Abstract
Background
Hiatal hernia is diagnosed by barium-swallow esophagogram or esophagogastroduodenoscopy, with possible suboptimal results. High-resolution manometry clearly identifies crural diaphragm and lower esophageal sphincter.
Objectives
To assess the diagnostic accuracy of high-resolution manometry in detecting hiatal hernia compared to esophagogram and esophagogastroduodenoscopy, using as reference the surgical in vivo measurement.
Methods
Patients were studied with esophagogram, esophagogastroduodenoscopy, high-resolution manometry and in vivo evaluation of the esophago-gastric junction. Esophago-gastric junction was classified as type I (no separation between crural diaphragm and lower esophageal sphincter); type II (≥1, ≤ 2 cm separation); type III (>2 cm). During in vivo measurement, distance between the esophago-gastric junction and crural diaphragm proximal border was recorded.
Results
Surgery identified 53 hiatal hernias in 100 patients. Forty-seven percent were classified as type I esophago-gastric junction, 35% type II and 18% type III. Referenced to in vivo evaluation, high-resolution manometry showed superior diagnostic sensitivity and specificity (94.3% and 91.5%, respectively) to esophagogram and esophagogastroduodenoscopy, with 92.6% predictive value of a positive test and 93.5% predictive value of a negative test. The kappa value for high-resolution manometry and in vivo evaluation was 0.85. High-resolution manometry showed optimal sensitivity and specificity in detecting types I, II and III esophago-gastric junction.
Conclusions
High-resolution manometry enables an accurate diagnosis of hiatal hernia and a better classification than endoscopy and radiology, reaching optimal agreement with in vivo assessment.
Keywords: Hiatal hernia, esophagogastric junction, high resolution manometry, upper endoscopy, barium esophagogram
Key summary
Summarise the established knowledge on this subject
Sliding HH presence is more frequently observed with the increasing severity of GERD and it has been associated with abnormal esophageal acid exposure, prolonged esophageal clearance and increased number of reflux episodes.
HHs can be diagnosed by barium swallow esophagogram and EGD; however, these two methods are impaired by the subjective and indirect evaluation of EGJ components.
A better diagnostic evaluation of HH presence and axial dislocation may be useful in the management of GERD patients.
What are the significant and/or new findings of this study?
HRM showed the highest correlation with surgical in vivo evaluation of HHs.
Referenced to in vivo evaluation, HRM showed superior diagnostic sensitivity and specificity (94.3% and 91.5%, respectively) than esophagogram and EGD.
HRM showed optimal sensitivity and specificity in detecting types I, II and III EGJ.
Introduction
Hiatal hernia (HH) occurs when the stability of the esophagogastric junction (EGJ) is impaired, allowing upward dislocation of the lower esophageal sphincter (LES) and stomach through the crural diaphragm (CD). Generally, four types of HH are described: sliding HH (type I), paraesophageal (type II), mixed (type III) and massive (type IV). Type I HH is by far the most common type, accounting for 95% of instances.
Sliding HH has been associated with increased reflux exposure1–3 and increasing severity of gastroesophageal reflux disease (GERD), from non-erosive compared to erosive esophagitis and Barrett’s esophagus.4–12 Sliding HH diagnosis is classically made by barium-swallow esophagogram,13 and/or during esophagogastroduodenoscopy (EGD).14 However, these methods are impaired by subjective and indirect evaluation of LES and CD location.
Previous studies with conventional manometry documented two separate pressure zones at the EGJ level in HH patients, representing the spatial separation of LES and CD, but failed to reach optimal sensitivity.9,15 High-resolution manometry (HRM) clearly identifies CD and LES and evaluates their anatomical relationship. At HRM, three EGJ subtypes are described based on LES–CD separation.16 However, its diagnostic accuracy has been poorly investigated. Only two studies have measured the agreement between HRM and conventional techniques, i.e. esophagogram and EGD, in detecting HH,17,18 and HRM findings were never compared with in vivo measurements.
We aimed to assess the HRM diagnostic value in HH detection, in comparison with esophagogram and EGD, assuming the in vivo direct measurement during open surgery of the distance between EGJ and diaphragmatic hiatus as the golden standard for a sliding HH presence.
Materials and methods
Study population
We enrolled at the University of Campania consecutive patients undergoing open foregut surgery, between May 2009 and December 2015. The study protocol was approved by the internal review board (University of Campania, part of protocol no. 608/26-10-2017).
Exclusion criteria were: the presence of paraesophageal, mixed and type IV HH, a past history of thoracic, esophageal, or gastric surgery; primary motility disorders; pregnancy.
All subjects underwent esophagogram, EGD, HRM (anatomical preoperative assessment) and EGJ morphology in vivo evaluation during surgery in which EGJ needed to be dissected (total fundoplication for refractory GERD, Barrett’s esophagus, bariatric surgery). At first visit, demographics and clinical history were recorded. Patients were asked to discontinue any medication influencing esophageal motor function 5–7 days prior to testing.
EGJ appearance
In order to optimise concordance between HH grades assessed by different exams, we arbitrarily decided to adopt a unique nomenclature, as follows: type I, type II and type III EGJ appearance.
Barium esophagogram
Esophagogram was performed in fasting conditions, following a standardised protocol (drinking 200 ml of diluted barium, in upright, supine, and prone positions, with and without gas powders). Determination of sliding HH presence was based on the EGJ appearance determined by two skilled radiologists blinded to the patient’s symptoms and other findings. A radiological B ring was defined as a smooth, symmetric ring-like indentation at EGJ. The HH axial length was measured in centimetres. We classified radiological appearance as follows: type I, normal EGJ without B ring or gastric fold evidence; type II, sliding HH 2 cm or less; type III, sliding HH greater than 2 cm, when B ring or gastric folds demarcated the EGJ displaced above the diaphragm proximal border.
Esophagogastroduodenoscopy
During EGD, two landmarks were recorded: the position of the crural impression proximal border and EGJ. Proximal gastric rugal folds end defined EGJ.19 The distance in centimetres from incisors to the diaphragmatic pinch proximal border and to the top of proximal gastric folds was recorded. Endoscopic type I morphology indicated a normal position of EGJ (superimposed to diaphragmatic pinch); type II, a HH diagnosed with a difference of 1 cm or greater and 2 cm or less between the position of the crural impression proximal border and EGJ and type III, with a difference greater than 2 cm.
High-resolution manometry
HRM was performed with a 4.2 mm diameter solid-state assembly with 32 circumferential sensors spaced at 1-cm intervals (HRiM catheter InSight; Sandhill Scientific Inc., USA). Examinations were done in the supine position after fasting and manometric assemblies were positioned with at least five intragastric sensors. EGJ was assessed during a 5-minute baseline recording, then at least 10 single water swallows (5 mL) evaluated esophageal peristalsis.20–22
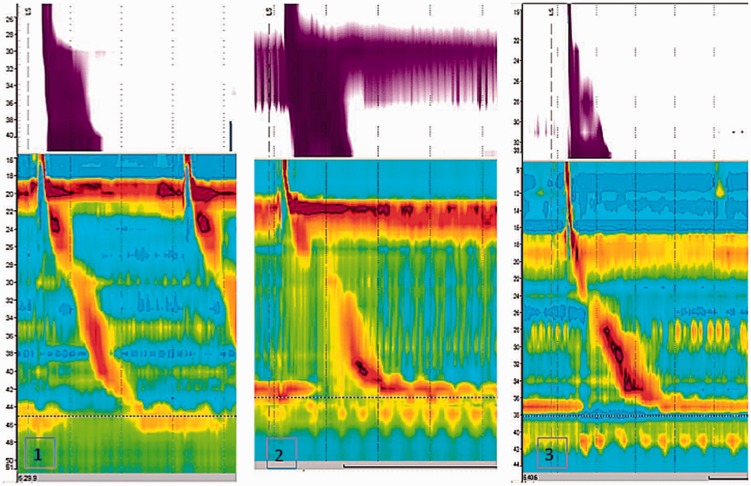
Analysis was performed using Sandhill Bioview software, after thermal compensation. EGJ was localised and its pressure and relaxations evaluated; proximal and distal borders were marked according to intraesophageal and intragastric pressures. CD was marked as the axial level characterised by maximal inspiratory pressure augmentation. The distance in centimetres between the maximal LES pressure peak and the maximal CD pressure peak was measured directly from isocontour plots. Two blinded investigators independently analysed EGJ morphology for each subject. Patients were then classified to have three morphological types of EGJ, based on LES–CD axial separation, measured in centimetres, and classified as: type I, no separation between LES and CD; type II, minimal separation (>1 and ≤ 2 cm); type III, greater than 2 cm of separation (Figure 1).23
Figure 1.
Examples of high resolution manometry traces in (1) esophagogastric junction (EGJ) morphology type I (complete overlap between lower esophageal sphincter and crural diaphragm); (2) EGJ morphology type II (separation > 1 but ≤ 2 cm); (3) EGJ morphology type III (separation > 2 cm).
In vivo measurement
In vivo HH measurement was performed during elective open surgery; laparoscopic measurements were not considered to exclude diaphragmatic overdistension due to pneumoperitoneum. After phrenoesophageal membrane incision, complete EGJ and CD isolation was obtained. When necessary, hernia sac and anterior fat pad resection was performed. Mediastinal esophageal dissection was strictly avoided before measurement and no gastric tension was applied. Then, an endoscope was inserted orally up to the proximal gastric folds (EGJ level). A large hemoclip marked the EGJ position. Endoscope deflated the stomach and then retracted to the clip (Figure 2). Surgeons measured the distance in centimetres between the clip (helped by endoscopical trans-illumination) and CD proximal border (apex), with a ruler. We classified in vivo EGJ morphology as follows: type I, EGJ placed below or overlapping the CD, type II, EGJ above CD greater than 1 cm but 2 cm or less, and type III, EGJ laid down above CD greater than 2 cm, respectively.
Figure 2.
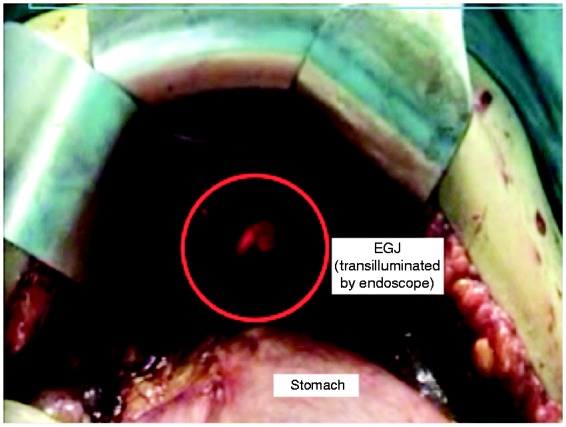
Intraoperative recording of esophagogastric junction (EGJ) landmarks (proximal rugal fold and crural diaphragm) by means of intraoperative endoscopy.
Statistical analysis
Statistical analysis was performed using SPSS (version 22; SPSS Inc.). Continuous data were expressed as median and interquartile (25–75th) range, unless otherwise indicated. To determine HRM, endoscopy and radiology diagnostic value in identifying HHs we used the EGJ morphology detected during an in vivo measurement as the reference standard. Sensitivity, specificity and predictive value of a positive test (PVPT) and a negative test (PVNT) were determined for each technique, using Baye’s theorem for the observed HH prevalence during in vivo study. Comparisons of probability of concordance (agreement between the positive results of two tests among the same patients) between the three tests were made using McNemar’s test. The chi-square test was used to compare the proportion of false positive and false negative results between the diagnostic tests. Receiver operating characteristic (ROC) analysis was used to determine the optimal length of LES–CD separation on HRM to predict HH presence at in vivo measurement, and to compare HRM to EGD and esophagogram in predicting HH presence. P values of 0.05 or less were considered statistically significant.
Results
We enrolled one hundred consecutive patients who all underwent surgery, and prior esophagogram, EGD and HRM. The baseline characteristics of patients are shown in Table 1.
Table 1.
Baseline characteristics of study population classified according to presence of a hiatal hernia identified during surgery.
| Features | Whole population | Patients with HH | Patients without HH | P value |
|---|---|---|---|---|
| Patients, n | 100 | 53 | 47 | |
| Male patients, n (%) | 42 (42%) | 22 (41.5%) | 20 (42.5%) | NS |
| Mean age (range) | 48.4 (37–71) | 53 (38–71) | 46 (37–67) | 0.032 |
| Mean BMI (range) | 25 (17–33) | 25 (19–33) | 24 (17–31) | NS |
| Alcohol consumption, n (%) | 20 (20%) | 12 (22.6%) | 8 (17%) | NS |
| Coffee consumption, n (%) | 47 (47%) | 24 (45.3%) | 23 (48.9%) | NS |
| Smoking, n (%) | 18 (18%) | 9 (16.9%) | 9 (19.1%) | NS |
| H. pylori infection, n (%) | 24 (24%) | 13 (24.5%) | 11 (23.4%) | NS |
| Esophagitis grade A, B (sec. Los Angeles), n (%) | 26 (26%) | 19 (35.8%) | 7 (14.8%) | 0.031 |
| Esophagitis grade C, D (sec. Los Angeles), n (%) | 6 (6%) | 5 (9.4%) | 1 (2.1%) | NS |
| Barrett’s esophagus (any), n (%) | 4 (4%) | 4 (7.5%) | 0 (0%) | NS |
HH: hiatal hernia; BMI: body mass index; NS: not significant.
In vivo
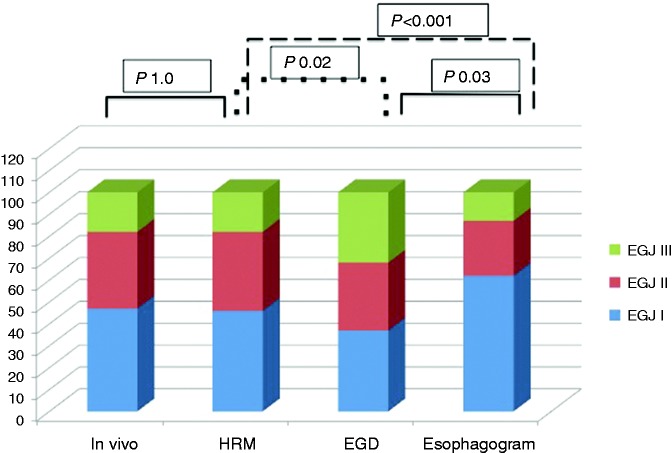
During surgery, 53 (53%) patients had HHs. Forty-seven (47%) patients were classified as type I EGJ, 35 (35%) type II and 18 (18%) type III. The mean LES–CD axial separation in HH patients was 2.13 ± 1.14 cm. HH patients were older than type I EGJ patients (P < 0.01).
High-resolution manometry
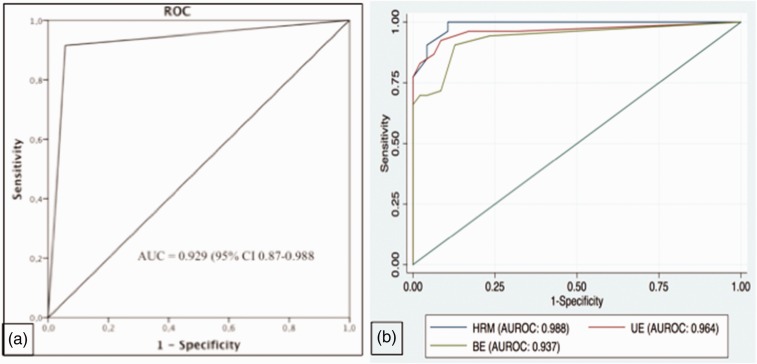
Fifty-four (54%) patients had a LES–CD axial separation higher than 1 cm, whereas the remaining 46 patients had a type I EGJ. Among the HHs identified, 36 (36%) were type II, and 18 (18%) type III EGJ. The mean LES–CD axial separation in HH patients was 2.11 ± 1.14 cm. Compared to in vivo evaluation, HRM showed a diagnostic sensitivity and specificity of 94.3% and 91.5%, respectively, in detecting HH presence, with 92.6% PVPT and 93.5% PVNT. The kappa value for HRM and in vivo evaluation was 0.85. McNemar’s test demonstrated a statistically significant concordance (P = 1.0). HRM showed optimal diagnostic sensitivity and specificity in detecting types I, II and III EGJ morphology (Figure 3). Detailed findings are shown in Tables 2 and 3. ROC analysis determined a cu-off value of LES–CD axial separation of 1.2 cm, as this yielded the optimal performance (sensitivity and specificity of 95.7% and 90.6%, respectively) in diagnosing HH presence (Figure 4).
Figure 3.
Hiatal hernia presence and esophagogastric junction appearance during in vivo examinations and during different diagnostic testing. EGJ: esophagogastric junction; HRM: high-resolution manometry; EGD: esophagogastroduodenoscopy.
Table 2.
Sensitivity, specificity, predictive values of positive and negative tests, and Cohen’s kappa agreement for HRM, endoscopy and barium esophagogram, using in vivo assessment as gold standard diagnostic reference for hiatal hernia.
| HRM | Endoscopy | Esophagogram | |
|---|---|---|---|
| Sensitivity (%) | 94.3 | 96.2 | 69.8 |
| Specificity (%) | 91.5 | 74.5 | 97.9 |
| PVPT (%) | 92.6 | 81 | 97.4 |
| PVNT (%) | 93.5 | 94.6 | 74.2 |
| Kappa values | 0.85 | 0.716 | 0.661 |
HRM: high-resolution manometry; PVPT: predictive value of a positive test; PVNT: predictive value of a negative test.
Table 3.
Sensitivity, specificity, predictive values of PVPT and PVNT, and Cohen’s kappa agreement for HRM, endoscopy and barium esophagogram, using in vivo assessment as gold standard diagnostic reference for HH with subgroups according to EGJ morphology.
| HRM type I | HRM type II | HRM type III | Endoscopy type I | Endoscopy type II | Endoscopy type III | Barium type I | Barium type II | Barium type III | |
|---|---|---|---|---|---|---|---|---|---|
| Sensitivity (%) | 91.5 | 91.4 | 100 | 74.5 | 54.3 | 100 | 97.9 | 54.3 | 72.2 |
| Specificity (%) | 94.3 | 93.8 | 100 | 96.2 | 81.5 | 82.9 | 69.8 | 90.8 | 100 |
| PVPT (%) | 93.5 | 88.9 | 100 | 94.6 | 61.3 | 56.3 | 74.2 | 76 | 100 |
| PVNT (%) | 92.6 | 95.3 | 100 | 81 | 76.8 | 100 | 97.4 | 78.7 | 94.3 |
| Kappa | 0.86 | 0.847 | 1.0 | 0.716 | 0.368 | 0.636 | 0.665 | 0.482 | 0.81 |
HH: hiatus hernia; EGJ: esophagogastric junction; HRM: high-resolution manometry; PVPT: predictive value of a positive test; PVNT: predictive value of a negative test.
Figure 4.
(a) Receiver operating characteristic (ROC) curve for the presence of hiatal hernia by high resolution manometry (HRM), using in vivo surgical assessment as diagnostic reference. AUC: area under the curve. (b) Area under receiver operating characteristic(AUROC) curve for the presence of hiatal hernia by the measurements of lower esophageal sphincter to crural diaphragm (LES–CD) length at high-resolution manometry (HRM), upper endoscopy (UE) and barium esophagogram (BE).
Esophagogastroduodenoscopy
EGD identified 63 (63%) patients with HH. Thirty-one (31%) had type II and 32 (32%) type III EGJ. Sensitivity was similar to HRM (96.2%), but specificity was lower (74.5%), with a 0.716 kappa value. McNemar’s test demonstrated a statistically significant discordance (P = 0.022) between endoscopy and HRM. EGD produced lower sensitivity in detecting type I EGJ, lower specificity in type III EGJ and both lower sensitivity and specificity in type II EGJ than HRM (Tables 2 and 3).
Barium esophagogram
In 38 (38%) patients a B ring was documented as sign of HH presence. Twenty-five (25%) patients were categorised as type II EGJ, whereas 13 (13%) were considered type III EGJ. The esophagogram ability in detecting HHs was similar to HRM, with a specificity of 97.9% but with lower sensitivity (69.8%). Compared to EGD, radiology showed higher specificity and lower sensitivity. McNemar’s test demonstrated a statistically significant discordance (P < 0.001) between radiology and HRM. According to morphological EGJ groups, esophagogram had similar specificity in identifying types II and III, and lower specificity in type I EGJ than HRM, whereas sensitivity was lower for types II and III than HRM. Compared to EGD, radiology had similar sensitivity for type II, but discordant values for both types I and III (Table 2 and 3).
Area under receiver operating characteristic comparison
Using HRM as reference standard, the predictability of HH presence was higher than endoscopy and esophagogram, even if there was an absence of statistical significance (Figure 4(b)).
Discussion
Recent studies demonstrated that HRM has a high sensitivity and specificity for sliding HH detection.13,14 In this study, we confirmed that HRM seems to detect HH presence accurately. In particular, we proved this ability using a reference standard, the surgical in vivo evaluation of LES–CD axial dislocation. HRM reached a sensitivity and specificity of HH diagnosis of 94.3% and 91.5%, respectively, exceeding the diagnostic ability of both endoscopy and radiography. HRM showed a ‘substantial’ agreement (kappa 0.85) and a statistically significant concordance (P = 1.0) with the in vivo assessment. Furthermore, we demonstrated that HRM could accurately define HH size (i.e. small when > 1 but ≤ 2 cm, and large when > 2 cm).
The HH role in GERD patients has been extensively investigated.24 EGJ migration can be responsible for a decreased barrier function,25–31 dysphagia4 and hernia symptoms. Also, HH axial extension is considered an important parameter to plan pharmacological, endoscopic or surgical therapies.32
Usually, HH diagnosis is performed during EGD or esophagogram, but the reported data on the sensitivity, specificity or reproducibility of these tests are limited,12,27 and are affected by various drawbacks, such as EGJ mobility.32–34 This makes it difficult to standardise the assessment and measurement of a sliding HH with snapshot techniques. EGD is influenced by additional confounding factors such as difficulty in marking the squamo-columnar junction in Barrett’s metaplasia, an extremely patulous hiatus, and excess gastric insufflation.35 In this study, endoscopy showed a lower specificity than HRM, whereas radiology had a lower sensitivity. This is in agreement with the limitations of these two techniques; a lower specificity of EGD can be explained by exaggerated gastric inflation or by low patient compliance that might exaggerate the apparent HH size. Instead, radiology was very accurate in excluding HH presence, but was unable to identify small hernias.
HRM allows a more dynamic evaluation of EGJ and its components and, theoretically, is not influenced by subjective landmarks (such as a damaged mucosa) or by position or exaggerated gastric filling. Also, HRM protocol is well standardised and is rigorous worldwide. We demonstrated that HRM reached both optimal sensitivity and specificity when a LES–CD axial separation greater than 1.2 cm was present, with optimal PVPT and PVNT values, suggesting its major role in HH diagnosis. These findings are consistent with a recent study by Weijenborg et al.,17 in which the authors compared HRM diagnostic power in identifying HHs with endoscopy and radiology either alone or combined. HRM showed 92% sensitivity and 93% specificity, exceeding the diagnostic power of the other two techniques alone. However, their study presented two important limitations: the reference standard applied was a subjective one, made by the combination of HH presence at endoscopy or radiology, and data from endoscopies and esophagograms were retrospectively collected.
In contrast to our results, Khajanchee et al.18 often reported false negative results with HRM (low sensitivity, 52.38%, but high specificity, 95.12%). Interestingly, they evaluated HH length assessment during laparoscopic surgery and established this measurement as the reference. However, as stated by the authors themselves, creating a pneumoperitoneum may have exaggerated the HH size. Pneumoperitoneum could also reduce LES–CD axial separation, due to the well-known induced diaphragmatic proximal overdistension.
A major strength of our study is that we used an in vivo assessment of HHs during open surgery as the reference standard, avoiding increasing the abdominal pressure and diaphragmatic overdistension. Also, all measurements, both in vivo and during all tests, were easily reproducible and performed according to standardised protocols under the same circumstances. Finally, we performed a diagnostic power analysis for detecting both small and large sliding HH. It is worthy of note that the lowest diagnostic test values belonged to endoscopy and radiology in classifying small HHs (>1 cm but ≤ 2 cm), whereas both techniques showed optimal diagnostic power in large HHs. These data seem to support the hypothesis that endoscopy usually tends to exaggerate HH size, while radiology underestimates small hernias. HRM, instead, showed an optimal performance in classifying various EGJ morphologies. Another potential reason for the better correlation of the surgically measured HHs with HRM measurements is that both these measurements are performed in the basal state of the EGJ, without swallows (as with barium studies) or air insufflation causing secondary peristalsis (as with EGD).
This study has some limitations. The patients were enrolled during a 5-year interval, in which software analyses were probably ameliorated. However, this long time lapse was due to the wide use of laparoscopy for upper gastrointestinal surgery, and this caused difficulty in enrolling an adequate number of subjects. This is the first study using in vivo evaluation as the reference for HH presence (even if classic reports in early 1900 verified the presence of HHs during surgical procedures after diagnostic attempts with X-rays), thus there is not yet a real validation. Another possible limitation is the subjective definition of small and large HHs. It is intuitive that with a strict definition of 1 cm and 2 cm as landmarks, some HHs (i.e. HHs measuring 0.9 cm or 1.9 cm) can be erroneously classified as no HH or both small and large HHs. However, we had a very low frequency of these ‘borderline’ cases. The role of a constant presence of LES–CD axial separation greater than 0 cm but less than 1 cm was not investigated. This can be explained by the catheter spatial sensor configuration (1 cm each apart), which renders it actually impossible to detect exact separations lower than 1 cm. Finally, this study was not intended to compare the pathophysiological significance of HH measured with any of the testing modalities. However, as the surgically identified HH is subject to repair during anti-reflux surgery, we feel that knowing which non-surgical measurement most accurately predicts surgical HH size is relevant to the treating physician and surgeon. Thus, in this context, our findings indicate that HH measurements on HRM best predict what the surgeon encounters during anti-reflux surgery.
In conclusion, HRM can accurately diagnose a sliding HH presence, with high sensitivity and specificity. HRM seems to classify HHs better (no HH, small or large size) than endoscopy and radiology, and reaches an optimal agreement with surgical in vivo assessment, thus suggesting its use in combination with esophagogram and EGD for HH evaluation, particularly when anti-reflux surgery is considered.
Author contribution
ST, ES and GZ: data collection and analysis, writing of the manuscript, approving final version. MF and NdB: writing of the manuscript; LF, GdG, GB and MF: data collection and analysis; VS and LD: approving final version.
Declaration of conflicting interests
The authors declared that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee.
Informed consent
Written informed consent was obtained from each patient included in the study.
References
- 1.Kahrilas PJ, Lin S, Chen J, et al. The effect of hiatus hernia on gastrooesophageal junction pressure. Gut 1999; 44: 476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med 1997; 336: 924–932. [DOI] [PubMed] [Google Scholar]
- 3.Bredenoord AJ, Weusten BLAM, Timmer R, et al. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology 2006; 130: 334–340. [DOI] [PubMed] [Google Scholar]
- 4.Savarino E, Zentilin P, Frazzoni M, et al. Characteristics of gastro-esophageal reflux episodes in Barrett's esophagus, erosive esophagitis and healthy volunteers. Neurogastroenterol Motil 2010; 22: 1061–e1280. [DOI] [PubMed] [Google Scholar]
- 5.Savarino E, Gemignani L, Pohl D, et al. Oesophageal motility and bolus transit abnormalities increase in parallel with the severity of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2011; 34: 476–486. [DOI] [PubMed] [Google Scholar]
- 6.Jones MP, Sloan SS, Jovanovic B, et al. Impaired egress rather than increased access: an important independent predictor of erosive oesophagitis. Neurogastroenterol Motil 2002; 14: 625–631. [DOI] [PubMed] [Google Scholar]
- 7.Savarino E, Zentilin P, Tutuian R, et al. Impedance-pH reflux patterns can differentiate non-erosive reflux disease from functional heartburn patients. J Gastroenterol 2012; 47: 159–168. [DOI] [PubMed] [Google Scholar]
- 8.Tolone S, de Cassan C, de Bortoli N, et al. Esophagogastric junction morphology is associated with a positive impedance-pH monitoring in patients with GERD. Neurogastroenterol Motil 2015; 27: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 9.Mittal RK, Lange RC, McCallum RW. Identification and mechanism of delayed esophageal acid clearance in subjects with hiatus hernia. Gastroenterology 1987; 92: 130–135. [DOI] [PubMed] [Google Scholar]
- 10.Van Herwaarden MA, Samsom M, Smout AJ. Excess gastroesophageal reflux in patients with hiatus hernia is caused by mechanisms other than transient LES relaxations. Gastroenterology 2000; 119: 1439–1446. [DOI] [PubMed] [Google Scholar]
- 11.Frazzoni M, Savarino E, Manno M, et al. Reflux patterns in patients with short-segment Barrett’s oesophagus: a study using impedance-pH monitoring off and on proton pump inhibitor therapy. Aliment Pharmacol Ther 2009; 30: 508–515. [DOI] [PubMed] [Google Scholar]
- 12.de Bortoli N, Frazzoni L, Savarino EV, et al. Functional heartburn overlaps with irritable bowel syndrome more often than GERD. Am J Gastroenterol 2016; 111: 1711–1717. [DOI] [PubMed] [Google Scholar]
- 13.Ott DJ, Gelfand DW, Chen YM, et al. Predictive relationship of hiatal hernia to reflux esophagitis. Gastrointest Radiol 1985; 10: 317–320. [DOI] [PubMed] [Google Scholar]
- 14.Hill LD, Kozarek RA, Kraemer SJ, et al. The gastroesophageal flap valve: in vitro and in vivo observations. Gastrointest Endosc 1996; 44: 541–547. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal A, Tutuian R, Hila A, et al. Identification of hiatal hernia by esophageal manometry: is it reliable? Dis Esophagus 2005; 18: 316–319. [DOI] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v 3.0. Neurogastroenterol Motil 2015; 27: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weijenborg PW, van Hoeij FB, Smout AJ, et al. Accuracy of hiatal hernia detection with esophageal high-resolution manometry. Neurogastroenterol Motil 2015; 27: 293–299. [DOI] [PubMed] [Google Scholar]
- 18.Khajanchee YS, Cassera MA, Swanström LL, et al. Diagnosis of type-I hiatal hernia: a comparison of high-resolution manometry and endoscopy. Dis Esophagus 2013; 26: 1–6. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus. The need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol 1998; 93: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 20.Pohl D, Ribolsi M, Savarino E, et al. Characteristics of the esophageal low-pressure zone in healthy volunteers and patients with esophageal symptoms: assessment by high-resolution manometry. Am J Gastroenterol 2008; 103: 2544–2549. [DOI] [PubMed] [Google Scholar]
- 21.Patel A, Ding A, Mirza F, et al. Optimizing the high-resolution manometry (HRM) study protocol. Neurogastroenterol Motil 2015; 27: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil 2015; 27: 1423–1431. [DOI] [PubMed] [Google Scholar]
- 23.Pandolfino JE, Kim H, Ghosh SK, et al. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol 2007; 102: 1056–1063. [DOI] [PubMed] [Google Scholar]
- 24.Kahrilas PJ, Shi G, Manka M, et al. Increased frequency of transient lower esophageal sphincter relaxation induced by gastric distention in reflux patients with hiatal hernia. Gastroenterology 2000; 118: 688–695. [DOI] [PubMed] [Google Scholar]
- 25.Bredenoord AJ, Weusten B, Timmer R, et al. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology 2006; 130: 334–340. [DOI] [PubMed] [Google Scholar]
- 26.Lord RV, DeMeester SR, Peters JH, et al. Hiatal hernia, lower esophageal sphincter incompetence, and effectiveness of Nissen fundoplication in the spectrum of gastroesophageal reflux disease. J Gastrointest Surg 2009; 13: 602–610. [DOI] [PubMed] [Google Scholar]
- 27.Bytzer P. Information bias in endoscopic assessment. Am J Gastroenterol 2007; 102: 1585–1587. [DOI] [PubMed] [Google Scholar]
- 28.Frazzoni M, De Micheli E, Zentilin P, et al. Pathophysiological characteristics of patients with non-erosive reflux disease differ from those of patients with functional heartburn. Aliment Pharmacol Ther 2004; 20: 81–88. [DOI] [PubMed] [Google Scholar]
- 29.Frazzoni M, Manno M, De Micheli E, et al. Pathophysiological characteristics of the various forms of gastro-oesophageal reflux disease. Spectrum disease or distinct phenotypic presentations? Dig Liver Dis 2006; 38: 643–648. [DOI] [PubMed] [Google Scholar]
- 30.Savarino E, Tutuian R, Zentilin P, et al. Characteristics of reflux episodes and symptom association in patients with erosive esophagitis and nonerosive reflux disease: study using combined impedance-pH off therapy. Am J Gastroenterol 2010; 105: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 31.Cameron AJ. Barrett’s esophagus: prevalence and size of hiatal hernia. Am J Gastroenterol 1999; 94: 2054–2059. [DOI] [PubMed] [Google Scholar]
- 32.Frazzoni M, De Micheli E, Grisendi A, et al. Hiatal hernia is the key factor determining the lansoprazole dosage required for effective intra-oesophageal acid suppression. Aliment Pharmacol Ther 2002; 16: 881–886. [DOI] [PubMed] [Google Scholar]
- 33.Pouderoux P, Lin S, Kahrilas PJ. Timing, propagation, coordination, and effect of esophageal shortening during esophageal peristalsis. Gastroenterology 1997; 112: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 34.Pandolfino JE, Zhang Q, Ghosh S, et al. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high resolution manometry. Gastroenterology 2006; 131: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 35.Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol 2008; 22: 601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]