Abstract
Background and study aims
Gastric cancer is known to reside in some gastric ulcers but what predicts this association is still unclear. Historically it has been thought that the increasing size of gastric ulcers may be a predictor for harbouring malignancy. Giant gastric ulcers are arbitrarily defined as ≥3 cm. The aim of this retrospective study was to examine patients with giant gastric ulcers within a single tertiary centre over a 10-year period. Our primary outcomes included the malignancy yield in giant gastric ulcers and to determine if any demographic, clinical or endoscopic predictors for malignancy exist. Secondary outcomes included the 30-day and 12-month mortality.
Method
Patients with giant gastric ulcers ≥3 cm presenting from September 2005 to December 2015 were included in the study. Malignancy yield was obtained by looking at histology reports. Predictors for malignancy were tested using binary logistic regression, after demographic, clinical and endoscopic variables were tested using univariate analysis and for collinearity.
Results
A cohort of 111 patients was included for the final analysis. Forty-two giant gastric ulcers were malignant, equating to a yield of 37.8% (95% CI 28.8–46.8). Binary logistic regression revealed predictors for malignancy included: ulcer location being within the fundus, cardia or incisura (odds ratio (OR) 4.417; 95% CI 1.10–17.76; P = 0.036); younger age of patient (OR 0.202; 95% CI 0.06–0.71; P = 0.013); and endoscopic ‘non-suspicion’ (OR 0.138; 95% CI 0.049–0.39; P < 0.001). Patient's 12-month mortality for giant gastric ulcer was 61.9% (26/42) for malignant and 21.9% (11/73) for benign histology.
Conclusion
We have shown a high malignancy yield of 37.8% (95% CI 28.8–46.8) and a 12-month mortality of 61.9% for malignant giant gastric ulcers and 21.9% for benign giant gastric ulcers. Predictors for malignancy in patients with giant gastric ulcers include ulcer location, patient's age and endoscopist's ‘suspicion’ during endoscopy.
Keywords: Giant gastric ulcers, gastric ulcer, gastric cancer, malignancy risk giant gastric ulcers, gastric ulcer mortality
Key points
What is already known:
A proportion of gastric ulcers are known to harbour malignancy
Historically data have been conflicting with regard to increasing size of gastric ulcer and cancer risk
New findings from the study:
Patients with giant gastric ulcers have a significant malignancy yield (37.8%).
Predictors for malignancy include ulcer location (fundus, cardia or incisura), patient's age and endoscopic suspicion.
Introduction
Gastric ulceration is a common finding during gastroscopy for patients presenting with symptoms such as dyspepsia, iron deficiency anaemia and gastrointestinal bleeding. Gastric cancer is known to reside in some gastric ulcers1 but what predicts this association is still unclear. As a result, biopsies from the edge and base of all gastric ulcers2 are recommended by most gastroenterological societies3,4 at the time of index gastroscopy, provided no contraindications are present. Seven biopsies from the ulcer seem to provide the highest sensitivity.5 If the histology proves to be benign, UK guidelines recommend repeat gastroscopy 6 to 8 weeks after the initial procedure.4 At Leeds Teaching Hospitals NHS Trust, UK, our departmental guidelines state we should be performing 6- to 8-weekly repeat gastroscopies (with biopsies) until the ulcer has fully healed, and if biopsies are not taken during index gastroscopy due to contraindications, a repeat gastroscopy is performed with biopsies before patient discharge.
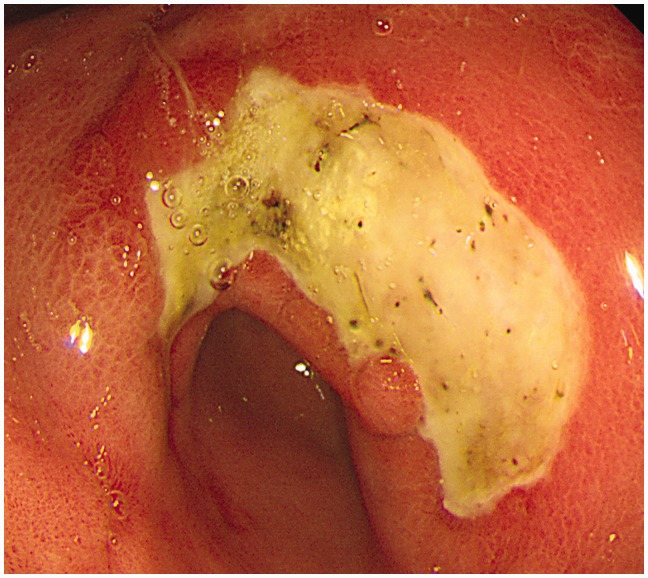
Giant gastric ulcers have arbitrarily been classified as being equal to or greater than 3 cm (see Figure 1 for photograph) in size; in old literature this is about the size of half a dollar.6 A recent study at Leeds Hospitals looked at malignancy yield in all size gastric ulcers from January 2012 to September 2013.7 A malignancy/dysplasia yield of 6% was reported, and smaller ulcer size was found to be a predictor for benign disease. Historically it has been thought that the increasing size of gastric ulcers may predict malignancy,6,8–11 though data have been conflicting. One study reported a risk of malignancy to be as low as 1.5%.8
Figure 1.
Photograph of a giant gastric ulcer.
The short- and long-term prognosis of patients with giant gastric ulcers is also uncertain. Only one study has looked at survival following diagnosis and found the 6-year survival to be much lower than the expected of a population matched for age and sex.8
The aim of this retrospective study was to examine patients with giant gastric ulcers (≥3 cm) within a single tertiary centre, over a 10-year period. Our primary outcomes included the malignancy yield in giant gastric ulcers and to determine if any potential demographic, clinical or endoscopic predictors for malignancy exist. Secondary outcomes included the 30-day and 12-month mortality of patients in both malignant and benign giant gastric ulceration.
Methods
This retrospective cohort study was conducted at Leeds Teaching Hospitals NHS Trust, located in the north of England, UK. It is a tertiary centre, with endoscopy units at two large teaching hospitals within close proximity of each other and a third endoscopy unit based in a rural outpatient setting.
Subject selection was acquired by using a search engine on a computer-based endoscopic reporting system (ADAM, Fujifilm Europe GmbH 2011). Search criteria involved reports coded as gastric ulcers dating from September 2005 to December 2015. BJR performed this process.
Next all reports were reviewed by ME. Patients were included in the cohort if the report showed gastric ulcers equal to or greater than 3 cm (ADAM gives the option of stating ulcer size on the report), with those less than 3 cm excluded. Other exclusion criteria were patients referred from outside this trust, if the size of the ulcer was not stated or absence of histology reports. Patient's demographic, clinical and endoscopic information was obtained via a computer-based patient record system and from endoscopy reports on ADAMs. This information was collated and recorded in a spreadsheet by LT, ME and RL. To measure the primary outcome malignancy yield, patient's histology for the corresponding endoscopy was reviewed, as were subsequent reports if initial histology was benign. For the second primary outcome, predictors for malignancy, variables were initially assessed using univariate analysis (Pearson chi-square for categorical data) to determine association. Variables showing statistically significant results, determined by P value < 0.05, were checked for multicollinearity. Independence of variables for predictors of malignancy was performed using binary logistic regression, calculating the odds ratio and associated P value. Again, a P value < 0.05 was determined as statistically significant. Secondary analysis for 30-day and 12-month mortality was calculated as proportions for both the malignant and benign cohort. All statistics were calculated using the computer statistical software package IBM SPSS version 24. This was performed by RL and verified by VS.
This retrospective study was performed as part of a clinical audit and therefore ethical approval and need for consent is not required under current8,12 UK regulations.
Results
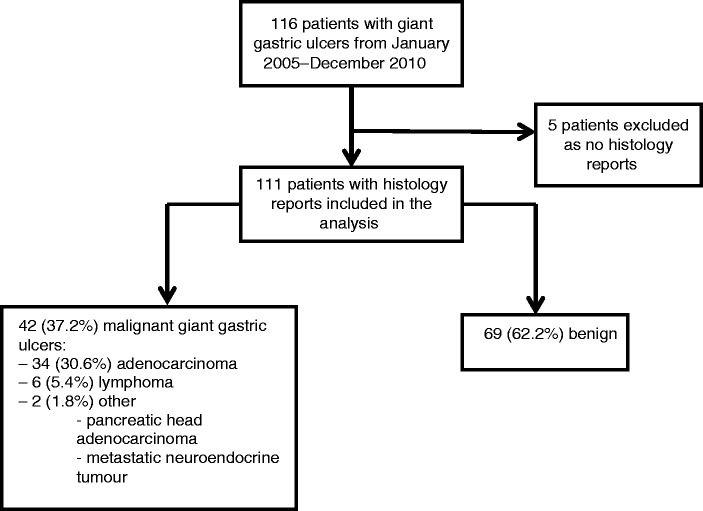
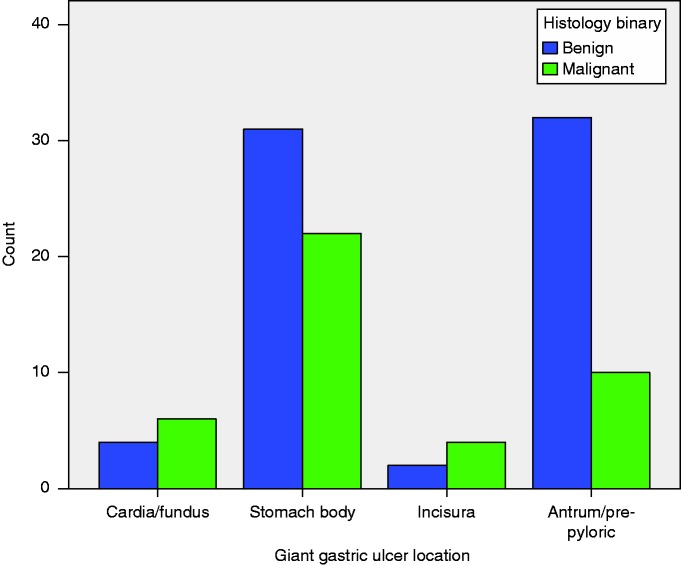
A total of 116 patients were found to have giant gastric ulcers between September 2005 and December 2015, averaging at around 11 giant gastric ulcers per year. Histology reports were found for only 111 patients; therefore, five patients were excluded from the results (see Figure 2 for flow diagram). Table 1 shows the cohort demographic, clinical and endoscopic features. The median age was 75 years; 52.3% being male and 96.4% of the cohort were Caucasian. The most common indication for gastroscopy was upper gastrointestinal bleeding accounting for 55% of referrals. The median number of ulcers per endoscopy was 1 (range 1–7), with the average size being 4 cm (IQR 3–5). The most common location for giant gastric ulcers was the body (50.9%) and antrum (37.1%), with the vast majority being cratered (82.9%). The number of malignant and benign giant gastric ulcers described according to location can be seen in Figure 3. In descending order of malignancy risk, malignancy accounted for 4/6 (90.5%) giant gastric ulcers at the incisura, 6/10 (60.4%) at the cardia, 22/53 (41.5%) at the body and 10/42 (23.8%) at the antrum/pre-pyloric region.
Figure 2.
Patient flow diagram for giant gastric ulcer cohort.
Table 1.
Demographic, clinical, endoscopic and histological breakdown of all patients within study cohort.
| Cohort variables | ||
| Age | Median 75 (IQR 60–82) | |
| Sex | ||
| Male | 59 (53.2%) | |
| Female | 52 (46.8%) | |
| Smoking status | ||
| Never | 34 (30.6%) | |
| Current | 26 (23.4%) | |
| Ex smoker | 16 (14.4%) | |
| Data missing | 36 (31.5%) | |
| Ethnicity | ||
| Caucasian | 107 (96.4%) | |
| Indian | 2 (1.8%) | |
| Arab | 1 (0.9%) | |
| Data missing | 1 (0.9%) | |
| Charlson Comorbidity Index Score | Median 5 (IQR 4–6) | |
| Indications for endoscopy | ||
| Dyspepsia | 10 (9.0%) | |
| Upper GI bleed | 61 (55.0%) | |
| Fe deficiency anaemia | 21 (18.9%) | |
| Weight loss | 7 (6.3%) | |
| Abdominal pain | 1 (0.9%) | |
| Vomiting/nausea | 6 (5.4%) | |
| Other | 2 (1.8%) | |
| No. of ulcers per index oesophago-gastroduodenoscopy (OGD) | Median 1.0 (Range 1–7) | |
| Size of gastric ulcer (cm) | Median 4.0 (IQR 3–5) | |
| Endoscopic appearance | ||
| Superficial | 16 (14.4%) | |
| Linear | 2 (1.8%) | |
| Cratered | 92 (82.9%) | |
| Superficial/linear | 1 (0.9%) | |
| Ulcer location | ||
| Cardia/Fundus | 6 (5.2%) | |
| Body | 59 (50.9%) | |
| Incisura | 8 (6.9%) | |
| Antrum/pre-pyloric | 43 (37.1%) | |
| Endoscopist's suspicion | ||
| Non-suspicious | 38 (34.2%) | |
| Suspicious | 65 (58.6%) | |
| Data missing | 8 (7.2%) | |
| H. pylori status | ||
| Negative | 55 (49.5%) | |
| Positive | 26 (23.4%) | |
| Not tested | 30 (27.0%) | |
| Histological diagnosis | ||
| Benign | 69 (62.2%) | |
| Adenocarcinoma | 34 (30.6%) | |
| Lymphoma | 6 (5.4%) | |
| Other neoplasia | 2 (1.8%) | |
| - Pancreatic head adenocarcinoma - Neuroendocrine tumour | ||
Figure 3.
Distribution of giant gastric ulcers defined as benign or malignant.
Of the 111 patients, 42 had malignant ulcers. This equates to a malignant yield of 37.8% (95% CI 28.8–46.8). Of these 42 ulcers, 34 (30.6%) were gastric adenocarcinomas, 5 (5.4%) were lymphomas and 2 (1.8%) were other neoplastic lesions (one being a pancreatic head adenocarcinoma and the other a metastatic neuroendocrine tumour). Most cancers (83.3% n = 35) were diagnosed on the first set of biopsies taken; 14.3% (n = 6) required two oesophago-gastroduodenoscopies (OGDs) and 2.4% (n = 1) required four OGDs (this being a lymphoma) for histological confirmation. The mean number of samples taken to obtain a diagnosis in the malignant group was 7.9 biopsies/OGD.
The results for the univariate analysis to determine association of demographic, clinical and endoscopic variables with that of histology are shown in Table 2. Statistically significant variables included age (P = 0.005), number of ulcers found per patient (P = 0.038), increasing size of the ulcer (P = 0.002), location (P = 0.028), endoscopist's ‘non-suspicion’ (P < 0.001) and presence of atrophic gastritis (P = 0.021). On running a correlation matrix, significant collinearity was found between two variables: the number of ulcers and the size of the ulcers. The number of ulcers was used in the binary logistic regression model as this gave a higher predictor percentage in combination with other significant variables. Binary logistic regression results are shown in Table 3, presented as odds ratio and associated P value. Atrophic gastritis was not used within the model despite univariate significance due to more than 50% of data being absent.
Table 2.
P values for the association of independent variables with that of malignant histology as the dependent variable. P value calculated using Pearson chi-square for categorical data.
| Demographic, clinical, endoscopic & histological independent variables | Univariate association analysis |
|---|---|
| Sex | P = 0.149 |
| Age | P = 0.005 |
| Ethnicity | P = 0.692 |
| Smoking status | P = 0.801 |
| Charlson Comorbidity Index Score | P = 0.583 |
| Indication for endoscopy | P = 0.613 |
| Number of ulcers per endoscopy | P = 0.038 |
| Location | P = 0.028 |
| Size of largest ulcer | P = 0.002 |
| Endoscopic non-suspicion | P < 0.001 |
| Gastric ulcer appearance | P = 0.059 |
| Presence of atrophic gastritis | P = 0.021 |
| H. pylori status | P = 0.395 |
Table 3.
Results of binary logistic regression testing association of statistically significant variables to that of predicting malignant ulcer histology. Data presented as odds ratios with its associated P value.
| Odds ratios (95% confidence intervals) | P value | |
|---|---|---|
| Age (20–59) | 0.202 (0.06–0.71) | P = 0.013 |
| No. of ulcers (single ulcer) | 1.514 (0.85–2.70) | P = 0.161 |
| Location combined (fundus/cardia/incisura) | 4.417 (1.10–17.76) | P = 0.036 |
| Endoscopic ‘non-suspicion’ | 0.138 (0.05–0.39) | P < 0.001 |
Predictors for malignant ulcer when all other variables held constant included location of the ulcer, with an odds ratio (OR) of 4.417 (95% CI 1.10–17.76) if the ulcer was located in the fundus, cardia or incisura as compared with that of the body and antrum. Endoscopist's ‘non-suspicion’ was associated with a significant reduction in the odds of being malignant with an OR of 0.138 (95% CI 0.05–0.39). Age of the patient was also associated with a significant reduction in the odds of malignancy with an OR of 0.202 (95% CI 0.06–0.71) if the patient was aged between 20 and 59 years, compared with that for ages 60–99 years. The number of ulcers per patient was not found to be a significant predictor for malignancy when adjusted for other variables in the model.
Patients with a malignant gastric ulcer had a 30-day mortality of 11.9% (5/42) and a 12-month mortality of 61.9% (26/42) compared with a 30-day mortality of 9.6% (7/73), and a 12-month mortality of 21.9% (11/73) in patients with benign ulcers. Patients with adenocarcinoma who underwent surgery, with or without adjuvant chemoradiotherapy, had a 12-month mortality of 36.4%. Of those who died within 12 months with malignant gastric ulcers, 57.8% (15/26) were related to advanced cancer, 38.5% (10/26) were the result of comorbidities and one patient died at the time of surgery. Of those who died within 12 months from benign giant gastric ulcers, 54.5% (6/11) died relating to complications from the ulcer, with the remaining 45.5% (5/11) due to comorbidities.
Discussion
This is the largest study looking at patients with giant gastric ulcers in the era of proton pump inhibitors. We have demonstrated that the risk of cancer within giant gastric ulcers is higher than previously recognised, approaching 40%. Predictors for cancer were ulcer location, patient's age and the endoscopist's diagnosis of ‘suspicious’ ulceration.
Previous studies looking at giant gastric ulcers have been principally case series10,13 reporting a much lower risk of cancer.7,8 We analysed predictors for malignancy using logistic regression analysis to improve the accuracy for predicting malignancy. Independence of variables that looked to predict malignancy in our cohort of patients included ulcer location (fundal or incisura) and age of the patient (>60), whilst if the ulcer looked endoscopically ‘non-suspicious’ then it was less likely to be malignant. Of those patients with malignant ulcers, 16.7% of these required at least one additional gastroscopy with biopsies before histology confirmed malignancy. They waited on average 23 days until repeat gastroscopy was performed for diagnosis. All of these patients on initial gastroscopy had suspicious-looking lesions and a median age of 80, being higher than our cohort average.
Long-term data for prognosis of patients diagnosed with giant gastric ulcers were previously unknown. However, here we have illustrated that for patients with a malignant gastric ulcer, the 12-month prognosis is poor, with a high mortality rate of 61.9% in our cohort. The 12-month mortality for benign giant gastric ulcers is also surprisingly high, with around a quarter dead at 1 year. This is likely to reflect the population's age and increasing comorbidities. We used a scoring system called the Charlson Comorbidity Index Score, which is a scoring system based on age and comorbidities, helping to predict a patient's 10-year survival. The median score in our cohort was 5 (IQR 4–6), equating to a 10-year survival of 23.36%, which matches the results in this study.
There are several limitations to our study. First, this is a retrospective study. This type of study is known to have inherent difficulties in patient and data acquisition. Patients were enrolled after searching through a computer-based endoscopy search engine and therefore patients potentially could have been missed. Information bias is another problem, but this was minimised by extensively searching hospital electronic records. However, some important variables had more than 50% of the data missing, including Helicobacter pylori status, smoking, presence of atrophic gastritis and intestinal metaplasia, all of which are known to influence the risk for developing gastric cancer. Atrophic gastritis during univariate analysis showed significant association for malignant histology; however, when applied to the regression model it negatively impacted on its predicting potential because large quantities of data were missing. A complete data set with the above variables may create a more powerful predictor model, although this is only likely to be acquired by long prospective cohort studies.
Another limitation to our study was the subjectivity of the variable ‘endoscopist's suspicion’. What defines ‘suspicion’ or ‘non-suspicion’ is not clearly recorded within the endoscopy reports and is difficult in general to quantify. If there is any suggestion of an ulcer looking ‘suspicious’, endoscopists are likely to err on the side of caution and call it ‘suspicious’. An endoscopist is only likely to describe an ulcer as ‘non-suspicious’ if they are extremely confident that it looks benign. Being able to clearly grasp what defines this dichotomous decision is challenging and is likely to be dependent on the endoscopist's experience.
Despite being a single centre study, Leeds NHS Trust covers a large area serving a population of over 770,000 people. Caucasians accounted for 96.4% of the study's ethnicity, which is around 10% higher than that for the whole of England. Whether these findings can be extrapolated worldwide is unlikely in view of the strong association of certain ethnicities with gastric cancer. Although Leeds Hospital serves as a tertiary referral centre, we excluded patients who were referred from other hospitals in order to reduce referral bias, thereby not falsely inflating our yield.
In summary, this is the largest cohort of giant gastric ulcers studied. We have shown a high malignancy yield of 37.8% (95% CI 28.8–46.8) and 12-month mortality of 61.9% for malignant ulcers and 21.9% for benign giant gastric ulcers. Predictors for malignancy in patients with giant gastric ulcers in this cohort included ulcer location, patient's age and endoscopist's ‘suspicion’ during gastroscopy.
Author contributions
RL performed data gathering, statistical analysis, write up of initial draft and is the corresponding author. ME performed data gathering, write up of initial draft amd manuscript editing. LT performed data gathering and manuscript editing. NM performed study design, conceptualisation amd manuscript editing. VS performed statistical analysis and manuscript editing. BJR performed study design, conceptualisation, database search and manuscript editing.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethics approval
Ethics approval was not required as performed as part of a clinical audit.
Informed consent
Informed consent was not required.
References
- 1.Hansson L, Nyren O, Hsing AW, et al. The risk of stomach cancer in patients with gastric or duodenal ulcer disease. New Engl J Med 1996; 335: 242–249. [DOI] [PubMed] [Google Scholar]
- 2.Lv SX, Gan JH, Ma XG, et al. Biopsy from the base and edge of gastric ulcer healing or complete healing may lead to detection of gastric cancer earlier: an 8 years endoscopic follow-up study. Hepatogastroenterology 2012; 59: 947–950. [DOI] [PubMed] [Google Scholar]
- 3.ASGE Standards of Practice Committee. Banerjee S, Cash BD, et al. The role of endoscopy in the management of patients with peptic ulcer disease. Gastrointest Endosc 2010; 71: 663–668. [DOI] [PubMed] [Google Scholar]
- 4.Excellence NIfHaC. Dyspepsia and gastro-oeosphageal reflux disease. NICE Clinical Guidelines. 2014; 184.
- 5.Graham DY, Schwartz JT, Cain GD, et al. Prospective evaluation of biopsy number in the diagnosis of esophageal and gastric carcinoma. Gastroenterology 1982; 82: 228–231. [PubMed] [Google Scholar]
- 6.Cohn I, Sartin J. Giant gastric ulcers. Ann Surg 1958; 147: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selinger CP, Cochrane R, Thanaraj S, et al. Gastric ulcers: malignancy yield and risk stratification for follow-up endoscopy. Endosc Int Open 2016; 4: E709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raju GS, Bardhan KD, Phil D, et al. Giant gastric ulcer: its natural history and outcome in the H2RA era. Am J Gastroenterol 1999; 94: 3478–3486. [DOI] [PubMed] [Google Scholar]
- 9.Avery Jones F. Clinical and social problems of peptic ulcer. BMJ 1957; 1: u86–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaterka S, Filho JM, Pontes JF. Giant gastric ulcer. Am J Digest Dis 1962; 7: 236–249. [DOI] [PubMed] [Google Scholar]
- 11.Kocak E, Kilic F, Akbal E, et al. The usefulness of ulcer size and location in the differential diagnosis of benign and malignant gastric ulcer. Wien Klin Wochenschr 2013; 125: 21–25. [DOI] [PubMed] [Google Scholar]
- 12.Health Research Authority. Determine whether your study is research. 2016.
- 13.Alvarez WC, MacCarty WC. Size of resected gastric ulcers and gastric carcinomas. JAMA 1928; 91: 226–231. [Google Scholar]