Abstract
Background
The western diet high in fat and fructose may cause metabolic disorders and cardiovascular diseases.
Objective
To evaluate whether long-term daily vitamin D3 supplementation prevents hepatic steatosis and cardiovascular abnormalities and restores insulin sensitivity caused by fat diet in rats without vitamin D deficiency.
Methods
Three groups of rats were fed for 6 months with standard diet (SD), western diet (WD) or WD containing 23 IU/day/rat vitamin D3, respectively. Tail-cuff systolic blood pressure (SBP)measurements in conscious rats and transthoracic echocardiography were performed in basal condition, and after 3 and 6 months of diet. Hepatic steatosis and myocardial fibrosis were assessed in liver and cardiac tissues using standard methods. Serum insulin and 25(OH)D3 concentrations were determined using rat-specific ELISA kits. Insulin resistance was determined according to the homeostasis model assessment of insulin resistance (HOMA-IR) method.
Results
Sixty-one per cent of hepatocytes in WD rats had steatotic vacuoles compared with just 27% in rats on a WD plus vitamin D3 (p < 0.05).HOMA-IR was reduced in rats with vitamin D supplementation compared with WD alone (19.4 ± 5.2 vs 41.9 ± 8.9, p < 0.05). Rat blood pressure and left ventricular mass were both reduced by vitamin D3 supplementation.
Conclusion
In animal models of liver and cardiovascular metabolic damage, the supplementation of vitamin D3 shows liver and cardio-protective effects.
Keywords: Diabetes, insulin, HOMA, vitamin D, western diet, cardiovascular disease
Key summary
The novelty of this study is the demonstration that supplementation with vitamin D exerts its beneficial effects even in the absence of vitamin depletion.
Dietary supplementation with vitamin D abolishes the detrimental effects induced by a WD on the development of insulin resistance, liver steatosis and CV risk profile.
These results shed new light on the controversial role of vitamin D in liver and cardiac changes resulting from fatty diets.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of chronic liver disease in western countries,1,2 and is associated with insulin resistance, type 2 diabetes mellitus, dyslipidaemia and heart failure.3 Western diet (WD) and sedentary lifestyle increase cardiovascular (CV) risk profile through the development of hypertension and metabolic syndrome.4–6 One of the pathogenic mechanisms shared between the determinants of CV risk and the NAFLD is insulin resistance (IR). Clinical and experimental evidence show that IR is involved in the pathogenesis of essential hypertension, diabetes, obesity, metabolic syndrome and NAFLD.7–9 Vitamin D is a group of steroid molecules that play a canonical role in calcium and bone homeostasis. However, nowadays it is known that vitamin D regulates in many organs and tissues several biological functions through their receptors (VDR), which are widespread in the body.10 A growing body of experimental and clinical evidence indicates that an association exists between vitamin D deficiency and the prevalence of many chronic morbid conditions, including CV and liver diseases.11,12 In particular, an association between vitamin D deficiency and the occurrence of major adverse CV events was found among Framingham Offspring Study participants.13 Moreover, several studies showed an association between deficit of vitamin D, hypertension, diabetes14,15 and pathogenesis of NAFLD.12,15 Interestingly, a deficiency of vitamin D contributes also to the onset of IR.11 Furthermore, a recent paper shows a trophic action of vitamin D on muscle fibres, not only in rats fed with regular diet, but also in the case of a diet mimicking the Mediterranean diet.16 Nevertheless, the relationship among vitamin D, CV risk profile and liver damage is still a debated issue. In particular, it is unclear whether the supplementation of vitamin D interferes with the determinant of CV risk and the development of NAFLD even in the presence of physiological ranges of vitamin D. Therefore, we planned this study in an animal model in the presence of normal dietary intake of vitamin D to: (1) evaluate the effects of WD on the development of metabolic, hepatic and CV abnormalities; and (2) verify whether dietary supplementation with vitamin D, in this experimental setting, prevents the development of liver damage and ameliorates the CV risk profile.
Materials and methods
Study design
Eighteen male Wistar rats from Harlan Italy (Udine, Italy) weighing 120–150 g were randomly housed in wire-bottomed cages. Animals were maintained at a temperature of 21 ± 1℃ with a 12-hour light/dark cycle and had free access to food and liquids. We avoided the use of metabolic cages for two main reasons: first because we did not need to collect faeces and urine separately and second to avoid the stress caused to the animals by long-term housing in such cages.17 Three groups of six rats each were treated as follows: (i) standard diet (SD) containing 1000 IU/kg of vitamin D; (ii) WD containing 1000 IU/kg of vitamin D and a 12.5% w/v sweet drinking solution; and (iii) WD and a 12.5% w/v sweet drinking solution plus additional 750 IU/kg vitamin D (WD+VitD). The SD consisted of 18% fat, 24% proteins and 58% carbohydrates (3.3 kcal/g). The WD consisted of 58% energy fat, 18% proteins and 24% carbohydrates (5.6 kcal/g). The sweet solution was constituted of 55% fructose and 45% glucose. The diets were purchased from Harlan Laboratories (Udine, Italy). The experiment was conducted for 6 months. The weight and caloric intake of each rat were recorded weekly. Blood pressure and echocardiography were measured in basal conditions and after 3 and 6 months. The rats were painlessly killed with anaesthetics according to the approved protocol by the Ethics Committee of the University of Naples ‘Federico II’ (N. 0124285).
Serum measurements
At 6 months, vitamin D, glucose, total cholesterol and triglyceride concentrations were measured in the serum of rats using a Modular Autoanalyzer. Serum insulin and 25(OH)D3 concentrations were determined with rat-specific ELISA kits (EMD Millipore, USA, and DIAsource SA, Belgium, respectively). Homeostasis model assessment of insulin resistance (HOMA-IR) was evaluated as the product of fasting plasma glucose (mM) and insulin concentration (ng/ml): HOMA-IR = [glucose] × [insulin]/22.5.
Cardiovascular measurements
Systolic blood pressure and heart rate in conscious restrained rats were measured noninvasively by tail-cuff plethysmography (PE-300, Narco Biosystems Inc) using a heating pad to obtain a body temperature of 35–37℃ taking care to ensure minimal stress to the animals. We used the last 5 out of 10 measurements per cycle for our analyses.18 Transthoracic echocardiography was performed using a dedicated small-animal high-resolution ultrasound system (VeVo 770, Visualsonics Inc. Toronto, ON, Canada) equipped with a 7.5 MHz transducer (RMV-716). Left ventricular (LV) end-diastolic and end-systolic diameters (LVEDD and LVESD, respectively) were measured at the level of the papillary muscles from the parasternal short-axis view as recommended. Interventricular septal (IVS) and LV posterior wall thickness were measured at end diastole. Left ventricular mass (LVM) was calculated according to the following formula, representing the M-mode cubic method: LVM = 1.05 × [(IVS + LVEDD + LVPW)3 − (LVEDD)3]; LVM was corrected by body weight. All measurements were averaged on five consecutive cardiac cycles and analyzed by two experienced investigators blinded to treatment.
Tissue collection
At the time of sacrifice, hearts were harvested, rinsed in cold phosphate buffer saline (PBS) and blotted dry, weighed, divided into left and right ventricles, and then fixed in 10% formalin. The fixed tissues were dehydrated, embedded in paraffin and sectioned at 6 -µm thickness. Collagen analysis was determined using the Sirius red stained sections of LV myocardium using a Nikon E800 microscope connected to a computer with Metamorph image. Twenty fields from each of three LV sections (base, mid, apex) were examined, and collagen area percent for each animal was expressed as the average of all fields examined. The liver was collected, weighed and subjected to macroscopic evaluation. Liver biopsies were used for histology, and the remainder was frozen and stored at −80℃. Frozen liver sections were stained with Oil Red O to identify the presence of steatosis. Histology was evaluated by pathologists blinded to the source of the material. NAFLD was defined taking into account the presence of steatosis, lobular inflammation and ballooning degeneration. Fatty area percent for each animal was expressed as the average of all fields examined. Epididymal fat, reflecting the fat accumulated in response to a fatty diet,19 was surgically removed during the autopsy, weighted and expressed as a quotient with respect to the body weight.
Statistical analysis
Statistically significant differences between the three groups of rats were determined with one-way analysis of variance (ANOVA) followed by the Tukey’s multiple comparison test; p < 0.05 was considered statistically significant.
Results
Food intake, body weight and fat body storage
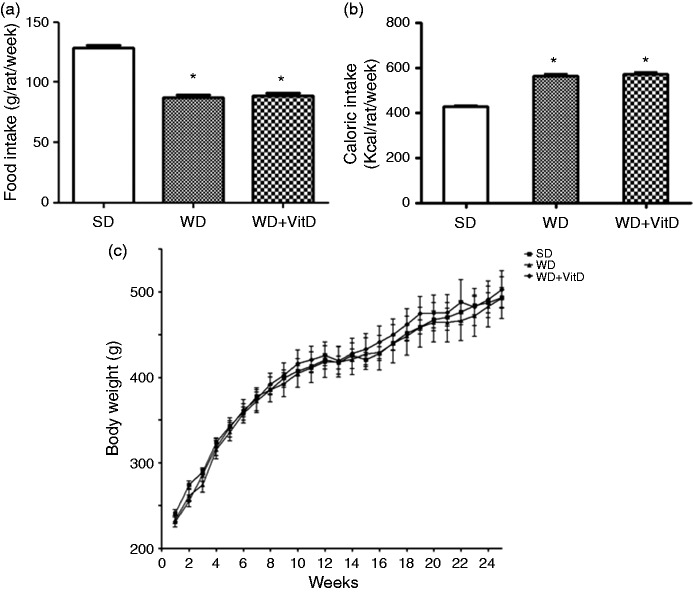
Food intake was significantly higher in the SD group than in WD and WD+VitD (p < 0.01 in both cases) (Figure 1(a)), nevertheless, caloric intake was significantly lower in SD than in WD and WD+VitD rats (p < 0.01 in both cases) (Figure 1(b)). The rat body weight did not differ significantly among the three groups (Figure 1(c)). The epididymal fat/body weight ratio was higher in WD than in SD rats (1.16 ± 0.03 vs 0.94 ± 0.05, p < 0.05) and was significantly lower in WD+VitD compared with WD rats (0.75 ± 0.07 vs 1.16 ± 0.03, p < 0.01).
Figure 1.
Food intake (a), calorie intake (b) and body weight (c) of three groups of rats fed different diets for 6 months. SD: standard diet; WD: western diet; WD+VitD: western diet plus vitamin D (*p < 0.05 vs SD).
Serum metabolic parameters
Despite a daily intake of the same adequate dose of vitamin D (1000 IU/kg), WD rats had lower serum levels of 25(OH)D3 than SD rats. As expected, the levels of 25(OH)D3 were significantly higher in WD + VitD rats than in WD rats (p < 0.01) (Figure 2).
Figure 2.
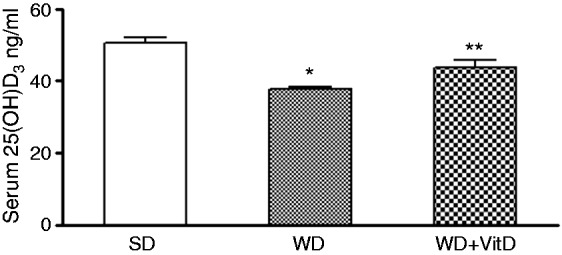
Serum 25-(OH) D3 in three groups of rats fed different diets for 6 months. SD: standard diet; WD: western diet; WD+VitD: western diet plus vitamin D (*p < 0.05 vs SD; **p < 0.05 vs WD).
Table 1 shows the rat serum lipid and metabolic profiles. The total cholesterol level did not differ among groups, whereas triglyceride levels were significantly higher in the WD than in the SD group. Serum triglyceride levels were reduced by WD+VitD compared with WD rats, although the difference was not statistically significant. Interestingly, all SD and WD+VitD rats had normal glycaemia values (<120 mg/dl), whereas all rats in the WD group were diabetic, and had significantly higher glycaemia values compared with SD rats. Insulin was higher in WD than in SD and WD+VitD rats. HOMA-IR was significantly higher in WD than in SD rats, and was significantly lower in WD+VitD than in WD rats (p < 0.05).
Table 1.
Serum lipid and metabolic profile of rats fed a standard diet, a western diet or a western diet plus vitamin Da
| SD | WD | WD + VitD | p-value | |
|---|---|---|---|---|
| Total cholesterol (mg/dl) | 92.3 ± 5.4 | 73.2 ± 6.6 | 83.7 ± 4.1 | NS |
| Triglyceride (mg/dl) | 46.8 ± 4.8 | 113.5 ± 19.1 | 94.8 ± 17.0 | SD vs WD p < 0.05 |
| Glycaemia (mg/dl) | 98.7 ± 8.1 | 139.0 ± 9.6 | 103.2 ± 6.1 | SD vs WD p < 0.01 WD vs WD + VitD p < 0.05 |
| Insulin (ng/ml) | 1.07 ± 0.21 | 5.37 ± 1.22 | 3.23 ± 0.80 | SD vs WD p < 0.01 |
| HOMA-IR | 6.17 ± 1.30 | 41.90 ± 8.89 | 19.38 ± 5.25 | SD vs WD p < 0.01 WD vs WD + VitD p < 0.05 |
Values are expressed as mean ± SE.
SD: standard diet; WD: western diet; WD + VitD: western diet plus vitamin D.
Liver status
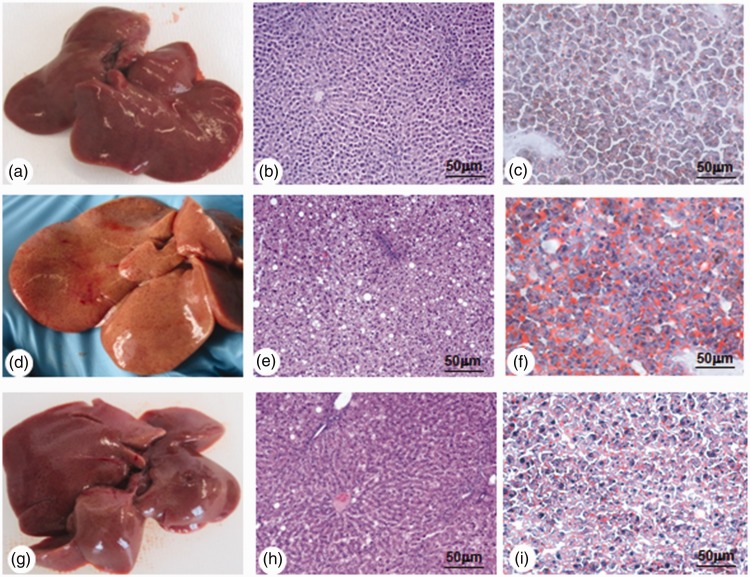
The liver of SD rats was of normal volume, smooth to the touch and brownish-red (Figure 3(a)). Rats fed a WD were affected by hepatomegaly, and the liver was largely pale yellow suggesting infiltration of adipose tissue in the parenchyma (Figure 3(d)). The livers of WD+VitD rats were, like the livers of WD rats, enlarged and pinkish in colour (Figure 3(g)). To avoid bias arising from intra-group differences with respect to liver weight, we calculated the ‘liver/body weight ratio’. This ratio was significantly lower in SD rats than in WD rats (2.01 ± 0.04% vs 2.90 ± 0.05%, p < 0.001). Moreover, it was lower in WD+VitD than in WD rats, although the difference was not significant (2.75 ± 0.07% vs 2.90 ± 0.05%; NS). Histology of the livers of SD rats was unremarkable: hepatocytes were free of fat, and lobules were visible, each of which was a hexagonal structure consisting of a central vein surrounded by radiating hepatocyte plates, and the nucleus (blue-stained) was located in the centre of each hepatocyte (Figure 3(b)). In the WD group, hepatocytes were disordered with reduced cytoplasm and ballooning degeneration, and they featured abundant lipid droplets and widespread vacuolation; the blue-stained nucleus was located in the border of the cell (Figure 3(e)). In WD+VitD animals, hepatocytes were similar to those in SD rats: the lipid droplets were clearly less abundant than in the WD group, the hepatocytes were less disordered and most had a normal aspect (Figure 3(h)). In the WD group, 61% of hepatocytes contained steatotic vacuoles versus 27% in the WD+VitD group (p < 0.05). Oil Red O staining of liver tissue was higher in the WD rats (Figure 3(f)) than in SD rats (Figure 3(c)) and was strongly reduced in WD+VitD rats (Figure 3(i)).
Figure 3.
Macroscopic appearance of the liver, histology and Oil Red O staining in three groups of rats fed different diets for 6 months. Rats fed a standard diet (a−c); rats fed a western diet (d−f); rats fed a western diet plus vitamin D (g−i). Original magnification × 40; scale bar: 50 µm.
Cardiovascular status
WD enhanced systolic blood pressure (SBP) by 27 ± 12%, p < 0.001, at 3 months, and by 47 ± 11%, p < 0.001, at 6 months. Supplementation with vitamin D abolished the WD-induced increase in SBP (Figure 4).
Figure 4.
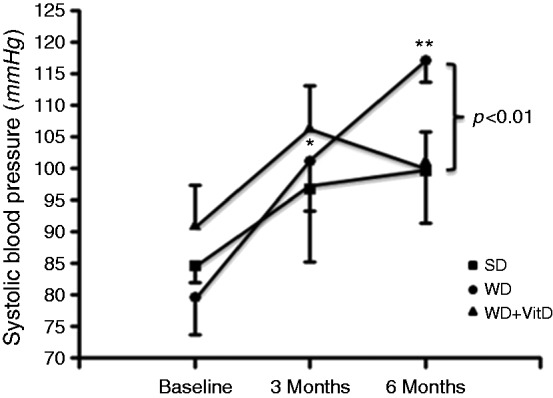
Changes of systolic blood pressure in the three study groups during the study. SD: standard diet (n = 6); WD: western diet (n = 6); WD+vitD: western diet + vitamin D supplement (n = 6). *p < 0.05 vs baseline, **p < 0.05 vs baseline and 3 months (by ANOVA). Other significance values are shown in the figure.
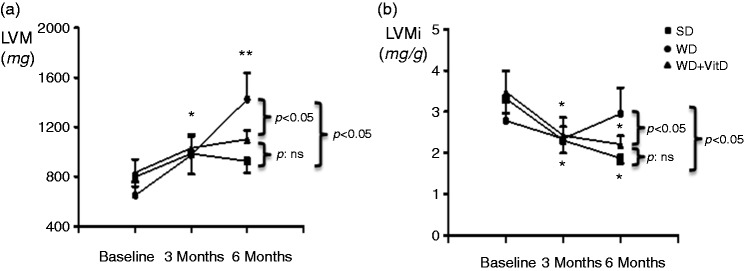
WD rats showed a significant increase of LVM during the study period while the supplement with vitamin D abolished this increase of LVM (Figure 5(a)). In basal conditions, indexed LVM (LVMi), was similar in the three groups. In SD and WD+VitD groups LVMi significantly decreased throughout the study period. On the contrary, it increased in the WD group, resulting, at 6 months, in higher values than in both SD and WD+VitD groups (Figure 5(b)).
Figure 5.
(a) Changes of left ventricular mass (LVM) and (b) indexed LVMi in the three study groups during the study. SD: standard diet (n = 6); WD: western diet (n = 6); WD+vitD: western diet + vitamin D supplement (n = 6). *p < 0.05 vs baseline, **p < 0.05 vs baseline and 3 months (by ANOVA). Other significance values are shown in the figure.
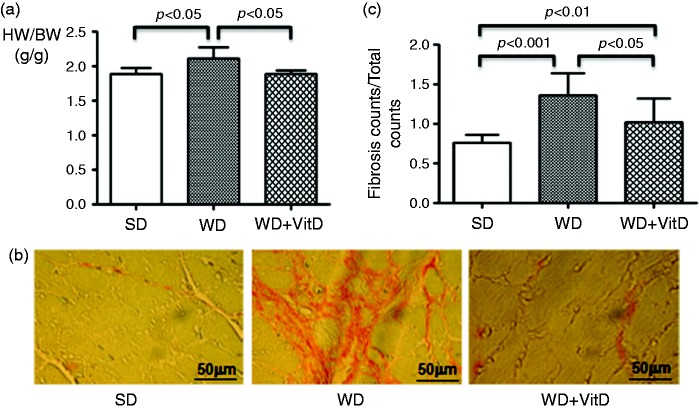
Heart weight/body weight ratio was enhanced by WD (Figure 6(a)) while no difference was detected between SD and WD+VitD groups.
Figure 6.
(a) Heart weight/body weight ratio in the three study groups detected at the end of the study. HW: heart weight; BW: body weight. (b) Myocardial fibrosis detected by Picric acid Sirius red staining of heart sections. In the Picric acid Sirius red staining evaluation, cardiomyocytes are coloured yellow, nucleus is dark blue and collagen is fuchsia. (c) Quantification of fibrosis. The significance values are shown in the figure. Original magnification × 40; scale bar: 50 µm.
Cardiac fibrosis is characterized by net accumulation of collagen and other extracellular matrix proteins in the cardiac interstitium and around the vessels. As shown in Figure 6(b), myocardial fibrosis was more evident in the WD group. There was a significant increase of interstitial fibrosis in the left ventricle in the WD group versus the SD group (1.36 ± 0.27% vs 0.76% ± 0.09%, respectively, p < 0.001) (Figure 6(c)). Interstitial fibrosis was significantly reduced by vitamin D supplementation in the WD group (1.02 ± 0.07%, p < 0.05 vs WD); however, fibrosis was still present in the WD+VitD vs SD group (Figure 6(b) and (c)).
Discussion
The main result of this study is that, in rats, dietary supplementation with vitamin D eliminates the detrimental effects induced by WD on the development of IR, liver steatosis and CV risk profile.
As expected, a western diet (WD) induces hypertriglyceridaemia, IR and liver steatosis, in addition to increasing systolic blood pressure, enhancing LVM and inducing myocardial fibrosis. These abnormalities, which resemble the metabolic syndrome, are prevented by dietary supplementation with vitamin D. The novelty of this study is the demonstration that vitamin D supplementation exerts its beneficial effects even in the absence of vitamin depletion.
It is interesting to note that the levels of vitamin D were lower in rats fed with a WD compared with rats fed with a SD. This result confirms similar recent findings of an association between a fatty diet, adiposity and lower serum levels of vitamin D.20 This phenomenon observed both in human studies and in animal models20,21 has been pathogenically interpreted as accumulation of vitamin D in adipose tissue.22 Nonetheless, the addition of a supplementary dose of vitamin D partly reverses this effect and reduces the metabolic abnormalities (hyperglycaemia, hypertriglyceridaemia, hyperinsulinaemia and IR) associated with a fatty diet. On the other hand, no effect has been observed on the levels of total cholesterol, even if recent observations suggest a role of vitamin D in modulating HDL-cholesterol also.23 The mechanism by which vitamin D exerts its beneficial effect is incompletely known, although oxidative stress leading to IR has been documented in hepatic cell lines with vitamin D-induced deficiency.24
Both histological analysis and staining with Oil Red O showed that WD leads to severe liver steatosis associated with altered hepatocyte structure. Daily administration of an additional dose of vitamin D induces a cytoprotective effect on hepatocytes in animals subjected to a typical WD. Altered serum levels of insulin, glucose and triglycerides following a WD are both the cause and consequence of IR, as they are biochemical precursors and metabolic signals of steatosis.25 Hyperinsulinaemia and hyperglycaemia activate transcription factors in the liver, namely, SREBP-lc (Sterol Regulator Element-Binding Protein) and ChREBP (Carbohydrate Response Element-Binding Protein), which, in turn, activate enzymes required for the conversion of excess glucose into fatty acids.26,27 The increased intrahepatic concentration of free fatty acids and triglycerides induces the synthesis and secretion of tumour necrosis factor alpha (TNF-α), which interferes with the signalling of insulin at receptor level;28 this, in turn, induces the IR characteristic of the metabolic syndrome associated with obesity.29 This cascade of events results in further accumulation of intrahepatic fat. In our experiment, the extra supplementation of vitamin D improves serum levels of glucose, triglycerides and insulin, and the HOMA-IR index, and reduces hepatic steatosis. In other words, vitamin D improves the biochemical and histological parameters of metabolic syndrome, probably by interfering with some metabolic pathways in the mechanisms of synthesis and accumulation of lipids in the liver. Furthermore, according to a previous study,30 it is likely that vitamin D downregulates the inflammatory pathway mediated by Toll-like receptor 4 reducing the expression of TLR4, NF-κB, and TNF-α in the liver tissue and attenuating the hepatic inflammation and fibrosis.
Our results are consistent with previous reports that demonstrated the association between WD, hypertension and hypertension-related target organ damage.31 In particular, at the end of our study, SBP in the group fed with a WD increased by 46% compared with the control group.Furthermore, WD elicited a significant increase of both LVM and interstitial fibrosis. It must be underlined that LVM increased by 123%. It is reasonable to hypothesize that this inappropriate growth together with the development of interstitial fibrosis is the result of the action of trophic factors rather than the slight increase in SBP. In this regard, the pivotal role of the renin-angiotensin-aldosterone system (RAAS) in the pathogenesis of LVH and myocardial fibrosis has been well documented.32 Interestingly, RAAS has a key role also in the development of hypercaloric diet-induced hypertension.33 It is noteworthy that vitamin D modulates the transcription of several genes including the gene that codes for renin. In particular, it has been reported that vitamin D inhibits the expression of the renin gene.34 For these reasons, the deficit of vitamin D is associated with the development of hypertension and ventricular hypertrophy. For instance, it has been demonstrated, in 1-alpha-hydroxylase knockout mice, that administration of activated vitamin D rescues the hypertensive phenotype and normalizes LVM.35 Therefore, it is reasonable to speculate that in our experimental model, supplementation with vitamin D, by blunting the WD-evoked activation of the RAAS, interferes with the increase in SBP and inhibits the development of LVH and myocardial fibrosis.
It is important to emphasize that this study was designed to evaluate whether the vitamin D supplement was able to reverse the detrimental effects of a WD, and not to explore the molecular mechanisms involved.
In conclusion, in an animal model of liver and cardiovascular metabolic damage supplementation with vitamin D3 shows liver and cardio-protective effects. Furthermore, two aspects of this study need to be underlined. First, the results of the present study indicate the existence, in response to a WD, of a tight association between cardiovascular and liver damage, which highlights the need for extensive investigations on the cross-talk between the cardiovascular system and liver in humans. Second, the strength of the present study is that the results can easily be translated to clinical practice. Furthermore, these results suggest that in clinical practice, the determination of vitamin D3 status should always be considered in patients at high risk for CV diseases and/or NAFLD, since its supplementation could ameliorate sub-clinical organ damage. Clinical studies in primary prevention are needed to test this hypothesis.
Acknowledgement
We thank Jean Ann Gilder (Scientific Communication srl., Naples, Italy) for substantive editing of the text.
Declaration of conflicting interests
None declared
Funding
This research received no specific grant from any funding.
Ethics approval
The study was approved by the Ethics Committee of the University of Naples “Federico II” (N. 0124285)
Informed consent
Not applicable
References
- 1.Tappy L, Le KA. Metabolic effect of fructose and the worldwide increase in obesity. Physiol Rev 2010; 90: 23–46. [DOI] [PubMed] [Google Scholar]
- 2.Masuoka HC, Chalasani N. Nonalcoholic fatty liver disease: an emerging threat to obese and diabetic individuals. Ann N Y Acad Sci 2013; 1281: 106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed MH, Husain NE, Almobarak AO. Nonalcoholic Fatty liver disease and risk of diabetes and cardiovascular disease: what is important for primary care physicians? J Fam Med Prim Care 2015; 4: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaguchi M, Kojima T, Takeda N, et al. Nonalcoholic fatty liver disease is a novel predictor of cardiovascular disease. World J Gastroenterol 2007; 13: 1579–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacifico L, Di Martino A, De Merulis M, et al. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology 2014; 59: 461–470. [DOI] [PubMed] [Google Scholar]
- 6.Lonardo A, Sookoian S, Pirola CJ, et al. Non-alcoholic fatty liver disease and risk of cardiovascular disease. Metabolism 2016; 65(8): 1136–1150. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabet Care 1991; 14(3): 173–194. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities: the role of insulin resistance and the sympathoadrenal system. N Engl J Med 1996; 334(6): 374–381. [DOI] [PubMed] [Google Scholar]
- 9.Anyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 2001; 120: 1183–1192. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357: 266–281. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, O'Keefe JH, Bell D, et al. Vitamin D deficiency: an important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol 2008; 52(24): 1949–1956. [DOI] [PubMed] [Google Scholar]
- 12.Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): is it more than just an association? Hepatology 2013; 58(3): 1166–1174. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008; 29 117(4): 503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol 2017; 70(1): 89–100. [DOI] [PubMed] [Google Scholar]
- 15.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004; 27: 2813–2818. [DOI] [PubMed] [Google Scholar]
- 16.Trovato F, Castrogiovanni P, Szychlinska MA, et al. Impact of Western and Mediterranean diets and vitamin D on muscle fibers of sedentary rats. Nutrients 2018; 10(2): 231–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalliokoski O, Jacobsen KR, Darusman HS, et al. Mice do not habituate to metabolism cage housing: a three week study of male BALB/c mice. Plos One 2013; 8(3): e58460–e58460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lembo G, Iaccarino G, Vecchione C, et al. Insulin modulation of vascular reactivity is already impaired in prehypertensive spontaneously hypertensive rats. Hypertension 1995; 26(2): 290–293. [DOI] [PubMed] [Google Scholar]
- 19.Mulder P, Morrison MC, Wielinga PY, et al. Surgical removal of inflamed epididymal white adipose tissue attenuates the development of non-alcoholic steatohepatitis in obesity. Int J Obes 2016; 40(4): 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore CE, Liu Y. Low serum 25-hydroxyvitamin D concentrations are associated with total adiposity of children in the United States: National Health and Examination Survey 2005 to 2006. Nutr Res 2016; 36(1): 72–79. [DOI] [PubMed] [Google Scholar]
- 21.Chou CL, Pang CY, Lee TJ, et al. Beneficial effects of calcitriol on hypertension, glucose intolerance, impairment of endothelium-dependent vascular relaxation, and visceral adiposity in fructose-fed hypertensive rats. PLoS One 2015; 10(3): e0119843–e0119843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordeiro A, Santos A, Bernardes M, et al. Vitamin D metabolism in human adipose tissue: could it explain low vitamin D status in obesity? Horm Mol Biol Clin Investig. Epub ahead of print 18 July 2017. DOI: 10.1515/hmbci-2017-0003. [DOI] [PubMed] [Google Scholar]
- 23.Faridi KF, Zhao D, Martin SS, et al. Serum vitamin D and change in lipid levels over 5 y: The Atherosclerosis Risk in Communities study. Nutrition 2017; 38: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao S, Yuan Q, Mao L, et al. Vitamin D deficiency causes insulin resistance by provoking oxidative stress in hepatocytes. Oncotarget 2017; 8(40): 67605–67613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6: 299–304. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Huang W, Gu J, et al. SREBP-1c overactivates ROS-mediated hepatic NF-κB inflammatory pathway in dairy cows with fatty liver. Cell Signal 2015; 27(10): 2099–2109. [DOI] [PubMed] [Google Scholar]
- 27.Yin Y, Yu Z, Xia M, et al. Vitamin D attenuates high fat diet-induced hepatic steatosis in rats by modulating lipid metabolism. Eur J Clin Invest 2012; 4211: 1189–1196. [DOI] [PubMed] [Google Scholar]
- 28.Bao S, Cao Y, Fan C, et al. Epigallocatechin gallate improves insulin signaling by decreasing toll-like receptor 4 (TLR4) activity in adipose tissues of high-fat diet rats. Mol Nutr Food Res 2014; 58(4): 677–686. [DOI] [PubMed] [Google Scholar]
- 29.Cosma A, Cecchet D, Gaiani S, et al. Clinical and biochemical determinants of the extent of liver steatosis in type 2 diabetes mellitus. Eur J Gastroenterol Hepatol 2015; 27(12): 1386–1391. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Zhang Q, Chai Y, et al. 1,25(OH)2D3 down-regulates the Toll-like receptor 4-mediated inflammatory pathway and ameliorates liver injury in diabetic rats. J Endocrinol Invest 2015; 38(10): 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panchal SK, Poudyal H, Iyer A, et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J Cardiovasc Pharmacol 2011; 57(5): 611–612. [DOI] [PubMed] [Google Scholar]
- 32.Ramires FJ, Sun Y, Weber KT. Myocardial fibrosis associated with aldosterone or angiotensin II administration: attenuation by calcium channel blockade. J Mol Cell Cardiol 1998; 30(3): 475–483. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi R, Nagano M, Nakamura F, et al. Role of angiotensin II in high fructose-induced left ventricular hypertrophy in rats. Hypertension 1993; 21: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 34.Li YC, Kong J, Wei M, et al. 1,25- Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 2002; 110: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou C, Lu F, Cao K, et al. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int 2008; 74: 170–179. [DOI] [PubMed] [Google Scholar]