Abstract
Background
In addition to visceral fat, peripheral ectopic fat accumulation is suggested to play a role in the pathophysiology of metabolic syndrome, which is known to be associated with not only cardiovascular diseases and type 2 diabetes mellitus but also colorectal cancer.
Objective
This study aims to clarify whether there is ectopic fat accumulation in human colorectal tissue in association with metabolic syndrome or its components such as abdominal obesity and insulin resistance.
Methods
Lipid contents of colorectal tissue were measured in 27 patients with colorectal polyp excised endoscopically. In addition, lipid droplets were immunohistochemically estimated using anti-perilipin antibody in 32 patients with colorectal cancer resected surgically.
Results
Increasing tissue triglyceride/phospholipid ratio was associated with increasing body mass index, fasting plasma insulin level and homeostasis model assessment as an index of insulin resistance (HOMA-IR), and also decreasing serum adiponectin level. Lipid droplets were observed in the submucosal region of colorectal tissue. The amount of lipid droplets was associated with increasing body mass index, waist circumference and visceral fat area.
Conclusion
This study showed the presence of submucosal fat accumulation in human colorectal tissue and its association with abdominal obesity and insulin resistance.
Keywords: Ectopic fat accumulation, colorectal tissue, lipid droplets, perilipin, abdominal obesity, insulin resistance, metabolic syndrome
Key summary
Metabolic syndrome is known to be associated with not only cardiovascular diseases and type 2 diabetes mellitus but also colorectal cancer.
In addition to visceral fat, ectopic fat accumulation in peripheral tissues such as the liver and skeletal muscle is also suggested to play a role in the pathophysiology of metabolic syndrome.
It has not been well known whether there is ectopic fat accumulation in colorectal tissue in association with metabolic syndrome or its components, or whether, if so, colorectal fat accumulation participates in colorectal carcinogenesis.
This study showed for the first time the presence of submucosal fat accumulation in human colorectal tissue in association with abdominal obesity and insulin resistance by measurement of the lipid contents and immunohistochemical evaluation of lipid droplets.
Introduction
Metabolic syndrome (MetS) is a cluster of metabolic abnormalities which increases the risk of cardiovascular diseases, type 2 diabetes mellitus and overall mortality, including abdominal obesity (increased waist circumference and visceral fat), elevated blood pressure, hyperglycaemia (raised fasting plasma glucose (FPG), insulin (FPI) and insulin resistance), and dyslipidaemia (high serum triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C)).1,2
Excessive visceral adipose tissue accumulation due to over-nutrition is suggested to play a central role in the pathophysiology of MetS with abdominal obesity by promoting pathogenic hypertrophic adipocytes and adipose tissue dysfunction with elevated levels of free fatty acids and altered levels of adipose tissue-derived substances called adipokines such as adiponectin, pro-inflammatory cytokines, growth factors and reactive oxygen species; these provoke lipotoxicity, insulin resistance and chronic low-grade inflammation in that adipose tissue and peripheral target organs.2–5
In addition to excessive visceral adipose tissue accumulation, ectopic lipid deposition in peripheral tissues or organs which do not typically accommodate fat, outside of the adipose tissue, such as the liver, skeletal muscle, kidney, pancreas, heart and vessels, is also suggested to play a role in the pathophysiology of MetS, as a locally acting endocrine and paracrine organ which has effects primarily on adjacent anatomic organs, directly via lipotoxicity and indirectly via aberrant secretion of adipokines.2–5
MetS is also shown to be associated with an increased risk of colorectal cancer incidence and mortality in both sexes.6,7 We also have shown that increasing the area of visceral fat assessed with computed tomography (CT) scans and a decreased concentration of plasma adiponectin are associated with the development of colorectal cancer and also adenoma, which is its precursor lesion.8,9 Hyperinsulinaemia due to insulin resistance and its influence in enhancing free or bioavailable concentrations of insulin-like growth factor-1, and altered levels of adipokines and their contribution to chronic low-grade inflammation are suggested to play a pivotal role in increasing colorectal cancer risk.5,10
However, it has not been well known whether there is ectopic lipid deposition in the colorectal tissue in association with MetS or its components such as abdominal obesity and insulin resistance, or whether, if so, colorectal lipid deposition participates in colorectal carcinogenesis.
To clarify whether there is MetS-associated lipid deposition in colorectal tissue, we measured lipid contents in colorectal tissues removed by endoscopy, and investigated the relationship of lipid contents with MetS or its components. In addition, to clarify the location of lipid deposition, we examined for the presence of lipid droplets in colorectal tissues obtained surgically by immunohistochemistry, and compared the grade of lipid droplet accumulation with the indices of obesity.
Materials and methods
Study patients
This study examined 27 consecutive patients with colorectal polyps at Hyogo Prefectural Nishinomiya Hospital (median 65 years; 35 to 86). The histological diagnosis was adenoma for 22 patients, in situ cancer in adenoma for four and juvenile polyp for one. All patients underwent polyp resection by endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). These patients were weight stable from diagnosis of colorectal polyp to endoscopic resection (<2% change). Exclusion criteria included a previous history of cancer, previous intestinal surgery, a history of inflammatory bowel diseases, a history suggestive of malabsorption, current treatment for weight loss, major medical problems, and consumption of medications with anti-inflammatory properties (e.g. nonsteroidal anti-inflammatory drugs, statins, glitazones, and cyclooxygenase-2 inhibitors).
To clarify the location of lipid deposition, we examined the non-tumorous colorectal tissue surrounding the tumour, which had been resected surgically from 32 patients with colorectal cancer. The median age was 71 years (34 to 90) for the 13 males and 19 females.
The study was approved by the Hyogo Prefectural Nishinomiya Hospital Ethical Committee on 17 January 2012 and 19 November 2013. Written informed consent was obtained from each patient before participation in the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Procedures and sample collection
Trained nurses measured waist circumference at the midpoint between the lower border of the rib cage and the iliac crest. Samples of venous blood were drawn from all patients after an overnight fast before the EMR or ESD procedure. These samples were immediately analysed for serum TG, total cholesterol (TC), HDL-C, low-density lipoprotein cholesterol (LDL-C), serum C-reactive protein (CRP), FPG, and FPI levels. The indices of insulin secretion and sensitivity were evaluated by homeostasis model assessment as an index of insulin resistance (HOMA-IR) = FPI (µIU/ml) × FPG (mg/dl)/405.11,12 Residual serum samples were immediately stored at –80℃ for serum adiponectin until further use.
The EMR and ESD procedures were carried out with modifications according to methods previously reported.13,14 The colorectal tissue surrounding the polyp resected from each patient was immediately stored at –80℃ for further use.
Assay of tissue lipid contents
About 30 mg of wet colorectal tissue resected from each patient was used for the assay of tissue TG, TC, and phospholipid (PL) contents. The tissue was minced with surgical scissors. Lipid extraction was carried out by a method previously described by Folch et al.15 The minced tissue was homogenised with methanol/chloroform (1/2 volume/volume, 20 ml/g tissue), and aliquots of the organic phase were evaporated under nitrogen gas. The dried lipid extracts were dissolved in isopropyl alcohol. The concentration of the tissue TC and TG within the lipid extracts was measured with a cholesterol assay kit and a TG assay kit using an enzyme method (Sekisui Medical Co Ltd, Tokyo, Japan) according to the manufacturer’s recommendations. The tissue PL concentration was measured with a PL assay kit using an enzyme method (Toyobo Co Ltd, Tokyo, Japan) according to the manufacturer’s recommendations. The tissue TC/PL and TG/PL ratios were used as substitutes for the TC/tissue weight and TG/tissue weight, respectively, to correct a possible increase of tissue weight by local injection of suitable amount of glucose or sodium hyaluronate solution used during endoscopic polyp resection because PL content was regarded as the indicator of cell membrane content, almost the total cell number, or nearly the original amount of tissue.
Measurement of visceral and subcutaneous fat areas
The intra-abdominal visceral and subcutaneous fat areas and waist circumference were measured at the level of the umbilicus by CT scan according to a procedure previously described.16 The border of the intra-abdominal cavity was outlined on the CT image, and visceral and subcutaneous fat areas were quantified using standard software. All CT scans were carried out using a CT scanner (General Electric Co, Fairfield, CT, USA) with participants in a supine position before operation.
Immunohistochemistry
For immunohistochemical examination of lipid droplet morphology, paraffin sections were stained with mouse monoclonal antibody against synthetic peptide of perilipin/PLIN1 (duplicated N-terminus of perilipin) (1:1, clone Peri112.17, Catalogue no. 651156; Progen Biotechnik, Heidelberg, Germany) as previously described.17 All sections were placed on an automated Ventana Benchmark XT platform (Roche Diagnostics) for detection of perilipin on the lipid droplets according to the manufacturer’s recommendations.
The contents of lipid droplets were divided into three grades (0 to 2) for comparison with factors such as body mass index (BMI) and visceral fat area associated with abdominal obesity.
Statistical analysis
Data are presented as the median and the range from the minimal and maximal value of each variable. The Wilcoxon signed rank test or the chi-square test was used to compare continuous or categorical variables, respectively, between colorectal polyp patients with and without MetS. Spearman rank test was used to evaluate correlations between tissue TG/PL values and continuous variables or categorical variables. The Kruskal–Wallis test or the chi-square test was used to compare continuous or categorical variables, respectively, in each of the tertile tissue TG/PL groups, and in each of the lipid droplet grades. Differences at a probability (p) value of less than 0.05 were considered to be significant. All statistical calculations were carried out using SAS Enterprise Guide, v. 4.2 (SAS Institute Inc, Cary, NC, USA).
Results
Baseline characteristics of the patients
The baseline characteristics of 27 colorectal polyp patients with or without MetS are summarised in Table 1. Thirteen of 27 patients fulfilled criteria for MetS. This group showed significantly higher values of BMI, FPI, FPG, HOMA-IR and diastolic blood pressure, and lower values of serum HDL-C and adiponectin than the rest. Four of the 27 patients had type 2 diabetes mellitus. There were no tendencies toward difference in the baseline characteristics between the diabetics and non-diabetics (p value ≥ 0.1).
Table 1.
Characteristics of 27 colorectal polyp patients with or without metabolic syndrome.
| Metabolic syndrome |
p value | |||
|---|---|---|---|---|
| (–) | (+) | |||
| n = 14 | n = 13 | |||
| Age | (Year) | 63 | 67 | 0.560 |
| (35–86) | (37–77) | |||
| Gender | (Male/Female) | 7/7 | 8/5 | 0.547 |
| Body mass indexa | (kg/m2) | 22.4 | 25.6 | 0.004 |
| (18.9–28.0) | (20.3–31.6) | |||
| Waist circumference | (cm) | 86 | 90 | 0.076 |
| (65–95) | (79–98) | |||
| Triglyceride | (mg/dl) | 83 | 132 | 0.052 |
| (48–313) | (85–248) | |||
| Total cholesterol | (mg/dl) | 198 | 198 | 0.466 |
| (159–265) | (147–280) | |||
| High-density lipoprotein cholesterol | (mg/dl) | 50 | 36 | 0.029 |
| (30–95) | (33–54) | |||
| Low-density lipoprotein cholesterol | (mg/dl) | 135 | 117 | 0.576 |
| (68–170) | (84–189) | |||
| Fasting plasma insulin | (µIU/ml) | 3.6 | 7.5 | 0.042 |
| (1.1–8.0) | (1.9–11.7) | |||
| Fasting plasma glucose | (mg/dl) | 86 | 110 | 0.002 |
| (61–95) | (70–199) | |||
| HOMA-IRb | 0.782 | 2.308 | 0.016 | |
| (0.166–1.719) | (0.525–3.459) | |||
| Adiponectin | (µg/dl) | 8.4 | 6.6 | 0.025 |
| (5.1–19.9) | (2.8–10.5) | |||
| C-reactive protein | (mg/dl) | 0.057 | 0.090 | 0.765 |
| (0.005–0.733) | (0.024–0.296) | |||
| Blood pressure | (mmHg) | |||
| Systolic | 128 | 147 | 0.159 | |
| (106–191) | (108–189) | |||
| Diastolic | 75 | 89 | 0.035 | |
| (61–112) | (67–121) | |||
| Number of positive factors under diagnostic criteria for MetSc | 1 | 4 | <0.001 | |
| (0–2) | (3–5) | |||
HOMA-IR: homeostasis model assessment as an index of insulin resistance; MetS: metabolic syndrome.
Results are expressed as the median and the range of minimal and maximal value of each variable or number. A p value was evaluated by the Wilcoxon signed rank test or, only for Gender, by the chi-square test of independence.
Body mass index is the weight in kilograms divided by the square of the height in meters.
HOMA-IR = fasting plasma insulin (µIU/ml) × fasting plasma glucose (mg/dl)/405.
According to the joint interim statement (Alberti et al.1), and only as to waist circumference, to Japanese riteria. Serum triglyceride ≥150 mg/dl, high-density lipoprotein cholesterol <40 mg/dl for male or < 50 g/dl for female, plasma fasting glucose ≥ 100 mg /dl, systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg, and waist circumference ≥ 85 cm for male or ≥ 90 cm for female. MetS is diagnosed when at least 3 of the above 5 conditions are fulfilled.
Table 2 shows the characteristics of 32 patients with colorectal cancer resected surgically.
Table 2.
Characteristics of 32 patients with colorectal cancer.
| Age | (Year) | 70.5 | (34–90) |
| Gender | (Male/Female) | 13/19 | |
| Body mass index | (kg/m2) | 21.9 | (14.7–31.0) |
| Body circumferencea | (cm) | 81 | (60–102) |
| Visceral fat areaa | (cm2) | 75 | (7–242) |
| Subcutaneous fat areaa | (cm2) | 95 | (0–266) |
Results are expressed as the median and the range of minimal and maximal values of each variable or number.
Body circumference, intra-abdominal visceral and subcutaneous fat areas were measured at the level of the umbilicus by computed tomography scan.
Tissue TG/PL and correlation with various factors
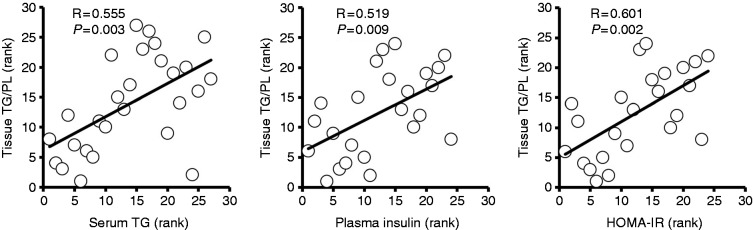
The median tissue TG/PL and TC/PL for the 27 patients were 0.22 (0.02–5.3) and 0.33 (0.26–0.40), respectively. The median tissue TG/PL ratios for the 13 patients with MetS and the 14 patients without MetS were 0.22 (0.07–2.7) and 0.26 (0.02–5.3), respectively, with no significant difference. The median tissue TG/PL ratios were 0.15 (0.02–2.12) for the four patients with type 2 diabetes, and 0.22 (0.08–5.3) for the 23 patients without diabetes, with no significant difference. Correlation coefficients of tissue TG/PL with various factors are shown in Table 3. Tissue TG/PL was significantly correlated with serum TG and FPI levels, and the value of HOMA-IR (Figure 1). Significant correlation was also found between tissue TG/PL and serum TC or LDL-C levels. Tissue TC/PL was not correlated with any factors.
Table 3.
Correlation of tissue triglyceride/phospholipid with various factors.
| Correlation coefficient | p value | ||
|---|---|---|---|
| Age | (Year) | –0.199 | 0.319 |
| Body mass index | (kg/m2) | 0.314 | 0.110 |
| Waist circumference | (cm) | 0.272 | 0.189 |
| Triglyceride | (mg/dl) | 0.555 | 0.003 |
| Total cholesterol | (mg/dl) | 0.515 | 0.006 |
| High-density lipoprotein cholesterol | (mg/dl) | –0.160 | 0.426 |
| Low-density lipoprotein cholesterol | (mg/dl) | 0.647 | <0.001 |
| Fasting plasma insulin | (µIU/ml) | 0.519 | 0.009 |
| Fasting plasma glucose | (mg/dl) | 0.359 | 0.072 |
| HOMA-IR | 0.601 | 0.002 | |
| Adiponectin | (µg/dl) | –0.335 | 0.118 |
| C-reactive protein | (mg/dl) | 0.196 | 0.394 |
| Blood pressure | (mmHg) | ||
| Systolic | 0.199 | 0.320 | |
| Diastolic | 0.181 | 0.368 | |
| Number of positive factors under diagnostic criteria for MetS | |||
| 0.257 | 0.195 |
Spearman rank test was used to evaluate correlations.
HOMA-IR: homeostasis model assessment as an index of insulin resistance; MetS: metabolic syndrome.
Figure 1.
Correlations of tissue triglyceride/phospholipid (TG/PL) ratio with serum triglyceride (TG), fasting plasma insulin levels, and homeostasis model assessment as an index of insulin resistance (HOMA-IR). Spearman rank test was used to evaluate correlations. R: correlation coefficient; P: p value; HOMA-IR = fasting plasma insulin (µIU/ml) × fasting plasma glucose (mg/dl)/405.
Tertile analysis of tissue TG/PL
Factors associated with increasing tissue TG/PL were serum TG, TC, LDL-C, FPI level, HOMA-IR and BMI (p = 0.025, p = 0.013, p = 0.001, p = 0.041, p = 0.013, p = 0.047, respectively), while serum adiponectin level was associated with decreasing tissue TG/PL (p = 0.046) (Table 4). On the other hand, none of the metabolic factors were associated with tissue TC/PL.
Table 4.
Tertile analysis of factors associated with tissue triglyceride/phospholipid value.
| Tertile 1 |
Tertile 2 |
Tertile 3 |
p value | ||
|---|---|---|---|---|---|
| Triglyceride/phospholipid | (<0.126) | (0.126 ≤ and < 1.095) | (≥1.095) | ||
| Age | (Year) | 65 | 69 | 62 | 0.274 |
| (35–86) | (50–73) | (37–72) | |||
| Gender | (Male/Female) | 4/5 | 7/2 | 4/5 | 0.259 |
| Body mass index | (kg/m2) | 22.2 | 24.9 | 24.5 | 0.047 |
| (18.9–25.6) | (21.8–31.6) | (20.1−30.1) | |||
| Waist circumference | (cm) | 83 | 89 | 90 | 0.237 |
| (65–96) | (83–92) | (82v98) | |||
| Triglyceride | (mg/dl) | 69 | 111 | 132 | 0.025 |
| (48–215) | (64–313) | (94–248) | |||
| Total cholesterol | (mg/dl) | 191 | 168 | 226 | 0.013 |
| (147–207) | (151–265) | (182–280) | |||
| High-density lipoprotein cholesterol | (mg/dl) | 50 | 43 | 37 | 0.235 |
| (35-95) | (30-61) | (33-64) | |||
| Low-density lipoprotein cholesterol | (mg/dl) | 114 | 117 | 144 | 0.001 |
| (68–135) | (83–160) | (121–189) | |||
| Fasting plasma insulin | (µIU/ml) | 3.4 | 5.3 | 7.2 | 0.041 |
| (1.1–11.7) | (1.9–7.6) | (5.9–10.3) | |||
| Fasting plasma glucose | (mg/dl) | 74 | 88 | 102 | 0.089 |
| (61–107) | (70–123) | (86–199) | |||
| HOMA-IR | 0.617 | 0.988 | 2.152 | 0.013 | |
| (0.166–3.091) | (0.426–2.308) | (1.32–3.46) | |||
| Adiponectin | (µg/dl) | 12.9 | 7.4 | 5.6 | 0.046 |
| (6.2–19.9) | (5.1–12.2) | (2.8–10.0) | |||
| C-reactive protein | (mg/dl) | 0.029 | 0.128 | 0.047 | 0.258 |
| (0.005–0.733) | (0.026–0.296) | (0.033–0.549) | |||
| Blood pressure | (mmHg) | ||||
| Systolic | 116 | 130 | 142 | 0.358 | |
| (106–154) | (120–191) | (113–189) | |||
| Diastolic | 73 | 86 | 76 | 0.067 | |
| (61–91) | (78-112) | (69–121) | |||
| Number of positive factors under diagnostic criteria for MetS | 1 | 3 | 2 | 0.130 | |
| (0–4) | (1–4) | (1–5) |
HOMA-IR: homeostasis model assessment as an index of insulin resistance; MetS: metabolic syndrome.
Results are expressed as the median and the range of minimal and maximal value of each variable or number. P value was evaluated by the Kruskal–Wallis test or, only for Gender, by the chi-square test of independence.
Localisation of lipid droplets
Perilipin-immunostaining was observed in the submucosal region of the colorectal tissue (Figure 2). Panel (A) is grade 0, showing no lipid droplet in a representative case (Figure 2(A)), and panel (B) (Figure 2(B)) and panel (C) (Figure 2(C)) are grade 1 and grade 2, respectively. High-power view of grade 2 of perilipin-immunostaining is seen in the colorectal submucosal region (Figure 2(D)).
Figure 2.
Lipid droplets in the submucosal region of colorectal tissue (immunohistochemical examination using anti-perilipin antibody). (A) Grade 0 of lipid droplet accumulation. (B) Grade 1 of lipid droplet accumulation. (C) Grade 2 of lipid droplet accumulation. (D) High-power view of grade 2 is seen in the colorectal submucosal region. Bar represents a length of 1 mm.
Association of lipid droplet accumulation with abdominal obesity
Lipid droplets were observed in the submucosal region of the colorectal tissue in 18 of the 32 individuals, grade 1 in 13 and grade 2 in five. Grade of lipid droplet accumulation was significantly associated with BMI, waist circumference, and visceral and subcutaneous fat areas (p = 0.039, p = 0.007, p = 0.002 and p = 0.039, respectively) (Table 5).
Table 5.
Factors associated with perilipin-staining grade of colorectal tissue.
| Perilipin-staining grade | 0 | 1 | 2 | p value | |
|---|---|---|---|---|---|
| (n = 14) | (n = 13) | (n = 5) | |||
| Age | (Year) | 71 | 73 | 45 | 0.124 |
| (40–89) | (61–90) | (34–83) | |||
| Gender | (Male/Female) | 3/11 | 6/7 | 4/1 | 0.063 |
| Body mass index | (kg/m2) | 20.7 | 21.8 | 24.2 | 0.039 |
| (14.7–31.0) | (19.9–30.1) | (22.0–28.1) | |||
| Waist circumference | (cm) | 78 | 83 | 90 | 0.007 |
| (60–90) | (72–102) | (82–98) | |||
| Visceral fat area | (cm2) | 30 | 98 | 150 | 0.002 |
| (7–186) | (17–202) | (106–242) | |||
| Subcutaneous fat area | (cm2) | 49 | 100 | 142 | 0.039 |
| (0–178) | (42–266) | (65–186) |
Results are expressed as the median and the range of minimal and maximal value of each variable or number.
P value was evaluated by the Kruskal–Wallis test or, only for Gender, by the chi-square test of independence.
Discussion
In the present study, we found that as the FPI level and HOMA-IR increased and the serum adiponectin level decreased, the tissue TG/PL ratio increased. However, the tissue TC/PL ratio was not correlated with any risk factors such as FPI and HOMA-IR. Our observation indicated that increased TG content in the colorectal tissue seemed to be associated with insulin resistance.
Lipid droplets are intracellular organelles that store neutral lipids within cells.18 Recently, understanding of lipid droplet biology in metabolic disease has advanced considerably.18 Lipid droplets are known to regulate the storage and hydrolysis of neutral lipids, including TG and cholesterol esters. Adipocytes, the major reservoir of TG in the body, store TG within lipid droplets, and the amount stored increases with obesity. The rates of lipolysis in the adipocytes in obesity are constitutively increased, resulting in elevated levels of circulating fatty acids. This seems to lead to their being stored as TG in lipid droplets within the liver, skeletal muscle, and other organs.
The TG droplets become coated with a monolayer of PL and a cadre of proteins that stabilise it within the cytoplasm of cells.19 Perilipin-1 (PLIN1) was identified as the first lipid droplet-associated protein.20 Tissue surveys revealed that PLIN1 is most highly expressed in adipocytes.21 PLIN1 is the most abundant and thoroughly studied perilipin/ADRP/TIP47 (PAT) family protein in adipocytes.22,23
In this study, to clarify the localisation of TG accumulation, we carried out immunohistochemical examination using anti-PLIN1 antibody and examined the presence of lipid droplets in the colorectal tissues obtained. Lipid droplets were observed in the submucosal region of the colorectal tissue in 18 of the 32 individuals examined (grade 1 in 13 and grade 2 in five). The grade of lipid droplet accumulation was significantly associated with BMI, waist circumference, and visceral fat area. These observations demonstrated for the first time that the lipid droplets were localised in the submucosal region of colorectal tissues in individuals with abdominal obesity.
From these observations, the presence of lipid droplets seems to be related to the TG accumulation in the colorectal tissues from individuals with abdominal obesity and insulin resistance. It is possible that in a state of abdominal obesity and insulin resistance, an increase in free fatty acid supply to colorectal tissues could lead to TG accumulation and the formation of lipid droplets. However, further study is needed to elucidate the mechanism(s) whereby lipid droplets accumulate in the submucosal region of the colorectal tissue in individuals with abdominal obesity and insulin resistance.
The selection of patients with colorectal polyp and cancer only is a limitation in this study. However, it was impossible to examine fat accumulation in colorectal tissues resected from individuals without colorectal neoplasia because of ethical reason and very few occasions of therapeutic colorectal resection in individuals without its inflammation or neoplasia. Development of a non-invasive system to detect submucosal fat accumulation in the colorectal tissue is expected. Moreover, a future problem not confirmed in the present study is whether submucosal lipid deposition is different among the parts of the colon and rectum, or associated with local pericolorectal fat adjacent to the outside of the colorectal wall, which seems difficult to measure in distinction from others, rather than total intra-abdominal visceral fat.
In conclusion, the presence of ectopic fat accumulation of colorectal tissues, similar to fat accumulation in liver and skeletal muscle, could be associated with abdominal obesity and insulin resistance. Although this study should be replicated with a larger number of patients, our observations warrant future study on the association of ectopic fat accumulation with colorectal diseases and pathophysiology, including inflammation and carcinogenesis.24
Acknowledgements
We thank Ms Rie Takeda, Ms Yoko Ueto, Mr Koichi Sanada, Ms Nami Uematsu and Ms Satomi Higashi, Division of Pathology, Hyogo Prefectural Nishinomiya Hospital, for their excellent technical assistance.
Declaration of conflicting interests
None declared.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
Ethics approval
This study was approved by the Hyogo Prefectural Nishinomiya Hospital Ethical Committee on 17 January 2012 and 19 November 2013. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Informed consent
Written informed consent was obtained from each patient before participation in this study.
References
- 1.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: Contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb 2011; 18: 629–639. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM. Overnutrition, ectopic lipid and the metabolic syndrome. J Investig Med 2016; 64: 1082–1086. [DOI] [PubMed] [Google Scholar]
- 4.Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol 2014; 34: 1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riondino S, Roselli M, Palmirotta R, et al. Obesity and colorectal cancer: Role of adipokines in tumor initiation and progression. World J Gastroenterol 2014; 20: 5177–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito K, Chiodini P, Capuano A, et al. Colorectal cancer association with metabolic syndrome and its components: A systematic review with meta-analysis. Endocrine 2013; 44: 634–647. [DOI] [PubMed] [Google Scholar]
- 7.Jinjuvadia R, Lohia P, Jinjuvadia C, et al. The association between metabolic syndrome and colorectal neoplasm: Systemic review and meta-analysis. J Clin Gastroenterol 2013; 47: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otake S, Takeda H, Fujishima S, et al. Decreased levels of plasma adiponectin associated with increased risk of colorectal cancer. World J Gastreoenterol 2010; 16: 1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: Evidence for participation of insulin resistance. Clin Cancer Res 2005; 11: 3642–3646. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: A review. Am J Clin Nutr 2007; 86: s836–s842. [DOI] [PubMed] [Google Scholar]
- 11.Fukushima M, Usami M, Ikeda M, et al. Insulin secretion and insulin sensitivity at different stages of glucose tolerance: A cross-sectional study of Japanese type 2 diabetes. Metabolism 2004; 53: 831–835. [DOI] [PubMed] [Google Scholar]
- 12.Kuroe A, Fukushima M, Usami M, et al. Impaired beta-cell function and insulin sensitivity in Japanese subjects with normal glucose tolerance. Diabetes Res Clin Pract 2003; 59: 71–77. [DOI] [PubMed] [Google Scholar]
- 13.Tada M. Endoscopic mucosal resection of the stomach: Initial description. Gastrointest Endosc Clin N Am 2001; 11: 499–510. [PubMed] [Google Scholar]
- 14.Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis 2005; 6: 119–121. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957; 226: 497–509. [PubMed] [Google Scholar]
- 16.Tokunaga K, Matsuzawa Y, Ishikawa K, et al. A novel technique for determination of body fat by computed tomography. Int J Obes 1983; 7: 437–445. [PubMed] [Google Scholar]
- 17.Lenz LS, Marx J, Chamulitrat W, et al. Adipocyte-specific inactivation of acyl-CoA synthetase fatty acid transporter protein 4 (Fatp4) in mice causes adipose hypertrophy and alterations in metabolism of complex lipids under high fat diet. J Biol Chem 2011; 286: 35578–35587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg AS, Coleman RA, Kraemer FB, et al. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest 2011; 121: 2102–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchette-Mackie EJ, Dwyer NK, Barber T, et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 1995; 36: 1211–1226. [PubMed] [Google Scholar]
- 20.Greenberg AS, Egan JJ, Wek SA, et al. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 1991; 266: 11341–11346. [PubMed] [Google Scholar]
- 21.Londos C, Brasaemle D, Schultz C, et al. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol 1999; 10: 51–58. [DOI] [PubMed] [Google Scholar]
- 22.Lu X, Gruia-Gray J, Copeland NG, et al. The murine perilipin gene: The lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene of ancient origin. Mamm Genome 2001; 12: 741–749. [DOI] [PubMed] [Google Scholar]
- 23.Miura S, Gan JW, Brzostowski J, et al. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem 2002; 277: 32253–32257. [DOI] [PubMed] [Google Scholar]
- 24.Pendyala S, Neff LM, Suárez-Fariñas M, et al. Diet-induced weight loss reduces colorectal inflammation: Implications for colorectal carcinogenesis. Am J Clin Nutr 2011; 93: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]