Abstract
Background
Colorectal cancer (CRC) is the second most common cancer in Germany. Screening colonoscopies are considered an effective tool for early detection and prevention of CRC and are recommended in Germany for citizens over the age of 55. To increase the participation rate for screening colonoscopies, an invitation procedure was initiated in parts of Germany for patients between the ages of 55 and 75 who had never undergone a screening colonoscopy before.
Methods
We examined the number of participating patients before, during, and after the invitation procedure and compared the number of the participating patients who received a cover letter with the participating patients from the control group. Additionally, we classified the findings of the colonoscopies including CRC, advanced adenomas, and polyps.
Results
During the invitation period, the participation rate of the invitation group increased from 220 patients to 531 patients compared to 1256 to 1693 in the control group. The increase was significantly greater in patients with cover letters (+141% vs.+35%, p < 0.0001). Also, significantly more polyps and adenomas were found in patients from the invitation letter group (254 (+102%) vs. 679 (–9%), p < 0.0001).
Conclusions
Our study clearly indicates that personal invitation letters are an effective measure to increase overall participation rates in screening colonoscopies.
Keywords: Screening colonoscopy, invitation procedure, participation rate, colorectal cancer, advanced adenomas
Key summary
- Summarize the established knowledge on this subject:
- Colorectal cancer is the second most common cancer in Germany.
- Screening colonoscopies are an effective tool for early detection and prevention of a colorectal carcinoma.
- Screening colonoscopies are recommended in European and German guidelines.
- There are low participation rates for screening colonoscopies.
- What are the significant and/or new findings of this study?
- Invitation letters lead to a significant increase in the participation rates for screening colonoscopies.
- The participation rate decreased after the invitation period ended.
- Advanced adenomas were found more often after the invitation letters had been sent out.
- Advanced adenomas were found significantly more often in men than in women.
Introduction and background
Colonoscopy screening of asymptomatic individuals is considered to be an effective tool for early detection of colorectal cancer (CRC) and its precursor lesions.1–5 There is a correlation between early detection and better chances of survival.1,6 Furthermore, CRC can be prevented by removal of its precursor lesions.1,7–10 In 2002, Germany was the first country worldwide to offer screening colonoscopies for citizens over the age of 55. The screening results are collected in a national registry.11–13
However, the participation rate in screening colonoscopy remains rather low—overall about 23.8% of eligible women and 21.3% of eligible men underwent a screening colonoscopy between 2003 and 2014.14 With the objective to increase the number of participating individuals undergoing a screening colonoscopy, the “Gastroenterology Hesse eG”—an association of established gastroenterologists in Hesse—started with the health insurance company AOK Hesse—an invitation procedure for screening colonoscopies for citizens over the age of 55 who had never had a screening colonoscopy before.
The aim of our study was to evaluate if invitations for screening colonoscopies increase the number of participants in a known effective screening measure for detection of CRC and its precursor lesions.
Methods
Patient cohort
In June 2014 the invitation procedure of the “Gastroenterology Hesse eG” and the health insurance company AOK Hesse was inaugurated in the north of Hesse, one of the 16 federal states in Germany, with more than 6 million inhabitants. The regions in Hesse taking part were the counties of Kassel, Werra-Meißner-Kreis, Waldeck-Frankenberg, Schwalm-Eder-Kreis, Hersfeld-Rotenburg, Marburg-Biedenkopf, Vogelsberg and Fulda. The potential participants—individuals ages 55 to 75 who had never undergone a colonoscopy (and therefore also no screening colonoscopy) before obtained a cover letter from AOK Hesse as well as an information sheet explaining the reasons for the pilot project. Established gastroenterologists performed the screening colonoscopies. The invitation period was carried out for 28 weeks—from June 16, 2014 until December 15, 2014—in which around 3000 letters were sent out on a weekly basis. In total, about 84,000 were sent out during the study period.
Our control population comprised individuals ages 55–75 in the Hessian population from health insurance companies other than AOK Hesse who were also undergoing a colonoscopy for the first time. These screening colonoscopies were performed by the same gastroenterologists. The results of the colonoscopies during the invitation period were compared to those of the colonoscopies nine months before and three months after, respectively, the invitation procedure.
In total, data from 859 screening colonoscopies from policyholders of AOK Hesse and 3470 from non-AOK policyholders were compared.
Study procedures
After the data had been collected, we examined retrospectively the number of participants before, during, and after the invitation period and compared the number of invited participants from AOK Hesse with the control group. We classified the time period before the invitation procedure as period 1 (P1, July 2013–March 2014), the time period during the invitation procedure including the following quarter as period 2 (P2, July 2014–March 2015) and the subsequent quarter as period 3 (P3, April–July 2015). We decided to add the first quarter of 2015 (the first quarter after the invitation procedure had stopped) to P2 because we assume that some screening colonoscopies took place after the invitation process had already been stopped.
Additionally, we classified the findings of the colonoscopies into the following groups: polyps (hyperplastic, other benign and adenomas), advanced adenomas (AA; >1 cm, villous components in histology—as villous or tubulovillous features, and high-grade intraepithelial neoplasia (IEN)2), and CRC.
Polyps were removed by snare or forceps polypectomy during colonoscopy and investigated by local pathologists.
The results of the participants undergoing screening colonoscopies in the north of Hesse before, during, and after the invitation period were transmitted to IOMTECH, Berlin, Germany, a company providing quality assurance and storage. IOMTECH collected the data from established gastroenterologists who were members of the association “Gastroenterology Hesse,” in which 70% of established gastroenterologists are registered. IOMTECH Berlin collected the data from the screening colonoscopies prospectively. We were able to investigate the data retrospectively after receiving the ethical approval.
Data analysis
Categorical variables were compared with the χ2 test or Fisher’s exact test, and quantitative variables with the nonparametric Kruskal–Wallis test, whereby post-hoc comparisons p values were adjusted applying the Bonferroni–Holm procedure.
All tests were two tailed and the level of significance was 5%. Statistical analysis was performed with R (version 3.2.4, R Foundation of Statistical Computing, Vienna, Austria).
Ethical considerations
The principle of a pseudonymized (each patient was encoded by sex, age and the first three digits of the postal code) documentation was approved by the local ethics committee of the medical department of Goethe University in Frankfurt on October 30, 2017 (Ethik-Kommission des Fachbereichs Medizin der Goethe Universität Frankfurt, 30.10.2017, 332/17).
The study has been registered under DRKS00012708 (http://www.drks.de).
All the principles outlined in the Declaration of Helsinki of 1975 have been followed in this retrospective study.
Informed consent
Since this was a retrospective study, no informed consent was obtained from the patients included.
Results
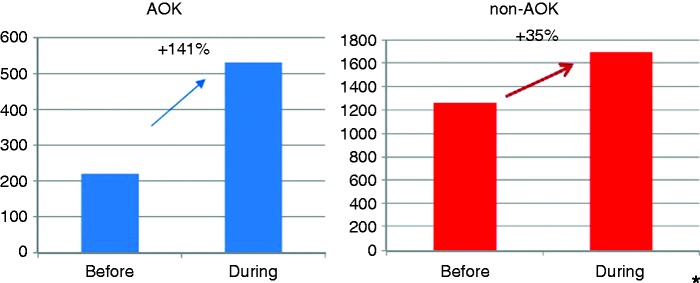
Before the invitation period, in P1, 220 participants from AOK Hesse from the north of Hesse underwent a screening colonoscopy compared to 1256 participants with other health insurance. In P2 the number rose to 531 individuals from AOK Hesse and to 1693 participants with other health insurance. The increase was significantly greater in AOK participants (+141% vs. +35%, p < 0.0001*, fig. 1.
Figure 1.
Number of colonoscopies before and during the invitation procedure, comparison AOK/non-AOK.
*p < 0.0001.
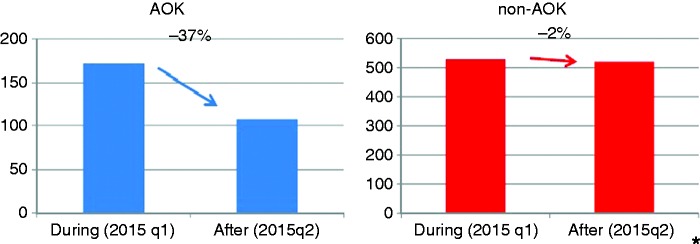
In comparison to the last quarter of P2, the number of participants decreased in P3 from 172 (2015q1) to 108 (2015q2) (AOK Hesse) and from 529 (2015q1) to 521 (2015q2) (other health insurance) (–37% vs. –2%; p = 0.0013*, fig. 2.
Figure 2.
Number of colonoscopies during (2015q1) and after (2015q2) the invitation procedure, comparison AOK/non-AOK.
*p = 0.0013.
Interestingly, significantly more findings (polyps, adenomas, AA, CRC and other benign findings) were detected in AOK Hesse participants compared to the control cohort during the invitation period (254 (+102%) AOK Hesse vs. 679 (–9%) others, p < 0.0001) (Table 1).
Table 1.
Number of findings before and during the invitation procedure, comparison AOK/non-AOK.
| Before (P1) | During (P2) | Changes (%) | |
|---|---|---|---|
| AOK | 126 | 254 | 101.59 |
| non-AOK | 743 | 679 | –8.61 |
p < 0.0001.
After the invitation period, significantly fewer findings were detected and removed in the AOK Hesse cohort. Fewer findings were also reported in the control group (Table 2).
Table 2.
Number of findings during and after the invitation procedure, comparison AOK/non-AOK.
| During invitation (2015q1) | After invitation (2015q2) | Changes (%) | |
|---|---|---|---|
| AOK | 76 | 43 | –43.42 |
| non-AOK | 224 | 212 | –5.35 |
p = 0.0204.
Findings of CRC, AA and polyps (Table 3)
AA are characterized as adenomas ≥1 cm, or with villous features (villous or tubulovillous), or with high-grade severe dysplasia, respectively (Table 4).15
Table 3.
Findings (colorectal cancer (CRC), advanced adenoma and polyps) before, during and after the invitation process in AOK and non-AOK participants, in total.
| Before (n) AOK/non-AOK | During (n) AOK/non-AOK | After (n) AOK/non-AOK | |
|---|---|---|---|
| CRC | 2/10 | 1/6 | 0/3 |
| High-grade dysplasia | 0/4 | 2/4 | 1/0 |
| Villous adenomas | 0/2 | 1/0 | 0/1 |
| Tubulovillous adenomas | 12/48 | 36/56 | 9/18 |
| Mixed polyps | 0/0 | 0/1 | 0/0 |
| Tubular adenoma | 59/404 | 118/358 | 23/113 |
| Traditional serrated adenoma | 0/1 | 0/0 | 0/0 |
| Sessile-serrated adenoma | 0/0 | 1/4 | 0/0 |
| Hyperplastic polyp | 59/330 | 105/269 | 12/87 |
| Other benign | 3/25 | 9/26 | 0/6 |
Table 4.
Numbers of advanced adenomas (AA) before, during and after the invitation procedure in AOK and non-AOK participants, in total.
| Before (n) AOK/non-AOK | During (n) AOK/non-AOK | After (n) AOK/non-AOK | |
|---|---|---|---|
| AA, total | 17/84 | 52/111 | 13/33 |
| – Adenoma ≥ 1 cm | 5/30 | 13/51 | 3/14 |
| – Villous features | 12/50 | 37/56 | 9/19 |
| – High-grade dysplasia | 0/4 | 2/4 | 1/0 |
Before the invitation letters were sent, AA could be found in 6.84% in total (7.72% of AOK participants and 6.69% of non-AOK participants); during the invitation process AA were detected in 7.33% in total (9.79% in AOK and 6.5% in non-AOK participants); and after AA were found in 7.31% in total (12.03% of AOK participants and 6.33% of non-AOK participants). Altogether, more AA were detected after invitation letters had been sent (Table 5).
Table 5.
Comparing histological findings (especially advanced adenomas (AA)) in women and men in all health insurance programs (AOK and non-AOK).
| Male | Female | |
|---|---|---|
| Histological findings | 1597 | 1799 |
| AA | 191 | 109 |
p < 0.0001.
AA were found significantly more often in men than in women (p < 0.0001).
Localization
As AA have a different risk of degradation in different colonic areas,15 we also examined the localization of AA.
In total, 350 AA were detected. A total of 51.71% were located in the colon (no differentiation between single sections), and 48.29% were located in the proximal sigmoid and sigmoid/rectum.
No significant differences in localization were observed between individuals from AOK Hesse and participants from other health insurance (p > 0.2).
Discussion
This study shows for the first time that a personal invitation to a screening colonoscopy leads to (1) significantly higher participation rates in male as well as female healthy individuals and (2) an improved endoscopic performance as indicated by higher detection rates of CRC and its precursor lesions in invited participants in comparison to their noninvited counterparts.
It has been hypothesized before that remarkable effects in screening colonoscopies may be achieved by high attendance and participation rates, which may be increased by an invitation procedure.16 The adoption rate among the general public for screening programs—in our case CRC screening programs—is certainly a coefficient for its efficacy and effectiveness.17 A national polyp study in the United States (US) showed a reduction of CRC mortality of about 53% after a median follow-up of 15 years after adenomatous polyps had been removed colonoscopically.10 This study indicates, in accordance with other studies, that patients without polyps or with nonadenomatous polyps have low rates of development of colorectal neoplasia after undergoing a colonoscopy.10,18–20
In our study, we show a significant rise in the number of performed colonoscopies after an invitation procedure. Accordingly, there was a significant reduction in the participation rate after the invitation procedure had stopped. We conclude that the supporting and the “awareness-raising” effect of the invitation letter are transient. Another study from 2017 showed similar results regarding an increased participation rate in undergoing screening colonoscopy after a written invitation.21
A study by Brenner et al. from 2017—in which a personal invitation for 50- to 54-year-old citizens had taken place—indicates that screening colonoscopies should already be offered, at least for men, from age 50 onward, as the prevalence of advanced neoplasia for men is twice as much as in women.22 In our study, we investigated patients only over the age of 55. However, we also showed that AA were found significantly more often in men than in women (p < 0.0001), which may support the idea that screening colonoscopies should be offered at the age of 50, at least for men.
The adoption rate among the general public of screening colonoscopies is important for their efficacy.17 Sending invitation letters may be one tool to increase the attendance rate. This has been shown in screening programs for several forms of cancer,23 including CRC.24
Adenomatous polyps can degenerate. The risk of degeneration and malignancy correlates with size, histologic type, and degree of dysplasia.15 AA are characterized as adenomas ≥1 cm, or with villous features (villous or tubulovillous), or with high-grade or severe dysplasia.15 Given the higher risk of degradation in AA, detection and removal of adenomas, and especially AA, seems to be essential for the prevention of CRC.9,10,25
In this study, we investigated the detection rate of AA. In the AOK patient cohort, AA could be found in 7.72% of patients before (P1) and in 9.79% of patients during (P2) the invitation period. We theorize that the knowledge of an invitation process led to greater attention and alertness in the gastroenterologists performing the colonoscopies. Another possible factor might be that people who had a higher risk for CRC (maybe because of a positive family history) or who already experienced unspecific symptoms (e.g. blood in the stool) felt more motivated to undergo a colonoscopy after receiving the invitation letter. Nevertheless, there was a high prevalence of AA found during the invitation process, which supports the idea of raising the attendance for screening colonoscopies by sending invitation letters.
We found that more than 50% of AA were detected in the right colon, compared to the other half, which was located in the proximal sigmoid and sigmoid/rectum. Considering complete colonoscopies seem to be essential to reducing the rate of AA. Additionally, a prospective nationwide study in Germany (including 2,821,392 asymptomatic patients) showed that about one-third of the detected cancers were located proximal to the sigmoid colon and would not have been diagnosed by a sigmoidoscopy.13 However, there is an absence of data demonstrating the effectiveness of the use of screening colonoscopies for prevention of CRC, whereas for sigmoidoscopy there are randomized, controlled studies showing a significant reduction in the incidence of and mortality from CRC.26,27 It has also been indicated that sigmoidoscopy may be an equivalent and even more cost-effective tool for screening.1,26–28 By contrast, a retrospective, population-based case-control study by Brenner et al.29 hypothesizes that the risks and costs of a colonoscopy—in comparison to a sigmoidoscopy—are justified by a significant advantage in the distal and proximal colon. In addition, in the US,30,31 and internationally,32,33 a shift is described toward right-sided or proximal colon cancers, with the highest relative increase in incidence located in the coecum.34,35 This may be caused by improvements in diagnosis and by increased screening by sigmoidoscopy with removing suspect polyps; however, there does seem to be a real increase in right-sided cancers.33
Interpretation of our data might be limited by the fact that there is no distinction between symptomatic and asymptomatic participants or between high-risk (e.g. positive family history of CRC or inflammatory bowel disease) and no-high-risk individuals. Furthermore, we did not investigate aspects such as levels of education or lifestyle habits that may influence colonoscopy utilization. To reduce the effect of these limitations, only patients who had never undergone a colonoscopy before received an invitation letter for the screening colonoscopy. But, effects from the pooling of symptomatic and asymptomatic or of high-risk or no-high-risk patients cannot be ruled out.
Another aspect that may limit the interpretation of our data is that we included only the data from gastroenterologists who are members of the association “Gastroenterology Hesse.” There may be other internal specialists who may also have performed screening colonoscopies that could not be considered in our investigation. As the majority of gastroenterologists are in “Gastroenterology Hesse,” we assume that most of the performed screening colonoscopies were collected in our data.
In summary, our study clearly indicates that personal invitation letters are an effective measure to increase overall participation rates in screening colonoscopies. However, further studies are needed to confirm our results in a larger, nationwide screening cohort.
Acknowledgments
The authors would like to thank all the gastroenterologists and patients who participated in the study. The authors would also like to thank the Gastroenterologie Hessen eG as well as the AOK Hessen for initiating the study.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Since this was a retrospective study, no informed consent was obtained from the patients included.
Ethics approval
The principle of a pseudonymized (each patient was encoded by sex, age and the first three digits of the postal code) documentation was approved by the local ethics committee of the medical department of Goethe University in Frankfurt on October 30, 2017 (Ethik-Kommission des Fachbereichs Medizin der Goethe Universität Frankfurt, 30.10.2017, 332/17). All the principles outlined in the Declaration of Helsinki of 1975 have been followed in this retrospective study.
References
- 1.Selby JV, Friedman GD, Quesenberry CP, Jr, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med 1992; 326: 653–657. [DOI] [PubMed] [Google Scholar]
- 2.Imperiale TF, Wagner DR, Lin CY, et al. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med 2000; 343: 169–174. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000; 343: 162–168. [DOI] [PubMed] [Google Scholar]
- 4.Imperiale TF, Wagner DR, Lin CY, et al. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med 2002; 346: 1781–1785. [DOI] [PubMed] [Google Scholar]
- 5.Ransohoff DF. Colon cancer screening in 2005: Status and challenges. Gastroenterology 2005; 128: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 6.Gatta G, Capocaccia R, Sant M, et al. Understanding variations in survival for colorectal cancer in Europe: A EUROCARE high resolution study. Gut 2000; 47: 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329: 1977–1981. [DOI] [PubMed] [Google Scholar]
- 8.Newcomb PA, Storer BE, Morimoto LM, et al. Long-term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. J Natl Cancer Inst 2003; 95: 622–625. [DOI] [PubMed] [Google Scholar]
- 9.Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001; 48: 812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pox C, Schmiegel W. Colorectal screening in Germany. Z Gastroenterol 2008; 46(Suppl 1): S31–S32. [DOI] [PubMed] [Google Scholar]
- 12.Pox C, Schmiegel W, Classen M. Current status of screening colonoscopy in Europe and in the United States. Endoscopy 2007; 39: 168–173. [DOI] [PubMed] [Google Scholar]
- 13.Pox CP, Altenhofen L, Brenner H, et al. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology 2012; 142: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 14.Altenhofen L. Projekt Wissenschaftliche Begleitung von Früherkennungs-Koloskopien in Deutschland Berichtszeitraum 2014. 28-1-2016. https://www.zi-dmp.de/Files/Koloskopie/Jahresbericht_2014_Darmkrebs_Frueherkennung.pdf.
- 15.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975; 36: 2251–2270. [DOI] [PubMed] [Google Scholar]
- 16.Riemann JF, Maar C, Betzler M, et al. Early detection of colonic cancer in the National Cancer Program—Present status and recommendations [article in German]. Z Gastroenterol 2011; 49: 1428–1431. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA. Cost-effectiveness model for colon cancer screening. Gastroenterology 1995; 109: 1781–1790. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman DA, Weiss DG, Harford WV, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology 2007; 133: 1077–1085. [DOI] [PubMed] [Google Scholar]
- 19.Imperiale TF, Glowinski EA, Lin-Cooper C, et al. Five-year risk of colorectal neoplasia after negative screening colonoscopy. N Engl J Med 2008; 359: 1218–1224. [DOI] [PubMed] [Google Scholar]
- 20.Brenner H, Haug U, Arndt V, et al. Low risk of colorectal cancer and advanced adenomas more than 10 years after negative colonoscopy. Gastroenterology 2010; 138: 870–876. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmeister M, Holleczek B, Zwink N, et al. Screening for bowel cancer: Increasing participation via personal invitation. Dtsch Arztebl Int 2017; 114: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner H, Zwink N, Ludwig L, et al. Should screening colonoscopy be offered from age 50? Dtsch Arztebl Int 2017; 114: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giorgi Rossi P, Camilloni L, Cogo C, et al. Methods to increase participation in cancer screening programmes [article in Italian]. Epidemiol Prev 2012; 36(1 Suppl 1): 1–104. [PubMed] [Google Scholar]
- 24.Segnan N, Senore C, Andreoni B, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132: 2304–2312. [DOI] [PubMed] [Google Scholar]
- 25.Brenner H, Hoffmeister M, Stegmaier C, et al. Risk of progression of advanced adenomas to colorectal cancer by age and sex: Estimates based on 840,149 screening colonoscopies. Gut 2007; 56: 1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoff G, Grotmol T, Skovlund E, et al. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: Randomised controlled trial. BMJ 2009; 338: b1846–b1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: Follow-up findings of the Italian Randomized Controlled Trial—SCORE. J Natl Cancer Inst 2011; 103: 1310–1322. [DOI] [PubMed] [Google Scholar]
- 28.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet 2010; 375: 1624–1633. [DOI] [PubMed] [Google Scholar]
- 29.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Intern Med 2011; 154: 22–30. [DOI] [PubMed] [Google Scholar]
- 30.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: An update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer 1999; 85: 1670–1676. [PubMed] [Google Scholar]
- 31.Jessup JM, McGinnis LS, Steele GD, Jr, et al. The National Cancer Data Base. Report on colon cancer. Cancer 1996; 78: 918–926. [DOI] [PubMed] [Google Scholar]
- 32.Thörn M, Bergström R, Kressner U, et al. Trends in colorectal cancer incidence in Sweden 1959–93 by gender, localization, time period, and birth cohort. Cancer Causes Control 1998; 9: 145–152. [DOI] [PubMed] [Google Scholar]
- 33.Stewart RJ, Stewart AW, Turnbull PR, et al. Sex differences in subsite incidence of large-bowel cancer. Dis Colon Rectum 1983; 26: 658–660. [DOI] [PubMed] [Google Scholar]
- 34.Mamazza J, Gordon PH. The changing distribution of large intestinal cancer. Dis Colon Rectum 1982; 25: 558–562. [DOI] [PubMed] [Google Scholar]
- 35.Vukasin AP, Ballantyne GH, Flannery JT, et al. Increasing incidence of cecal and sigmoid carcinoma. Data from the Connecticut Tumor Registry. Cancer 1990; 66: 2442–2449. [DOI] [PubMed] [Google Scholar]