaling resulting from prenatal SSRI exposure may alter fetal brain development and subsequent functioning.6
Animal studies support this idea. Perinatal SSRI exposure in rodent studies is associated with delayed motor development, reduced pain sensitivity, disrupted thalamocortical organization, reduced dorsal raphe neuronal firing, reduced arborization of 5-HT neurons, and altered limbic and cortical circuit functioning.7,8 Rodent studies also suggest behavioral consequences of early-life SSRI exposure, including increases in anxiety and depression-like behaviors in adulthood (eg, impaired stress response and grooming, decreased play),3,9 and suggest that early perturbations in 5-HT signaling may be associated with neurodevelopment, giving rise to atypical emotion-related behaviors later in life.
Literature on prenatal SSRI exposure in humans is limited and mixed. Studies have most consistently reported that prenatal SSRI exposure is associated with a shorter gestational period, lower birth weight, lower Apgar scores, and neonatal abstinence syndrome.10,11 Initial studies on longer-term neurodevelopmental consequences have yielded mixed findings; some studies suggest increased internalizing and externalizing behaviors during early childhood,11,12 whereas others fail to find such associations.13 However, consistent with animal studies,3 a recent national registry study (including >15 000 prenatally SSRI-exposed offspring) found increased rates of depression in early adolescence in youth with prenatal SSRI exposure.14
Brain imaging provides a window into neurodevelopment, yet human infant and fetal imaging studies of prenatal SSRI exposure are scarce. A recent electroencephalography study found reduced interhemispheric connectivity and lower cross-frequency integration in SSRI-exposed infants, suggesting uncoupling of frontal circuitry.15 Two infant magnetic resonance imaging (MRI) studies documented changes in gray matter (GM) and white matter (WM) tissue properties in prenatally SSRI-exposed infants (eg, altered fractional anisotropy of the thalamostriatal GM and superior WM fascicule16 and increased mean diffusivity in several major fasciculi17). Although these studies suggest an association between prenatal SSRI exposure and variation in fetal brain development, they are confounded by sample characteristics (eg, inclusion of very preterm-born infants) or the lack of an untreated PMD comparison group.
Based on prior animal studies, we hypothesized that prenatally SSRI-exposed infants would demonstrate altered GM morphology and WM connectivity within the corticolimbic circuit. To test this, we used deformation-based GM morphometry and diffusion probabilistic WM tractography. To more accurately assess the association between prenatal SSRI exposure and the infant brain, we considered the following methodologic advances: 2 comparison groups (healthy controls [HCs]) and infants exposed to untreated PMD), optimization of image analytics for the infant brain, and enhanced connectivity measures.
Methods
Participants
Data were collected between January 6, 2011, and October 26, 2016. Participants (pregnant women, aged 18-45 years) were recruited through obstetricians, midwives, and psychiatrists. A total of 204 mothers were recruited; 103 infants underwent an MRI scan (eMethods and eTable 1 in the Supplement). Group membership was determined after mothers completed a prenatal mood and medication assessment (between 19 and 39 weeks’ gestation). Mothers were assigned to the SSRI group if they self-reported receiving an SSRI at some point in their pregnancy. Sleeping, nonsedated infants underwent an MRI session when they were approximately 3.43 (SD 1.50) weeks of age. The New York State Psychiatric Institute Institutional Review Board approved all procedures and participants provided written informed consent. Participants received financial compensation for their participation.
PMD and Psychiatric Symptoms
Group membership (PMD vs HC) was determined during the prenatal assessment based on the mother’s depression scores, assessed via the Center for Epidemiological Studies Depression19 scale (scores ≥16 were considered indicative of clinically significant depression). Women completed the Schedule for Affective Disorders and Schizophrenia (SADS), a semistructured diagnostic interview.18 Owing to time limitations, 20 of the 98 mothers with usable infant MRI data did not complete the SADS (SSRI, 2; PMD, 5; and HC, 13). Postnatal depression was assessed via the CES-D, which was completed by mothers again at the time of their infant’s MRI session.
Infant 5-HT Transporter–Linked Polymorphic Region Genotype
To determine infant 5-HT transporter–linked polymorphic region (5-HTTLPR) genotype, saliva samples were collected and genotyped (eMethods in the Supplement). Genotype data were missing for 9 infants, who were excluded from 5-HTTLPR analyses.
MRI Acquisition and Analysis
Structural and diffusion MRI was acquired on a whole-body scanner (MR 750 3T; GE Healthcare) with an 8-channel head coil; eMethods in the Supplement provides details.
A T2-weighted structural MRI (single run) and diffusion-weighted images (2 runs) were obtained from 98 infants; 80 infants had usable T2-weighted scans and 91 had at least 1 usable diffusion-weighted imaging run (eTable 2 in the Supplement). The T2-weighted images underwent voxel-based morphometry. After preprocessing, diffusion-weighted imaging underwent probabilistic tractography and a recently developed filtering algorithm to curtail false-positive streamline estimates and improve the quantitative interpretability of streamline-based connectivity measures (eMethods in the Supplement provide details).
Statistical Analysis
For both GM morphometry and WM connectivity, linear regression with permutation testing (nonparametric) was used. To examine the effects of SSRI exposure beyond the effects of PMD exposure, primary contrast maps compared the SSRI group vs both PMD and HC infants. Three follow-up contrasts were then conducted, comparing (1) SSRI-exposed vs HC infants, (2) SSRI- vs PMD-exposed infants, and (3) PMD-exposed vs HC infants. The following covariates were included in the initial regression model: infant sex, age at scan, birth weight, and mother's postnatal depression score, indexed via the CES-D. Significance of effects was determined using nonparametric permutation tests, which do not assume Gaussian distributions. To control for type I error in voxel-based morphometry, we used conditional Monte Carlo permutation testing (randomize program in Functional MRI of Brain Software Library [FSL] v5.0; 10 000 permutations) with the cluster-extent threshold option (a cluster-forming threshold of z = 3.1; whole-brain correction).
For WM connectivity data, we used both connection-level and whole-brain connectome-level analysis. Connection-level analysis used the same linear regression model as described above with exhaustive permutation testing in the ImPerm r package (https://cran.r-project.org/web/packages/lmPerm/index.html). Whole-brain connectome-level analysis was done with the Network-Based Statistics Toolbox (NBS, version 1.2).20 We used 2 methods to control for type I error: false discovery rate and network-based statistics. These methods are complementary because false discovery rate tests the null hypothesis at the individual connection level, whereas network-based statistics tests at the network level using family-wise error. False discovery rate is more sensitive to focal effects, while network-based statistics is more sensitive to distributed network effects; 10 000 permutations were used to determine significance.
Results
Demographics
Magnetic resonance imaging scans were collected from 103 term infants, 98 of whom had usable MRI data. Five infants were excluded owing to apparent imaging artifacts resulting from excessive head motion. Specific subsamples for each imaging modality were structural MRI in 80 infants (SSRI, 14; PMD, 19; and HC, 47) and diffusion MRI in 92 infants (SSRI, 14; PMD, 20; and HC, 58). Groups did not differ significantly on infant gestational age at birth, sex, and birth weight (all P > .05; analysis of variance) (Table 1). No significant group differences were detected in the number of nondepressive psychiatric disorders documented with the SADS measure. Group differences were detected for infant age at MRI scan, maternal age, maternal race/ethnicity, and total family income (Table 1).
Table 1. Demographic Data.
| Characteristic | SSRIa (n = 16) |
PMD (n = 21) |
HC (n = 61) |
Test Statistic (df) | P Value |
|---|---|---|---|---|---|
| Age at scan, mean (SD), wk | 4.29 (1.81) | 3.03 (1.65) | 3.30 (1.27) | F2,95 = 3.82 | .02 |
| Gestational age at birth, mean (SD), wk | 38.71 (1.00) | 39.32 (1.04) | 39.43 (1.06) | F2,95 = 3.08 | .05 |
| Sex | χ22 = 0.84 | ||||
| Male | 8 | 8 | 30 | .65 | |
| Female | 8 | 13 | 31 | ||
| Infant birth weight, mean (SD), g | 3754.19 (1320.23) | 4000.57 (755.81) | 3888.78 (746.28) | F2,95 = 0.37 | .69 |
| Maternal age, mean (SD), y | 33.12 (4.20) | 27.55 (6.57) | 31.04 (5.75) | F2,95 = 4.72 | .01 |
| Maternal race/ethnicity | χ26 = 20.91 | ||||
| Hispanic/Latina | 0 | 7 | 19 | .002 | |
| White | 12 | 3 | 16 | ||
| Black/African American | 1 | 8 | 17 | ||
| Other | 3 | 3 | 9 | ||
| Total family income, $ | χ26 = 37.98 | ||||
| 0-25 000 | 1 | 13 | 13 | <.001 | |
| 26 000-50 000 | 1 | 4 | 16 | ||
| 51 000-100 000 | 2 | 2 | 20 | ||
| >100 001 | 12 | 2 | 11 | ||
| Infant SERT genotype | χ26 = 2.66 | ||||
| Long/long | 4 | 8 | 21 | .61 | |
| Short/long | 4 | 7 | 28 | ||
| Short/short | 4 | 2 | 9 | ||
| Depressive disorder | 7b | 16c | |||
| Women with nondepressive disorder | 4d | 3e | 5f | χ22 = 2.91 | .23 |
| Maternal CES-D, prenatal, mean (SD) | 12.63 (12.12) | 24.33 (7.22) | 7.22 (3.99) | F2,95 = 51.80 | <.001 |
| Maternal CES-D, postnatal, mean (SD) | 10.84 (11.44) | 15.8 (9.39) | 6.98 (4.86) | F2,95 = 11.54 | <.001 |
Abbreviations: CES-D, Center For Epidemiologic Studies Depression scale; HC, healthy control; MDD, major depressive disorder; PMD, prenatal maternal depression; SADS, Schedule for Affective Disorders and Schizophrenia; SERT, serotonin transporter; SSRI, selective serotonin reuptake inhibitor.
Exposure to low-level (n = 4), midlevel (n = 7), and high-level (n = 5) SSRI dosage (eMethods in the Supplement) in the first (n = 1), second (n = 2), and third (n = 13) trimesters.
With diagnosis based on SADS score18: major depressive disorder (n = 3) and depressive disorder not otherwise specified (n = 3).
With diagnosis based on SADS score: major depressive disorder (n = 4) and depressive disorder not otherwise specified (n = 12).
With diagnosis based on SADS score: bulimia (n = 1), panic disorder (n = 2), generalized anxiety disorder (n = 1), and obsessive-compulsive disorder (n = 1).
With diagnosis based on SADS score: posttraumatic stress disorder (n = 1), panic disorder (n = 2), agoraphobia (n = 1), obsessive-compulsive disorder (n = 1), and simple phobia (n = 1).
With diagnosis based on SADS score: posttraumatic stress disorder (n = 1) and simple phobia (n = 4).
SSRI Exposure and GM Volume
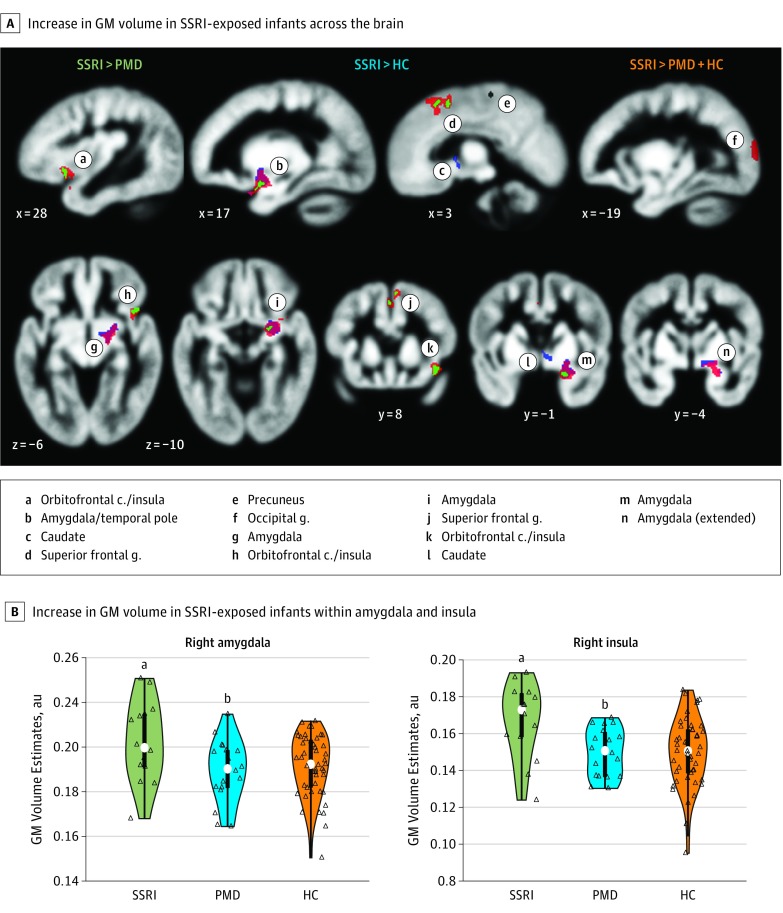
Compared with infants not exposed to SSRIs (ie, PMD and HC), SSRI-exposed infants showed a significant GM volume expansion in the right amygdala and insula with medium to large effect sizes (SSRI vs PMD and HC: right amygdala, Cohen d = 0.65; 95% CI, 0.06-1.23; right insula, Cohen d = 0.86; 95% CI, 0.26-1.14) as well as in the right superior frontal gyrus and the left occipital gyrus at a whole-brain corrected P value <.05 (randomization permutation; adjusted for standard covariates) (Figure 1 and Table 2). An unadjusted model also showed a significant increase in volume in the right amygdala and right insula at whole-brain corrected P < .05.
Figure 1. Brain Region Volumes in Infants With Prenatal Selective Serotonin Reuptake Inhibitor (SSRI) Exposure.
A, Significant group volume differences in infant brains (mean, 4 weeks). Regression analyses were conducted on gray matter (GM) volume maps, estimated from T2-weighted magnetic resonance imaging and through voxel-based morphometry, using a whole-brain corrected P < .05 (randomization permutation; cluster-extent based correction). The colored areas show an increase in volume in SSRI-exposed infants relative to prenatal maternal depression (PMD) without SSRI exposure (green), healthy controls (HC) (blue), and both groups combined (orange) (SSRI, n = 14; PMD, n = 19; HC, n = 47). Compared with the PMD, HC, and both groups combined, the SSRI group showed significant expansion in volume in the right amygdala and insula compared with the PMD group and combined groups only in the superior frontal gyrus, and compared with combined groups only, the occipital gyrus. B, Distribution (colored area), quartiles (thick bar), 95% CIs (thin line), and medians (white dots). Open triangles represent individual infant values. The significance of group differences was based on voxelwise analysis (whole-brain corrected using randomization permutation) from the 2 separate clusters in the right amygdala and the anterior insula. au indicates arbitrary unit; c, cortex; g, gyrus.
aP = .03 compared with both the PMD group, P = .02 compared with the HC group, and P = .01 compared with the PMD and HC groups combined, all significant results.
bP = .34 compared with the HC group.
Table 2. Group Comparison of Gray Matter Volumes (Voxel-Based Morphometry).
| Brain Region | Coordinates x, y, z, mm |
Whole-Brain Corrected P Valuea | Cluster Size, mm3 |
|---|---|---|---|
| Contrast: SSRI>PMD + HC | |||
| Right amygdala | 17, −1, −11 | = .01 | 397 |
| Right insula/orbitofrontal cortex | 28, 8, −5 | .01 | 50 |
| Right superior frontal gyrus | 3, 7, 36 | .03 | 129 |
| Left occipital gyrus | −20, −63, 9 | .04 | 116 |
| Contrast: SSRI>PMD | |||
| Right amygdala | 16, −2, −12 | .03 | 65 |
| Right insula/orbitofrontal cortex | 29, 9, −6 | .03 | 70 |
| Right superior frontal gyrus | 3, 10, 35 | .03 | 35 |
| 0, 14, 29 | .03 | 19 | |
| Right precuneus | 29, 9, −6 | .03 | 70 |
| Contrast: SSRI>HC | |||
| Right amygdala/insula | 15, −2, 08 | .02 | 211 |
| Right caudate | 7, 0, −2 | .02 | 26 |
| Contrast: SSRI>PMD + HC | |||
| Right amygdala | 17, −1, −11 | .01 | 397 |
| Right insula/orbitofrontal cortex | 28, 8, −5 | .01 | 50 |
| Right superior frontal gyrus | 3, 7, 36 | .03 | 129 |
| Left occipital gyrus | −20, −63, 9 | .04 | 116 |
Abbreviations: HC, healthy control; PMD, prenatal maternal depression; SSRI, selective serotonin reuptake inhibitor.
Significance was determined using randomization permutation.
Compared with PMD infants alone, SSRI-exposed infants showed a significant increase in volume in the right amygdala, right insula, right superior frontal gyrus, and right precuneus at whole-brain corrected P < .05 (Figure 1 and Table 2). Furthermore, compared with HC infants alone, SSRI-exposed infants showed a significant increase in volume in the right amygdala, right insula, and right caudate. No regions showed a decrease in GM intensity in the SSRI-exposed group at whole-brain corrected P < .05. There were no significant differences in GM volumes between the PMD and HC groups.
SSRI Exposure and WM Connectivity
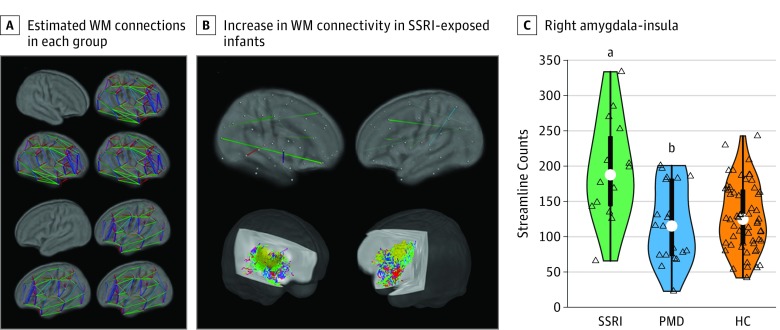
Linear regression of the structural connectome (connectivity was defined as streamline counts) revealed a significant increase in connectivity in the SSRI group relative to all infants not exposed to SSRIs (SSRI vs PMD and HC) in the following 4 connections: right amygdala-right insula, left anterior cingulate cortex-left thalamus, right precentral gyrus-right cuneus, and left insula-right precuneus at P < .05 (permutation testing; null hypothesis testing at the individual connection level using false discovery rate). An unadjusted model similarly showed a significant increase in connectivity between the right amygdala and right insula and between the left insula and right precuneus at P < .05. However, at the network level (network-based statistics), the structural connectome showed no significant group differences, suggesting similar topology of the structural connectomes across the 3 groups (Figure 2A). Fractional anisotropy and mean diffusivity of the structural connectomes showed no significant effects of group at P < .05.
Figure 2. White Matter (WM) Structural Connections in Infants With Prenatal Exposure to Selective Serotonin Reuptake Inhibitors (SSRIs).
A, White matter structural connectomes (90 regions) estimated using diffusion tractography; across both hemispheres, similar connectome organization was evident in each study group: healthy control (HC) infants, SSRI-exposed infants, and prenatal maternal depression (PMD)-exposed infants without SSRI exposure (permutation tests against 1000 randomized connections; P < .05; SSRI, n = 14; PMD, n = 20; HC, n = 58). B, Upper row shows a map of significant group differences in WM connectivity. Regression analyses were conducted on the connectivity matrix using a whole-brain-corrected P < .05 (randomization permutation; false discovery rate control). Lower row shows a representative WM pathway connecting the right amygdala (red) and the right insula (yellow) color-coded by direction. C, Distributions (colored area), quartiles (thick bar), 95% CIs (thin line), and medians (white dots). Open triangles represent infants. The significance of the group differences was based on a regression model performed on the right amygdala-right amygdala connectivity (exhaustive permutations).
aP < .001 compared with the PMD group, P < .001 compared with the HC group, and P = .001 compared with the PMD and HC groups combined.
bP = .80 compared with the HC group.
Given the significant increase in GM volumes in the right amygdala and right insula in the SSRI group (Figure 2B and C), we performed a separate analysis focusing on WM connectivity between the 2 regions to better estimate the effect size. With a large effect size, linear regression showed an increase in structural connectivity in the SSRI-exposed group relative to all non–SSRI-exposed infants (SSRI vs PMD and HC: t = 4.82; P < .001; Cohen d = 0.99; 95% CI, 0.40-1.57; adjusted for the aforementioned covariates). Also with large effect sizes, the SSRI group showed an increase compared separately with either HC (SSRI vs HC: t = 4.68; P < .001; Cohen d = 0.97; 95% CI, 0.36-1.57) or PMD (SSRI vs PMD: t = 3.82; P < .001; Cohen d = 1.16; 95% CI, 0.41-1.89). We confirmed these results in an additional tractography analysis with a larger total streamline count (ie, 100 million for initial tractography and 5 million for the streamline filtering analysis).
Potential Confounders
Effects of SSRIs on GM volume expansion and increased WM connectivity remained significant after adjusting for maternal age, maternal race/ethnicity, total household income, maternal education, maternal comorbid psychiatric disorders, and infant 5-HTTLPR genotype (eTable 3 and eResults in the Supplement).
Prediction of Brain Changes Associated With Prenatal SSRI Exposure
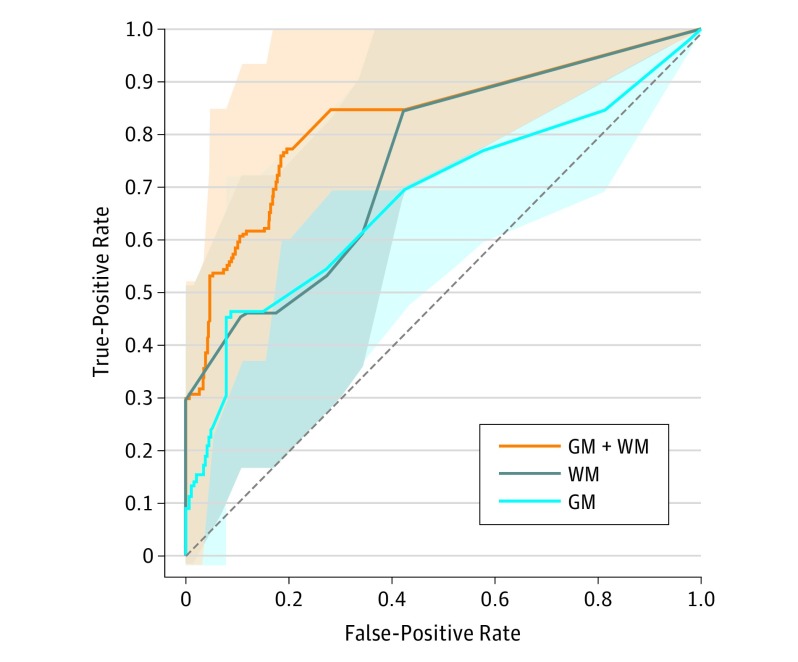
We assessed the capability of the selected GM and WM measures (amygdala volume, insula volume, and amygdala-insula tract estimate) to predict prenatal SSRI exposure (Figure 3). A logistic regression model with both GM and WM (GM and WM model) showed the greatest area under the curve (AUC) of 0.83 (95% CI, 0.63-0.93; leave-1-out cross-validation), significantly greater than models with either WM (P = .008, Wilcoxon rank sum test; AUC, 0.74; 95% CI, 0.51-0.87) or GM (P = .003; AUC, 0.67; 95% CI, 0.45-0.84) alone. The GM and WM model with randomly permuted group labels showed an AUC of 0.41 (1000 iterations).
Figure 3. Selected Gray Matter (GM) and White Matter (WM) Measures Associated With Brain Changes After Prenatal Exposure to Selective Serotonin Reuptake Inhibitors (SSRIs).
Receiver operating characteristics analysis curve showing cross-validated diagnostic accuracy of a linear regression model predicting SSRI exposure (leave-1-out cross validation). We tested 3 logistic regression models with different sets of the selected brain measures as predictors: GM+WM (orange), WM (gray), and GM (blue) models (predictors: for GM, right amygdala volume and right insula volume; for WM, amygdala-insula connectivity). Shaded areas are confidence bounds (estimated in 5000 bootstrap samples).
Discussion
To our knowledge, this is the first study to report increased volumes of the amygdala and insular cortex, as well as increased WM connection strength between these 2 regions, in prenatally SSRI-exposed infants. Our findings suggest a potential association between prenatal SSRI exposure, likely via aberrant serotonin signaling, and the development of the amygdala-insula circuit in the fetal brain.
Expression patterns of the 5-HT transporter in the developing brain may underlie the association of prenatal SSRI exposure with altered brain morphology. Exclusively during the prenatal period, 5-HT transporter is expressed in serotonergic neurons (eg, dorsal raphe) as well as nonserotonergic regions across the brain, such as corticolimbic and sensorimotor systems, as commonly seen in rodents,21 nonhuman primates,22 and humans.5 Expression in nonserotoninergic cells is then repressed after birth.23 It is thus possible that the transient prenatal expression of the 5-HT transporter in nonserotonergic systems may render the fetal vs postnatal brain differentially sensitive to SSRI exposure.
Our finding of increased GM volume in the amygdala, anterior insula, and superior frontal gyrus in prenatally SSRI-exposed infants is in line with findings from animal studies. In 5-HT transporter knockout mice, studies have shown increased dendritic spine density in the amygdala24,25 and increased dendritic branching of pyramidal neurons of the infralimbic cortex.25 Studies also point to effects of pharmacologic blockade of the 5-HT transporter in rodents during the immediate postnatal period (ie, comparable to third-trimester gestation in humans) with subsequent increased anxiety and depression-like behaviors.3,26 However, these behavioral sequelae emerged only after the rodents entered puberty,3 much like what has been observed in a birth cohort study of children with gestational SSRI exposure.14
Coordination of the amygdala and insula during threat awareness is essential to adaptive fear regulation.27,28 A meta-analysis of functional brain imaging studies indicates that fear conditioning is associated with task-related activations in both the amygdala and insula in healthy individuals,29 and analogous findings are reported in individuals with high trait anxiety.30 Similarly, the coordination of the amygdala and insula is essential not only for fear conditioning, but also for anticipatory anxiety, particularly under conditions of uncertainty.31,32
Abnormalities in the amygdala-insula circuitry may be associated with anxiety and depression.33 Increased amygdala volumes are seen in both children and adults with anxiety disorders,34,35,36 heightened amygdala and insula task-related functional MRI activations are evident in adults with anxiety disorders during the presentation of fearful stimuli,29 and resting-state functional MRI studies show increased functional connectivity between the amygdala and insula in generalized anxiety and posttraumatic stress disorder.37,38 Similar functional connectivity findings are reported in children and adolescents across a range of anxiety disorders and symptoms.34,39,40 This abnormal amygdala-insula circuitry may be associated with increased vulnerability to anxiety and/or other mood disorders. Amygdala-insula hyperactivity to threats is reported in people who are at risk but not yet meeting criteria for anxiety disorders, suggesting that hyperactivity in this circuit may index increased susceptibility to anxiety disorders.30 Taken together, the structurally primed circuit in the infant brains could lead to maladaptive fear processing in their later life, such as generalization of conditioned fear or negative attention bias.
Prior infant neuroimaging studies report seemingly mixed findings: increased fractional anisotropy of the superior WM pathway16 and decreased fractional anisotropy and increased mean diffusivity 17 of the WM pathways. However, the first study had no direct comparison of exposure to both SSRI and PMD vs exposure with PMD alone and, in the second study, the association between prematurity and neurodevelopment might confound the results.
The effects of SSRIs were present only in the right hemisphere. One study hints at asymmetric distributions of 5-HT receptors. In healthy adults, 5-HT1A receptor binding estimated by positron emission tomography is higher in the right frontal gyri relative to their left hemispheric counterparts (and higher in the left auditory cortex).41 It remains to be determined whether the asymmetric expression pattern of 5-HT transporter in adults is also present during the fetal period.
Limitations
Our findings should be interpreted in the context of a few limitations. First, because participants were not randomly assigned to the PMD or SSRI group, there could be unmeasured sample differences. It is possible that women who received an SSRI during pregnancy were more severely depressed than were those with PMD. Because our assessments of depression occurred when women were already receiving the SSRI, this hypothesis requires further investigation. Future human studies could include randomization and placebo control (eg, ClinicalTrials.gov Identifier: NCT02185547). Second, the groups in our study differed in sociodemographics (maternal education, income, race/ethnicity, and birth weight). Although we statistically adjusted for these potential confounding variables, future research will be needed to conclusively disentangle SSRI and PMD exposure from these sociodemographic variables. Third, the behavioral and psychological correlates of our volumetric and connectivity findings need to be determined and longitudinal studies will need to examine whether developmental trajectories are affected. Last, the accuracy (eg, sensitivity, but perhaps not reliability42) of the fiber orientation estimates in diffusion probabilistic tractography might be partially limited by a relatively small number of gradient directions (n = 11). However, our selection of scanning parameters was based on multiple factors, not solely on the number of gradient directions, such as spatial resolution (submillimeter in-plane resolution for the small neonatal brains), scan duration, and signal-to-noise ratio that decreases as the number of gradient directions increases.
Conclusions
Use of SSRIs during pregnancy has increased in recent decades,43 yet their association with fetal neurodevelopment continues to be a topic of considerable debate. Because untreated PMD poses risks to both the infant and mother,2,44,45,46 the decision to initiate, continue, or suspend SSRI treatment remains a clinical dilemma. Preclinical studies of rodents indicate that dose, timing, and mechanism of action (5-HT augmenting or not) all contribute to outcomes in later life.3,9,10,47 Further study is required to better elucidate the effects of gestational SSRI exposure on fetal brain development and later life susceptibility to depressive, cognitive, and motor abnormalities. Such information may eventually allow more informed clinical decisions about how to best treat psychiatric disorders during pregnancy for the benefit of both mother and fetus.
eMethods. Detailed Methodology
eResults. Further Results
eTable 1. Comparison of Demographic Variables Between Subjects With and Without Infant MRI Scans (Related to Participants)
eTable 2. Number of Infants Having Unusable Volumes of DWI
eTable 3. Parameter Estimates Adjusting for Confounding Variables
References
- 1.Andrade SE, Reichman ME, Mott K, et al. . Use of selective serotonin reuptake inhibitors (SSRIs) in women delivering liveborn infants and other women of child-bearing age within the US Food and Drug Administration’s Mini-Sentinel program. Arch Womens Ment Health. 2016;19(6):969-977. [DOI] [PubMed] [Google Scholar]
- 2.Gentile S. Untreated depression during pregnancy: short- and long-term effects in offspring—a systematic review. Neuroscience. 2017;342:154-166. [DOI] [PubMed] [Google Scholar]
- 3.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879-881. [DOI] [PubMed] [Google Scholar]
- 4.Kiryanova V, McAllister BB, Dyck RH. Long-term outcomes of developmental exposure to fluoxetine: a review of the animal literature. Dev Neurosci. 2013;35(6):437-439. [DOI] [PubMed] [Google Scholar]
- 5.Verney C, Lebrand C, Gaspar P. Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. Anat Rec. 2002;267(2):87-93. [DOI] [PubMed] [Google Scholar]
- 6.Hermansen TK, Melinder A. Prenatal SSRI exposure: Effects on later child development. Child Neuropsychol. 2015;21(5):543-569. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Sari Y, Zhou FC. Selective serotonin reuptake inhibitor disrupts organization of thalamocortical somatosensory barrels during development. Brain Res Dev Brain Res. 2004;150(2):151-161. [DOI] [PubMed] [Google Scholar]
- 8.Rebello TJ, Yu Q, Goodfellow NM, et al. . Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Neurosci. 2014;34(37):12379-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier JDA, Vallès A, van Heesch F, et al. . Fluoxetine administration to pregnant rats increases anxiety-related behavior in the offspring. Psychopharmacology (Berl). 2011;217(3):419-432. [DOI] [PubMed] [Google Scholar]
- 10.Malm H, Sourander A, Gissler M, et al. . Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population-based national register data. Am J Psychiatry. 2015;172(12):1224-1232. [DOI] [PubMed] [Google Scholar]
- 11.Casper RC, Fleisher BE, Lee-Ancajas JC, et al. . Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402-408. [DOI] [PubMed] [Google Scholar]
- 12.Oberlander TF, Reebye P, Misri S, Papsdorf M, Kim J, Grunau RE. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch Pediatr Adolesc Med. 2007;161(1):22-29. [DOI] [PubMed] [Google Scholar]
- 13.Suri R, Hellemann G, Stowe ZN, Cohen LS, Aquino A, Altshuler LLA. A prospective, naturalistic, blinded study of early neurobehavioral outcomes for infants following prenatal antidepressant exposure. J Clin Psychiatry. 2011;72(7):1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malm H, Brown AS, Gissler M, et al. . Gestational exposure to selective serotonin reuptake inhibitors and offspring psychiatric disorders: a national register-based study. J Am Acad Child Adolesc Psychiatry. 2016;55(5):359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Videman M, Tokariev A, Saikkonen H, et al. . Newborn brain function is affected by fetal exposure to maternal serotonin reuptake inhibitors. Cereb Cortex. 2017;27(6):3208-3216. [DOI] [PubMed] [Google Scholar]
- 16.Podrebarac SK, Duerden EG, Chau V, et al. . Antenatal exposure to antidepressants is associated with altered brain development in very preterm-born neonates. Neuroscience. 2017;342:252-262. [DOI] [PubMed] [Google Scholar]
- 17.Jha SC, Meltzer-Brody S, Steiner RJ, et al. . Antenatal depression, treatment with selective serotonin reuptake inhibitors, and neonatal brain structure: A propensity-matched cohort study. Psychiatry Res. 2016;253:43-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35(7):837-844. [DOI] [PubMed] [Google Scholar]
- 19.Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and revision (CESD and CESD-R) In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment Instruments for Adults, 3rd ed Mahwah, NJ: Lawrence Erlbaum; 2004:363-377. [Google Scholar]
- 20.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197-1207. [DOI] [PubMed] [Google Scholar]
- 21.Narboux-Nême N, Pavone LM, Avallone L, Zhuang X, Gaspar P. Serotonin transporter transgenic (SERTcre) mouse line reveals developmental targets of serotonin specific reuptake inhibitors (SSRIs). Neuropharmacology. 2008;55(6):994-1005. [DOI] [PubMed] [Google Scholar]
- 22.Lebrand C, Gaspar P, Nicolas D, Hornung JP. Transitory uptake of serotonin in the developing sensory pathways of the common marmoset. J Comp Neurol. 2006;499(4):677-689. [DOI] [PubMed] [Google Scholar]
- 23.Berbel P, Ausó E, García-Velasco JV, Molina ML, Camacho M. Role of thyroid hormones in the maturation and organisation of rat barrel cortex. Neuroscience. 2001;107(3):383-394. [DOI] [PubMed] [Google Scholar]
- 24.Nietzer SL, Bonn M, Jansen F, et al. . Serotonin transporter knockout and repeated social defeat stress: impact on neuronal morphology and plasticity in limbic brain areas. Behav Brain Res. 2011;220(1):42-54. [DOI] [PubMed] [Google Scholar]
- 25.Wellman CL, Izquierdo A, Garrett JE, et al. . Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J Neurosci. 2007;27(3):684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popa D, Léna C, Alexandre C, Adrien J. Lasting syndrome of depression produced by reduction in serotonin uptake during postnatal development: evidence from sleep, stress, and behavior. J Neurosci. 2008;28(14):3546-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Critchley HD, Mathias CJ, Dolan RJ. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33(4):653-663. [DOI] [PubMed] [Google Scholar]
- 28.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189-195. [DOI] [PubMed] [Google Scholar]
- 29.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164(10):1476-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein MB, Simmons AN, Feinstein JS, Paulus MP. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 2007;164(2):318-327. [DOI] [PubMed] [Google Scholar]
- 31.Sarinopoulos I, Grupe DW, Mackiewicz KL, et al. . Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20(4):929-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation. Soc Cogn Affect Neurosci. 2011;6(1):74-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct Funct. 2010;214(5-6):451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin S, Young CB, Duan X, Chen T, Supekar K, Menon V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol Psychiatry. 2014;75(11):892-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361-1372. [DOI] [PubMed] [Google Scholar]
- 36.Schienle A, Ebner F, Schäfer A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261(4):303-307. [DOI] [PubMed] [Google Scholar]
- 37.Baur V, Hänggi J, Langer N, Jäncke L. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry. 2013;73(1):85-92. [DOI] [PubMed] [Google Scholar]
- 38.Rabinak CA, Angstadt M, Welsh RC, et al. . Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2(62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamm LL, Jacobs RH, Johnson MW, et al. . Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 2014;4(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu WJ, Yin DZ, Cheng WH, et al. . Abnormal functional connectivity of the amygdala-based network in resting-state fMRI in adolescents with generalized anxiety disorder. Med Sci Monit. 2015;21:459-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fink M, Wadsak W, Savli M, et al. . Lateralization of the serotonin-1A receptor distribution in language areas revealed by PET. Neuroimage. 2009;45(2):598-605. [DOI] [PubMed] [Google Scholar]
- 42.Heiervang E, Behrens TE, Mackay CE, Robson MD, Johansen-Berg H. Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage. 2006;33(3):867-877. [DOI] [PubMed] [Google Scholar]
- 43.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196(6):544.e1-544.e5. [DOI] [PubMed] [Google Scholar]
- 44.Monk C, Sloan RP, Myers MM, et al. . Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43(3):283-290. [DOI] [PubMed] [Google Scholar]
- 45.O’Connor TG, Monk C, Fitelson EM. Practitioner review: maternal mood in pregnancy and child development—implications for child psychology and psychiatry. J Child Psychol Psychiatry. 2014;55(2):99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posner J, Cha J, Roy AK, et al. . Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry. 2016;6(11):e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bairy KL, Madhyastha S, Ashok KP, Bairy I, Malini S. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology. 2007;79(1):1-11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed Methodology
eResults. Further Results
eTable 1. Comparison of Demographic Variables Between Subjects With and Without Infant MRI Scans (Related to Participants)
eTable 2. Number of Infants Having Unusable Volumes of DWI
eTable 3. Parameter Estimates Adjusting for Confounding Variables