Abstract
Background
Studies of the use of CO2 insufflation during endoscopic resection of large colorectal polyps (LCPs) are lacking.
Objective
We evaluated the effect of CO2 insufflation on pain after endoscopic resection of LCPs.
Methods
In a prospective randomized controlled trial (RCT), 132 patients were randomly assigned to groups who underwent endoscopic resection with CO2 insufflation (CO2 group, n = 66) or air insufflation (air group, n = 66). The primary outcome was abdominal pain post-procedure (PP). The secondary outcomes were abdominal distension, rates of technical success, amounts of sedatives prescribed, use of analgesics, and adverse events.
Results
Baseline patient characteristics were similar between the groups. The mean abdominal pain score was 12.3 in the CO2 group vs. 17.5 in the air group at 1 h PP (p = 0.047). Also, the proportion of patients without pain was significantly higher in the CO2 group at 1 h PP (p = 0.008). The pain score differed more in the endoscopic submucosal dissection group and long-time group. The secondary outcomes were not significantly different between the two groups.
Conclusions
The results of this RCT demonstrate the superiority of CO2 insufflation for endoscopic resection of LCPs in terms of decreasing PP abdominal pain (KCT0001636).
Keywords: Carbon dioxide, colorectal polyp, colonoscopy, endoscopic mucosal resection, endoscopic submucosal dissection
Key summary
1. Summarize the established knowledge on this subject
• Compared with air insufflation, carbon dioxide (CO2) insufflation reduces abdominal pain after colonoscopy.
• Understanding of the effects of CO2 insufflation during endoscopic resection of large colorectal polyps (LCPs) is lacking.
2. What are the significant and/or new findings of this study?
• CO2 insufflation during endoscopic resection of LCPs leads to a significant reduction in post-procedure abdominal pain.
• We found that the abdominal pain score differed more in the endoscopic submucosal dissection group and long-time group compared with the whole group.
• We suggest that CO2 insufflation should be used routinely during endoscopic resection of LCPs.
Introduction
Endoscopic resection of colorectal polyps reduces the risk and mortality rate of colorectal cancer.1 For large colorectal polyps (LCPs; diameter, ≥20 mm), endoscopic resection is more important because the risk of malignancy increases with increasing polyp size.2,3 Insufflation is needed during endoscopic resection to facilitate visualization and make the technique more exact. Air insufflation is the standard method; however, excessive air insufflation results in greater expansion of the bowel lumen, causing abdominal pain or discomfort.4–7 Generally, endoscopic resection takes longer to perform for LCPs than for small colorectal polyps; thus, a considerable amount of air enters the intestinal lumen with the former.6,8–12 Compared with air, carbon dioxide (CO2) is readily absorbed by the intestinal mucosa and eliminated via the respiratory tract. Therefore, CO2 insufflation can reduce post-procedure (PP) intestinal distension and abdominal pain.4–7
CO2 insufflation reportedly decreases colonoscopy-related abdominal fullness and pain compared with air insufflation.4,6,13–17 However, most previous studies have focused on the effects of pain relief by CO2 insufflation during diagnostic or routine colonoscopy, and so data on therapeutic colonoscopy are limited.6,16,18 Furthermore, clinical studies on the efficacy of CO2 insufflation for endoscopic resection (endoscopic submucosal dissection [ESD] or endoscopic mucosal resection [EMR]) of LCPs have focused on safety rather than pain.8,9,19,20 A PubMed search yielded only one observational cohort study of pain following endoscopic resection of LCPs.9 Therefore, we performed a prospective, double-blind, randomized controlled trial (RCT) to assess the effects of CO2 insufflation on the PP pain of patients undergoing endoscopic resection of LCPs.
Materials and methods
This was a prospective, double-blind RCT conducted to assess the efficacy of CO2 insufflation during endoscopic resection of LCPs.
Patients
From October 2015 to April 2017, we prospectively recruited consecutive patients scheduled for endoscopic resection of ≥20 mm colorectal polyps at the Gil Medical Center. Polyp size was assessed visually during colonoscopy. All procedures were performed by five expert endoscopists. The exclusion criteria were as follows: chronic obstructive pulmonary disease with retention of CO2, moderate or severe heart failure (New York Heart Association classification III or IV), long-term (>3 months) analgesic use in the past 6 months, and refusal to participate. In total, 132 patients were enrolled in the study, and all underwent bowel preparation with 2 l polyethylene glycol solution (Coolprep; Taejoon Pharm Inc, Seoul, South Korea) or 4 l polyethylene glycol solution (Colyte; Taejoon Pharm Inc.).
Written informed consent was obtained from all enrolled patients before the procedure. We informed participants of the study aims, methods, and possible adverse events. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution’s human research committee. The Institutional Review Board of Gil Medical Center approved this study (GCIRB2015-211, approved date: 4 August 2015) and this study was registered in the Clinical Research Information Service (KCT0001636).
Blinding and endoscopic resection procedure
Participants were randomly assigned to the CO2 insufflation group (CO2 group) or air insufflation group (air group) by a clinical research coordinator who was not associated with the study. The randomization was performed using computer-generated random numbers. All endoscopists, recovery-room nurses, and study subjects were blinded to the type of insufflation used. The endoscopy nurse switched the CO2 equipment “on” and “off” as dictated by the randomization. To maintain blinding of the endoscopists, the CO2 equipment was hidden under a fabric cover at all times.
All endoscopic procedures were performed using a CF-Q260AL or CF-H260AL colonoscope (Olympus Medical Systems Corp, Tokyo, Japan). The methods of endoscopic resection (ESD, EMR, endoscopic piecemeal mucosal resection [EPMR], and EMR after precutting [EMR-P]) were standardized.21–25 For ESD procedures, sodium hyaluronate (Endo-MucoUp 20, BMI Korea Corp., Uiwang, South Korea) was locally injected into the submucosa. After injection, a circumferential incision was made in the mucosa, and the submucosal layer was dissected using a Dual Knife (KD-650U, Olympus Medical Systems Corp., Tokyo, Japan). The electrocautery unit (VIO 300 D; ERBE, Tübingen, Germany) was operated in endo-cut mode (effect 2, cut duration 3, cut interval 2) to make the incision and in the 40 W forced-coagulation mode to perform dissection. For EMR procedures, a solution of glycerol, epinephrine, and methylene blue was used to generate a submucosal layer cushion. After injection, a snare was used to resect the lifted lesion. During EPMR procedures, the number of resections was minimized. The most suspicious portion of malignant and submucosal invasion was resected preferentially, and the remainder was resected so that no residual tissue remained. In the case of EMR-P, an incision was made around the lesion using a knife before resecting using a snare. All procedures were performed on an inpatient basis and the durations of all procedures were recorded.
Intra- and post-procedure management
In the CO2 group, CO2 was administered using a CO2-efficient endoscopic insufflator (Colosence Pro-500; Miraemedics Inc, Sung Nam, South Korea) connected to a CO2 bottle. In the air group, air was administered using an ordinary air insufflation system.
The patients received intravenous sedation with midazolam and propofol. The target sedation level was moderate sedation as defined by the practice guidelines of the American Society of Anesthesiologists Task Force.26 In the following cases, unsedated colonoscopy was performed: the patient refused sedated colonoscopy or a considerable number of posture changes was required due to polypectomy of difficult or defiant polyps. Pulse oxygen saturation, respiration rate, heart rate, and blood pressure were monitored during the procedure.
Chest and abdominal radiographs were obtained immediately after completion of the procedure. If adverse events such as colon perforation occurred, abdominal–pelvic computed tomography (APCT) was performed. In addition, laboratory tests were conducted pre-procedure and on day 1 PP. Follow-up colonoscopy was not performed PP; however, emergency colonoscopy was performed if a large amount of hematochezia was present. All patients were fasted on the day of the procedure and started a liquid diet on day 1 PP if there were no adverse events. After the procedure, if the patient complained of severe abdominal pain, an analgesic (tramadol, Tridol; Yuhan Corporation, Seoul, South Korea or propacetamol hydrochloride, Denogan; Yungjin Pharmaceutical Corporation, Seoul, South Korea) was prescribed.
Study outcomes
The primary outcome was the degree of abdominal pain at 1 h PP. Pain was recorded on a 100 mm visual analog scale (VAS). The VAS ranged from 0 (left) to 100 (right), where 0 designated no pain and 100 designated the worst pain imaginable. Abdominal pain was evaluated at 1, 3, 6, and 24 h PP. Also, subgroup analyses were performed for treatment method and procedure time.
The secondary outcomes were the extent of abdominal distension (waist circumference at the level of the umbilicus was estimated immediately before and after the procedure using a tape measure), rates of technical success (en bloc resection rate, complete resection rate [en bloc resection with histologically confirmed tumor-free lateral and vertical resection margins], and successful EMR rate [complete endoscopic lesion removal with no remnant lesion visible]), amounts of sedative drugs, frequency of analgesic prescription PP, and rates of adverse events.
Sample size calculation and statistical analysis
We carried out a prospective power analysis to determine the required sample size. The sample size calculation was based on differences in the VAS scores of the CO2 and air groups at 1 h PP. Based on previous studies, we hypothesized that the mean VAS score of patients in the CO2 group would be 10 mm and that that of patients in the air group would be 30 mm.13,27,28 A sample size of 59 patients per group was required for a significance level of 5% and power of 80%. Thus, assuming a 10% dropout rate, 132 patients were required.
Statistical analyses were performed using SPSS software (ver. 23.0; IBM, Armonk, New York, USA). None of the variables exhibited deviation from a normal distribution. Categorical variables were compared using a chi square test (or Fisher’s exact test) and are expressed as percentages and frequencies. Continuous variables were compared using a t-test and are presented as means ± standard deviation. A p-value <0.05 was considered indicative of statistical significance.
Results
Patients
During the study period, 140 patients were screened for eligibility. Of them, 8 patients were excluded, and thus 132 patients were assigned randomly to receive insufflation with CO2 (n = 66) or air (n = 66). Patients who did not complete endoscopic resection (n = 9) were excluded from the final analyses: four patients had non-lifting colorectal lesions, four had huge colorectal lesions (involving more than three-quarters of the circumference of the colorectal lumen), and one patient had a colorectal lesion with extensive fibrosis. Finally, data from 123 patients (CO2 group, 62; air group, 61) were analyzed. Group allocations and reasons for exclusion of patients are shown in Figure 1.
Figure 1.
Patients’ flow-chart. COPD: chronic obstructive pulmonary disease; CO2: carbon dioxide.
There were no significant differences in age, sex ratio, body mass index, medical history, or history of abdominal surgery between the two groups. The most common lesion location was the right colon in both groups (p = 0.441). The groups were similar in terms of mean tumor size (26.1 mm in the CO2 group; 28.2 mm in the air group, p = 0.217) and mean procedure time (38.1 min in the CO2 group; 34.4 min in the air group, p = 0.354). Bowel preparation quality, laterally spreading tumor (LST), type of endoscopic resection, histology, and frequency of sedation did not differ significantly between the two groups (Table 1).
Table 1.
Clinical characteristics of patients.
| CO2 group | Air group | p-value | |
|---|---|---|---|
| Patients, no. | 62 | 61 | N/A |
| Lesions, no. | 69 | 67 | N/A |
| Age, mean ± SD, years | 63.9 ± 8.9 | 61.2 ± 11.0 | 0.141 |
| Sex (male/female) | 41/21 | 35/26 | 0.318 |
| BMI, mean ± SD, kg/m2 | 23.9 ± 3.3 | 24.4 ± 3.3 | 0.382 |
| History of smoking | 17 (27.4%) | 13 (21.3%) | 0.430 |
| History of alcohol | 26 (41.9%) | 25 (41.0%) | 0.915 |
| Hypertension | 25 (40.3%) | 22 (36.1%) | 0.627 |
| Diabetes mellitus | 15 (24.2%) | 10 (16.4%) | 0.282 |
| Pulmonary disease historya | 1 (1.6%) | 2 (3.3%) | 0.619 |
| Cardiovascular disease historyb | 4 (6.5%) | 1 (1.6%) | 0.365 |
| Abdominal surgery historyc | 16 (25.8%) | 14 (23.0%) | 0.712 |
| Laparoscopic/open | 10/6 | 7/7 | 0.737 |
| Bowel preparationd | 0.759 | ||
| Excellent | 47 | 47 | |
| Good | 12 | 10 | |
| Fair | 3 | 3 | |
| Poor | 0 | 1 | |
| Location of lesion | 0.441 | ||
| Right colon | 34 | 27 | |
| Left colon | 23 | 23 | |
| Rectum | 12 | 17 | |
| Laterally spreading tumor | 40 (58.0%) | 39 (58.2%) | 0.978 |
| Tumor size, mean ± SD, mm | 26.1 ± 7.5 | 28.2 ± 11.2 | 0.217 |
| Type of endoscopic resection | 0.849 | ||
| ESD | 22 | 17 | |
| EPMR | 23 | 23 | |
| EMR-P | 2 | 2 | |
| Conventional EMR | 22 | 25 | |
| Histology | 0.252 | ||
| Adenoma | 46 | 48 | |
| Adenocarcinoma (well-differentiated/ moderately-differentiated) | 20 (12/8) | 13 (10/3) | |
| Hyperplastic polyp | 3 | 3 | |
| Otherse | 0 | 3 | |
| Procedure time, mean ± SD, minutes | 38.1 ± 21.9 | 34.4 ± 22.2 | 0.354 |
| Sedated/unsedated | 51/11 | 53/8 | 0.478 |
SD: standard deviation; BMI: body mass index; ESD: endoscopic submucosal dissection; EPMR: endoscopic piecemeal mucosal resection; EMR-P: endoscopic mucosal resection after precutting; EMR: endoscopic mucosal resection.
Of the three patients with a history of pulmonary disease, all had an old tuberculosis scar.
Of the patients with a history of cardiovascular disease, three had coronary artery obstructive disease, one had angina pectoris, and one patient had atrial fibrillation.
Abdominal surgeries included cholecystectomy, caesarean section, hysterectomy, appendectomy, radical subtotal gastrectomy, and ovarian cystectomy.
The Aronchick bowel preparation scale (ABPS) was used to evaluate bowel preparation quality.
Other histology findings included lipoma, juvenile polyp, and lymphangioma.
Post-procedure pain measurements
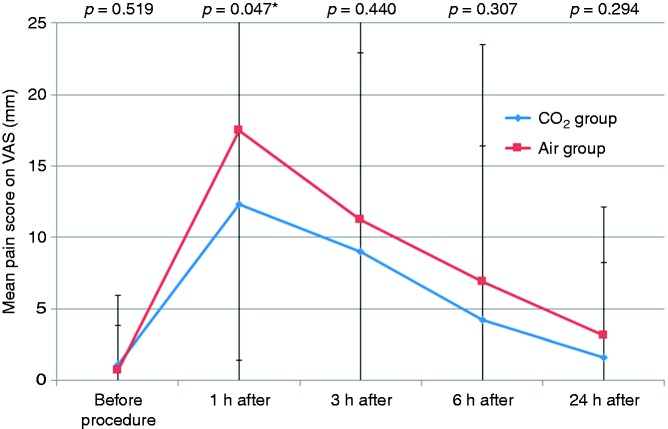
Figure 2 shows mean PP pain scores. Pain scores in the CO2 group were lower than those in the air group at all time points. The mean pain score at 1 h was significantly lower in the CO2 group than in the air group (scores at 1 h PP: 12.3 ± 13.0 vs. 17.5 ± 16.1, p = 0.047). Figure 3 shows the proportion of patients who did not experience pain (VAS score of 0). Compared with the CO2 group, the air group had a lower frequency of no pain at all time points. The difference was significant at 1 h PP (CO2 vs. air group: 41.9% vs. 19.7%, p = 0.008).
Figure 2.
Visual analog scale scores for abdominal pain of the CO2 and air groups after endoscopic resection of large colorectal polyps. Patients in the CO2 group had significantly lower pain scores 1 hour after the procedure.
Figure 3.
Proportion of patients free of pain. The proportion of patients free of pain was higher in the CO2 group than in the air group at all time points. The difference was significant 1 hour after the procedure.
Subgroup analyses were performed for treatment method and procedure time. Table 2 shows the mean abdominal pain scores within each subgroup (ESD group, EMR group, short-time group, and long-time group). We note that the effect of CO2 insufflation on abdominal pain at 1 h PP was statistically significant in the ESD group. Subgroup analysis on the long-time group also revealed pain scores that were significantly lower in the CO2 group than in the air group at 1, 6, and 24 h PP.
Table 2.
Subgroup analysis of the treatment method and procedure time.
| ESD groupa | CO2 group (n = 22) | Air group (n = 17) | p-value |
|---|---|---|---|
| Abdominal pain, mean ± SD | |||
| Before procedure | 1.8 ± 5.0 | 1.8 ± 5.3 | 0.974 |
| 1 h after | 13.6 ± 11.8 | 26.5 ± 22.9 | 0.029 |
| 3 h after | 11.8 ± 15.6 | 17.1 ± 23.4 | 0.407 |
| 6 h after | 5.9 ± 15.0 | 10.6 ± 18.5 | 0.389 |
| 24 h after | 2.7 ± 8.8 | 5.9 ± 14.2 | 0.399 |
| EMR groupb |
CO2 group (n = 40) |
Air group (n = 44) |
p-value |
| Abdominal pain, mean ± SD | |||
| Before procedure | 0.8 ± 4.7 | 0.2 ± 1.5 | 0.490 |
| 1 h after | 11.5 ± 13.7 | 14.1 ± 11.1 | 0.341 |
| 3 h after | 7.5 ± 12.8 | 8.9 ± 12.2 | 0.619 |
| 6 h after | 3.3 ± 10.5 | 5.5 ± 15.8 | 0.457 |
| 24 h after | 1.0 ± 5.0 | 2.1 ± 5.9 | 0.386 |
| Short-time group ( ≤ 30 minutes)c |
CO2 group (n = 24) |
Air group (n = 31) |
p-value |
| Abdominal pain, mean ± SD | |||
| Before procedure | 0.8 ± 2.8 | 0.3 ± 1.8 | 0.418 |
| 1 h after | 9.6 ± 12.7 | 13.2 ± 11.7 | 0.274 |
| 3 h after | 6.7 ± 13.7 | 6.1 ± 8.4 | 0.859 |
| 6 h after | 5.0 ± 13.5 | 1.3 ± 4.3 | 0.206 |
| 24 h after | 2.5 ± 8.5 | 0.3 ± 1.8 | 0.227 |
| Long-time group ( > 30 minutes)d |
CO2 group (n = 38) |
Air group (n = 30) |
p-value |
| Abdominal pain, mean ± SD | |||
| Before procedure | 1.3 ± 5.8 | 1.0 ± 4.0 | 0.800 |
| 1 h after | 14.0 ± 13.1 | 22.0 ± 18.8 | 0.041 |
| 3 h after | 10.5 ± 13.9 | 16.3 ± 20.6 | 0.192 |
| 6 h after | 3.7 ± 11.5 | 12.7 ± 22.0 | 0.049 |
| 24 h after | 1.1 ± 5.1 | 6.0 ± 12.2 | 0.044 |
ESD: endoscopic submucosal dissection; SD: standard deviation; EMR: endoscopic mucosal resection.
The median procedure time of ESD group was 50 minutes.
The median procedure time of EMR group was 26 minutes.
The median procedure time of short-time group was 18 minutes.
The median procedure time of long-time group was 46 minutes.
Secondary outcomes
Table 3 shows the secondary outcomes. There were no significant differences in the amount of sedative administered, en bloc resection rate, complete resection rate, or successful EMR rate between the two groups. The mean abdominal circumference before and after the procedure was 85.4 ± 10.5 and 85.9 ± 9.9 cm in the CO2 group and 85.3 ± 7.8 and 86.6 ± 8.3 cm in the air group, respectively. The mean increase in abdominal circumference was greater in the air group than in the CO2 group; however, there were no significant differences between the two groups (CO2 vs. air group; 0.5 ± 2.6 vs. 1.3 ± 2.5; p = 0.196). The frequency of need for analgesics PP was also not significantly different between the groups (CO2 vs. air group; 9.7% [6/62] vs. 11.5% [7/61], p = 0.746).
Table 3.
Secondary outcomes measurement in the study.
| CO2 group (n = 62) | Air group (n = 61) | p-value | |
|---|---|---|---|
| Sedative drugs, mean ± SD, mg | |||
| Midazolam | 2.6 ± 1.2 | 2.8 ± 1.0 | 0.444 |
| Propofol | 210.6 ± 148.2 | 181.9 ± 101.4 | 0.250 |
| Procedure data | |||
| En bloc resection rate | 19/22 (86.4%) | 14/17 (82.4%) | 0.731 |
| Complete resection rate | 10/22 (45.5%) | 7/17 (41.2%) | 0.789 |
| Successful EMR | 41/47 (87.2%) | 46/50 (92.0%) | 0.440 |
| Abdominal circumference, mean ± SD, cm | |||
| Before procedure | 85.4 ± 10.5 | 85.3 ± 7.8 | 0.977 |
| Post-procedure | 85.9 ± 9.9 | 86.6 ± 8.3 | 0.747 |
| Change | 0.5 ± 2.6 | 1.3 ± 2.5 | 0.196 |
| Number of patients receiving analgesic Adverse events | 5 (8.1%) | 8 (13.1%) | 0.362 |
| Perforation | 0 (0.0%) | 3 (4.9%) | 0.119 |
| Post-procedure bleeding | 0 (0.0%) | 1 (1.6%) | 0.496 |
SD: standard deviation; EMR: endoscopic mucosal resection.
Procedure-related perforation occurred in three patients (CO2 vs. air group; 0.0% [0/62] vs. 4.9% [3/61], p = 0.119) and PP bleeding occurred in one patient (CO2 vs. air group; 0.0% [0/62] vs. 1.6% [1/61], p = 0.496). All of the patients with perforation had LST; of these, two patients underwent ESD and one patient underwent EPMR. All patients had the perforation discerned at the time of endoscopic resection, and endoscopic clipping was attempted immediately. However, extensive pneumoperitoneum findings on APCT continued and perforation could not be resolved. Therefore, we performed the laparotomy with surgical suturing in all patients with perforation, and as a result, we could treat the perforation. The patient with PP bleeding was treated by endoscopic hemostasis. No deaths or other serious cardiopulmonary adverse events occurred.
Discussion
This is the first RCT to report that CO2 insufflation during endoscopic resection of LCPs leads to a significant reduction in PP abdominal pain based on a VAS. The pain level at 1 h PP was significantly lower in the CO2 group than in the air group. In addition, the frequency of no pain at 1 h PP was significantly higher in the CO2 group than in the air group. Since the introduction of CO2 insufflation during gastrointestinal endoscopy, its efficacy during colonoscopy has been demonstrated.5,6,13,16,18,27 Colonoscopy usually requires at least 20 min, so a relatively large amount of air is insufflated. In addition, the entire colon is in contact with the insufflated air, which occupies a large portion of the abdomen. Therefore, CO2 insufflation during colonoscopy has a marked effect on the abdomen. Endoscopic resection of LCPs is technically more challenging and requires more time; thus, CO2 insufflation may have a greater impact on patients undergoing such procedures. However, few studies have assessed CO2 insufflation during endoscopic resection of LCPs,8,9,19,20 and most have focused on safety rather than pain. To the best of our knowledge, only one observational study has evaluated the effects of CO2 insufflation on procedure-related pain after endoscopic resection of LCPs.9
Few studies have addressed the reduction in abdominal pain after CO2 insufflation in patients undergoing colorectal polypectomy. To date, two studies of colorectal polypectomy have been published,9,29 only one of which involved LCPs.9 Bassan et al. reported that CO2 insufflation significantly reduces the rate of admission due to PP abdominal pain; our findings are in concordance, but our data differ for several reasons.9 First, the study design was different. Bassan and colleagues employed a prospective observational cohort design, whereas we conducted a prospective double-blind RCT; the latter is the optimum design for comparing interventions.30 Therefore, our findings not only validate the results of Bassan et al. but also provide more reliable data. Second, Bassan et al. analyzed only the proportion of patients with abdominal pain sufficient to result in hospitalization, whereas we quantified changes in abdominal pain using a VAS. This method could show more clearly the effect of CO2 insufflation on abdominal pain. Finally, they evaluated only EMR, where we compared various methods, including ESD.
We also performed subgroup analyses for treatment method and procedure time. We found that the abdominal pain scores differed more in the ESD group and the long-time group compared with the whole group. In the ESD group, the effect of CO2 insufflation on abdominal pain at 1 h PP was greater than that in the whole group. Similarly, in the long-time group, pain scores were significantly lower in the CO2 group than in the air group, not only at 1 h PP but also at 6 and 24 h PP. The total volume of gas used to complete a colonoscopy may be influenced by the type of procedure or procedure duration, and this volume of insufflated gas is one of the important factors affecting abdominal pain. Therefore, the pain-reduction effect of CO2 insufflation is more remarkable when the procedure time is prolonged or when complicated procedures such as ESD are performed.
In our study, the difference in pain scores between CO2 group and air group was modest and limited to 1 h after the procedure. There are two reasons for this. First, endoscopic resection of LCPs in this study was performed only by selected expert endoscopists (no involvement of fellow or less-experienced endoscopists). Therefore, endoscopists conducted only the necessary amount of insufflations for patients, and did not cause excessive pain. Both groups had relatively low levels of abdominal pain, which may have led to the modest difference in pain levels between the two groups. Second, when the subgroup analysis was carried out, the effect of reducing CO2 insufflation in the ESD group was greater compared with the EMR group. Although this study only targeted the endoscopic resection for LCPs, the treatment methods were heterogeneous. This heterogeneity may have also affected the modest difference in pain scores in the whole group.
Residual gas in the bowel lumen is a major cause of abdominal pain after colonoscopy. To evaluate bowel gas retention, we assessed the extent of abdominal distension by measuring the change in waist circumference. The increase in waist circumference PP was lower in the CO2 group than in the air group, albeit not significantly so. Previous studies have evaluated the volume of gas insufflated in the intestinal lumen by abdominal radiography or abdominal circumference measurements.14,31,32 In two previous studies, the amount of residual bowel gas in the colon and small bowel was significantly less with CO2 insufflation.14,31 However, another study reported that there were no significant differences in waist circumference between CO2 and air insufflation, with which our findings are consistent.32 The discrepancies among studies are likely due to the use of different measurement methods. Abdominal circumference measurement is an indirect indicator of residual gas; therefore, it is less accurate than abdominal radiography. We elected to measure waist circumference because this method is simple, low cost, and does not involve exposure to radiation.
There were no significant differences between the groups in the rates of en bloc resection, complete resection, and successful EMR. The effects of type of insufflation on the success rate of LCP removal are unclear. Bassan et al. reported that there were no significant differences in the overall EMR success rate between air and CO2 insufflation.9 Moreover, Saito et al. demonstrated that there were no differences in the en bloc resection rate between air and CO2 insufflation; however, the free margin rate was significantly higher in the air group.8 The type of insufflation had little impact on the procedure success rate, likely because while polyp size, shape, and location are determinants of successful endoscopic resection, insufflation is not.12,33,34
No severe cardiopulmonary adverse events occurred. The safety of CO2 insufflation during colonoscopy has already been confirmed in many previous studies.6,18,35 In our study, all adverse events occurred in the air group; however, there were no significant differences in the rate of perforation or bleeding. These adverse events are not thought to be directly related to excessive insufflation, that is, barotrauma. In our opinion, most perforations were due to direct thermal or mechanical injury to the muscular layer by knives and snares; therefore, perforations were recognized during the endoscopic resection procedures. Nevertheless, we believe that CO2 insufflation during endoscopic resection of LCPs affords a clear advantage. Colorectal polypectomy is associated with a risk of perforation, which may cause pneumoperitoneum, severe abdominal pain, or abdominal compartment syndrome.3,6,10,33,34 Escape of insufflated gas through a perforation into the abdominal cavity can create cardiopulmonary distress associated with a degenerated tension pneumoperitoneum. CO2 is absorbed quickly, thus reducing any secondary detrimental effects of perforation.6 Therefore, we recommend that CO2 insufflation should be routine during endoscopic procedures associated with a high perforation risk such as endoscopic resection of LCPs.
Several limitations of the present study need to be considered. First, it involved only a single center. Second, sedation was not performed in some patients (15.4%). However, there were no significant differences in the proportion of sedated patients between the two groups. The results demonstrate that CO2 insufflation during endoscopic resection of LCPs significantly reduces PP abdominal pain. Moreover, CO2 insufflation is simple and did not adversely affect outcomes. Our results and other recent studies suggest that CO2 insufflation should be used routinely during endoscopic resection of LCPs.
Conflict of interest
The authors declare no conflict of interest.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI16C2319).
Ethics approval
The study was registered in the Clinical Research Information Service (KCT0001636).
Informed consent
Written, informed consent was obtained from each patient
References
- 1.Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975; 36: 2251–2270. [DOI] [PubMed] [Google Scholar]
- 3.Kedia P, Waye JD. Routine and advanced polypectomy techniques. Curr Gastroenterol Rep 2011; 13: 506–511. [DOI] [PubMed] [Google Scholar]
- 4.Dellon ES, Hawk JS, Grimm IS, et al. The use of carbon dioxide for insufflation during GI endoscopy: A systematic review. Gastrointest Endosc 2009; 69: 843–849. [DOI] [PubMed] [Google Scholar]
- 5.Wang WL, Wu ZH, Sun Q, et al. Meta-analysis: The use of carbon dioxide insufflation vs. room air insufflation for gastrointestinal endoscopy. Aliment Pharmacol Ther 2012; 35: 1145–1154. [DOI] [PubMed] [Google Scholar]
- 6.Committee AT, Lo SK, Fujii-Lau LL, et al. The use of carbon dioxide in gastrointestinal endoscopy. Gastrointest Endosc 2016; 83: 857–865. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Chung JW, Park DK, et al. Efficacy of carbon dioxide insufflation during gastric endoscopic submucosal dissection: A randomized, double-blind, controlled, prospective study. Gastrointest Endosc 2015; 82: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 8.Saito Y, Uraoka T, Matsuda T, et al. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc 2007; 65: 537–542. [DOI] [PubMed] [Google Scholar]
- 9.Bassan MS, Holt B, Moss A, et al. Carbon dioxide insufflation reduces number of postprocedure admissions after endoscopic resection of large colonic lesions: A prospective cohort study. Gastrointest Endosc 2013; 77: 90–95. [DOI] [PubMed] [Google Scholar]
- 10.Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010; 72: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 11.Overhiser AJ, Rex DK. Work and resources needed for endoscopic resection of large sessile colorectal polyps. Clin Gastroenterol Hepatol 2007; 5: 1076–1079. [DOI] [PubMed] [Google Scholar]
- 12.Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc 2012; 76: 255–263. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Chung JW, Park DK, et al. Comparison of carbon dioxide and air insufflation during consecutive EGD and colonoscopy in moderate-sedation patients: A prospective, double-blind, randomized controlled trial. Gastrointest Endosc 2017; 85: 1255–1262. [DOI] [PubMed] [Google Scholar]
- 14.Chen SW, Hui CK, Chang JJ, et al. Carbon dioxide insufflation during colonoscopy can significantly decrease post-interventional abdominal discomfort in deeply sedated patients: A prospective, randomized, double-blinded, controlled trial. J Gastroenterol Hepatol 2016; 31: 808–813. [DOI] [PubMed] [Google Scholar]
- 15.Bretthauer M, Lynge AB, Thiis-Evensen E, et al. Carbon dioxide insufflation in colonoscopy: Safe and effective in sedated patients. Endoscopy 2005; 37: 706–709. [DOI] [PubMed] [Google Scholar]
- 16.Sajid MS, Caswell J, Bhatti MI, et al. Carbon dioxide insufflation vs conventional air insufflation for colonoscopy: A systematic review and meta-analysis of published randomized controlled trials. Colorectal Dis 2015; 17: 111–123. [DOI] [PubMed] [Google Scholar]
- 17.Bourke MJ, Rex DK. Tips for better colonoscopy from two experts. Am J Gastroenterol 2012; 107: 1467–1472. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Hu B. The role of carbon dioxide insufflation in colonoscopy: A systematic review and meta-analysis. Endoscopy 2012; 44: 128–136. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi T, Fu KI, Saito Y, et al. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: A prospective study. Surg Endosc 2010; 24: 2231–2235. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida M, Imai K, Hotta K, et al. Carbon dioxide insufflation during colorectal endoscopic submucosal dissection for patients with obstructive ventilatory disturbance. Int J Colorectal Dis 2014; 29: 365–371. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: Possibility of standardization. Gastrointest Endosc 2007; 66: 100–107. [DOI] [PubMed] [Google Scholar]
- 22.Tamegai Y, Saito Y, Masaki N, et al. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 2007; 39: 418–422. [DOI] [PubMed] [Google Scholar]
- 23.Conio M, Repici A, Demarquay JF, et al. EMR of large sessile colorectal polyps. Gastrointest Endosc 2004; 60: 234–241. [DOI] [PubMed] [Google Scholar]
- 24.Khashab M, Eid E, Rusche M, et al. Incidence and predictors of “late” recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc 2009; 70: 344–349. [DOI] [PubMed] [Google Scholar]
- 25.Sakamoto T, Matsuda T, Nakajima T, et al. Efficacy of endoscopic mucosal resection with circumferential incision for patients with large colorectal tumors. Clin Gastroenterol Hepatol 2012; 10: 22–26. [DOI] [PubMed] [Google Scholar]
- 26.American Society of Anesthesiologists Task Force on S, Analgesia by N-A. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002; 96: 1004–1017. [DOI] [PubMed] [Google Scholar]
- 27.Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, et al. NORCCAP (Norwegian colorectal cancer prevention): A randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut 2002; 50: 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geyer M, Guller U, Beglinger C. Carbon dioxide insufflation in routine colonoscopy is safe and more comfortable: Results of a randomized controlled double-blinded trial. Diagn Ther Endosc 2011; 2011: 378906–378906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami K, Kataoka H, Hayano J, et al. Autonomic nervous responses in colorectal polypectomy: Randomized controlled trial comparing air and carbon dioxide insufflation. Dig Endosc 2016; 28: 203–209. [DOI] [PubMed] [Google Scholar]
- 30.Gluud C, Gluud LL. Evidence based diagnostics. BMJ 2005; 330: 724–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumanac K, Zealley I, Fox BM, et al. Minimizing postcolonoscopy abdominal pain by using CO(2) insufflation: A prospective, randomized, double blind, controlled trial evaluating a new commercially available CO(2) delivery system. Gastrointest Endosc 2002; 56: 190–194. [DOI] [PubMed] [Google Scholar]
- 32.Homan M, Mahkovic D, Orel R, et al. Randomized, double-blind trial of CO2 versus air insufflation in children undergoing colonoscopy. Gastrointest Endosc 2016; 83: 993–997. [DOI] [PubMed] [Google Scholar]
- 33.Monkemuller K, Neumann H, Fry LC, et al. Polypectomy techniques for difficult colon polyps. Dig Dis 2008; 26: 342–346. [DOI] [PubMed] [Google Scholar]
- 34.Monkemuller K, Neumann H, Malfertheiner P, et al. Advanced colon polypectomy. Clin Gastroenterol Hepatol 2009; 7: 641–652. [DOI] [PubMed] [Google Scholar]
- 35.Memon MA, Memon B, Yunus RM, et al. Carbon dioxide versus air insufflation for elective colonoscopy: A meta-analysis and systematic review of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 2016; 26: 102–116. [DOI] [PubMed] [Google Scholar]