Osteochondritis dissecans (OCD) is a joint condition in which bone underneath the cartilage loses its vitality owing to a lack of blood flow. This disease mainly affects young people practicing sports activities, and the elbow is the second-most affected site in the body (after the knee), representing 6% of overall OCD.8,16 Indications for surgical treatment include the presence of loose bodies, mechanical symptoms (eg, articular locking), unstable lesions, and stable lesions still symptomatic after 6 months of conservative management.24,27 The ideal surgical treatment has not yet been identified, and the currently performed procedures are borrowed from experience with other major joints, such as the knee or ankle.
Several treatment techniques have been described for OCD, such as debridement, drilling, microfracture, fragment fixation, osteochondral autografting or allografting, and autologous chondrocyte implantation (ACI).9 These techniques show well-known positive aspects but also some important drawbacks: (1) the lack of restoration of high-quality cartilaginous tissue (ie, for microfracture), (2) the high costs and patient discomfort (ie, ACI requires 2-step surgery and a dedicated laboratory for the cell culture), (3) donor site morbidity attributed to the plug’s harvest from a healthy joint,6 and (4) limited donor availability (ie, for autologous or homologous osteochondral grafts). Recent acquisitions in the field of regenerative medicine have demonstrated that bone marrow–derived cells (BMDCs) on a scaffold are able to replicate and regenerate bone as well as cartilaginous tissue, without any need for laboratory treatment.1,10,13,21 BMDC transplantation was proposed and successfully performed for the treatment of knee and ankle OCD. Owing to the multipotential ability of bone marrow nucleated cells, in association with platelet-rich fibrin (PRF), the osteochondral layer may regenerate and show properties similar to those of the original hyaline cartilage.2,3,29,30
The aim of this case report is to describe the surgical technique and clinical outcomes, at a mean 4 years of follow-up, for the first 3 patients affected by elbow OCD and treated by BMDC transplantation with a 1-step technique. All patients showed closed physes and had experienced no relief after 6 months of conservative management. This study was approved by an institutional review board, and all 3 patients provided informed consent.
Cases
Case 1
A 15-year-old boy came to our institution with right elbow pain that had developed 2 years previously; the pain had become more intense in the past months, limiting his ability to play water polo. Clinical examination showed pain in the capitellar area and a range of movement from 15° to 130° in flexion and extension, with no limitations in pronation and supination.
Radiograph, computed tomography scan, and magnetic resonance imaging showed signs of OCD, with no clear signs of fluid infiltrate underlying the cartilage.
Case 2
The second case involved a 12-year-old boy who had experienced elbow pain for about 7 months, with significantly worse pain in the past 2 months. He was a competitive gymnast who had to stop the agonistic activity because of the intense pain during weightbearing on the elbow: this activity, uncommon for other athletes, is often performed during gymnastics training and competitions. Pain was reported in the posterior area of the right lateral epicondyle, decreasing with nonsteroidal anti-inflammatory drugs but returning after moderate activity. He showed no range of motion limitation, and imaging reported signs of capitellar OCD.
Case 3
The third case involved a 17-year-old patient who had experienced pain in his right elbow for about 3 years. The pain resulted in a reduction of volleyball from a competitive to a recreational level, and conservative therapy did not improve the symptoms over time. There was persistent pain and range of movement restrictions, and OCD was documented on imaging.
Surgical Technique
The surgical technique for the BMDCs consisted of several phases, all performed during the same surgical session. The procedure was performed by full arthroscopy in 2 cases and with combined arthroscopy and a mini-open procedure in 1 case.
PRF Gel Production
Autologous platelet gel was used to provide direct, in situ additional growth factors for stem cell proliferation and differentiation, being an “accelerator” for healing processes25 and containing several types of molecules that promote bone and cartilage regeneration.18,20 Moreover, PRF is rich in fibrin and is able to coagulate faster than regular platelet-rich plasma.
The PRF was produced on the day before the operation. Peripheral venous blood (120 mL) was harvested and processed with the Vivostat System (Vivolution A/S) to obtain 6 mL of PRF, which was cryopreserved at –30°C until the time of surgery.
Bone Marrow Aspiration
Bone marrow was aspirated from the posterior superior iliac crest after preparation of a sterile surgical field with the patient lying in the lateral decubitus position. The posterior superior location is preferable to the anterior iliac crest because of the higher number of available cells.22 The equipment for the iliac crest harvest comes with a dedicated kit for osteochondral regeneration (IOR-G1; Novagenit).
A total of 60 mL of whole bone marrow was harvested and then concentrated in the surgical theater by eliminating most of the plasma and erythrocytes. Using a cell separator-concentrator (Res-Q; ThermoGenesis) and its related sterile and disposable kit allowed for an increased concentration of nucleated cells. After a 15-minute working cycle, 6 mL of concentrated cells were obtained.
Elbow Arthroscopy
The patient was positioned in lateral decubitus (Figure 1), with the arm free to be moved on an arm holder and a dedicated sterile ischemic tourniquet at the limb’s root inflated to 250 mm Hg. Sterile saline solution (20 mL) was then injected into the joint from the center of the triangle composed by the olecranon, radial head, and lateral epicondyle.
Figure 1.
The patient is positioned in lateral decubitus. (A) The bony landmarks are highlighted on the skin. (B) An optical instrument and a probe were inserted through the posterior portals. The arrow points to the anterolateral portal.
Anterior Phase
The elbow joint was first approached by standard anteromedial and anterolateral arthroscopic portals (Figure 2). The radial head, capitulum humeri, and lateral articular capsule were then inspected: the coronoid, coronoid fossa, and anteromedial surface of the articular capsule from the anterolateral portal, with the capitulum humeri from the anteromedial portal. By means of an accessory anterior portal (medial or lateral), the capsule was retracted for better visualization. With the help of a probe, the osteochondral fragment was identified and the indication confirmed. The fragment was then removed and the subchondral bone curetted below the subchondral plate until bleeding bone was reached.
Figure 2.

Standard arthroscopic portals are performed for the elbow arthroscopy: (A) anteromedial and anterolateral and (B) posterolateral and midlateral portals. (C) Specific instruments already developed for arthroscopic chondrocyte implantation were used: a flat probe, different-size windowed cannulas, and a dedicated trocar for the biomaterial insertion. (D) Arthroscopic biomaterial insertion via the windowed cannula through the posterolateral portal.
Posterolateral Phase
This phase was used to better perform the surgical procedure on the capitulum humeri, with posterolateral and midlateral portals (Figure 2). With the elbow flexed to 90°, the osteochondral fragment was detected and removed, shaving the damaged subchondral bone with standard instruments, including a bur and curette. At this point, in cases 2 and 3, surgery was performed by full arthroscopy, and in case 1, the surgery was converted into a mini-open procedure to better perform the biomaterial implant. In this last case, the 2 portals (midlateral and posterolateral) were joined by a skin incision, and the capsule was approached and sectioned in the interval between the anconeus and extensor carpi ulnaris. The lesion size was measured, and the biomaterial to be implanted was then cut accordingly to better reproduce the lesion shape (Figures 3 and 4).
Figure 3.

In case 1, once the lesion was detected and curetted, a mini-open procedure was performed connecting the 2 posterior portals. The lesion was exposed without detaching the lateral collateral ligament complex.
Figure 4.

The lesion size is accurately measured with the help of an (A) aluminum phantom, exactly contoured on (B) the lesion site.
The collagen membrane included within the IOR-G1 kit was used for cell support. Approximately 2 mL of marrow concentrate was loaded onto the highly hydrophilic membrane and quickly absorbed (Figure 5).
Figure 5.

The membrane is shaped according to the phantom shape and loaded with around 2 mL of cell concentrate, which is fully absorbed in approximately 2 minutes.
The biomaterial was loaded into the lesion site directly in the mini-open procedure or by sliding it to the edge of the lesion with the help of a special windowed cannula (Figure 2).14 A flat probe then helped to position the biomaterial. To provide a growth factor supplement and to improve the stability of the implant, the PRF was then applied to cover the lesion with a dedicated spray pen (Figure 6). The biomaterial, given its soft consistency, does not aim to immediately restore the articular congruity; this is supposed to happen with the subsequent activity of the multipotent cells. The biomaterial should be positioned slightly below the adjacent cartilage level, to avoid its accidental dislocation with postoperative elbow movements.
Figure 6.
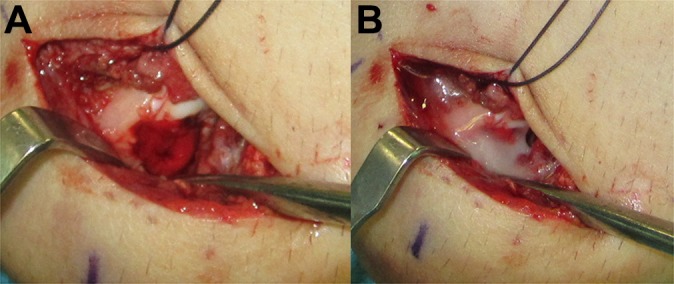
(A) The biomaterial is positioned onto the lesion site with the help of a flattened probe, and (B) a layer of platelet gel is sprayed on the biomaterial to help the stability of the patch and to provide supplemental growth factors (case 1).
Implant stability was checked with multiple elbow flexions and extensions, and a gravity articular drain was positioned in the anterior chamber with the elbow flexed at 90° far from the lesion site. It is important to respect these indications for drainage positioning to prevent biomaterial dislocation. A proper skin closure was then performed.
Postoperative Care
A restriction splint at 90° of flexion, with neutral rotation and pronation-supination blocked, was positioned postoperatively. The articular drain was removed the day after surgery. From the second day after surgery, patients were allowed to remove the splint twice a day to perform auto-assisted exercises, including flexion-extension and pronation-supination movements. One month after surgery, patients began progressive mobilization and exercises in the pool assisted by a therapist, and at 2 months a dry rehabilitation protocol was allowed, avoiding exercises causing elbow compression. From the fourth to sixth month, patients could perform joint reinforcement, and sport-specific exercises (eg, throwing or swimming) were allowed at 6 months postoperatively. After good progress through the rehabilitation protocol and the absence of pain, patients were allowed full competitive athletic activities at 9 months.
Results
Clinical
Lesion sizes measured in the 2 main and perpendicular axes were 1.5 × 1 cm (case 1), 1.2 × 1.3 cm (case 2), and 1 × 1.3 cm (case 3). No minor or major complications were reported. No donor site morbidity was experienced by the patients with regard to iliac crest harvest. All 3 patients showed clinical improvement, with slight range of motion improvement at maximum follow-up (the full data set is reported in Table 1). All patients returned to play the competitive sports activity they participated in before surgery, without symptoms or limitations, starting from 9 months after surgery. The Mayo Elbow Performance Score improved from 78.3 to 93.3 at follow-up. The Oxford Elbow Score increased from 40.0 to 47.6.
TABLE 1.
Patient Demographics and Clinical Resultsa
| Grading | Preoperative ROM | ROM at Follow-up | OES | MEPS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age, y | MRI | ICRS | FLEX | EXT | PRON | SUP | FLEX | EXT | PRON | SUP | PRE | POST | PRE | POST | Recovery, mo | Follow-up, mo |
| 1 | 15 | 3 | 3 | 130 | 15 | 90 | 90 | 145 | 0 | 90 | 90 | 39 | 47 | 80 | 95 | 6 | 82 |
| 2 | 13 | 3 | 3 | 140 | 0 | 90 | 90 | 140 | 0 | 90 | 90 | 41 | 48 | 80 | 95 | 6 | 34 |
| 3 | 17 | 4 | 4 | 130 | 10 | 80 | 80 | 140 | 0 | 85 | 90 | 41 | 46 | 75 | 90 | 6 | 30 |
aEXT, extension; FLEX, flexion; ICRS, International Cartilage Repair Society; MEPS, Mayo Elbow Performance Score; MRI, magnetic resonance imaging; OES, Oxford Elbow Score; POST, postoperative; PRE, preoperative; PRON, pronation; ROM, range of motion; SUP, supination.
Imaging
The surgical procedures were followed up with serial radiographs and magnetic resonance imaging at 6, 12, and 36 months. The lesion site progressed with the regenerative process over time, with the formation of regenerated tissue and with the aspect of bone in the deep portion covered by soft tissue similar to adjacent cartilage in the articular surface previously affected by OCD (Figures 7 –9).
Figure 7.
(A-C) Preoperative computed tomography scan showing the area of osteochondritis dissecans. (D-F) Same area at 3-year follow-up: a regeneration of the bony layer is evident, even if a small spot of nonossified subchondral bone is present (case 1).
Figure 8.
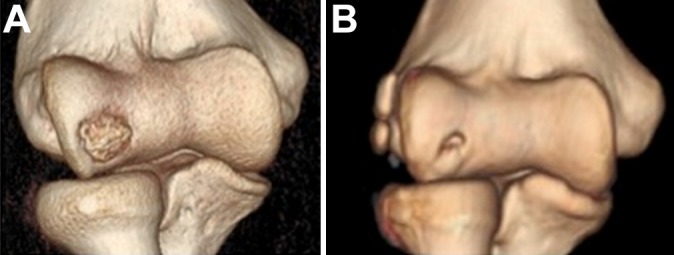
Three-dimensional computed tomography scan (A) preoperatively and (B) at 3-year follow-up (case 1).
Figure 9.

Magnetic resonance imaging showing the osteochondritis dissecans of (A) the capitulum humeri and (B) the good defect filling with restoration of capitellar convexity at 3-year follow-up (case 1).
Discussion
OCD of the capitellum is a frequent and disabling injury among young athletes.26 The treatment is usually guided by clinical findings, radiographic appearance, status of the overlying articular cartilage, and position of the involved segment. Nonsurgical treatment is typically selected for patients with early-grade stable lesions, and it is based on activity restriction and rest. Nevertheless, long-term studies have reported poor results with conservative treatment,19,26,27 with an early development of osteoarthritis in >50% of patients and with residual symptoms affecting the quality of life. Takahara et al26 observed a greater chance of healing of these lesions among patients with open physes, whereas Ruch al23 did not find the same correlation.
Surgical indications include the presence of loose bodies, mechanical symptoms, unstable lesions, and stable lesions that have failed 6 months of nonsurgical management.24,27 Many surgical options are described in the literature, with positive and negative aspects. Bauer et al4 and Harada et al15 indicated open fragment excision and debridement only for defects involving <50% of the articular surface. Fragment fixation has been performed via several techniques, from Herbert screws to reabsorbable pins or bone peg fixation.15,17,26,28 Takahara et al,26 in a recent review of the authors’ own cases, recommended fixation for lesions of grade II (International Cartilage Repair Society) and grafting in grade III. Numerous investigators have studied the role of arthroscopic debridement in OCD, showing an improvement in pain and function after the procedure. However, after debridement, recurrence of loose bodies often affected return to sport, and an increasing presence of osteoarthritic joint degeneration was observed.26 Drilling and microfracture techniques have led to positive results, but Bojanić et al7 observed a lesion filling with fibrocartilage, with lower mechanical properties than those of hyaline cartilage.
Based on experience gained on lower limb surgical techniques, osteochondral autograft transfer was recently introduced for elbow OCD.5,26 Despite encouraging results, there are still many disadvantages with the use of this technique in the elbow—the need for a donor site, for example, leading to local secondary morbidity6 and the technical difficulty of proper plug insertion, which often involves a noncongruence in graft placement. Finally, the cartilage composition and the curvature of the surface are different between the donor site of the knee and the recipient elbow, reducing the possibilities for an optimal result.
In this scenario, we decided to follow the experience acquired in our hospital with regenerative treatment13 via a 1-step technique on osteochondral lesions of the lower limb, performing the same technique for injuries affecting the elbow and evaluating the results for a mean of 4 years. When the same technique was applied in the ankle joint, the qualitative analysis performed on the regenerated cartilage showed regenerated tissue with T2 values of 35 to 45 milliseconds, similar to hyaline cartilage, in a mean ± SD of 78% ± 16% of the repaired lesion area.11 The BMDC technique described here shows several advantages when compared with conventional osteochondral repair techniques: there is no donor site morbidity (reported with mosaicplasty), there is no problem of limited availability (seen with allograft), the tissue quality seems to be similar to the native tissue (different from microfracture11), and it is possible to obtain regenerated bone and cartilage at a lower cost (less than half of an arthroscopic ACI procedure12).
This technique, being a 1-step procedure, does not require a cell culture phase in a laboratory and second-step surgery to implant the biomaterial. Furthermore, it is possible to perform the technique with an exclusively arthroscopic approach, thereby limiting the surgical exposure. Even in the case of difficult management, arthroscopy allows the surgeon to have a first look at the lesion, confirm the indication, and perform part of the surgery, minimizing tissue damage and avoiding additional iatrogenic ligament weakening (as in the extensile Kocher approach) that would lengthen the postoperative rehabilitation. If necessary, the surgical exposure can be extended to continue the operation as a conventional open procedure. Because of the low stiffness of the biomaterial, it must be performed in contained stable or unstable lesions, with the presence of a capitellar intact lateral wall providing implant lateral stability. It is important to observe that with this procedure, harm to the tissue is limited: the BMDC transplantation does not preclude the possibility of revision surgery such as mosaicplasty or osteochondral graft in case of failure.
The major limitation of this study is the small number of patients, which does not allow us to draw conclusions about the safety and efficacy of this treatment for OCD of the elbow joint.
Conclusion
The described cases indicate that the 1-step BMDC technique is a regenerative procedure that may be performed for the treatment of elbow OCD. This technique, which has proven to be effective for other joints, overcomes several drawbacks of different state-of-the-art techniques for the treatment of OCD. Furthermore, the possibility to perform it arthroscopically reduces the risk of infection and tissue damage and accelerates postoperative rehabilitation protocols.
Footnotes
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Bashir J, Sherman A, Lee H, Kaplan L, Hare JM. Mesenchymal stem cell therapies in the treatment of musculoskeletal diseases. PM R. 2014;6(1):61–69. [DOI] [PubMed] [Google Scholar]
- 2. Battaglia M, Rimondi E, Monti C, et al. Validity of T2 mapping in characterization of the regeneration tissue by bone marrow derived cell transplantation in osteochondral lesions of the ankle. Eur J Radiol. 2011;80(2):e132–e139. [DOI] [PubMed] [Google Scholar]
- 3. Battaglia M, Vannini F, Buda R, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1376–1384. [DOI] [PubMed] [Google Scholar]
- 4. Bauer M, Jonsson K, Josefsson PO, Linden B. Osteochondritis dissecans of the elbow: a long-term follow-up study. Clin Orthop Relat Res. 1992;284:156–160. [PubMed] [Google Scholar]
- 5. Baumgarten TE, Andrews JR, Satterwhite YE. The arthroscopic classification and treatment of osteochondritis dissecans of the capitellum. Am J Sports Med. 1998;26(4):520–523. [DOI] [PubMed] [Google Scholar]
- 6. Bexkens R, Ogink PT, Doornberg JN, et al. Donor-site morbidity after osteochondral autologous transplantation for osteochondritis dissecans of the capitellum: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bojanić I, Ivković A, Borić I. Arthroscopy and microfracture technique in the treatment of osteochondritis dissecans of the humeral capitellum: report of three adolescent gymnasts. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):491–496. [DOI] [PubMed] [Google Scholar]
- 8. Chen NC. Osteochondritis dissecans of the elbow. J Hand Surg Am. 2010;35(7):1188–1189. [DOI] [PubMed] [Google Scholar]
- 9. Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471(4):1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galois L, Freyria AM, Grossin L, et al. Cartilage repair: surgical techniques and tissue engineering using polysaccharide- and collagen-based biomaterials. Biorheology. 2004;41(3-4):433–443. [PubMed] [Google Scholar]
- 11. Giannini S, Buda R, Battaglia M, et al. One-step repair in talar osteochondral lesions: 4-year clinical results and T2-mapping capability in outcome prediction. Am J Sports Med. 2013;41(3):511–518. [DOI] [PubMed] [Google Scholar]
- 12. Giannini S, Buda R, Cavallo M, et al. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41(11):1196–1203. [DOI] [PubMed] [Google Scholar]
- 13. Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36(5):873–880. [DOI] [PubMed] [Google Scholar]
- 15. Harada M, Ogino T, Takahara M, Ishigaki D, Kashiwa H, Kanauchi Y. Fragment fixation with a bone graft and dynamic staples for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2002;11(4):368–372. [DOI] [PubMed] [Google Scholar]
- 16. Kenniston JA, Beredjiklian PK, Bozentka DJ. Osteochondritis dissecans of the capitellum in fraternal twins: case report. J Hand Surg Am. 2008;33(8):1380–1383. [DOI] [PubMed] [Google Scholar]
- 17. Kuwahata Y, Inoue G. Osteochondritis dissecans of the elbow managed by Herbert screw fixation. Orthopedics. 1998;21(4):449–451. [DOI] [PubMed] [Google Scholar]
- 18. Mascarenhas R, Saltzman BM, Fortier LA, Cole BJ. Role of platelet-rich plasma in articular cartilage injury and disease. J Knee Surg. 2015;28(1):3–10. [DOI] [PubMed] [Google Scholar]
- 19. Mitsunaga MM, Adishian DA, Bianco AJ. Osteochondritis dissecans of the capitellum. J Trauma. 1982;22(1):53–55. [DOI] [PubMed] [Google Scholar]
- 20. Nair MB, Varma HK, John A. Platelet-rich plasma and fibrin glue-coated bioactive ceramics enhance growth and differentiation of goat bone marrow-derived stem cells. Tissue Eng Part A. 2009;15(7):1619–1631. [DOI] [PubMed] [Google Scholar]
- 21. Oreffo ROC, Cooper C, Mason C, Clements M. Mesenchymal stem cells: lineage, plasticity, and skeletal therapeutic potential. Stem Cell Rev. 2005;1(2):169–178. [DOI] [PubMed] [Google Scholar]
- 22. Pierini M, Di Bella C, Dozza B, et al. The posterior iliac crest outperforms the anterior iliac crest when obtaining mesenchymal stem cells from bone marrow. J Bone Joint Surg Am. 2013;95(12):1101–1107. [DOI] [PubMed] [Google Scholar]
- 23. Ruch DS, Cory JW, Poehling GG. The arthroscopic management of osteochondritis dissecans of the adolescent elbow. Arthroscopy. 14(8):797–803. [DOI] [PubMed] [Google Scholar]
- 24. Ruchelsman DE, Hall MP, Youm T. Osteochondritis dissecans of the capitellum: current concepts. J Am Acad Orthop Surg. 2010;18(9):557–567. [DOI] [PubMed] [Google Scholar]
- 25. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 18(1):93–103. [PubMed] [Google Scholar]
- 26. Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205–1214. [DOI] [PubMed] [Google Scholar]
- 27. Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728–732. [DOI] [PubMed] [Google Scholar]
- 28. Takeda H, Watarai K, Matsushita T, Saito T, Terashima Y. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent baseball players. Am J Sports Med. 2002;30(5):713–717. [DOI] [PubMed] [Google Scholar]
- 29. Vannini F, Battaglia M, Buda R, Cavallo M, Giannini S. “One step” treatment of juvenile osteochondritis dissecans in the knee: clinical results and T2 mapping characterization. Orthop Clin North Am. 2012;43(2):237–244. [DOI] [PubMed] [Google Scholar]
- 30. Vannini F, Cavallo M, Baldassarri M, et al. Treatment of juvenile osteochondritis dissecans of the talus: current concepts review. Joints. 2015;2(4):188–191. [PMC free article] [PubMed] [Google Scholar]




