Abstract
Background:
Fatigue is a well-recognized symptom in patients with inflammatory bowel disease and irritable bowel syndrome (IBS), and has been associated with psychological comorbidity and impaired quality of life in both. However, features associated with fatigue in patients with microscopic colitis (MC) are less clear.
Materials and methods:
We conducted a cross-sectional survey of patients with a new diagnosis of MC including levels of anxiety, depression, somatization, quality of life, and IBS-type symptoms. Levels and impact of fatigue were assessed using the Inflammatory Bowel Disease Fatigue self-assessment scale. Mean scores were compared against various patient characteristics, and were also correlated with anxiety, depression, somatization, and quality-of-life scores.
Results:
In total, 129 patients with MC diagnosed between 2010 and 2015 returned completed postal questionnaires. Common histological subtypes were collagenous colitis (53.5%, n = 69) and lymphocytic colitis (38.8%, n = 50). Higher mean fatigue severity and impact scores were associated with the presence of irritable-bowel-syndrome-type symptoms, abnormal levels of anxiety and depression, and high levels of somatization (p < 0.0001 for all), but those reporting ongoing symptoms attributable to MC did not report significantly higher scores. There were significant positive correlations between total anxiety, depression, or somatization scores and fatigue severity and impact scores, and significant negative correlations with quality-of-life measures (p < 0.001 for all).
Conclusions:
Fatigue in MC appears to be associated with reporting IBS-type symptoms, psychological comorbidity and impaired quality of life. It may therefore represent an important target for treatment.
Keywords: fatigue, irritable bowel syndrome, microscopic colitis, psychological health, quality of life
Introduction
Fatigue is a distressing and pervasive symptom, reported in up to 80% of patients with chronic inflammatory disorders, such as rheumatoid arthritis, systemic lupus erythematosus or chronic liver disease.1–3 This issue is also relevant to gastrointestinal (GI) diseases. Patients with inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) or Crohn’s disease,4–9 and irritable bowel syndrome (IBS)10–12 frequently report severe fatigue. In both IBD and IBS, those reporting fatigue often have reduced quality of life,6,13,14 and it has also been shown that psychological factors, such as depression or anxiety, are predictors for increased levels of fatigue in patients with IBS.15
Microscopic colitis (MC), often described as either collagenous (CC) or lymphocytic colitis (LC) is a less well-recognized form of IBD but is also associated with impaired quality of life.16,17 Given that patients with MC experience chronic diarrhoea, similar to patients with IBD and those with diarrhoea-predominant IBS, it is probable that fatigue will also be a pertinent issue in MC. However, data assessing levels of fatigue in MC are sparse. In one previous cross-sectional study, the symptom of fatigue was more prevalent in both LC and CC compared with controls,16 but this was not assessed using a validated fatigue questionnaire. This study also demonstrated an increased prevalence of fatigue even among those classed as having quiescent MC, based on self-reported absence of diarrhoea.16 Similar findings have been reported in classical IBD, where fatigue was reported in comparable numbers of patients with both active and inactive disease.4,5
It has therefore been hypothesized that fatigue in patients with IBD may relate to the presence of concomitant functional symptoms.18,19 There are data to support the fact that patients with IBD in remission, assessed using faecal calprotectin, but who have ongoing IBS-type symptoms, may have marked psychological comorbidity and impaired quality of life.20 This study was therefore designed to examine patient characteristics associated with fatigue in patients with a histological diagnosis of MC, using a validated fatigue assessment tool.
Materials and methods
Participants and setting
Participants were identified from a cohort of adult patients with a new histological diagnosis of MC at the Leeds Teaching Hospitals Trust, United Kingdom, between January 2010 and December 2015. Patients were invited to participate once survival status was confirmed, a diagnosis of cognitive impairment excluded, and a valid contact address obtained. Those with a known prior histologic diagnosis of MC were excluded. Potentially eligible participants were sent study documents, including a questionnaire and written consent form by post, and initial nonresponders received a second postal questionnaire. The study was approved by the local research ethics committee (Yorkshire and The Humber, Leeds West, United Kingdom) in January 2016, and the postal surveys took place between June 2016 and February 2017.
Diagnosis of microscopic colitis
A diagnosis of CC was made in the presence of a subepithelial collagen band of ⩾10 µm in thickness, with evidence of diffuse chronic inflammation, and a diagnosis of LC using a threshold of >20 intra-epithelial lymphocytes per 100 epithelial cells, also with evidence of diffuse chronic inflammation, but no thickening of the subepithelial collagen band. In patients where the recorded pathology diagnosis did not clearly specify the subtype of MC, we recorded a diagnosis of ‘MC, not otherwise specified (MC-NOS)’ as, at our centre, the diagnoses of incomplete CC or LC are not made. It has previously been demonstrated that there is little interobserver variability in the diagnosis of MC.21
Clinical and demographic data
All participants were asked to provide the following demographic data: sex, age, ethnicity, marital status, educational level, tobacco and alcohol use, and weight (in kilograms) and height (in metres), which were used to calculate body mass index (BMI). We also asked participants to complete a checklist of medications previously reported as being implicated in the development of MC,22 and to respond to questions regarding the presence of common autoimmune diseases associated with MC,23 including coeliac disease, thyroid disease, rheumatoid arthritis, psoriasis, autoimmune hepatitis, and type 1 diabetes. Patients were asked to report their stool frequency, as well as if they had ongoing GI symptoms that they attributed to MC. The latter was recorded as a dichotomized outcome as either ‘yes’ or ‘no’.
IBS-type symptoms, according to the Rome III criteria,24 included abdominal pain or discomfort occurring at least 3 days per month over the past 3 months, with the onset of discomfort at least 6 months previously, associated with two or more of the following: an improvement of pain or discomfort with the passage of stool, more or less frequent stools, or looser or harder stools.
To assess for the presence of either anxiety or depression, participants were asked to complete the validated 14-item Hospital Anxiety and Depression Scale (HADS) questionnaire.25 This includes seven questions screening for the presence of anxiety, and another seven questions for depression. Each question is scored from 0 to 3, resulting in a maximum potential score of 21 for anxiety or depression, separately. The severity of anxiety and depression symptoms was then graded according to three categories: normal (total depression or anxiety scores 0–7), borderline abnormal (8–10), and abnormal (⩾11).
In order to assess for the presence of somatization, we used the Patient Health Questionnaire 15 (PHQ-15), a validated questionnaire enquiring about the presence of 15 specific somatic symptoms occurring within the previous 4 weeks.26 Symptoms were graded into three levels of severity; ‘not bothered at all’ (scored as 0), ‘bothered a little’ (scored as 1), or ‘bothered a lot’ (scored as 2), giving a total possible score of 30. The severity of somatization was categorized into high (total score ⩾15), medium (10–14), low (5–9), and minimal (⩽4) levels, as has been previously recommended.27
Finally, the validated medical outcomes study 36-item Short Form (SF-36) score was used to assess health-related quality of life.28 The 36 questions are grouped into eight domains: physical functioning, role limitations due to physical health, role limitations due to emotional health, energy or fatigue, emotional well-being, social functioning, pain, and general health. Participants were asked to score each question from 0 to 100, with higher scores indicating more favourable quality of life.
Assessment of fatigue severity and impact
Participants were asked to complete a scoring tool known as the Inflammatory Bowel Disease Fatigue Assessment Scale (IBD-F). This was designed using cohorts of patients with either Crohn’s disease or UC,29 and has been validated for use in IBD.30,31 It is composed of two sections, with scores for each calculated separately. The first section (fatigue severity component) is designed as a baseline assessment of presence and severity of fatigue, and includes five questions measured on a Likert scale (from 0–4) with a maximum score of 20. The second section (fatigue impact component) includes 30 questions, again measured on a Likert scale (from 0–4) with a maximum score of 120, and assesses the impact of fatigue on the patient’s activities of daily living. Patients can score 0 in either section, indicating the absence of fatigue or impact on activities of daily living.
Statistical analysis
Mean levels of fatigue severity and impact were compared according to baseline demographic characteristics, including medications and coexistent autoimmune disease, the presence of IBS-type symptoms, presence or absence of abnormal HADS anxiety or depression scores, and presence or absence of high levels of somatization using a student’s t test. The relationship between fatigue severity and impact scores and age, BMI, HADS scores, PHQ-15 scores, and SF-36 scores were assessed using Pearson’s correlation coefficient. A two-tailed p value of <0.01 was considered to be statistically significant for all analyses, due to multiple comparisons. All statistical analyses were performed using SPSS for Windows version 21.0 (SPSS Inc., Chicago, IL, USA).
Results
Postal questionnaires were distributed to 478 potentially eligible patients, all of whom were diagnosed with MC over the 6-year study period. Of 157 (32.8%) responders, 6 patents were found to have a prior histological diagnosis of MC and were therefore excluded (Figure 1). In total, 129 (85.4%) of 151 eligible responders completed the IBD-F questionnaire fully, and were included in the analysis. In terms of subtype, 69 patients (53.5%) had CC, 50 (38.8%) had LC, and 10 (7.8%) had MC-NOS. Only a few patients reported previous autoimmune diseases including coeliac disease (n = 10), thyroid disease (n = 19), and rheumatoid arthritis (n = 8), but none reported type 1 diabetes, autoimmune liver disease, or psoriasis. Comparing characteristics of responders to the questionnaire with nonresponders revealed no significant differences in MC subtype or sex, but there was a trend towards responders being older [mean age 68.0 years (standard deviation, SD 9.8) versus 65.4 (SD 12.6), p = 0.03].
Figure 1.
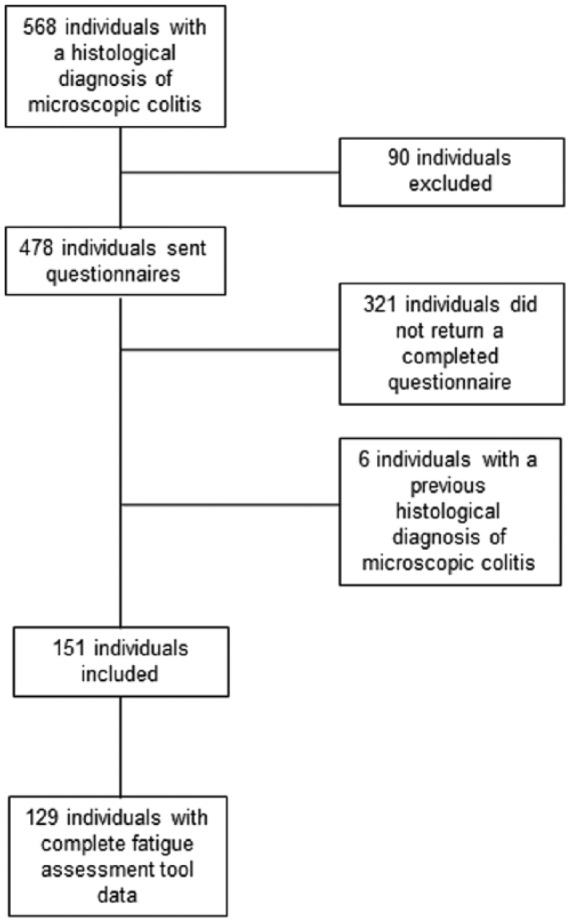
Flow chart of patients recruited into the study.
Fatigue severity and its associated features in microscopic colitis
For the fatigue severity assessment component, the mean score in all patients was 8.2 (SD ± 4.9), and the median score was 9 [interquartile range (IQR) 4–34]. Mean fatigue severity scores according to patient demographics, clinical data, presence or absence of abnormal anxiety or depression scores, and presence or absence of high levels of somatization are provided in Table 1. There were trends towards patients using proton pump inhibitors (PPIs) reporting more severe fatigue. Patients with IBS-type symptoms had significantly higher mean fatigue severity scores, and higher scores were also significantly associated with presence of abnormal anxiety and depression scores, and high levels of somatization (p < 0.001 for all). Of note, mean fatigue severity scores in those reporting ongoing symptoms that they felt were related to their MC were not significantly higher. There were significant positive correlations between HADS scores and PHQ-15 scores and fatigue severity scores, and significant negative correlations between quality-of-life scores across all domains of the SF-36 and fatigue severity (Table 2).
Table 1.
Associations between fatigue severity and impact and demographic features of patients with microscopic colitis.
| Mean fatigue severity score (SD) | p value* | Mean fatigue impact score (SD) | p value* | |
|---|---|---|---|---|
| Type of MC | ||||
| Collagenous colitis | 8.3 (5.0) | 23.1 (27.6) | ||
| Lymphocytic colitis | 7.5 (4.8) | 0.37 | 25.6 (25.0) | 0.61 |
| Sex | ||||
| Female | 8.4 (4.8) | 26.3 (26.8) | ||
| Male | 7.3 (5.0) | 0.26 | 20.8 (25.8) | 0.32 |
| Married or cohabiting | ||||
| Yes | 7.7 (4.6) | 22.0 (23.3) | ||
| No | 8.9 (5.2) | 0.18 | 30.5 (31.1) | 0.08 |
| University graduate | ||||
| Yes | 6.7 (4.3) | 18.2 (17.7) | ||
| No | 8.7 (5.0) | 0.03 | 27.6 (28.8) | 0.07 |
| Current smoker | ||||
| Yes | 11.0 (5.0) | 46.1 (32.3) | ||
| No | 7.8 (4.8) | 0.03 | 22.7 (24.9) | 0.002 |
| Using PPIs | ||||
| Yes | 9.2 (5.1) | 33.6 (29.8) | ||
| No | 7.2 (4.4) | 0.02 | 17.3 (20.5) | <0.001 |
| Using NSAIDs | ||||
| Yes | 7.9 (5.3) | 25.7 (27.4) | ||
| No | 8.3 (4.7) | 0.71 | 24.7 (26.2) | 0.83 |
| Using ranitidine | ||||
| Yes | 9.6 (5.0) | 32.0 (26.1) | ||
| No | 7.9 (4.8) | 0.15 | 23.7 (26.5) | 0.20 |
| Using aspirin | ||||
| Yes | 8.3 (4.8) | 27.0 (24.5) | ||
| No | 8.1 (4.9) | 0.81 | 24.4 (27.3) | 0.63 |
| Using selective serotonin reuptake inhibitors | ||||
| Yes | 9.7 (5.2) | 35.4 (29.0) | ||
| No | 7.8 (4.7) | 0.08 | 22.6 (25.5) | 0.03 |
| Using statins | ||||
| Yes | 8.3 (4.6) | 25.1 (24.1) | ||
| No | 8.1 (5.0) | 0.87 | 25.0 (27.8) | 0.03 |
| Autoimmune thyroid disease | ||||
| Yes | 7 (5.2) | 20.7 (24.1) | ||
| No | 8.4 (4.8) | 0.26 | 25.0 (27.8) | 0.44 |
| Rheumatoid arthritis | ||||
| Yes | 9.8 (5.6) | 36.5 (31.2) | ||
| No | 8.1 (4.8) | 0.34 | 24.3 (26.1) | 0.59 |
| Coeliac disease | ||||
| Yes | 8.0 (5.0) | 29.4 (32.4) | ||
| No | 9.6 (3.5) | 0.33 | 24.7 (26.1) | 0.59 |
| Rome III IBS-type symptoms | ||||
| Present | 10.4 (4.6) | 38.2 (30.4) | ||
| Absent | 7.0 (4.6) | <0.001 | 17.9 (21.2) | <0.001 |
| Stool frequency | ||||
| 1–3 times per day | 6.9 (4.0) | 17.0 (19.6) | ||
| 4–6 times per day | 8.8 (4.7) | 26.3 (26.3) | ||
| 7–9 times per day | 6.3 (4.0) | 17.0 (27.6) | ||
| >10 times per day | 9.6 (6.1) | 0.06 | 38.6 (31.1) | 0.01 |
| Described ongoing symptoms of MC | ||||
| Present | 8.4 (4.6) | 28.6 (27.4) | ||
| Absent | 7.8 (5.2) | 0.52 | 20.7 (25.0) | 0.09 |
| Abnormal anxiety scores | ||||
| Present | 10.8 (4.6) | 42.6 (30.7) | ||
| Absent | 6.5 (4.3) | <0.001 | 14.1 (16.1) | <0.001 |
| Abnormal depression scores | ||||
| Present | 13.8 (3.1) | 61.1 (26.0) | ||
| Absent | 6.7 (4.1) | <0.001 | 15.1 (15.5) | <0.001 |
| High levels of somatization | ||||
| Present | 13.9 (4.3) | 67.3 (26.4) | ||
| Absent | 7.2 (4.3) | <0.001 | 18.2 (19.3) | <0.001 |
All comparisons made using student t tests.
IBS, irritable bowel syndrome; MC, microscopic colitis; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor; SD, standard deviation.
Table 2.
Correlations between age, body mass index, Hospital Anxiety and Depression Scale scores, PHQ-15 scores, quality-of-life scores, and fatigue severity and impact in patients with microscopic colitis.
| Fatigue severity score |
Fatigue impact score |
|||
|---|---|---|---|---|
| R score | p value | R score | p value | |
| Age | −0.21 | 0.02 | −0.21 | 0.02 |
| Body mass index | 0.1 | 0.45 | 0.15 | 0.11 |
| Total HADS anxiety score | 0.58 | <0.001 | 0.65 | <0.001 |
| Total HADS depression score | 0.71 | <0.001 | 0.80 | <0.001 |
| Total PHQ-15 score | 0.69 | <0.001 | 0.71 | <0.001 |
| SF-36 physical functioning | −0.48 | <0.001 | −0.58 | <0.001 |
| SF-36 role limitations physical health | −0.58 | <0.001 | −0.58 | <0.001 |
| SF-36 role limitations emotional health | −0.59 | <0.001 | −0.67 | <0.001 |
| SF-36 energy/fatigue | −0.76 | <0.001 | −0.70 | <0.001 |
| SF-36 emotional well-being | −0.69 | <0.001 | −0.76 | <0.001 |
| SF-36 social functioning | −0.70 | <0.001 | −0.72 | <0.001 |
| SF-36 pain | −0.54 | <0.001 | −0.57 | <0.001 |
| SF-36 general health | −0.66 | <0.001 | −0.69 | <0.001 |
All correlations made using Pearson’s correlation coefficient.
HADS, Hospital Anxiety and Depression Scale; PHQ-15, Patient Health Questionnaire 15; SF-36, Short Form 36 health survey.
Fatigue impact and its associated features in microscopic colitis
For the fatigue impact-on-daily-life component, the mean score in all patients was 25.0 (SD ± 26.5), and the median score was 16 (IQR 4–35). Details of mean fatigue impact scores according to patient demographics, clinical data, presence or absence of abnormal anxiety or depression scores, and presence or absence of high levels of somatization are provided in Table 1. Patients with IBS-type symptoms, smokers, those taking PPIs, those with abnormal anxiety or depression scores, and those with high levels of somatization had significantly higher fatigue impact scores. There was also a trend towards higher mean fatigue impact scores in those with a higher stool frequency. Again, there were significant positive correlations between HADS scores and PHQ-15 scores and fatigue impact scores, and significant negative correlations between quality-of-life scores across all domains of the SF-36 and fatigue impact (Table 2).
Discussion
This cross-sectional survey has demonstrated that patients with MC often report high levels of fatigue severity and impact as assessed by the IBD-F self-assessment tool. Patients with abnormal anxiety and depression scores and high levels of somatization reported higher mean fatigue severity scores, and a greater impact on their daily lives. Fatigue severity and impact scores were also significantly higher among those patients with IBS-type symptoms, but not among those with ongoing GI symptoms that they attributed to their diagnosis of MC. There was also a trend towards higher mean fatigue impact scores in those with a higher stool frequency. Finally, higher levels of both the impact and severity of fatigue led to reduced quality of life, as measured by the SF-36.
To our knowledge, this is the first study to use a specific fatigue assessment questionnaire to examine the severity of fatigue in patients with MC and to study the impact of fatigue in such a population. We attempted to contact all patients with a new histological diagnosis of MC diagnosed in a secondary care setting over a 6-year period, and therefore our findings are likely to be generalizable to other patients with MC in usual clinical practice. Data on psychological health, somatization, and quality of life were collected using validated questionnaires. We also collected data on functional GI symptoms, and self-reported symptoms suggestive of active MC, allowing us to assess whether fatigue was related to possible disease activity, or rather just GI symptom-reporting per se.
There are some limitations to this study. We lacked a control group of individuals without MC in order to assess levels of fatigue compared with a background population. We also used the Rome III criteria for IBS,24 which have been superseded by the Rome IV criteria,32 because our study was designed and conducted prior to the publication of the latest iteration of these criteria. A further potential limitation is the recruitment of patients diagnosed with MC as long ago as 2010. This may have led to recall bias affecting the accuracy of reporting of clinical information, such as medication use. However, all of the validated questionnaires we administered, including the IBD-F and Rome III criteria, required contemporaneous answers. Participants were also asked to complete several different questionnaires, each with multiple questions, and this may have been burdensome, perhaps explaining why 22 patients did not fully complete the IBD-F. All participants were sent postal questionnaires, and this may have introduced volunteer bias in to our study; the implication would be those responding may have been intrinsically different to nonresponders. Unfortunately, as we did not have detailed demographic information on all patients with MC prior to study commencement, we were unable to compare all characteristics of responders and nonresponders, so we are uncertain whether those who took part are representative of the original population of 478 eligible patients. However, an analysis based on age, sex, and MC subtype did not reveal any significant differences between responders and nonresponders. It is unlikely that patients with higher levels of fatigue were more likely to take part, as this was only one aspect of our study, and the participant information sheet did not focus specifically on the issue of fatigue in MC.
It should also be noted that disease activity was not assessed by either a specific MC disease activity score, or by endoscopic or histologic assessment, as the recently proposed MC disease activity index was not published at the time we conducted this study.33 Together with the fact that the average age of patients was >60 years, this could mean that those reporting higher levels of fatigue severity and fatigue impact had either active MC, or other unrelated comorbidities that contributed to their fatigue, although those with symptoms attributable to their MC did not have higher mean scores, and there was no significant correlation between age and either fatigue impact or severity scores. Finally, we should reflect on the use of the IBD-F score. This was developed in patients with ‘classical’ IBD diagnoses of UC or Crohn’s disease, rather than MC. The benefits of this scale include the robust, patient-led development, which was well received by participants in the derivation study,29 and the fact that it has been shown to be comparable to other fatigue assessment tools.30 However, further work is needed to confirm the validity of this tool in MC.
Previous data on the prevalence of fatigue in MC are limited. Nyhlin and colleagues conducted a cross-sectional survey in patients with both CC and LC, using recent watery diarrhoea as a marker of disease activity, and assessing for the presence of fatigue using a specific question within their administered questionnaire.16 They suggested that fatigue was more prevalent in those felt to have active disease, but even those with inactive disease were twice as likely to report fatigue, compared with the background population. Although we cannot compare our data to a background population without MC, our use of a structured questionnaire allowed us to assess both the severity and impact of fatigue. We have also shown that the severity and impact of fatigue was not significantly affected by the presence of ongoing symptoms suggestive of active MC. This finding is supported by evidence of a high prevalence of fatigue in patients with both inactive UC and Crohn’s disease,13,19 and although an increase in both the severity and impact of fatigue has been observed in patients with IBD, this did not correlate with objective markers of disease activity, such as faecal calprotectin or haemoglobin.34
Making a direct comparison between patients with MC and classical IBD is difficult, due to the absence of a comparable biomarker for disease activity, and widely accepted clinical disease activity indices are lacking. In terms of data on IBD-F scores in patients with UC or Crohn’s, Vestergaard and colleagues reported median fatigue scores of 9 (IQR 5–11) and 21 (IQR 4–34) for fatigue severity or impact, respectively,31 and Ratnakumaran and colleagues reported mean fatigue severity scores of 11.5 (SD 3.0) in UC and 12.9 (3.6) in Crohn’s, and mean fatigue impact scores of 52.0 (28.7) and 53.5 (22.9) in UC and Crohn’s, respectively.34 However, stratification of either median or mean levels of fatigue severity or impact has not been performed. Some studies in patients with classical IBD have provided data concerning the impact of fatigue on health-related quality of life. One study that used clinical disease activity indices demonstrated that patients with inactive IBD and fatigue also had significant impairment of quality of life, based on the SF-36 scoring system.13 The greatest impact was seen in the domains relating to role limitations in physical health and general health in Crohn’s disease, and role limitation due to physical health in UC.35 In contrast, the significant negative correlations between quality-of-life scores and both fatigue severity and impact we observed were across all domains of the SF-36.
A remaining uncertainty relates to the aetiology of fatigue in those with quiescent ‘classical’ IBD or MC. Piche and colleagues assessed a cohort of patients with quiescent Crohn’s disease, reporting that almost 50% met criteria for IBS according to the Rome III criteria, and that these patients had greater impairments in quality of life, higher rates of depression and higher levels of fatigue.19 More recent data, using faecal calprotectin as a biomarker for disease activity, have shown that patients with quiescent IBD who meet Rome III criteria for IBS report increased levels of anxiety, depression, somatization and impaired quality of life.20 Other authors have concluded that the presence of concomitant IBS-type symptoms, in those with quiescent IBD, might be a key factor in the development of fatigue.18 Similarly, in our study, IBS-type symptoms were significantly associated with higher mean fatigue severity and impact scores. Although we should note the limitations of our study, particularly in determining if participants were suffering from recurrent or active MC, there was no such association demonstrated for symptoms suggestive of ongoing MC activity. Furthermore, there may be a role for low-grade inflammation or chronic immune activation in the development of fatigue, which is distinct from active MC,36,37 although we did not observe an association with specific autoimmune diseases in our study cohort. In contrast, given the observed association of fatigue with psychological comorbidity, our results suggest that central effects occurring due to brain–gut axis activation, rather than mucosal inflammation and disease activity, or somatoform-type behaviour, may be involved in the aetiology of fatigue in MC.
In summary, to our knowledge, this study represents the first attempt to examine factors associated with fatigue severity and impact in MC. It is clear that, regardless of the aetiology of fatigue in patients with a prior diagnosis of MC, coexistent anxiety, depression or somatization are associated with higher levels of fatigue severity, with a significant impact on patients’ lives, and detrimental effects on quality of life. Future prospective studies of fatigue in MC that help to determine the severity and impact of fatigue at diagnosis, after treatment, and in the longer term, are required. Furthermore, there is a growing interest in developing strategies to target fatigue in both UC and Crohn’s disease,38 and our data would suggest that the design of such intervention studies in patients with MC may also be required.
Acknowledgments
JSK and ACF devised the study. JSK and ACF drafted the manuscript. All authors contributed to and approved the final draft of the manuscript.
Footnotes
Funding: This work was supported by an investigator-initiated grant from Dr Falk Pharma UK Ltd. (Grant number: 108649).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: John S. Kane  https://orcid.org/0000-0003-1066-2115
https://orcid.org/0000-0003-1066-2115
Contributor Information
John S. Kane, Leeds Gastroenterology Institute, 4th Floor, Bexley Wing, St. James’s University Hospital, Beckett Street, Leeds LS9 7TF, UK.
Andrew J. Irvine, Leeds Gastroenterology Institute, St. James’s University Hospital, Leeds, UK
Yannick Derwa, Leeds Gastroenterology Institute, St. James’s University Hospital, Leeds, UK.
Alexander C. Ford, Leeds Gastroenterology Institute, St. James’s University Hospital, Leeds, UK Leeds Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, UK
References
- 1. Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 1996; 23: 1407–1417. [PubMed] [Google Scholar]
- 2. Tench CM, McCurdie I, White PD, et al. The prevalence and associations of fatigue in systemic lupus erythematosus. Rheumatology (Oxford) 2000; 39: 1249–1254. [DOI] [PubMed] [Google Scholar]
- 3. Newton JL, Jones DE, Henderson E, et al. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut 2008; 57: 807–813. [DOI] [PubMed] [Google Scholar]
- 4. Van Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther 2010; 32: 131–143. [DOI] [PubMed] [Google Scholar]
- 5. Graff LA, Vincent N, Walker JR, et al. A population-based study of fatigue and sleep difficulties in inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 1882–1889. [DOI] [PubMed] [Google Scholar]
- 6. Romberg-Camps MJ, Bol Y, Dagnelie PC, et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm Bowel Dis 2010; 16: 2137–2147. [DOI] [PubMed] [Google Scholar]
- 7. Minderhoud IM, Oldenburg B, Van Dam PS, et al. High prevalence of fatigue in quiescent inflammatory bowel disease is not related to adrenocortical insufficiency. Am J Gastroenterol 2003; 98: 1088–1093. [DOI] [PubMed] [Google Scholar]
- 8. Villoria A, Garcia V, Dosal A, et al. Fatigue in out-patients with inflammatory bowel disease: prevalence and predictive factors. PLoS One 2017; 12: e0181435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huppertz-Hauss G, Hoivik ML, Jelsness-Jorgensen LP, et al. Fatigue in a population-based cohort of patients with inflammatory bowel disease 20 years after diagnosis: the IBSEN study. Scand J Gastroenterol 2017; 52: 351–358. [DOI] [PubMed] [Google Scholar]
- 10. Han CJ, Yang GS. Fatigue in irritable bowel syndrome: a systematic review and meta-analysis of pooled frequency and severity of fatigue. Asian Nurs Res (Korean Soc Nurs Sci) 2016; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 11. Janssens KA, Zijlema WL, Joustra ML, et al. Mood and anxiety disorders in chronic fatigue syndrome, fibromyalgia, and irritable bowel syndrome: results from the lifelines cohort study. Psychosom Med 2015; 77: 449–457. [DOI] [PubMed] [Google Scholar]
- 12. Frandemark A, Jakobsson Ung E, Tornblom H, et al. Fatigue: a distressing symptom for patients with irritable bowel syndrome. Neurogastroenterol Motil. Epub ahead of print 11 July 2016. DOI: 10.1111/nmo.12898. [DOI] [PubMed] [Google Scholar]
- 13. Jelsness-Jorgensen LP, Bernklev T, Henriksen M, et al. Chronic fatigue is associated with impaired health-related quality of life in inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33: 106–114. [DOI] [PubMed] [Google Scholar]
- 14. Tang YR, Yang WW, Wang YL, et al. Sex differences in the symptoms and psychological factors that influence quality of life in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 2012; 24: 702–707. [DOI] [PubMed] [Google Scholar]
- 15. Lackner JM, Gudleski GD, Dimuro J, et al. Psychosocial predictors of self-reported fatigue in patients with moderate to severe irritable bowel syndrome. Behav Res Ther 2013; 51: 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nyhlin N, Wickbom A, Montgomery SM, et al. Long-term prognosis of clinical symptoms and health-related quality of life in microscopic colitis: a case-control study. Aliment Pharmacol Ther 2014; 39: 963–972. [DOI] [PubMed] [Google Scholar]
- 17. Hjortswang H, Tysk C, Bohr J, et al. Health-related quality of life is impaired in active collagenous colitis. Dig Liver Dis 2011; 43: 102–109. [DOI] [PubMed] [Google Scholar]
- 18. Jelsness-Jorgensen LP, Bernklev T, Moum B. Fatigue and disease-related worries among inflammatory bowel disease patients in remission; is it a reflection of coexisting IBS-like symptoms? A short report. J Psychosom Res 2012; 73: 469–472. [DOI] [PubMed] [Google Scholar]
- 19. Piche T, Ducrotte P, Sabate JM, et al. Impact of functional bowel symptoms on quality of life and fatigue in quiescent Crohn disease and irritable bowel syndrome. Neurogastroenterol Motil 2010; 22: 626-e174. [DOI] [PubMed] [Google Scholar]
- 20. Gracie DJ, Williams CJ, Sood R, et al. Negative effects on psychological health and quality of life of genuine irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2017; 15: 376–384.e375. [DOI] [PubMed] [Google Scholar]
- 21. Limsui D, Pardi DS, Smyrk TC, et al. Observer variability in the histologic diagnosis of microscopic colitis. Inflamm Bowel Dis 2009; 15: 35–38. [DOI] [PubMed] [Google Scholar]
- 22. Beaugerie L, Pardi DS. Review article: drug-induced microscopic colitis - proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 2005; 22: 277–284. [DOI] [PubMed] [Google Scholar]
- 23. Macaigne G, Lahmek P, Locher C, et al. Microscopic colitis or functional bowel disease with diarrhea: a French prospective multicenter study. Am J Gastroenterol 2014; 109: 1461–1470. [DOI] [PubMed] [Google Scholar]
- 24. Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 25. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 26. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999; 282: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 27. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002; 64: 258–266. [DOI] [PubMed] [Google Scholar]
- 28. McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 29. Czuber-Dochan W, Norton C, Bassett P, et al. Development and psychometric testing of inflammatory bowel disease fatigue (IBD-F) patient self-assessment scale. J Crohns Colitis 2014; 8: 1398–1406. [DOI] [PubMed] [Google Scholar]
- 30. Norton C, Czuber-Dochan W, Bassett P, et al. Assessing fatigue in inflammatory bowel disease: comparison of three fatigue scales. Aliment Pharmacol Ther 2015; 42: 203–211. [DOI] [PubMed] [Google Scholar]
- 31. Vestergaard C, Dahlerup JF, Bager P. Validation of the Danish version of inflammatory bowel disease self-assessment scale. Dan Med J 2017; 64: pii: A5394. [PubMed] [Google Scholar]
- 32. Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407.e1395. [DOI] [PubMed] [Google Scholar]
- 33. Cotter TG, Binder M, Loftus EV, Jr, et al. Development of a microscopic colitis disease activity index: a prospective cohort study. Gut 2018; 67: 441–446. [DOI] [PubMed] [Google Scholar]
- 34. Ratnakumaran R, Warren L, Gracie DJ, et al. Fatigue in inflammatory bowel disease reflects mood and symptom-reporting behavior rather than biochemical activity or anemia. Clin Gastroenterol Hepatol 2018; 16: 1165–1167. [DOI] [PubMed] [Google Scholar]
- 35. Jelsness-Jorgensen LP, Bernklev T, Henriksen M, et al. Chronic fatigue is more prevalent in patients with inflammatory bowel disease than in healthy controls. Inflamm Bowel Dis 2011; 17: 1564–1572. [DOI] [PubMed] [Google Scholar]
- 36. Pardi DS. Diagnosis and management of microscopic colitis. Am J Gastroenterol 2017; 112: 78–85. [DOI] [PubMed] [Google Scholar]
- 37. Munch A, Aust D, Bohr J, et al. Microscopic colitis: current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis 2012; 6: 932–945. [DOI] [PubMed] [Google Scholar]
- 38. Vogelaar L, Van’t Spijker A, Timman R, et al. Fatigue management in patients with IBD: a randomised controlled trial. Gut 2014; 63: 911–918. [DOI] [PubMed] [Google Scholar]


