Abstract
Stereotactic radiosurgery provides effective local control, but high recurrence rate are observed while ipilimumab have shown promising improvements in survival in the treatment of melanoma brain metastases. This meta-analysis was done to review the clinical evidence regarding the combination of stereotactic radiosurgery and ipilimumab in the treatment of brain metastases from melanoma. Comprehensive research of the electronic databases (PubMed and Cochrane Library) was carried out in April 2017. Different combination of MESH headings and words were used. Review Manager was used to analyze the outcome data of interest. According to heterogeneity, fixed effects model or random effects model was adapted. Six retrospective studies comparing stereotactic radiosurgery plus ipilimumab with stereotactic radiosurgery alone were found. Total of 411 participants were included in this meta-analysis. Of that, 128 patients had received stereotactic radiosurgery + ipilimumab, while 283 patients had received stereotactic radiosurgery only. Stereotactic radiosurgery plus ipilimumab significantly improved survival when compared to stereotactic radiosurgery alone (hazard ratio: 0.74 [95% confidence interval: 0.56-0.99, P = .04]), with no significant increase in the incidence of adverse events (odds ratio 0.57 [95% confidence interval: 0.28-1.17, P = .12]). Stereotactic radiosurgery with ipilimumab is safe and effective treatment option and can be recommended for the treatment of brain metastases in patients with melanoma.
Keywords: melanoma brain metastases, ipilimumab, stereotactic radiosurgery, overall survival
Introduction
Melanoma is the third leading cause of brain metastases (BM).1 Prognosis is poor, and median survival for patients developing BM is merely 17 to 22 weeks.1,2 Neurologic death is the main cause for patients with BM from melanoma; 20% to 54% of patients with melanoma BM die from BM.3,4
Management of BM includes surgery, whole-brain radiotherapy, and stereotactic radiosurgery (SRS).2–7 However the optimal choice of treatment is controversial. Whole-brain radiation therapy (WBRT) provides less tumor control in the treatment of BM from melanoma and is considered to be radioresistant.1,6 Radiosurgery, on the other hand, has overcome this limitation by providing high doses of radiation, and so a high tumor control has been reported between 73% and 90%.7–10
Systemic therapies are getting importance, as more and more drugs are approved for the treatment of metastatic melanoma and has shown improved progression-free survival and overall survival (OS). However, in brain, their activity might be compromised due to blood–brain barrier.2,3,11 Immunotherapy has reported positive impact on OS in patients with BM from melanoma primarily the immune checkpoint inhibitors: anti-cytotoxic T lymphocytic antigen 4 (CTLA)-4 antibody and recently anti-programmed death 1 (PD-1) antibody.12,13 Recent studies suggest the combination of local radiotherapy and immunotherapy producing synergistic response in local control as well as abscopal effect (activated T cells attacking cancer cells outside the irradiated area) is of immense importance for the treatment outcome of melanoma BM.14–19
A number of studies has already reported better response from this combination in melanoma BM, and hence, this meta-analysis was undertaken in an effort to combine the results of these studies and provide a better conclusive evidence for future.1,20–25
Methods
Research Strategy
A comprehensive literature search (PubMed database and Cochrane Library database) was carried out for studies comparing the clinical outcome of SRS alone and in combination with ipilimumab (IPI). Various searching terms were used: ((Stereotactic Radiosurgery OR SRS) AND (immune checkpoint inhibitors OR immunotherapy OR Anti-CTLA-4* OR Ipilimumab)) AND (Metastatic brain melanoma OR Metastatic melanoma*). In addition, in an attempt to broaden the search, the related articles as well as the reference lists were also searched manually for all available articles.
Eligibility Criteria and Outcomes of Interest
An eligibility criterion was set up for studies to be included: (1) studies comparing SRS plus IPI (anti-CTLA4 antibody) versus SRS alone, (2) patients with melanoma having BM, (3) published in English, and (4) no restriction on study designs. Primary outcomes of interest assessment were OS and adverse events, while secondary outcome of interest was brain tumor control. Additionally, the sequence of treatment induction was assessed as well.
Data Extraction and Quality Assessment
Survival graphs as well as other outcome of data were extracted by 2 reviewers. Results were verified by reviewers. If discrepancy was present, the authors had a discussion and drew a final decision. The quality of included studies was estimated according to the Newcastle-Ottawa Scale.26
Statistical Analysis
This meta-analysis was carried out using the Review Manager (RevMan) software, version 5.2. Pooled hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for time to event data using fixed-effects model.27 Continuous variables using weighted mean difference and a 95% CI; dichotomous variables were analyzed using odds ratios and 95% CI. A fixed effect model or a random effects model mode was applied. Heterogeneity was evaluated by chi square (χ2) and I2. Heterogeneity was considered to be present if the I2 statistic was >50%, and a random effect model was adopted. However, if I2 statistic was <50%, a fixed effect model was used. A value of P < .05 was considered to be significant. Funnel plot was used to evaluate publication of bias.
Results
Characteristics of the Included Studies
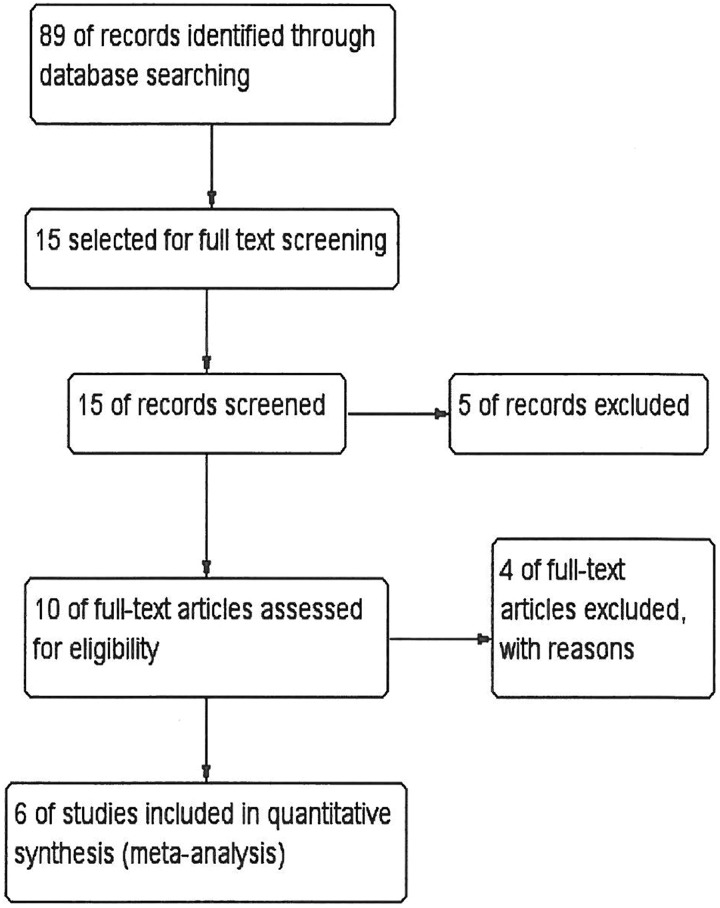
A total of 89 reports were identified; following the exclusion process, 6 retrospective studies1,20–24 involving 411 patients with melanoma having BM were included in the final analysis (Figure 1). The main characteristics of the included studies are listed in Table 1. Stereotactic radiosurgery doses were approximately same across studies depending on the lesion mass (15 Gy-24 Gy). Fractionation was not reported in most of the studies except for Patel et al.22 Only 4 patients had received fractionated SRS (3-5) and were associated with positive response (single fraction (50) versus 3-5f; HR 0.35; P = .04). Fractionation is believed to have positive influence on immune response when used in combination with IPI.
Figure 1.
Flow diagram of the literature research and study selection process.
Table 1.
General Characteristics of the Included Studies.a
| Study | Year | Design | Treatment | Dosage | No. of points | Age (Mean/Median) | Median Survival | QS |
|---|---|---|---|---|---|---|---|---|
| Knisely et al 1 | 2012 | Retrospective | SRS + IPI | NA | 27 | 53.2 | 21.3 | 7 |
| SRS | NA | 50 | 59.3 | 4.9 | ||||
| Silk et al 20 | 2013 | Retrospective | SRS + IPI | 3 mg/kg, every 3 weeks | 17 | 56.6 | 18.3 | 7 |
| SRS | 14-24 Gy-1-5f | 16 | 57.7 | 5.3 | ||||
| Sana Shoukat et al 23 | 2013 | Retrospective | SRS + IPI | NA | 11 | 43.16 | 28.3 | 6 |
| SRS | NA | 124 | 54.84 | 6.8 | ||||
| Mathew et al 21 | 2013 | Retrospective | SRS + IPI | 3 mg/kg, every 3 weeks | 25 | 62 med | 5.9 | 7 |
| SRS | 20 Gy | 33 | 57 med | 4.3 | ||||
| Patel et al 22 | 2015 | Retrospective | SRS + IPI | 3 mg/kg within 4 mts | 20 | 56.5 med | 5.9 | 7 |
| SRS | 15-20 Gy-3-5 f (4) | 34 | 60.2 med | |||||
| Choong et al 24 | 2017 | Retrospective | SRS + IPI | NA | 28 | NA | 7.5 | 5 |
| SRS | NA | 26 | NA | 10.8 |
Abbreviations: IPI, ipilimumab; NA, not applicable; QS, quality score; SRS, Stereotactic radiosurgery.
a Table 1 shows the general characteristics from the studies included in this meta-analysis. Median survival is shown in months. Some ages were reported in median age only (med = median). The quality of included studies was estimated according to the Newcastle-Ottawa Scale.
Meta-Analysis
Baseline characteristics
Five studies reported baseline characteristics for treatment comparison. A meta-analysis was done for common baseline characteristics (specifically baseline characteristics prognostic of survival outcome) to seek any significant differences between the studies. Odds ratio of all characteristics are given in the Table 2. Participants included in the studies were not significantly different from each other.
Table 2.
Comparison of Baseline Characteristics of the Included Studies.
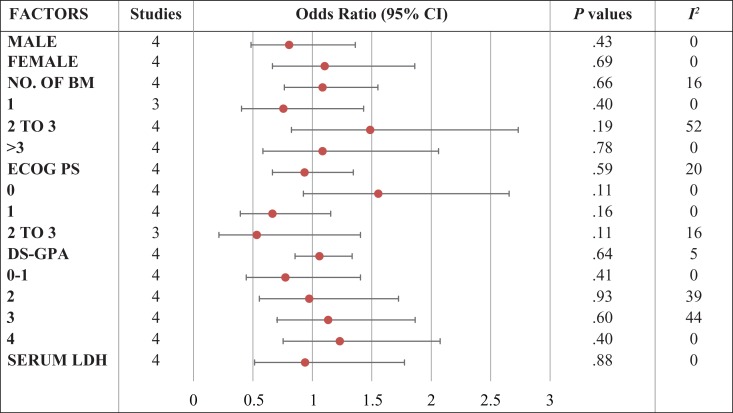
|
Abbreviations: BM, brain metastases; CI, confidence interval; DS-GPA, Diagnosis Specific Graded Prognostic Assessment; ECOG PS, Eastern Cooperative Oncology Group Performance Status; LDH, lactate dehydrogenase.
Overall survival
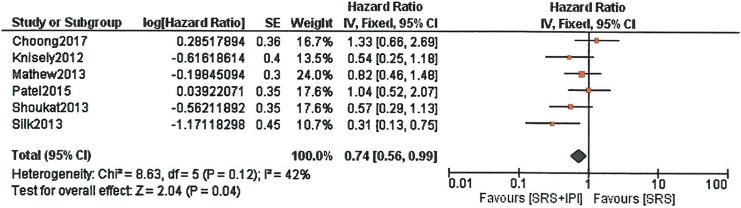
Meta-analysis of the survival outcome revealed significant survival for patients receiving SRS with IPI. Hazard ratio was 0.74 (95% CI: 0.56-0.99, P = .04) based on 6 retrospective studies (Figure 2).
Figure 2.
Forest plot of overall survival with stereotactic radiosurgery (SRS) plus ipilimumab versus SRS only. Hazard ratios with 95% confidence interval (CI) are given.
Sequencing of IPI and SRS
Four studies, using various outcomes (survival, brain tumor control, and tumor response), assessed the difference in response of patients receiving IPI before SRS, during SRS, and after SRS. Knisely et al 1 reported no difference in the hazard for death whether drug was started before or after the first SRS. Median survival for patients receiving IPI before SRS was 19.8 months, and for patients receiving after the SRS, the median survival was 21.3 months. Silk et al 20 found no significant difference in the responses of patients who received IPI before versus after the radiotherapy (P = .224). Assessing by the incidence of new BM, Mathew et al 21 found no significant difference between those who received IPI before (n = 4) versus concurrently (n = 7) with radiation therapy (RT; P = .224), those who received IPI concurrently versus after (n = 10) RT (P = .907), or those who received IPI before versus after RT (P = .109). Patel et al 22 reported 1-year and 2-year survival with IPI administered within 14 days of SRS (33.8% and 16.9%) and >14 days but within 4 months (38.5% and 25.7%). The results didn’t differ greatly.
Local and distant tumor control
Three studies reported the brain tumor control (local and distant) for the treatment difference. No difference was reported in local tumor control and distant brain recurrence. Mathew et al 21 reported 63% and 65% six-month local control for patients receiving IPI plus SRS and SRS only (P = .55). This study also revealed 6-month freedom from new BM (SRS + IPI vs SRS, 35% vs 47%, P = .48). Second study (n = 54)22 reported similar 1-year local control rate for IPI plus SRS and SRS alone (71.4% vs 92.3%, P = .40). Distant intracranial-free (DIF) survival was also not significantly different between the groups; median DIF survival was 4.2 versus 3.1 months, and 1-year free survival was 12.7% versus 29.1%, P = .592. Third study24 reported the median duration of brain control (BC) with patients receiving IPI within 6 weeks of SRS was 7.5 months while for that with SRS only was 10.8 months. These differences were significant.
Adverse events
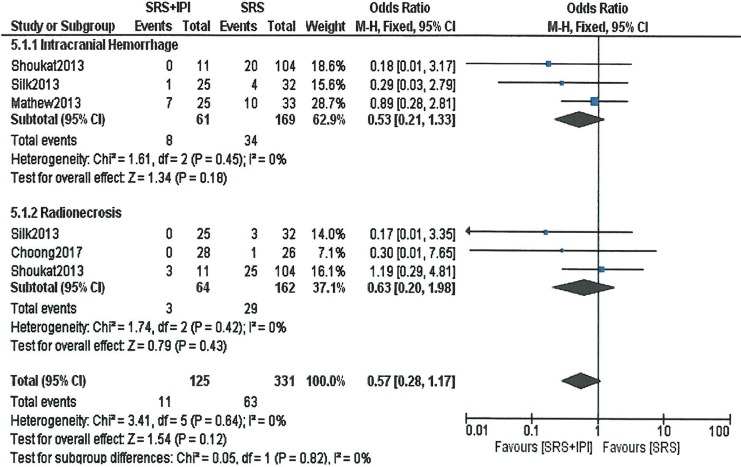
Based on the 3 studies that reported the toxicities, a meta-analysis was carried out to observe the overall effect. No difference in the adverse events (intracranial hemorrhage, radionecrosis) was observed in the treatment groups. The odds ratio was 0.57 (95% CI: 0.28-1.17, P = .12) for overall toxicity (Figure 3).
Figure 3.
Forest plot of toxicity (intracranial hemorrhage, radionecrosis) with stereotactic radiosurgery (SRS) plus ipilimumab versus SRS only. Odds ratios with 95% confidence interval (CI) are given.
Another study (n = 54)22 reported trend toward developing higher rates of radiation necrosis at 1 year in the IPI and SRS cohort (30.0% vs 20.92%, P = .078). One year rates of hemorrhage between the IPI plus SRS cohort and the SRS-alone cohort demonstrated no statistical difference (15% vs 14.7%, P = 1.00).
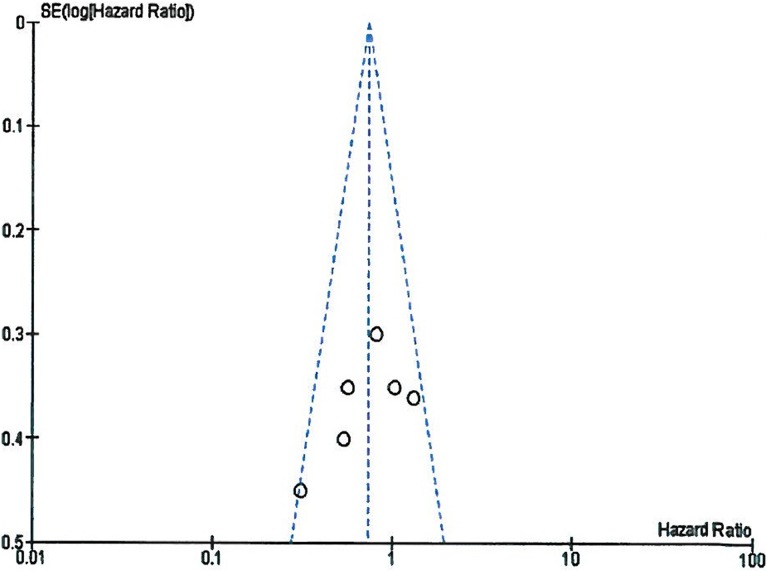
Publication of bias
Funnel plot indicated no apparent publication bias existed in this meta-analysis. All included studies were inside the 95% CI and symmetrical around the vertical as shown in Figure 4.
Figure 4.
Funnel plot illustrating the publication bias of the included studies.
Discussion
Clinical management of BM from melanoma is complex and controversial. It includes surgery, SRS and WBRT, or combination of these treatments.2–8 Chemotherapy role is limited as its penetration to brain is limited due to blood–brain barrier. B-rapidly accelerated fibrosarcoma (BRAF)-targeted therapy has shown some success, but it is limited due to its specificity against specific mutations.28 Moreover, high rate of distant brain recurrence and brain was the common site of treatment failure when used for patients without BM. Probably, this failure comes from inability of these drugs to potentially penetrate blood–brain barrier.2,3
Recent advancements in immunotherapy have opened a new dimension of treatment in coping with BM from melanoma. Unlike cytotoxic chemotherapy and targeted therapies, immunotherapeutic agents does not need to cross blood–brain barrier to be effective.29 Of these agents, the anti-CTLA-4 antibody have shown greater potency in achieving survival advantage. Cytotoxic T-lymphocytes antigen 4 expressed on T cells causing inhibition of T cell activation by antigen presenting cells (APCs) act as immune checkpoint. Ipilimumab (a humanized monoclonal antibody), an antibody to CTLA-4 antigen thereby allowing continued activation of T cells by APCs. In 2011, it was approved by the Food and Drug Administration for treatment of metastatic melanoma after it showed improved survival in an randomized clinical/controlled trial.30 Initially IPI use was restricted to patients with melanoma with no active BM. After discovering its activity in few patients, a clinical trial was started to assess its efficacy in the BM. A positive response (objective response of 15% and stable disease at 12 weeks) was reported with IPI in the BM from melanoma.31 Immunomodulatory effects, observed with RT including upregulation of antigen presentation, cytokine release, and activation of immune response could be augmented by the addition of immune checkpoint inhibitors producing more strong synergistic antitumor response. This synergism had been reported in preclinical research and clinical reports have also confirmed the combination of these modalities for better antitumor immune response.13–19 Number of studies has reported this concomitant or sequential combination of these 2 treatment modalities in patients with melanoma. Filippie et al have reported a great account of evidence in this direction.32 We have attempted to gather the evidence specifically limited to SRS and IPI in patients with BM from melanoma.
Prognostic factors associated with survival for melanoma BM are reported in several studies. Increased number of parenchymal BM, leptomeningeal involvement, and development of BM after receiving systemic therapy for extracranial metastases were identified to be significant prognostic factors for OS in a study done by Davies et al.4 Number of brain metastasis and pretreatment level of lactate dehydrogenase (LDH) were identified in a separate study to be the prognostic factors affecting OS.33 Similarly, melanoma-specific graded prognostic assessment by Sperduto et al 34 also identified Karnofsky Performance Scale and number of BM to be of prognostic value in these patients. Hence, a meta-analysis of the baseline characteristics was carried out to improve the validity of the included studies for survival outcome. No significant differences were found in the baseline characteristics of the patients included in each study.
This meta-analysis results showed a significantly better survival from the combination of SRS and immune checkpoint inhibitors in the treatment of BM from melanoma when compared to SRS only. Tazi et al 35 reported a median survival of 29.3 months from date of stage IV for cohort A (10 patients of stage IV melanoma with BM) endorsing our results. Kiess et al 36 study also demonstrated better OS of 12.4 months when compared to previous studies reporting survival for each treatment alone. Additionally, Choong et al 24 reported a better response with SRS plus anti-PD1 antibody when compared to SRS plus anti-CTLA4 antibody (median survival, 20.4 vs 7.5 months). This result was further endorsed by Ahmed et al 25 reporting a significantly better survival with anti-PD1 (6- and 12-month) OS rates following SRS were 76%/48% (anti-PD-1 therapy) and 68%/41% (anti-CTLA-4 therapy). These results showed that anti-PD1 seems to be more potent.
High brain recurrence is reported in patients with melanoma after SRS or surgery, despite having greater local control.8–10 In this meta-analysis, 3 studies21,22,24 reported local as well as distant brain control, and no difference was observed between the treatment arms. Kiess et al reported a regional recurrence of 69% and 64% for patients receiving IPI during or after SRS, suggesting immunomodulatory effects. Choong et al 24 study revealed median BC with anti-PD-1 therapy (12.7 months) when compared to anti-CTLA-4 therapy (7.5 months). Similarly, Ahmed et al 25 study reported enhanced 6- and 12-month distant melanoma BM control rates with anti-PD-1 therapy (61%/38%) when compared to anti-CTLA-4 therapy (26%/21%). However, local control rates were not different between the treatments.
The optimal timing of the 2 treatment modalities is an important aspect. Different pros and cons for administering radiotherapy before or after immunotherapy has been discussed. For example, if there is already immunoadjuvant present in the tumor microenvironment, it could maximize the radiation induced effects. On the other hand, radiation cytotoxicity could disrupt as ongoing antitumor immune response. Administration after RT; it has been argued that RT could generate de novo antigens and break any preexisting peripheral tolerance for targeted immune checkpoints of T-cell activation to be more effective.14 Studies included in this meta-analysis used different sequence induction (IPI before, during, and after SRS) of these modalities and used various outcomes to judge the effectiveness of the combination. Nonetheless, none of the studies favored any single sequence to be more effective, and responses from different timings were almost similar. Kiess et al reported better survival as well as tumor control for patients receiving IPI during or after SRS. In the study done by Qian et al 37, assessing the concurrent induction of IPI with SRS versus after SRS reported that the administration within 4 weeks of SRS (concurrent) results in an improved lesional response of melanoma BM in comparison with treatment separated by longer than 4 weeks. Local recurrence-free duration was longer with SRS and IPI when SRS was performed before or during the IPI treatments in the study done by Cohen-Inbar et al.38 Based on these results, it can be said that IPI is more effective given concurrently or after the induction of SRS, but duration between the treatments should not be longer. Further investigation is needed to validate the optimal timing of treatments.
This met-analysis has some limitations. First, the small sample sizes of the included studies. Second, all the studies were retrospective studies and one23 with historical controls. Due to retrospective nature of the studies, it is likely to have some degree of selection bias. Participants receiving IPI in addition to SRS were comparatively younger in the study done by Sana Shoukat et al.23 Choong et al’s 24 study didn’t provide the baseline characteristics for each treatment group separately and so more susceptible for selection bias. Furthermore, Knisley’s et al 1 didn’t account for prognostic patient characteristics (LDH, active systemic disease, and primary controlled) in their analysis. B-rapidly accelerated fibrosarcoma inhibitor use was also not recorded in this study. Eastern Cooperative Oncology Group Performance Status, subsequent radiotherapy, and BRAF inhibitor treatment was significantly more common in combined treatment arm in Silk et al’s study20 potentiating selection bias. These 2 studies have shown significant survival advantage could possibly be due to selection bias mainly from BRAF inhibitor use.
A previous study on the same topic has been published before with survival analysis based only on the data collected from 3 studies.39 We have included more studies in number to endorse the results making it more comprehensive report. We have also included a meta-analysis of baseline characteristics of prognostic importance in order to exclude bias reported with retrospective studies. Also, the inclusion of sequence analysis make this meta-analysis more detailed and comprehensive.
Recent trend of using SRS alone in order to preserve patients’ neurocognitive function could be endorsed with the addition of IPI, as significant survival is achieved with this combination. Another aspect that remains to be addressed is if this combination could also control the intracranial microscopic disease in order to replace WBRT use in this group of patients and when presented with multiple BM. At present, few clinical trials are already underway to evaluate the efficacy of this combination (NCT01950195, NCT01703507, and NCT02097732).3
Conclusions
Stereotactic radiosurgery in combination with IPI is an effective treatment combination producing survival advantage in patients with melanoma BM with no increase in toxicity.
Stereotactic radiosurgery in combination with IPI significantly improved OS when compared to SRS alone.
Regional recurrence as well as development of distant new metastases in brain didn’t differ between the treatments.
Assessing by various treatment outcomes, IPI induction before, during, or after SRS didn’t affect treatment outcomes.
Addition of IPI didn’t increase the incidence of adverse events.
Acknowledgments
The authors acknowledge the support from the National Natural Science Foundation of China Grants (81572964, 81773354, 81502342 and 81502194) and Guangzhou Key Medical Discipline Construction Project.
Abbreviations
- APCs
antigen presenting cells
- BM
brain metastases
- BRAF
B-rapidly accelerated fibrosarcoma
- CI
confidence interval
- CTLA-4
cytotoxic T lymphocytic antigen 4
- HR
hazard ratio
- IPI
ipilimumab
- LDH
lactate dehydrogenase
- OS
overall survival
- PD-1
programmed death 1
- RT
radiation therapy
- SRS
stereotactic radiosurgery
- WBRT
whole brain radiation therapy.
Authors’ Note: All authors have contributed equally to this work. As this study is a meta-analysis of randomized control trials, IRB approval was not necessary.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China Grants (81572964, 81773354, 81502342 and 81502194) and Guangzhou Key Medical Discipline Construction Project.
ORCID iD: Muhammad Khan, MBBS, MD  http://orcid.org/0000-0002-2207-4823
http://orcid.org/0000-0002-2207-4823
References
- 1. Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramanujam S, Schadendorf D, Long GV. Systemic therapies for melanoma brain metastases: which drug for whom and when? Chin Clin Oncol. 2015;4(2):25. [DOI] [PubMed] [Google Scholar]
- 3. Goyal S, Silk AW, Tian S, et al. Clinical management of multiple melanoma brain metastases: a systematic review. JAMA Oncol. 2015;1(5):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies MA, Liu P, McIntyre S, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer. 2011;117(8):1687–1696. [DOI] [PubMed] [Google Scholar]
- 5. Rauschenberg R, Tabatabai G, Troost EG, Garzarolli M, Beissert S, Meier F. Melanoma brain metastases: treatment options [in German]. Hautarzt. 2016;67(7):536–543. [DOI] [PubMed] [Google Scholar]
- 6. Jang S, Atkins MB. Treatment of melanoma CNS metastases. Cancer Treat Res. 2016;167:263–279. [DOI] [PubMed] [Google Scholar]
- 7. Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lippitz B, Lindquist C, Paddick I, Peterson D, O’Neill K, Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40(1):48–59. [DOI] [PubMed] [Google Scholar]
- 9. Gaudy-Marqueste C, Regis JM, Muracciole X, et al. Gamma-knife radiosurgery in the management of melanoma patients with brain metastases: a series of 106 patients without whole-brain radiotherapy. Int J Radiat Oncol Biol Phys. 2006;65(3):809–816. [DOI] [PubMed] [Google Scholar]
- 10. Mathieu D, Kondziolka D, Cooper PB, et al. Gamma knife radiosurgery in the management of malignant melanoma brain metastases. Neurosurgery. 2007;60(3):471–481; discussion 481–472. [DOI] [PubMed] [Google Scholar]
- 11. Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book. 2013:393–398. [DOI] [PubMed] [Google Scholar]
- 12. Cohen JV, Kluger HM. Systemic immunotherapy for the treatment of brain metastases. Front Oncol. 2016;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gerber NK, Young RJ, Barker CA, et al. Ipilimumab and whole brain radiation therapy for melanoma brain metastases. J Neurooncol. 2015;121(1):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013;123(7):2756–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel KR, Lawson DH, Kudchadkar RR, et al. Two heads better than one? Ipilimumab immunotherapy and radiation therapy for melanoma brain metastases. NeuroOncol. 2015;17(10):1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walshaw RC, Honeychurch J, Illidge TM. Stereotactic ablative radiotherapy and immunotherapy combinations: turning the future into systemic therapy? Br J Radiol. 2016;89(1066):20160472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franceschini D, Franzese C, Navarria P, et al. Radiotherapy and immunotherapy: can this combination change the prognosis of patients with melanoma brain metastases? Cancer Treat Rev. November 2016;50:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899–906. doi:10.1002/cam4.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mathew M, Tam M, Ott PA, et al. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013;23(3):191–195. [DOI] [PubMed] [Google Scholar]
- 22. Patel KR, Shoukat S, Oliver DE, et al. Ipilimumab and stereotactic radiosurgery versus stereotactic radiosurgery alone for newly diagnosed melanoma brain metastases. Am J Clin Oncol. 2017;40(5):444–450. [DOI] [PubMed] [Google Scholar]
- 23. Shoukat S, Marcus DM, Rizzo M, Lawson DH, Liu Y, Khan MK. Outcome with stereotactic radiosurgery and ipilimumab for malignant melanoma brain metastases. J Clin Oncol. 2013;31(suppl). Abstract 3032. [Google Scholar]
- 24. Choong ES, Lo S, Drummond M, et al. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer. 2017;75:169–178. [DOI] [PubMed] [Google Scholar]
- 25. Ahmed KA, Abuodeh YA, Echevarria MI, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol. 2016;27(12):2288–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wells G, Shea B, O’Connell D, et al. NewCastle-Ottawa Quality Assessment Scale —Cohort Studies[EB/OL]. 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed April 15, 2017.
- 27. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 doi:10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moraes FY, Taunk NK, Marta GN, Suh JH, Yamada Y. The rationale for targeted therapies and stereotactic radiosurgery in the treatment of brain metastases. Oncologist. 2016;21(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12(9):623–635. [DOI] [PubMed] [Google Scholar]
- 30. Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 32. Filippi AR, Fava P, Badellino S, Astrua C, Ricardi U, Quaglino P. Radiotherapy and immune checkpoints inhibitors for advanced melanoma. Radiother Oncol. 2016;120(1):1–12. [DOI] [PubMed] [Google Scholar]
- 33. Eigentler TK, Figl A, Krex D, et al. Number of metastases, serum lactate dehydrogenase level, and type of treatment are prognostic factors in patients with brain metastases of malignant melanoma. Cancer. 2011;117(8):1697–1703. [DOI] [PubMed] [Google Scholar]
- 34. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 35. Tazi K, Hathaway A, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med. 2015;4(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122(19):3051–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurg. 2017;127(5):1–8. [DOI] [PubMed] [Google Scholar]
- 39. Nguyen SM, Castrellon A, Vaidis O, Johnson AE. Stereotactic radiosurgery and ipilimumab versus stereotactic radiosurgery alone in melanoma brain metastases. Cureus. 2017;9(7):e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]