Abstract
Non-monotonic dose response curves (NMDRCs) occur in cells, tissues, animals and human populations in response to nutrients, vitamins, pharmacological compounds, hormones and endocrine disrupting chemicals (EDCs). Yet, regulatory agencies have argued that NMDRCs are not common, are not found for adverse outcomes, and are not relevant for regulation of EDCs. Under the linear dose response model, high dose testing is used to extrapolate to lower doses that are anticipated to be ‘safe’ for human exposures. NMDRCs that occur below the toxicological no-observed-adverse-effect level (NOAEL) would falsify a fundamental assumption, that high dose hazards can be used to predict low dose safety. In this commentary, we provide examples of NMDRCs and discuss how their presence in different portions of the dose response curve might affect regulatory decisions. We provide evidence that NMDRCs do occur below the NOAEL dose, and even below the ‘safe’ reference dose, for chemicals such as resveratrol, permethrin, chlorothalonil, and phthalates such as DEHP. We also briefly discuss the recent CLARITY-BPA study, which reported mammary adenocarcinomas only in rats exposed to the lowest BPA dose. We conclude our commentary with suggestions for how NMDRCs should be acknowledged and utilized to improve regulatory toxicity testing and in the calculation of reference doses that are public health protective.
Keywords: biphasic, endocrine disruptor, linear dose response, reference dose, risk assessment, test guideline
Introduction
Nonmonotonic dose–response curves (NMDRCs) are mathematically defined as a change in the sign (positive/negative) of the slope of a dose–response relationship over the range of doses tested.1 Numerous studies have recognized the occurrence of NMDRCs in organisms’ responses to nutrients, vitamins, pharmacological compounds, and other small molecules that interact with receptors including hormones.2 As the study of endocrine-disrupting chemicals (EDCs) has developed over the last 2 decades, many examples of NMDRCs have been identified in the peer-reviewed literature and include studies conducted in cultured cells, laboratory animals, and human populations.2–4
Prior to an international scientific meeting held in Berlin in 2012,5 the US Environmental Protection Agency (EPA) did not consider the issue of nonmonotonicity when establishing regulatory standards. Discussions at this meeting led the agency to collaborate on a review of nonmonotonicity, the draft of which was released in June 2013.6 The report concluded that NMDRCs occur for estrogen, androgen, and thyroid hormone receptor pathways and are not unexpected in vitro. It also concluded that NMDRCs are not common in vivo and that there is no evidence that NMDRCs occur for adverse outcomes in humans or wildlife. The EPA and others7 have also suggested that while NMDRCs may occur in EDC-exposed animals and human populations, the outcomes demonstrating nonmonotonicity do not represent “adverse effects” and therefore are not relevant for chemical safety regulation.8 It has also been suggested that NMDRCs only occur at high-dose ranges and thus do not influence the setting of “safe” levels of exposure.7
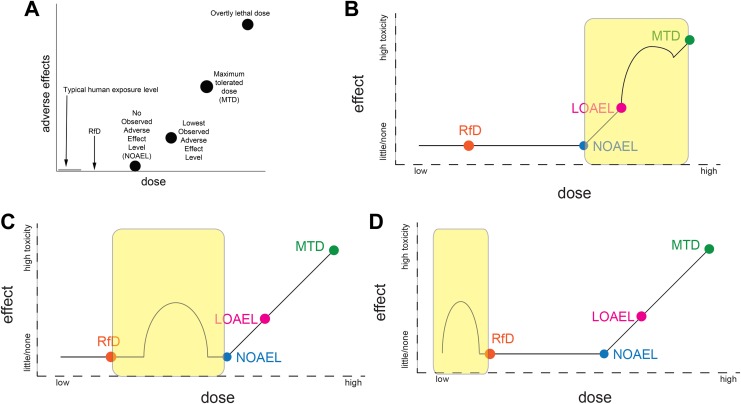
Under the linear dose–response model, high-dose testing is used to extrapolate to lower doses that are anticipated to be “safe” for human exposures (Figure 1A).9 The existence of NMDRCs in the range of human exposure levels would therefore appear to falsify a fundamental assumption, that high-dose hazards can be used to predict low dose safety. Here, we examine the relevance of nonmonotonicity to the process of setting chemical safety standards, specifically to determine whether the existence of NMDRCs in 3 different portions of the dose–response curve might affect regulatory decisions.
Figure 1.
Examples illustrating nonmonotonic dose–response curves (NMDRCs) at different portions of the dose–response curve relative to the no observed adverse effect level (NOAEL) and reference dose (RfD). A, Circles indicate doses typically examined in conventional toxicity tests, which are used to calculate the RfD. This figure shows the expected relationship between human exposure levels, the RfD, and the NOAEL; no adverse effects are expected at human exposure levels, the RfD, or the NOAEL, if the NOAEL is a “true” threshold for adverse effects. B, Case 1, where nonmonotonicity is observed above the NOAEL. C, Case 2, where nonmonotonicity occurs at exposures between the RfD and the NOAEL. D, Case 3, where nonmonotonicity occurs at exposures below the RfD.
Case 1: Nonmonotonicity Is Observed at High Levels of Exposure
Nonmonotonic dose–response curves might be detected by conventional testing protocols, although the likelihood of detection would be affected by the number and range of doses examined.4 Conventional toxicity studies (eg, Organisation for Economic Co-operation and Development test guidelines), examining traditional end points of toxicity, are used to establish no observed adverse effect levels (NOAELs). Considering how the NOAEL dose is used in risk assessment (ie, it is typically divided by a number of uncertainty factors to determine the acceptable daily intake or reference dose, RfD), the appearance of NMDRCs above the NOAEL is not expected to influence risk assessments (Figure 1B); while identifying NMDRCs may provide mechanistic insight into the actions of specific chemicals, or at least the pathways operating at overtly toxic doses, NMDRCs in the high-dose range (eg, at doses above the NOAEL) appear to have little consequence for the identification of a RfD. Thus, NMDRCs that occur above the NOAEL are irrelevant when drawing extrapolations to “safe” doses.
We encountered several examples of NMDRCs at high levels of exposure (above the NOAEL) in the peer-reviewed literature. One example revealed an inverted U-shaped relationship between 2,3,7,8-tetrachlorodibenzodioxin (TCDD) exposures and cell-mediated immunity in male rats.10 These effects were observed at 1 to 90 µg/kg/d TCDD, with significant effects at 10 and 20 µg/kg/d, above the toxicological NOAEL (<0.02 ng/kg/d). Another example of nonmonotonicity above the NOAEL was observed in male mice exposed to dichlorodiphenyltrichlorethane (DDT) during development.11 Anogenital distance was significantly affected at 200 and 100 000 µg/kg/d, but not 20, 2000, or 20 000 µg/kg/d. Both doses with significant effects were higher than the toxicological NOAEL of 50 µg/kg/d. These examples provide evidence that NMDRCs occur at high doses above the set NOAEL. However, as noted above, their presence is not likely to influence the establishment of the RfD.
Case 2: Nonmonotonicity Occurs Below the NOAEL, but Above the RfD
If nonmonotonicity occurs at a dose above the NOAEL, but no effects are observed at the NOAEL (case 1), it can be concluded that the NOAEL is “true,” for example, without adverse effects at lower doses. However, if NMDRCs occur between the NOAEL and the RfD (Figure 1C), they would likely not be revealed by conventional testing, which rarely examines sufficient doses below the NOAEL. More importantly, because risk assessments depend on an expectation of monotonicity (at least below the NOAEL dose), it would suggest that the RfD should not be calculated or extrapolated from the toxicological NOAEL, and the “true” NOAEL dose may be much lower. As noted elsewhere, it is important to evaluate each study individually and determine whether the significant effects are statistical artifacts12; of course, considering the known mechanisms by which NMDRCs manifest, it should not be a default assumption that these phenomena are not “real.”2,3
We identified multiple examples in the peer-reviewed literature where NMDRCs occur in the range of doses between the NOAEL and the RfD. One example demonstrates a nonmonotonic relationship between exposure to resveratrol and the severity of stomach lesions after treatment with an ulcer-inducing chemical.13 Doses in the range of 1 to 2 mg/kg/d decreased the ulcer index, whereas higher doses, 5 and 10 mg/kg/d, increased the ulcer index in mice. With a NOAEL of 300 mg/kg/d and an RfD of 0.3 mg/kg/d, this example illustrates an NMDRC occurring in between these toxicological dose markers. Another example showed that 1.5 mg/kg/d permethrin altered dopamine transport in mice, whereas higher (3 mg/kg/d) and lower (0.4, 0.8 mg/kg/d) doses were ineffective.14 These effects were observed above the RfD of 0.005 mg/kg/d and below the NOAEL of 5 mg/kg/d.
Case 3: Nonmonotonicity Occurs Below the RfD Established by High-Dose Experiments and/or in the Range of Known Human Exposures
Doses at or below the RfD are rarely directly tested in conventional toxicity studies; studies utilizing standard test guidelines typically aim to identify an NOAEL and examine only 3 or 4 doses—usually higher than the NOAEL. If NMDRCs are observed below the RfD (Figure 1D), the RfD (and, by extrapolation, the NOAEL) would be scientifically flawed and insufficiently protective of public health.
The more than 30 NMDRCs observed in human epidemiology studies fall into this category2 and provide evidence that human exposure levels, which are likely below the RfD, can result in adverse health outcomes. It is important to view these findings in light of the limitations of epidemiological studies (eg, there is often limited power to perform multiple comparisons between groups) but also acknowledge that many epidemiology studies are not designed to evaluate NMDRCs, meaning they likely go undetected.
We also identified multiple examples of NMDRCs occurring at or below the RfD in controlled animal studies. One study assessed exposure to chlorothalonil, a fungicide found in water at concentrations of approximately 0.2 ppm, which has a NOAEL of 60 ppm and an RfD of 0.6 ppm.15 NMDRCs were observed for survival of amphibians at concentrations in the range of 0.0000164 to 0.0164 ppm, well below both the RfD and environmentally relevant concentrations. Another example comes from a study of Di(2-ethylhexyl)phthalate (DEHP), a phthalate widely used in flexible plastics, and its effects on a number of endocrine-sensitive end points including maternal serum testosterone where significant effects were observed at 0.5, 1, and 5 µg/kg/d, and anogenital distance, where significant effects were observed at 5 µg/kg/d, below the RfD of 20 µg/kg/d.16 Multiple additional examples have been discussed elsewhere.2,17
Conclusions
Examples that fit into case 2 and case 3 indicate that nonmonotonicity occurs at doses/concentrations that are overlooked by regulatory toxicology as it is commonly practiced today. The examples above (and many more described in the published literature)2 illustrate the occurrence of NMDRCs at low dose ranges, for example, below the toxicological NOAEL and even below the RfD. This information is problematic for regulatory decision-making as it presents one of many challenges to the current practice of using high-dose studies to extrapolate to so-called “safe” doses.3,18
A recent example that is relevant to regulatory decision-making sheds light on how expanded guideline studies could be useful for addressing NMDRCs. The Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) study, a guideline study combined with additional end points examined in academic laboratories, revealed a nonmonotonic relationship between BPA and mammary adenocarcinoma.19 Similar nonmonotonic relationships have been documented elsewhere.20,21 Moving forward, we propose that this example and others like it should be used for several purposes: (1) to develop agreed upon methods, using best practices, for how nonmonotonic relationships should be evaluated statistically; (2) to consider increasing the number of doses, covering wider ranges, in studies used for regulatory purposes; (3) to utilize adverse effects (like mammary adenocarcinoma) observed at low, but not higher doses, to calculate RfDs that are public-health protective.
Acknowledgements
The authors are grateful to current and former members of the Vandenberg lab for feedback on this project. LNV acknowledges funding support from the National Institute of Environmental Health Sciences of the National Institutes of Health (Award Number K22ES025811).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: LNV acknowledges funding support from the National Institute of Environmental Health Sciences of the National Institutes of Health (Award Number K22ES025811). JPM is employed by Environmental Health Sciences, Charlottesville, VA, which has received support from the Broad Reach Fund, the Marisla Foundation, the Foundation for the Carolinas, the Cornell Douglas Foundation, the Forsythia Foundation and the Wallace Genetic Foundation. Additionally, Myers’s travel to speak about endocrine disruption at scientific meetings has been reimbursed by the Food Packaging Forum, ShiftCon, Advancing Green Chemistry, Made Safe and the Righospitalet of Copenhagen. LNV has received grants from the National Institutes of Health and funding from the Cornell Douglas foundation. She has been reimbursed for travel expenses by numerous organizations including SweTox, Israel Environment Fund, the Mexican Endocrine Society, Advancing Green Chemistry, ShiftCon, US EPA, CropLife America, BeautyCounter, and many universities, to speak about EDCs. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Kohn MC, Melnick RL. Biochemical origins of the non-monotonic receptor-mediated dose-response. J Mol Endocrinol. 2002;29(1):113–123. [DOI] [PubMed] [Google Scholar]
- 2. Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33(3):378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zoeller RT, Vandenberg LN. Assessing dose-response relationships for endocrine disrupting chemicals (EDCs): a focus on non-monotonicity. Environ Health. 2015;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam Horm. 2014;94:129–165. [DOI] [PubMed] [Google Scholar]
- 5. Beausoleil C, Ormsby JN, Gies A, et al. Low dose effects and non-monotonic dose responses for endocrine active chemicals: science to practice workshop: workshop summary. Chemosphere. 2013;93(6):847–856. [DOI] [PubMed] [Google Scholar]
- 6. United States Environmental Protection Agency. State of the science evaluation: nonmonotonic dose responses as they apply to estrogen, androgen, and thyroid pathways and EPA testing and assessment procedures. 2013.
- 7. Rhomberg LR, Goodman JE. Low-dose effects and nonmonotonic dose-responses of endocrine disrupting chemicals: Has the case been made? Regul Toxicol Pharmacol. 2012;64(1):130–133. [DOI] [PubMed] [Google Scholar]
- 8. Vandenberg LN, Colborn T, Hayes TB, et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol. 2013;38:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beronius A, Vandenberg LN. Using systematic reviews for hazard and risk assessment of endocrine disrupting chemicals. Rev Endocr Metab Disord. 2016;16(4):273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fan F, Wierda D, Rozman KK. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on humoral and cell-mediated immunity in Sprague-Dawley rats. Toxicology. 1996;106(1-3):221–228. [DOI] [PubMed] [Google Scholar]
- 11. Palanza P, Parmigiani S, vom Saal FS. Effects of prenatal exposure to low doses of diethylstilbestrol, o, p‘DDT, and methoxychlor on postnatal growth and neurobehavioral development in male and female mice. Horm Behav. 2001;40(2):252–265. [DOI] [PubMed] [Google Scholar]
- 12. Lagarde F, Beausoleil C, Belcher SM, et al. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ Health. 2015;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dey A, Guha P, Chattopadhyay S, Bandyopadhyay SK. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem Biophys Res Commun. 2009;381(1):90–95. [DOI] [PubMed] [Google Scholar]
- 14. Bloomquist JR, Barlow RL, Gillette JS, Li W, Kirby ML. Selective effects of insecticides on nigrostriatal dopaminergic nerve pathways. NeuroToxicology. 2002;23(4-5):537–544. [DOI] [PubMed] [Google Scholar]
- 15. McMahon T, Halstead N, Johnson S, et al. The fungicide chlorothalonil is nonlinearly associated with corticosterone levels, immunity, and mortality in amphibians. Environ Health Perspect. 2011;119(8):1098–1103. Epub Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA. Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses. Reprod Toxicol. 2012;34(4):614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vandenberg LN, Ehrlich S, Belcher SM, et al. Low dose effects of Bisphenol A: An integrated review of in vitro, laboratory animal and epidemiology studies. Endocrine Disruptors. 2013;1(1):e25078. [Google Scholar]
- 18. Maffini MV, Vandenberg LN. Closing the gap: improving additives safety evaluation to reflect human health concerns. Environ Risk Assess Remediat. 2017;1(3):26–33. [Google Scholar]
- 19. National Toxicology Program. Draft NTP research report on the CLARITY-BPA core study: a perinatal and chronic extended-dose-range study of bisphenol A in rats. 2018. [PubMed]
- 20. Vandenberg LN, Prins GS. Clarity in the face of confusion: new studies tip the scales on bisphenol A (BPA). Andrology. 2016;4(4):561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mandrup K, Boberg J, Isling LK, Christiansen S, Hass U. Low-dose effects of bisphenol A on mammary gland development in rats. Andrology. 2016;4(4):673–683. [DOI] [PubMed] [Google Scholar]