Abstract
Background
The 360 Express balloon catheter (360 Express) has the ability to self-adjust to the esophageal lumen, ensuring optimal tissue contact.
Objective
The objective of this article is to evaluate the efficacy and safety of the 360 Express for radiofrequency ablation (RFA) treatment of Barrett's esophagus (BE).
Methods
BE patients with low-grade dysplasia (LGD), high-grade dysplasia (HGD) or early cancer (EC) were included. Visible lesions were removed by endoscopic resection (ER) prior to RFA. RFA was performed with the 360 Express using the standard ablation regimen (12J/cm2–clean–12J/cm2). Primary outcome: BE regression percentage at three months. Secondary outcomes: procedure time, adverse events, complete eradication of dysplasia (CE-D) and intestinal metaplasia (CE-IM).
Results
Thirty patients (median BE C4M6) were included. Eight patients underwent ER prior to RFA. Median BE regression: 90%. Median procedure time: 31 minutes. Adverse events (13%): laceration (n = 1); atrial fibrillation (n = 1); vomiting and dysphagia (n = 1); dysregulated diabetes (n = 1). After subsequent treatment CE-D and CE-IM was achieved in 97% and 87%, respectively. In 10% a stenosis developed during additional treatment requiring a median of one dilation.
Conclusion
This study shows that circumferential RFA using the 360 Express may shorten procedure time, while maintaining efficacy compared to standard circumferential RFA.
Keywords: Barrett's esophagus, endoscopic treatment, radiofrequency ablation, low-grade dysplasia, high-grade dysplasia, early cancer
Key summary
What is known on this subject?
Radiofrequency ablation (RFA) has proven to be highly effective and safe in the treatment of dysplastic Barrett's esophagus (BE).
Circumferential RFA (c-RFA) with the Barrx 360 system using the standard ablation regimen results in a median procedure duration of 39 minutes and in median 83% BE regression after a single procedure.
What are the significant and/or new findings of this study?
C-RFA with the 360 Express using the standard ablation regimen results in shorter procedures (median 31 minutes), while maintaining efficacy when compared to c-RFA using the Barrx 360 system (90%).
Introduction
Currently advised therapy for patients with Barrett's esophagus (BE) and confirmed low-grade dysplasia (LGD), high-grade dysplasia (HGD) or mucosal cancer consists of endoscopic resection (ER) for visible abnormalities, followed by radiofrequency ablation (RFA) to eradicate all Barrett's mucosa. This approach has shown to be highly effective and safe. Complete eradication of dysplasia is achieved in 96% to 100% and complete eradication of intestinal metaplasia in 93% to 96% of patients.1–3 Most patients undergo primary circumferential RFA (c-RFA) with a balloon-based electrode, followed by additional focal RFA sessions using a cap-based electrode. Originally, a circumferential ablation procedure started with the introduction of a sizing balloon to measure the esophageal inner diameter (EID) at multiple levels. Subsequently, the sizing balloon was removed and an ablation balloon with the appropriate diameter was selected and introduced. The ablation balloon was available in five sizes ranging from 18 mm to 31 mm, and a diameter smaller than the smallest measured diameter was selected to minimize risk of laceration and perforation. A disadvantage of this approach was the need for multiple introductions with the sizing catheter, ablation catheter and endoscope. Furthermore, one ablation catheter with a fixed diameter was used to ablate the entire Barrett's segment, although the EID may differ within the same patient. By incorporating the sizing balloon and the ablation balloon into a single device, the use of a separate sizing balloon is eliminated, and a compliant balloon will adjust to different EIDs resulting in optimal mucosal contact with the electrode.
The new Barrx™ 360 Express RFA balloon catheter (360 Express) is a circumferential balloon catheter that contains a 4 cm long bipolar electrode that is wrapped around a balloon and features the ability to self-adjust to the esophageal lumen.
We hypothesized that by incorporating the sizing and ablation balloon into a single device, procedure time will be shortened without affecting the safety of the procedure. The efficacy of the procedure may be increased by the improved adjustment of the ablation balloon catheter to the esophageal mucosa. The aim of this pilot study was to evaluate the efficacy and safety of the 360 Express RFA balloon catheter.
Materials and methods
Study design
This was an uncontrolled, prospective, multicenter pilot study conducted at four collaborating Dutch tertiary referral centers: Academic Medical Center Amsterdam, Catharina Hospital Eindhoven, Erasmus Medical Center Rotterdam, and St. Antonius Hospital Nieuwegein. Study data were collected on standardized case record forms and entered into a central database.
Patient selection
Patients were eligible if they met all of the following inclusion criteria:
(a) age 18 to 85 years;
(b) BE segment between 2 cm and 10 cm at baseline, prior to any ER;
(c) biopsy-proven LGD, HGD or cancer confirmed by an expert pathologist;
(d) in case of prior ER for early cancer or visible lesions, the resection had to be limited to <2 cm in length and <50% of the circumference; and
(e) written informed consent.
Patients were excluded if they had any of the following exclusion criteria:
(a) prior endoscopic ablation treatment;
(b) significant esophageal stenosis preventing passage of a diagnostic endoscope or any prior endoscopic dilation for esophageal stenosis;
(c) in case of prior ER: positive vertical resection margins, deep submucosal invasion (>T1sm1), poorly or undifferentiated cancer, or lymphatic/vascular invasion;
(d) in case of prior ER: no invasive cancer in any of the biopsies obtained from residual BE;
(e) an interval of > 6 months between the last high-resolution endoscopy with biopsies and the RFA treatment, or an interval <6 weeks between ER and RFA; and
(f) contraindications for RFA treatment.
Work-up endoscopy prior to RFA
Patients underwent a high-resolution endoscopy within six months prior to c-RFA treatment. Biopsies were obtained according to the Seattle protocol (4Q/2 cm).
In case of visible lesions, ER was performed using the ER cap technique (Olympus GmbH; Hamburg, Germany) or multiband mucosectomy (Duette MBM system, Cook Endoscopy, Limerick, Ireland). Immediately after the ER or during a separate endoscopy, biopsies were obtained from the residual Barrett's segment (4Q/2 cm). In case no cancer was found in the residual BE, the c-RFA procedure was scheduled at least six weeks after ER.
RFA treatment
Study device
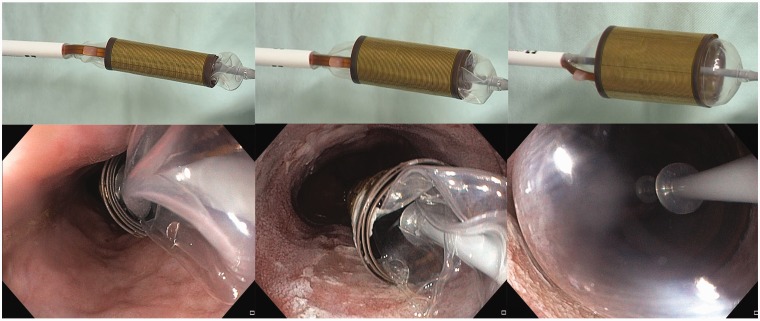
Circumferential ablation was performed using the Barrx™ 360 Express RFA balloon catheter (Medtronic, Sunnyvale, CA, USA) (Figure 1). Details on the 360 Express system are provided in Appendix A.
Figure 1.
Wrap/unwrap electrode design of the 360 Express RFA balloon catheter.
RFA procedure
Procedures were performed under conscious sedation with midazolam and/or fentanyl or under monitored deep sedation with propofol. Prior to ablation, the esophagus was inspected using white-light high-resolution endoscopy (WLE) and narrow-band imaging (NBI) to exclude presence of (residual) visible abnormalities. The extent of columnar-lined esophagus was documented according to the Prague C&M classification4 and still images with WLE and NBI were taken at 1 cm intervals. Then, a guidewire was introduced and the endoscope removed. The 360 Express was introduced, followed by the endoscope. Under endoscopic visualization, the balloon was positioned at the proximal end of the Barrett's segment. Under visual control the BE was ablated (12 J/cm2) working from proximally to distally using visual repositioning. A small overlap (i.e. <1 cm) between ablation zones was allowed. After the first ablation pass, the endoscope was removed followed by removal of the ablation catheter. The coagulum was cleaned off the balloon catheter. The endoscope was reintroduced to clean the ablation zone, by irrigation and scraping off coagulum using the rim of a distal attachment cap placed on the tip of the endoscope. Then, the guidewire was reintroduced, the endoscope removed and the ablation catheter and endoscope were reintroduced to repeat ablation (12J/cm2).
Post-ablation care
All study patients received esomeprazole 40 mg twice a day (or an equivalent dose of proton pump inhibitor) as a maintenance dosage during the study. In addition patients were prescribed ranitidine 300 mg at bedtime and 5 ml sucralfate suspension for a period of two weeks following RFA.
Follow-up after treatment
Three months after the c-RFA procedure, the first post-treatment endoscopy was performed. Still images with WLE and NBI were obtained from every 1 cm of the original BE segment, to assess the percentage of endoscopically visible regression of BE and presence of esophageal stenosis. Biopsies were not routinely obtained during this session. Further treatment was performed according to standard guidelines. In general, additional focal RFA sessions using the standard ablation regimen (2 × 15 J/cm2 – clean – 2 × 15 J/cm2) or a simplified ablation regimen (3 × 15 J/cm2 – no clean or 3 × 12 J/cm2 – no clean) were scheduled approximately every three months until complete endoscopic eradication of all BE was achieved. After complete endoscopic and histological eradication of intestinal metaplasia (CE-IM) and dysplasia (CE-D), patients were scheduled for further follow-up.
Histological evaluation
All biopsies and ER specimens were routinely processed and evaluated by a local gastrointestinal expert pathologist. All biopsies were assessed for IM and grade of dysplasia according to the Vienna classification.5 ER specimens were evaluated for dysplasia, tumor infiltration depth, differentiation, presence of lymphatic or vascular invasion, and radicality.
Outcome parameters
Primary outcome
Percentage of endoscopically visual surface regression of BE epithelium at three months.
The percentage of BE regression was independently scored by two endoscopists (E.J.S. and B.L.A.M.W.) who reviewed the endoscopic images that were taken from every cm of the original BE segment immediately prior to the initial c-RFA procedure and during the first post-treatment endoscopy at three months.
Definitions of all outcome parameters are summarized in Table 1.
Table 1.
Definitions of outcome parameters.
| Outcome parameter | Definition |
|---|---|
| Percentage of endoscopically visual regression of BE epithelium at three months. | The mean percentage of BE surface regression of the two endoscopists who reviewed the endoscopic images. In case the BE regression percentage differed ≥30% between both endoscopists, a new score was established during a consensus meeting. |
| Duration of the 360 Express procedure. | 1. Total procedure time: time from the first introduction until the time of removal of the endoscope. 2. Ablation time: the period between the introduction and the removal of the 360 Express. |
| Adverse events. | Timing: - acute: during the procedure - early: ≤48 hours - late: >48 hours Severity: - mild: unscheduled hospital admission, hospitalization <3 days, hemoglobin drop < 3 g/dl, no need for transfusion. - moderate: hospitalization 4–10 days, ≤ 4 units blood transfusion, need for repeat endoscopic intervention, radiological intervention. - severe: hospitalization > 10 days, intensive care unit admission, need for surgery, >4 units blood transfusion; or in the case of stenosis >5 dilations, stent placement, or incision therapy. - fatal: death attributable to procedure <30 days or longer with continuous hospitalization.6 |
| Complete endoscopic and histological eradication of IM at the end of the treatment phase. | Absence of visible Barrett's epithelium at the first follow-up endoscopy and histologically confirmed absence of IM when biopsies were obtained during this endoscopy. |
| Complete endoscopic and histological eradication of dysplasia at the end of the treatment phase. | Absence of visible Barrett's epithelium at the first follow-up endoscopy and histologically confirmed absence of dysplasia when biopsies were obtained during this endoscopy. |
| Number of patients with a stenosis requiring an intervention at any time during the treatment phase. | Patients who developed a stenosis requiring a therapeutic intervention (dilation, incision therapy, stent placement) at any point in the period from the 360 Express procedure until the first follow-up endoscopy. |
BE: Barrett's esophagus; IM: intestinal metaplasia.
Secondary outcomes
Duration of the 360 Express ablation procedure.
Adverse events related to the 360 Express ablation procedure.
Rate of CE-D and CE-IM at the end of the treatment phase.
Number of patients with a stenosis requiring an intervention at any time during the treatment phase.
Statistical analysis and ethical considerations
Because this was a pilot study no sample size calculation was performed.
Data analysis was performed using SPSS statistical software (version 24, SPSS Inc, Chicago, IL, USA). The mean ( ± standard deviation) was used to describe variables with a normal distribution and the median (and interquartile range (IQR)) for variables with a skewed distribution. The study was approved by the institutional review board (IRB) of the Academic Medical Center Amsterdam (date of approval: September 24, 2014). The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the IRB. Written informed consent was obtained from all participants. This study was registered in the ISRCTN registry (www.isrctn.com, number: ISRCTN38642364).
Results
Patients
Between October 2014 and December 2014, 30 consecutive patients were included. Baseline characteristics are shown in Table 2.
Table 2.
Baseline characteristics.
| Patients (n) | 30 |
| Male:female | 24:6 |
| Median age, years (IQR) | 66 (62–73) |
| Median BE, cm (IQR) | C 4 (2–6) M 6 (4–8) |
| Worst overall histological diagnosis prior to treatment (biopsies or ER specimens), n | |
| LGD | 14 |
| HGD | 11 |
| EC | 5 |
| ER prior to RFA, n (%) | 8 (27) |
| Worst histology of ER specimens, n | |
| LGD | 1 |
| HGD | 2 |
| EC | 5 |
| Worst histological diagnosis prior to RFA (after any ER), n | |
| No dysplasia | 2 |
| LGD | 22 |
| HGD | 6 |
| EC | 0 |
BE: Barrett's esophagus; cm: centimeter; EC: early cancer; ER: endoscopic resection; HGD: high-grade dysplasia; IQR: interquartile range; LGD: low-grade dysplasia; min: minutes; n: number of patients; RFA: radiofrequency ablation.
Primary outcome
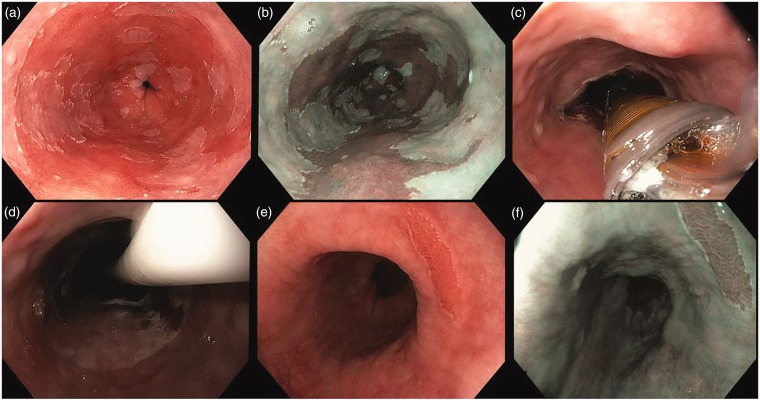
The median BE surface regression at three months was 90% (IQR 77–95) (Figure 2).
Figure 2.
Endoscopic images of a circumferential ablation procedure using the 360 Express RFA balloon catheter. (a) and (b) White-light high-resolution endoscopy (WLE) and narrow-band imaging (NBI) of a C6M10 Barrett's esophagus with low-grade dysplasia (LGD). (c) Deflated 360 Express balloon catheter in the esophageal lumen. (c) and (d) Ablation effect immediately after the ablation and after removal of the catheter. (e) and (f) Effect three months later during the first post-treatment endoscopy with WLE and NBI: 97% visual surface Barrett's epithelium regression.
In one patient a consensus meeting was required because the BE regression differed 30% between the two endoscopists (60% versus 90%). The consensus BE regression was 80%.
Secondary outcomes
1. Duration of the 360 Express procedure
The median total procedure time was 31 minutes (IQR 28–38). The median ablation time was 20 minutes (IQR 17–25).
2. Adverse events
No severe adverse events related to the use of the 360 Express device were observed. In four patients (13%) a mild or moderate adverse event occurred: mucosal laceration (n = 1); asymptomatic atrial fibrillation (n = 1); vomiting and dysphagia (n = 1); dysregulated diabetes (n = 1). Details of these adverse events are described in Appendix B.
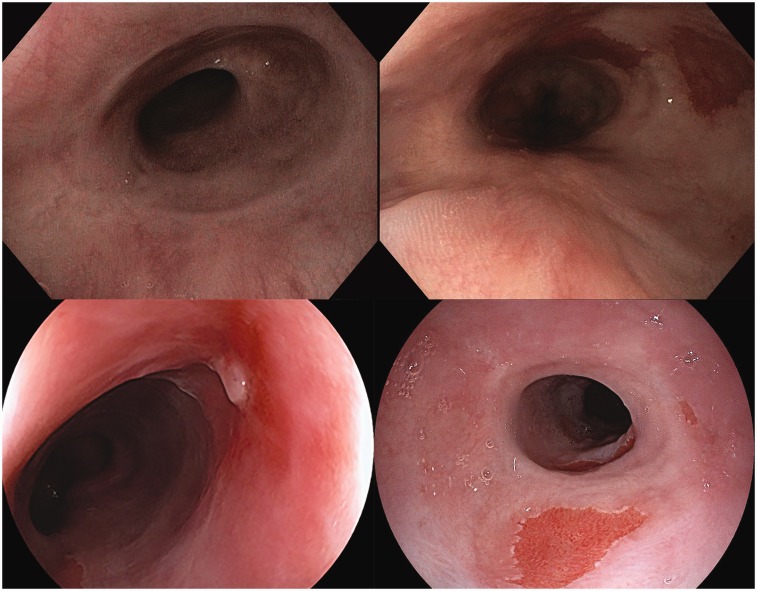
Esophageal scarring
None of the patients developed a symptomatic stenosis after initial RFA treatment with the 360 Express. Although not prespecified for this study, the endoscopists noted pronounced esophageal scarring in 7/30 patients (23%) at the three-month follow-up endoscopy (Figure 3). In 3/7 patients ER was performed prior to RFA treatment. There were no problems passing a diagnostic gastroscope in any of these patients.
Figure 3.
Endoscopic images of esophageal scarring seen during the three-month endoscopy in four cases.
3. Rate of CE-D and CE-IM at the end of the treatment phase
By December 2016, 28/30 patients completed treatment. In two patients further endoscopic treatment was discontinued after initial ablation with the 360 Express because of comorbidity. In the first patient, biopsies at the last endoscopy showed IM without dysplasia, and in the other patient no biopsies were obtained after 360 Express treatment.
According to an intention-to-treat analysis, CE-D was reached in 29/30 patients (97%) and CE-IM was reached in 26/30 patients (87%). In a per-protocol analysis, CE-D was reached in 28/28 patients (100%) and CE-IM in 26/28 patients (93%). One patient underwent escape ER of residual islands (maximum 10 ×10 mm) of non-dysplastic Barrett's mucosa; the other patient had IM just distal to a normal appearing neo-squamocolumnar junction.
The median total number of treatment sessions was three (IQR 2–4) (Table 3).
Table 3.
Outcomes.
| Outcomes of 360 Express procedure | |
| Patients (n) | 30 |
| Median percentage of Barrett's surface regression at three monthsa, % (IQR) | 90 (77–95) |
| Median total procedure time, min (IQR) | 31 (28–38) |
| Median ablation time, min (IQR) | 20 (17–25) |
| Adverse events of self-sizing session, n (%) | 4 (13) |
| Mild adverse events | 3 |
| - acute laceration | |
| - early atrial fibrillation | |
| - late dysregulation of diabetes mellitus | |
| Moderate complication | 1 |
| - early dysphagia, no endoscopic esophageal abnormalities | |
| Outcomes of entire treatment phase | |
| Patients (n) | 30 |
| Stenosis at any point during treatment phase requiring intervention, n (%) | 3 (10) |
| Complete eradication of dysplasia at end of the treatment phase, n (%) | |
| - Intention to treat analysis | 29/30 (97) |
| - Per protocol analysis | 28/28 (100) |
| Complete eradication of intestinal metaplasia at end of the treatment phase | |
| - Intention to treat analysis | 26/30 (87) |
| - Per protocol analysis | 26/28 (93) |
| Median total number of treatment sessionsb per patient, (IQR) | 3 (IQR 2–4) |
| Number of circumferential RFA sessions per patient, n | 1 |
| Median number of focal RFA sessions per patient, (IQR) | 1 (1–2) |
| Number of patients who underwent APCc, n | 13 |
| - One session | 8 |
| - Two sessions | 5 |
| Number of patients who underwent EMR, n | |
| - Escape treatment for residual IM after RFA (n = 1) | 2 |
| - Diagnosis of elevated BE islands (n = 1)d | |
Mean of two independent scores by expert endoscopists.
Treatment sessions included circumferential RFA, focal RFA, APC and EMR.
APC was used to treat small areas of Barrett's mucosa (islands/tongues < 10 mm).
Histology showed non-dysplastic IM.
APC: argon plasma coagulation; BE: Barrett's esophagus; EMR: endoscopic mucosal resection; IM: intestinal metaplasia; IQR: interquartile range; min: minutes; n: number of patients; RFA: radiofrequency ablation.
4. Number of patients with a stenosis requiring an intervention at any time during the treatment phase
Three patients (10%) developed a stenosis after additional focal RFA, for which they underwent endoscopic dilation (one to two sessions). None of the patients had a prior ER.
Discussion
This pilot study shows that c-RFA with the 360 Express is feasible and effective resulting in a median BE surface regression of 90% three months after treatment when using the standard ablation regimen (12 J/cm2 – clean – 12 J/cm2).
A prior randomized clinical trial comparing different regimens for c-RFA using the standard system showed that c-RFA with the standard ablation regimen results in a median BE surface regression of 83% (IQR 70–93) at three-month follow-up. The median total procedure time was 39 minutes.7 The median total procedure time in our study was 31 minutes indicating that c-RFA with the 360 Express likely results in a shorter procedure time compared with c-RFA using the standard system.
In our study we primarily assessed the efficacy of the 360 Express after a single RFA procedure by assessing the visual surface regression of BE three months after treatment. One may argue that obtaining biopsies during the three-month follow-up endoscopy would have led to more objectivity regarding the BE regression results. However, in clinical practice biopsies are not routinely obtained after a single RFA session and the decision to perform additional endoscopic treatment is usually guided by the presence of endoscopically visible Barrett's tissue. In our opinion, histological confirmation of absence or presence of IM does not add enough valuable information to the assessment of high-quality images to justify exposing patients to an extra endoscopic procedure. In addition, in prior studies evaluating circumferential RFA the BE regression was assessed using endoscopic images, which allow for comparison.7 Since efficacy of RFA is generally defined as histologically confirmed complete eradication of IM or dysplasia evaluated after multiple RFA sessions, we additionally evaluated CE-D and CE-IM rates at the end of the treatment phase. In this study, on an intention-to-treat basis 97% of patients achieved CE-D and 87% achieved CE-IM (per protocol: 100% and 93%, respectively). Our findings are in line with rates reported in the literature showing CE-IM rates ranging from 77% to 100% and CE-D ranging from 86% to 100% of patients.1–3,8–11
In seven patients (23%) pronounced esophageal scarring was noted during the three-month endoscopy. However, esophageal scarring was not a prespecified outcome and it is therefore difficult to quantify. Since none of the seven patients were symptomatic and required an intervention, the scarring did not qualify as stenosis. However, it was striking that all four experienced endoscopists who participated in this study noticed and recorded the esophageal scarring after a single session with the 360 Express in at least one of their patients. Upon inquiry the endoscopists mentioned they felt there was more fibrosis than what they would expect based on their clinical experience with the standard c-RFA system. Theoretically, the 360 Express may have a higher stenosis risk: The system results in optimal contact between the esophageal wall and the electrode because of the ability to self-adjust to the EID. Compared with c-RFA using the standard system in which the ablation catheter is chosen based on the smallest measured EID, the entire esophagus will receive more energy when the 360 Express is used. The use of 12 J/cm2 might therefore result in deeper mucosal damage than when using the traditional ablation catheter. During subsequent treatment 10% of patients developed a stenosis requiring median one dilation. Our findings are toward the high end of the spectrum of stenosis rates after RFA reported in the literature (0–14%).12 For follow-up studies we therefore advise an energy density setting of 10 J/cm2, as is now recommended in the instructions for use of the 360 Express, since we believe this may deliver a better balance of clinical efficacy and safety.
There are some limitations of this study that need to be addressed. First, this is a pilot study with a relatively small number of patients. Second, the study was conducted in four tertiary referral centers with extensive experience in the treatment of BE. The results may therefore not be automatically generalizable to standard clinical practice.
For the standard c-RFA system, a simplified no-cleaning ablation protocol was proven to be easier and faster, but equally safe and effective as the standard ablation protocol.7 To evaluate if a simplified regimen without a cleaning step is also safe when using the 360 Express, or if the stenosis risk is higher because of deeper ablation damage in addition to heat stacking, we initiated a randomized clinical trial (NTR5191, www.trialregister.nl). This trial compares the standard regimen (10 J/cm2–clean–10 J/cm2), a simplified double regimen (2 × 10 J/cm2 no clean) and a simplified single regimen (1 × 10 J/cm2). Although this study is still ongoing, an interim analysis showed an unacceptably high risk of severe stenosis (17%) in patients treated with the 360 Express using the simplified double regimen, which led to early closure of this arm after consultation with the data and safety monitoring board of the study.13 The standard regimen (10 J/cm2–clean–10 J/cm2) for the 360 Express, as advised in the instructions for use of the device, is therefore still the advised treatment regimen.
In conclusion, the results of this pilot study show that c-RFA with the 360 Express using an ablation regimen of 12 J/cm2–clean–12 J/cm2 leads to a decreased procedure time, but maintains efficacy and safety when compared to c-RFA using the standard system. Potentially, better contact of the electrode may result in more ablation damage to the esophageal wall, resulting in more scarring, and an ablation regimen with a lowered energy setting (10 J/cm2–clean–10 J/cm2) is therefore currently advised for RFA using the 360 Express.
Appendix A
Details on the Barrx™ 360 Express RFA balloon catheter
The 360 Express consists of a 4 cm long bipolar electrode array that is wrapped around a balloon. The Barrx Flex energy generator delivers radiofrequency energy in a bipolar mode to the balloon device. After placement over a guide wire, the device is inflated under endoscopic control, to a set pressure via the Barrx Flex energy generator. As the balloon inflates, the electrode unwraps until the electrode contacts the esophageal wall (figure 1). Upon activation with a foot paddle, energy is delivered using the identical energy algorithms (12 J/cm2, 40 W/cm2) as with the standard RFA Balloon Catheter, leading to controlled depth ablation of the Barrett's epithelium. After the ablation is complete, the generator automatically deflates the device causing the electrode to rewrap to its pre-expanded diameter. The Barrx Flex energy generator and the 360 Express have a CE mark for use in Europe.
Appendix B
Details on the four adverse events that occurred after a single 360 Express procedure.
Acute adverse event
A 2 cm long mucosal laceration just above the top of the gastric folds occurred during the first ablation pass in one patient. The zone proximal to the laceration was ablated for the second time according to protocol; the area with the laceration was not treated a second time. The laceration healed uneventfully and during the three-month follow-up no scarring was observed at the site of the laceration. The estimated regression percentage was 90%.
Early adverse events
A 65-year-old male developed asymptomatic atrial fibrillation after the RFA procedure, for which he was referred to a cardiologist. It was unclear whether this was a preexisting condition.
A 59-year-old female experienced dysphagia and vomiting one day after the procedure. She presented to the emergency room five days after RFA treatment and was hospitalized. Gastroscopy showed an ulcerated esophagus as expected after recent RFA treatment, without any signs of stenosis. A temporary duodenal feeding tube was placed; the patient recovered and was discharged home eight days later.
Late adverse event
One patient with known diabetes was hospitalized with dysregulation of glucose levels four days after the RFA procedure. She recovered after she was put on a new insulin regimen and was discharged after three days.
Declaration of conflicting interests
The conflict of interest statements of the authors are as follows (see also disclosure forms that were provided with the first submission): K. Belghazi: None Declared.R.E Pouw: None Declared. C.M.T. Sondermeijer: None Declared. S.L. Meijer: None Declared. E.J. Schoon: None Declared. A.D. Koch: Lecturing fees: Olympus Endoscopy; Cook Medical; Boston scientific; Erbe Medical. B.L.A.M. Weusten: Financial support for research: Covidien/Medtronic; C2Therapeutic. J.J.G.H.M. Bergman Financial support for research: Covidien/Medtronic; Olympus Endoscopy; Cook Medical; Boston scientific; Erbe Medical; C2Therapeutic; Ninepoint Medical, Consultancy: Boston Scientific; Cook Medical; Covidien/Medtronic.
Funding
This work was supported by Medtronic, Sunnyvale, CA, USA.
Ethics approval
This study was approved by the institutional review board (IRB) of the Academic Medical Center Amsterdam (date of approval: September 24, 2014). This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the IRB.
Informed consent
Written informed consent was obtained from all study participants.
References
- 1.Pouw RE, Wirths K, Eisendrath P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for Barrett's esophagus with early neoplasia. Clin Gastroenterol Hepatol 2010; 8: 23–29. [DOI] [PubMed] [Google Scholar]
- 2.van Vilsteren FG, Pouw RE, Seewald S, et al. Stepwise radical endoscopic resection versus radiofrequency ablation for Barrett's oesophagus with high-grade dysplasia or early cancer: A multicentre trial. Gut 2011; 60: 765–773. [DOI] [PubMed] [Google Scholar]
- 3.Phoa KN, Pouw RE, Bisschops R, et al. Multimodality endoscopic eradication for neoplastic Barrett oesophagus: Results of an European multicentre study (EURO-II). Gut 2016; 65: 555–562. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Dent J, Armstrong D, et al. The development and validation of an endoscopic grading system for Barrett's esophagus: The Prague C & M criteria. Gastroenterology 2006; 131: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 5.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pouw RE, Seewald S, Gondrie JJ, et al. Stepwise radical endoscopic resection for eradication of Barrett's oesophagus with early neoplasia in a cohort of 169 patients. Gut 2010; 59: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 7.van Vilsteren FG, Phoa KN, Alvarez Herrero L, et al. Circumferential balloon-based radiofrequency ablation of Barrett's esophagus with dysplasia can be simplified, yet efficacy maintained, by omitting the cleaning phase. Clin Gastroenterol Hepatol 2013; 11: 491–498.e1. [DOI] [PubMed] [Google Scholar]
- 8.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 2009; 360: 2277–2288. [DOI] [PubMed] [Google Scholar]
- 9.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: A randomized clinical trial. JAMA 2014; 311: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 10.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Stepwise circumferential and focal ablation of Barrett's esophagus with high-grade dysplasia: Results of the first prospective series of 11 patients. Endoscopy 2008; 40: 359–369. [DOI] [PubMed] [Google Scholar]
- 11.Gondrie JJ, Pouw RE, Sondermeijer CM, et al. Effective treatment of early Barrett's neoplasia with stepwise circumferential and focal ablation using the HALO system. Endoscopy 2008; 40: 370–379. [DOI] [PubMed] [Google Scholar]
- 12.Chadwick G, Groene O, Markar SR, et al. Systematic review comparing radiofrequency ablation and complete endoscopic resection in treating dysplastic Barrett's esophagus: A critical assessment of histological outcomes and adverse events. Gastrointest Endosc 2014; 79: 718–731. [DOI] [PubMed] [Google Scholar]
- 13.Pouw RE, Bergman JJ. Safety signal for the simple double ablation regimen when using the Barrx 360 Express radiofrequency ablation balloon catheter. Gastroenterology 2017; 15: 614. [DOI] [PubMed] [Google Scholar]