Abstract
Background
Sarcopenia has been associated with poor outcomes in patients with cirrhosis and solid tumours.
Objective
Analyse the influence of sarcopenia on survival and treatment duration in patients with advanced hepatocellular carcinoma (HCC) treated with sorafenib.
Methods
We conducted a multicentre, retrospective study on 96 patients with advanced HCC treated with sorafenib, all with available abdominal computed tomography (CT) scan within 30 days from treatment start. Anthropometric, laboratory, treatment and follow-up data were collected. Sarcopenia was defined by reduced skeletal muscle index calculated from an L3 section CT image.
Results
Sarcopenia was present in 49% of patients. Patients were divided into two groups according to sarcopenia: age was significantly higher in the sarcopenic group (SG) (66 years (31–87) versus 72 years (30–84), p = 0.04], with no difference in other baseline characteristics. The SG showed shorter overall survival (OS) (39 (95% confidence interval (CI) 26–50) versus 61 (95% CI 47–77) weeks (p = 0,01)) and shorter time on treatment (12.3 (95% CI 8–19) versus 25.9 (95% CI 15–33) weeks (p = 0.0044)). At multivariate analysis, sarcopenia was independently associated to reduced OS (p = 0.03) and reduced time on treatment (p = 0.001).
Conclusion
Sarcopenia is present in almost half of patients with advanced HCC, and is associated with reduced survival and reduced duration of oral chemotherapy.
Keywords: HCC, skeletal muscle index, CT scan, prognostic factor, performance status
Key summary
Sarcopenia has been associated with poor outcomes in patients with cirrhosis and hepatocellular carcinoma (HCC), and with reduced tolerance to chemotherapy.
Our study indicates that sarcopenia is present in almost half of cirrhotic patients with advanced HCC selected to undergo sorafenib treatment.
Sarcopenia was found to be independently associated with a twofold risk of mortality and reduced sorafenib treatment duration.
Baseline measurement of sarcopenia could improve prediction of mortality in patients with advanced HCC.
Introduction
Hepatocellular carcinoma (HCC) is the third cause of cancer related deaths, with rising incidence worldwide, with nearly 750,000 new cases per year.1 Epidemiological data suggest that the incidence of HCC is still similar to the death rate, and thus almost all patients who are diagnosed with HCC will die because of it.1 In developed countries, HCC predominantly affects the elderly, and is diagnosed at an advanced stage (Barcelona Clinic Liver Cancer (BCLC-C)) in up to 40% of all patients.2 At this stage, the only approved treatment in Italy is sorafenib (Nexavar®, Bayer), an orally administered multikinase inhibitor shown to improve survival, but burdened by relatively high costs and toxicity rates, limiting patients’ chances of receiving full dose treatment and hindering their quality of life.3
Therefore, the search for prognostic tools able to stratify risk, predict toxicity and allow for therapy tailoring for each patient is of major clinical interest.4
The condition of reduced muscle mass, defined as sarcopenia,5,6 has been identified as an independent predictor of reduced survival, tolerance to chemotherapy and higher rates of toxicity in patients with different neoplasias.7–10 In the research setting, imaging techniques such as computed tomography (CT) scanning are considered the gold standard for estimating muscle mass.6 In the field of hepatology, sarcopenia has been linked to reduced survival rates in different therapeutic settings such as liver transplantation, hepatic resection and intra-arterial treatments for HCC.11–15 Its presence has also been associated with increased toxicity in patients with HCC treated with sorafenib16 and reduced survival, but the few data available come mainly from oriental series, where ethnic and anthropometric differences limit reproducibility in a Western setting.17–19 Only one Western study is available, with a limited number of patients treated with various tyrosine kinase inhibitors.20
The aim of the present study was to identify the prevalence and influence of sarcopenia in a homogeneous population of European Caucasian patients with advanced HCC undergoing sorafenib therapy. We aimed to evaluate the influence of sarcopenia on patients’ survival, as well as on the duration and tolerance of sorafenib treatment.
Materials and methods
Study design
This is a multicentre, retrospective study to assess the added value of sarcopenia evaluation in patients with advanced HCC and patients with an intermediate HCC who were not eligible for or failed ablative therapies, treated with sorafenib according to international guidelines.2 Patients were followed and included in four different units in three hospital centres from 2008 to 2016.
Patients and data collection
All patients with advanced HCC, i.e. classified as BCLC stage C and those with a BCLC-B stage who were unfit to any or failed to respond to locoregional therapies,2 were enrolled. HCC was diagnosed by the criteria of the American Association for the Study of Liver Disease21 and staged by abdominal dynamic contrast-enhanced CT scan or gadolinium-enhanced magnetic resonance imaging (MRI). Each patient underwent also chest X-ray/CT scan and bone scanning as requested by the attending physicians.
The main inclusion criteria were those specified by the Italian Medicines Agency (AIFA) and the availability of an abdominal CT scan within 1 month before the start of sorafenib treatment. We analysed gender, age, pre-treatment anthropometric features, laboratory findings (including serum albumin, creatinine, international normality ratio (INR) and alpha-fetoprotein (AFP)), dry weight body mass index (BMI), macrovascular invasion and extra-hepatic spread. Cirrhosis, diagnosed either by histology or clinically, was graded according to the Child–Pugh and Model for End-stage Liver Disease (MELD) scores as a measures of liver impairment.22,23 Performance status (PS) was scored according to the Eastern Cooperative Oncology Group (ECOG).24 All data were obtained within 1 month before the start of sorafenib treatment. Follow-up was ended at patients’ death, last visit or on 10 December 2016.
After initial data collection, all patients were given an identification number to guarantee anonymity and information was entered in a dedicated database for statistical analysis. All patients gave informed consent for the use of clinical, laboratory and radiological data at the time of the first examination. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by local the institutional review board (Comitato Etico Milano Area 2, Università degli Studi di Milano, 3 December 2017).
Image analysis and treatment modality
CT scans were obtained routinely at baseline for tumour staging purposes within 1 month prior to initiation of sorafenib treatment and used to determine the skeletal muscle index (SMI). A transverse image at the level of the third lumbar vertebrae (L3) was selected from each scan. Muscles at this level include psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and the rectus abdominis.10,13,25 Images were analysed with SliceOmatic V 5.0 software (Tomovision, Montreal, Quebec, Canada), which enables specific tissue selection by using previously determined Hounsfield units (HU). Skeletal muscle is selected and quantified by thresholds between –29 to +150 HU.26 These specific thresholds permit evaluation of skeletal muscle regardless of ascites in patients with cirrhosis, excluding muscular fat infiltration in the selected section. All CT images were analysed by two trained observers (GA and PB). Cross-sectional areas were calculated and normalized for height (cm2/m2) as reported elsewhere.27 The MRI imaging data were excluded to avoid methodological heterogeneity. L3 SMI was expressed in cm2/m2. L3 SMI was considered to be indicative of sarcopenia if ≤53 cm2/m2 for male patients with a BMI ≥25 and ≤43 cm2/m2 in male patients with a BMI <25 and ≤41 cm2/m2 for women, independently of BMI.9
Sorafenib treatment was administered according to guidelines. Therapy was discontinued whenever the patient developed unacceptable toxicity, when radiological or symptomatic progression of HCC occurred, or by investigator’s judgment of the patient being unlikely to benefit from further treatment with sorafenib. Tumour progression was defined either radiologically by modified response evaluation criteria in solid tumours (mRECIST)28 or clinically in terms of worsening of PS or onset of symptoms unrelated to liver failure.29 Another reason for treatment discontinuation was unacceptable treatment toxicity, i.e. grade 2–4 adverse events (AEs) not responding to dose reductions and/or temporary interruption of treatment as suggested by the manufacturer.24,30 Toxicity was assessed in all patients who received at least one dose of sorafenib; AEs were graded according to the National Cancer Institute’s Common Terminology Criteria (version 3.0).31
Statistical analysis
Patients were divided in two groups according to the presence of sarcopenia and characteristics of the two groups were then compared. Continuous data were reported as mean ± SD or as median (range) and compared using a Student’s t-test or Mann–Whitney test as appropriate. Categorical variables were expressed as number (percentage) and compared using the χ2 test with Fisher’s exact test, with Yates’ correction as appropriate. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Overall survival (OS) time was defined as the period between treatment start and patient death, and the time on treatment was intended as time from treatment beginning to its end. Univariate analyses were performed using Cox’s proposal hazards model and hazard ratios (HRs) of independent predictive factors were obtained. The variables significantly associated with endpoints in univariate analysis were entered in a sex- and age-adjusted multivariate model. All p-values were two sided and the level of significance was set at p < 0.05. Results are reported as HR and 95% confidence interval (CI). All statistical analyses were carried out on MedCalc® 12.5 statistical software. Data are expressed as percentage (number/total) median (range) for demographic and laboratory data in tables and text unless otherwise indicated.
Results
Clinical and biochemical characteristics of advanced HCC patients treated with sorafenib
Of the 119 initial patients selected for the study, 15 patients were excluded since they had only a baseline MRI available, 4 were excluded since CT imaging and/or required clinical/laboratory data were missing, and 4 patients were excluded since treatment was administered in an off-label schedule. Therefore, 96 Caucasian patients treated with sorafenib were included in the final analysis: 75 were men (78%), with a median age of 69 years (30-87); 21 patients were female (22%), all post-menopausal. The most prevalent cause of cirrhosis was chronic viral infection (hepatitis C virus and hepatitis B virus, 62%), while alcohol abuse ranked second (17%). Other baseline characteristics are summarized in Table 1.
Table 1.
Baseline clinical and biochemical characteristics of 96 patients with advanced hepatocellular carcinoma treated with sorafenib.
| Characteristics | N = 96 |
|---|---|
| Men, number | 75 (78%) |
| Age, yearsa | 69 (30–87) |
| Aetiology, number | |
| HCV | 46 (48%) |
| HBV | 13 (14%) |
| Alcohol | 16 (17%) |
| NASH | 11 (11%) |
| Other | 10 (10%) |
| BMI, kg/m2a | |
| Overall | 24.5 (16.2–38.7) |
| Male | 24.7 (19.6–38.7) |
| Female | 22.7 (16.2–30) |
| <25 kg/m2, number | 58 (60%) |
| >25 kg/m2, number | 38 (40%) |
| INR | 1.10 (0.88–2.6) |
| Albumin, g/dLa | 3.7 (2.7–5.0) |
| Bilirubin, mg/dLa | 0.94 (0.3–5.0) |
| Creatinine, mg/dLa | 0.8 (0.4–1.7) |
| Alpha-fetoprotein, ng/mla | 68 (1–26941) |
| Encephalopathy, number | 0 |
| Ascites, number | 20 (21%) |
| Child–Pugh score, number | |
| A | 80 (83%) |
| B | 16 (17%) |
| MELD score | 8 (6–21) |
| Tumour vascular invasion | 18 (19%) |
| Metastases, number | 20 (21%) |
| ECOG PS, number | |
| 0 | 36 (37.5%) |
| 1 | 36 (37.5%) |
| 2 | 24 (25%) |
| BCLC stage, number | |
| B | 22 (23%) |
| C | 74 (77%) |
| L3 SMI, cm2/m2a | |
| Overall | 47.2 (23.4–70) |
| Male | 49.7 (33.3–70) |
| Female | 36 (23.4–49.8) |
| Sarcopenia, number | 47 (49%) |
HCV: hepatitis C virus; HBV: hepatitis B virus; NASH: non-alcoholic steatohepatitis; BMI: body mass index; INR: international normalized ratio; MELD: Model for End-stage Liver Disease; ECOG PS: Eastern Cooperative Oncology Group performance status; BCLC: Barcelona Clinic Liver Cancer; L3 SMI: Muscle Skeletal Index at the third lumbar vertebra.
Median (range).
Lumbar SMI and sarcopenia distribution
At baseline, the median value of the L3 SMI was 47.2 cm2/m2 (range 23.4–70). The median SMI L3 value was 49.7 cm2/m2 (range 33.3–70) in males and 36 cm2/m2 (range 23.4–49.8) in females: 47 (49%) patients were defined sarcopenic. Inter-observer concordance on L3 SMI values was >95%.
Sarcopenia was significantly more prevalent in women (91% versus 37%, p = < 0.0001) and in older patients (66 (range 31–87) versus 72 years (range 30–84), p = 0.04]. Grades of hepatic dysfunction expressed by the Child–Pugh and MELD scores were not significantly different in the two groups, as well as serum albumin, creatinine, sodium, total bilirubin, INR and AFP (data available for 84 patients). Data describing the differences between the two groups are detailed in Table 2. In particular, median BMI values were not different (24.6 (range 20.8–38.7) versus 24.4 (range 16.2–30), p = not significant). Among the 58 patients with BMI < 25, 28 (48%) were sarcopenic, while among the 38 patients with a BMI ≥25, 50% were sarcopenic (p = not significant).
Table 2.
Comparison of clinical and biochemical characteristics among sarcopenic and non-sarcopenic patients.
| Characteristics | Sarcopenic number 47 (49%) | Non-sarcopenic number 49 (51%) | p-value |
|---|---|---|---|
| Male | 28 (60%) | 47 (96%) | <0.0001a |
| Age, yearsb | 72 (30–84) | 66 (31–87) | 0.04c |
| L3 SMI, cm2/m2b | 38.94 (23.42–52.62) | 52.85 (43.1–70.1) | <0.0001c |
| BMI, kg/m2b | 24.4 (16.2–30) | 24.6 (20.8–38.7) | 0.07c |
| Albumin, g/dLb | 3.6 (2.7–4.3) | 3.8 (2.7–5.0) | 0.2c |
| Bilirubin, mg/dLb | 1.05 (0.3–5.0) | 0.9 (0.3–3.4) | 0.5c |
| Creatinine, mg/dLb | 0.8 (0.4–2.3) | 0.8 (0.55–1.7) | 0.7c |
| Alpha-fetoprotein, ng/ml | 47 (1–14662) | 61 (1–26941) | 0.2c |
| Aetiology | 0.5 | ||
| HCV | 18 (38%) | 28 (57%) | |
| HBV | 9 (19%) | 4 (8%) | |
| Alcohol | 11 (23%) | 5 (10%) | |
| NASH | 5 (11%) | 8 (17%) | |
| Other | 4 (9%) | 4 (8%) | |
| Ascites, number | 11 (23%) | 8 (16%) | 0.4a |
| Child–Pugh score A, number | 37 (79%) | 43 (88%) | 0.1c |
| MELD scoreb | 9 (6–21) | 10 (6–15) | 0.4c |
| BCLC, number | 0.7c | ||
| B | 11 (23%) | 11 (22%) | |
| C | 36 (77%) | 38 (78%) | |
| ECOG PS (0/1/2) | 17/20/10 | 19/16/14 | 0.2c |
| Toxicity, number | 29 (62) | 20 (40) | 0.04a |
| Overall survival, weeksb | 32 (3.1–171.2) | 63 (6.5–298.1) | 0.02c |
| Time on treatment, weeksb | 12.3 (1.1–103.1) | 25.8 (2–134.2) | 0.01c |
L3 SMI: Muscle Skeletal Index at 3rd lumbar vertebra; BMI: body mass index; INR: international normalized ratio; HCV: hepatitis C virus; HBV: Hepatitis B virus; NASH: non-alcoholic liver disease; MELD: Model for End-stage Liver Disease; BCLC: Barcelona Clinic Liver Cancer; ECOG PS: Eastern Cooperative Oncology Group performance status.
Fisher’s exact test.
Median (range).
Mann–Whitney test.
Factors influencing survival in advanced HCC patients treated with sorafenib
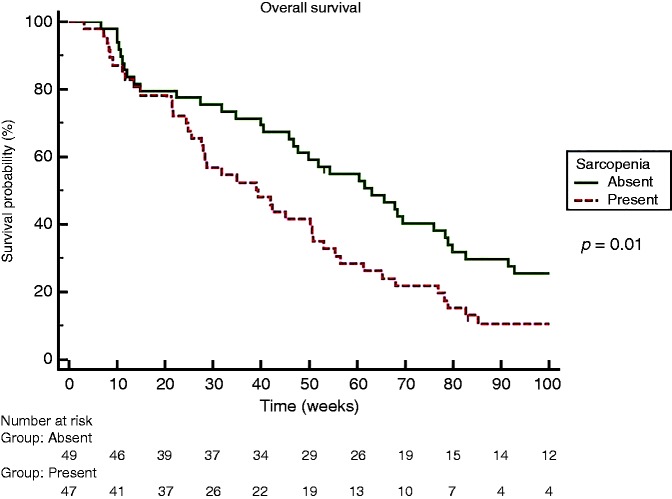
Overall survival in our cohort was 50 (3.1–298.1) weeks. According to the Kaplan–Meier method, median OS was significantly shorter among sarcopenic patients (63 versus 32 weeks, HR 1.69, p = 0.02) (Figure 1).
Figure 1.
Kaplan Meier Curve for Overall Survival comparing sarcopenic and non sarcopenic patients.
By univariate Cox analysis, factors associated with an increased mortality after sorafenib treatment were bilirubin level (p = 0.02; HR 1.75) (cut-off 1.4 mg/dL), vascular invasion (p = 0.03; HR 1.76), MELD (p = 0.01; HR 1.08) and sarcopenia defined by L3 SMI (p = 0.01; HR 1.71), while male gender, age, BMI, presence of ascites, INR, AFP, creatinine, albumin, sodium and Child–Pugh score were not associated with a higher mortality rate. In sex- and age-adjusted multivariate analysis using a Cox regression model (bilirubin was not entered to avoid colinearity with MELD score), the only independent factor significantly associated with an increased mortality was sarcopenia (p = 0.03; HR 1.63). Univariate and multivariate analyses for OS are shown in Table 3.
Table 3.
Univariate and multivariate analysis of risk factors for overall survival of hepatocellular carcinoma patients treated with sorafenib by the Cox proportional hazards model.
| Univariate |
Multivariatea |
|||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age (years) | 0.98 (0.96–1.01) | 0.36 | ||
| Albumin (mg/dL) | 0.71 (0.45–1.14) | 0.16 | ||
| Ascites | 0.90 (0.53–1.54) | 0.72 | ||
| Sex (male) | 1.04 (0.62–1.75) | 0.86 | ||
| Creatinine (mg/dL) | 1.78 (0.74–4.25) | 0.2 | ||
| Sarcopenia | 1.71 (1.12–2.71) | 0.01 | 1.63 (1.05–2.53) | 0.03 |
| INR | 1.17 (0.6–2.25) | 0.63 | ||
| Vascular invasion | 1.76 (1.03–3.00) | 0.03 | 1.65 (0.96–2.84) | 0.07 |
| Bilirubin (mg/dL) | 1.75 (1.22–2.51) | 0.02 | ||
| AFP (ng/ml) | 1.12 (0.78–2.11) | 0.44 | ||
| MELD score | 1.08 (1.01–1.15) | 0.01 | 1.01 (0.93–1.10) | 0.13 |
| Metastasis | 0.86 (0.51–1.43) | 0.57 | ||
| ECOG PS | 1.19 (0.93–1.52) | 0.16 | ||
| BMI (kg/m2) | 1.00 (0.94–1.06) | 0.9 | ||
| Child–Pugh score | 1.32 (0.98–1.78) | 0.07 | ||
HR: hazard ratio; CI: confidence interval; INR: international normalized ratio; AFP: alpha-fetoprotein; MELD: Model for End-stage Liver Disease; ECOG PS: Eastern Cooperative Oncology Group performance status; BMI: body mass index.
Bilirubin was not included in the multivariate analysis to avoid colinearity, since it was included in the MELD score.
Factors influencing time on treatment and sorafenib tolerance
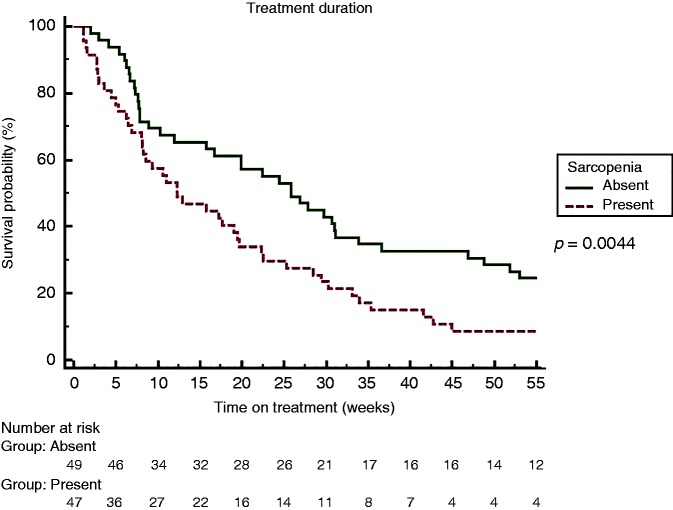
Overall, in our cohort, time on treatment was 19.2 (1.1–134.2) weeks. By Kaplan–Meier analysis, time on treatment was significantly shorter among sarcopenic patients (25.8 versus 12.3 weeks, HR 1.75, p = 0.0044) (Figure 2). All patients at the end of follow-up had discontinued sorafenib: 47 (49%) for disease progression, 42 (44%) for toxicity and 7 (7%) for liver decompensation. Sarcopenic patients significantly suffered sorafenib-related toxicity (AEs grade 3 and 4) as compared to non-sarcopenic patients (62% versus 40%, p = 0.04). Information concerning drug dose reduction was available for 55 patients. When available, there was no difference in dose reduction between sarcopenic and non-sarcopenic patients (43% versus 37%, p=not significant).
Figure 2.
Kaplan Meier Curve for Sorafenib treatment duration comparing sarcopenic and non sarcopenic patients.
By univariate Cox analysis, factors significantly associated with a reduced time on treatment were bilirubin level (p = 0.001; HR 1.66), vascular invasion (p = 0.04; HR 1.69), Child–Pugh score (p = 0.007; HR 1.43), creatinine (p = 0.04; HR 2.56), albumin (p = 0.03; HR 1.62), MELD (p = 0.0009; HR 1.13) and sarcopenia defined by L3 SMI (p = 0.005; HR 1.82). Male gender, age, BMI, presence of ascites, INR, AFP and sodium were not associated with reduced time on treatment. In sex- and age-adjusted multivariate analysis using the Cox regression model (albumin, bilirubin and creatinine were not entered to avoid colinearity with Child–Pugh and MELD scores), sarcopenia (p = 0.001; HR 1.72), MELD (p = 0.03; HR 1.08), Child–Pugh (p = 0.04; HR 1.34) and vascular invasion (p = 0.04; HR 1.73) were shown to be independent factors significantly associated with a reduced time on treatment. Detailed univariate and multivariate analyses for time on treatment are shown in Table 4.
Table 4.
Univariate and multivariate analysis of risk factors for time on treatment of hepatocellular carcinoma patients treated with sorafenib by the Cox proportional hazards model.
| Univariate |
Multivariatea |
|||
|---|---|---|---|---|
| Characteristics | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age (years) | 1.00 (0.98–1.02) | 0.68 | ||
| Albumin (mg/dL) | 1.62 (1.4–1.95) | 0.03 | ||
| Sex (male) | 1.06 (0.64–1.74) | 0.81 | ||
| MELD score | 1.13 (1.05–1.21) | 0.0009 | 1.08 (1.00–1.16) | 0.03 |
| Bilirubin | 1.66 (1.22–2.26) | 0.001 | ||
| AFP (ng/ml) | 1.09 (0.71–1.84) | 0.31 | ||
| INR | 1.44 (0.58–3.58) | 0.42 | ||
| ECOG PS | 1.06 (0.84–1.35) | 0.58 | ||
| Vascular invasion | 1.69 (1.01–2.85) | 0.04 | 1.73 (1.02–2.93) | 0.04 |
| Creatinine | 2.56 (1.04–6.2) | 0.04 | ||
| BMI (kg/m2) | 0.98 (0.93–1.04) | 0.71 | ||
| Child–Pugh score | 1.43 (1.10–1.87) | 0.007 | 1.34 (1.01–1.77) | 0.04 |
| Metastasis | 0.87 (0.53–1.41) | 0.57 | ||
| Sarcopenia | 1.82 (1.20–2.76) | 0.005 | 1.72 (1.12–2.64) | 0.001 |
| Ascites | 1.46 (0.88–2.44) | 0.14 | ||
HR: hazard ratio; CI: confidence interval; INR: international normalized ratio; AFP: alpha-fetoprotein; MELD: Model for End-stage Liver Disease; ECOG PS: Eastern Cooperative Oncology Group performance status; BMI: body mass index.
Bilirubin, creatinine and albumin were not included in the multivariate analysis to avoid colinearity, since they were included in either the Child–Pugh or MELD scores.
Discussion
To the best of our knowledge, this is the largest multicentre study addressing the relationship between sarcopenia and survival in a homogeneous cohort of European Caucasian cirrhotic patients with advanced HCC treated with sorafenib. Our study indicates that sarcopenia is present in almost half of cirrhotic patients with advanced HCC selected to undergo sorafenib treatment. In our cohort, sarcopenia was independently associated with a twofold risk of mortality and reduced treatment duration, and inclusion of its measurement at baseline could be associated with an improvement in the prediction of mortality in patients with advanced HCC.
Available data demonstrate that sarcopenia is commonly observed among patients with cirrhosis and associated with significant morbidity and mortality, contributing to reduced quality of life. Among cancer patients, prevalence of pre-therapeutic sarcopenia was found to be 38.6% in a recent comprehensive review of 32 studies.32
In our population of cirrhotic patients with advanced HCC, the prevalence of sarcopenia, as derived by a measurable and reproducible technique, was even higher, probably due to the association of advanced stage cancer and underlying liver disease. This percentage is in line with those observed among HCC cirrhotic patients and in our previous paper in Caucasian patients with HCC using similar L3 SMI thresholds.11,15 As expected, sarcopenia was linked to gender and age. A paper from Japan previously reported a higher prevalence of sarcopenia in patients treated with sorafenib (close to 65%), probably linked to different thresholds chosen from Asian series published in the literature, and as suggested by the authors, could be unfit for Caucasian patients.17 At odds with oriental data, sarcopenia was less represented in our patients, further suggesting a role of ethnicity in influencing results in this setting.
Unexpectedly, sarcopenia was more prevalent in female patients, a discordant result when compared to most of the available literature. Our finding may be due to the homogeneous composition of our cohort, which was comprised exclusively of patients of Caucasian-Mediterranean origin, again suggesting a possible role of ethnicity in determining the development of sarcopenia, and to the generalized post-menopausal state of our female patients, as it is well known that the protective effect of oestrogens in counteracting sarcopenia development is lost after menopause.33
Sarcopenia measurement can be obtained at marginally increased costs from CT scans already available at baseline for staging purposes, providing a further independent prognostic index to plan appropriate patient management. Furthermore, the dedicated software used overcomes the intrinsic limits of bioimpedance and anthropometric measurement, hampered by elevated BMI and ascites.6,34,35 In addition, since the measurements we performed were not limited to the psoas only, but comprised all the muscles represented in the transverse image selected, it maximized the opportunity to obtain a representative sample of muscle mass.
Sarcopenic patients experienced more serious AEs and remained on treatment for a shorter period of time, which suggests that sarcopenia could assist in performance status evaluation, normally applied to HCC patients for staging and treatment allocation.2 The role of reduced muscle mass in exposing patients to an increased risk of developing sorafenib-related side effects has been reported in a previous study.16 Our study, conducted in a larger and homogeneous sample representative of advanced HCC, including different degrees of liver functional reserve and performance status, confirms and completes these findings.
An additional relevant finding of our study is that sarcopenia was prevalent in 49% of overweight/obese patients (BMI ≥ 25). This observation confirms the warning that sarcopenia is not exclusively to be sought in patients with low BMI, strengthening the importance and prevalence of sarcopenic obesity.10,11,15
Our data further contributes to the definition of the role of sarcopenia as a possible predictor of reduced survival and cancer treatment toxicity, as has been previously shown in other oncological settings.10 Accurate measurement tools are also needed in HCC, and CT-based technologies provide measurable and repeatable muscle mass measurement. We believe that sarcopenia assessment can certainly be an additional component in the complex decision-making process surrounding therapeutic choices in advance HCC.
The main limitation of our study is the retrospective design, which cannot exclude selection bias, that was nonetheless minimized by cross-referencing clinical and radiological databases, and the unavailability of full data regarding sorafenib initial dose and dose reduction.
In conclusion, our data show that European Caucasian cirrhotic patients with advanced HCC have a high prevalence of sarcopenia, and that its measurement by CT scan could be a readily available tool to identify them, improving prognostic evaluation at minimally increased costs.
Declaration of conflicting interests
Massimo Iavarone is on the speaker bureau for Bayer. Angelo Sangiovanni is on the speaker bureau for Bayer. All other authors declare no conflict of interest.
Funding
No funding was obtained for this study.
Authors’ contributions
GA, EG and MM conceived the study, enrolled patients, performed statistical analysis, and wrote and revised the manuscript. MI, AS and PL enrolled patients and wrote and revised the manuscript. GA, PB, EI and PB retrieved and elaborated CT scan data. AMP, LM and PM enrolled patients. All authors read and approved the final version of the manuscript.
Informed consent
Written informed consent was obtained from each patient included in this study.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by local institutional review board (Comitato Etico Milano Area 2, Universita` degli Studi di Milano, 3 December 2017).
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2.European Association for Study of Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. Eur J Cancer 2012; 48: 599–641. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. New Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 4.Di Marco V, De Vita F, Koskinas J, et al. Sorafenib: From literature to clinical practice. Ann Oncol 2013; 24: ii30–ii37. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoun S, Baracos VE, Birdsell L, et al. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol 2010; 21: 1594–1598. [DOI] [PubMed] [Google Scholar]
- 8.Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care 2013; 7: 383–389. [DOI] [PubMed] [Google Scholar]
- 9.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013; 31: 1539–1547. [DOI] [PubMed] [Google Scholar]
- 10.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol 2008; 9: 629–635. [DOI] [PubMed] [Google Scholar]
- 11.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013; 47: 861–870. [DOI] [PubMed] [Google Scholar]
- 12.Itoh S, Shirabe K, Matsumoto Y, et al. Effect of body composition on outcomes after hepatic resection for hepatocellular carcinoma. Ann Surg Oncol 2014; 21: 3063–3068. [DOI] [PubMed] [Google Scholar]
- 13.Tandon P, Ney M, Irwin I, et al. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl 2012; 18: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 14.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg 2010; 211: 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begini P, Gigante E, Antonelli G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol 2017; 16: 107–114. [DOI] [PubMed] [Google Scholar]
- 16.Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PloS One 2012; 7: e37563–e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa H, Nishijima N, Enomoto H, et al. Prognostic significance of sarcopenia in patients with hepatocellular carcinoma undergoing sorafenib therapy. Oncol Lett 2017; 14: 1637–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashima M, Miyaaki H, Honda T, et al. Significance of psoas muscle thickness as an indicator of muscle atrophy in patients with hepatocellular carcinoma treated with sorafenib. Mol Clin Oncol 2017; 7: 449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiraoka A, Hirooka M, Koizumi Y, et al. Muscle volume loss as a prognostic marker in hepatocellular carcinoma patients treated with sorafenib. Hepatol Res 2017; 47: 558–565. [DOI] [PubMed] [Google Scholar]
- 20.Nault JC, Pigneur F, Nelson AC, et al. Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Dig Liver Dis 2015; 47: 869–876. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Sherman M. American Association for the Study of Liver Disease. Management of hepatocellular carcinoma: An update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60: 646–649. [DOI] [PubMed] [Google Scholar]
- 23.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33: 464–470. [DOI] [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982; 5: 649–655. [PubMed] [Google Scholar]
- 25.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol 2004; 97: 2333–2338. [DOI] [PubMed] [Google Scholar]
- 26.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1998; 85: 115–122. [DOI] [PubMed] [Google Scholar]
- 27.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008; 33: 997–1006. [DOI] [PubMed] [Google Scholar]
- 28.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Seminars in liver disease 2010; 30: 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 2011; 54: 2055–63. [DOI] [PubMed] [Google Scholar]
- 30.EU summary of Product Characteristics for Nexavar, Bayer Schering Pharma AG, Berlin, Germany, 2010.
- 31.Cancer Therapy Evaluation Program. Common terminology criteria for adverse events, version 3.0, DCTD, NCI, NIH, DHHS. http://ctep.cancer.gov. (2006, accessed September 2011).
- 32.Pamoukdjian F, Bouillet T, Levy V, et al. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin Nutr 2017; S0261–5614: 30249–30252. [DOI] [PubMed] [Google Scholar]
- 33.Messier V, Rabasa-Lhoret R, Barbat-Artigas S, et al. Menopause and sarcopenia: A potential role for sex hormones. Maturitas 2011; 68: 331–336. [DOI] [PubMed] [Google Scholar]
- 34.Heymsfield SB. Development of imaging methods to assess adiposity and metabolism. Int J Obes (Lond) 2008; 32: S76–S82. [DOI] [PubMed] [Google Scholar]
- 35.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010; 51: 1820–1832. [DOI] [PubMed] [Google Scholar]