Abstract
The molecular mechanisms responsible for the development of proteinuria and glomerulosclerosis in radiation nephropathy remain largely unknown. Podocytes are increasingly recognized as key players in the pathogenesis of proteinuria in primary and secondary glomerular disorders. The lipid-modulating enzyme sphingomyelin phosphodiesterase acid-like 3B (SMPDL3b) is a key determinant of podocyte injury and a known off target of the anti-CD20 antibody rituximab (RTX). The current study investigates the role of sphingolipids in radiation-induced podocytopathy. After a single dose of radiation (8 Gy), several ceramide species were significantly elevated. In particular, C16:00, C24:00, and C24:1 ceramides were the most abundant ceramide species detected. These changes were paralleled by a time-dependent drop in SMPDL3b protein, sphingosine, and sphingosine-1-phosphate levels. Interestingly, SMPDL3b-overexpressing podocytes had higher basal levels of sphingosine-1-phosphate and maintained basal ceramide levels after irradiation. Morphologically, irradiated podocytes demonstrated loss of filopodia and remodeling of cortical actin. Furthermore, the actin binding protein ezrin relocated from the plasma membrane to the cytosol as early as 2 h after radiation. In contrast, SMPDL3b overexpressing podocytes were protected from radiation-induced cytoskeletal remodeling. Treatment with RTX before radiation exposure partially protected podocytes from SMPDL3b loss, cytoskeletal remodeling, and caspase 3 cleavage. Our results demonstrate that radiation injury induces early cytoskeletal remodeling, down-regulation of SMPDL3b, and elevation of cellular ceramide levels. Overexpression of SMPDL3b and pretreatment with RTX confer a radioprotective effect in cultured podocytes. These findings indicate a potential role for SMPDL3b and RTX in radiation-induced podocytopathy.—Ahmad, A., Mitrofanova, A., Bielawski, J., Yang, Y., Marples, B., Fornoni, A., Zeidan, Y. H. Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes.
Keywords: lipids, cancer, ceramide, radioprotection
Radiation nephropathy is a consequence of excessive radiation doses delivered to the kidneys during management of abdominal or paraspinal tumors or total body irradiation (1, 2). Radiation exposure induces changes in various components of the kidney, including glomeruli, blood vessels, tubular epithelium, and interstitium (3). Excessive bilateral kidney irradiation eventually leads to radiation nephropathy, which is a syndrome of chronic renal failure (4). The size of the kidneys is reduced by 5% within a year of exposure if irradiated with a dose higher than 10 Gy (5, 6). The degree of damage to the kidney is associated with the volume and dose of radiation (7). Clinically, radiation nephropathy manifests in an acute phase with proteinuria followed by reduced glomerular filtration rate and progression to end-stage renal disease requiring dialysis or kidney transplantation.
The molecular mechanisms responsible for the development of proteinuria and glomerulosclerosis in radiation nephropathy remain largely unknown. Prior work identified the glomerulus as a key target for radiation-induced renal damage (8). Among glomerular cells, podocytes are highly specialized terminally differentiated cells and are heavily dependent on the integrity of the actin cytoskeleton to grant proper function (9). Podocytes are increasingly recognized as key players in the pathogenesis of proteinuria in primary or secondary glomerular disorders such as focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy. Interestingly, radiation nephropathy represents a secondary cause of FSGS (10). However, whether and how radiation affects podocyte function remain unknown.
Recent studies highlighted the importance of sphingolipids in podocyte function (11–13). In particular, podocyte-specific expression of a sphingomyelinase-like protein (SMPDL3b) was shown to be suppressed in FSGS and to facilitate podocyte actin remodeling and cell migration (14). SMPDL3b is localized in the lipid rafts of podocytes, where it may play a role in the conversion of sphingomyelin to ceramide, although the intrinsic enzymatic activity of SMPDL3b remains to be established (14). Several lines of evidence support a critical role for SMPDL3b in maintaining normal podocyte function. Using biopsies from patients with recurrent FSGS, Fornoni et al. (14) discovered that these patients have podocyte-specific deficiency in SMPDL3b and altered sphingomyelinase activity. This deficiency results in an altered pattern of podocyte actin stress fibers, which in turn can be reversed by using the anti-CD20 antibody rituximab (RTX). Interestingly, RTX has been previously shown to bind SMPDL3b through a phage library screen (15). Moreover, RTX immunoprecipitates SMPDL3b in pig glomeruli and prevents early development of proteinuria in baboons after kidney transplantation (16). These studies indicate that alteration of SMPDL3b levels influences podocyte function in various renal glomerular diseases. The goal of the current project is to identify the role of SMPDL3b in radiation-induced podocytopathy.
MATERIALS AND METHODS
Materials
RPMI 1640 medium, PBS, penicillin-streptomycin, bovine serum albumin, RIPA lysis and extraction buffer, and a Vybrant MTT Cell Proliferation Assay kit were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Flowery Branch, GA, USA). Collagen type-1 was obtained from Corning (Corning, NY, USA). Anti-ezrin rabbit and caspase-3 antibodies were from Cell Signaling Technology (Danvers, MA, USA). Anti-SMPDL-3b was obtained from GenWay (San Diego, CA, USA). Rhodamine-phalloidin, Alexa 488 anti-rabbit, and DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride) were from Thermo Fisher Scientific. Mouse monoclonal anti-actin was from Abcam (Cambridge, MA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit was from Promega (Madison, WI, USA). HRP-conjugated anti-mouse IgG, monoclonal anti-GAPDH antibody, protease, and phosphatase inhibitor cocktails were purchased from Calbiochem (San Diego, CA, USA). RNA extraction mini kit was from Qiagen (Valencia, CA, USA). Superscript III first-strand synthesis kit was from Invitrogen (Carlsbald, CA, USA). Green SuperMix with ROX was from QuantaBio (Beverly, MA, USA). D-erythro-sphingosine-1-phosphate and C12-NBD ceramide were from Avanti Polar Lipids (Alabaster, AL, USA). Detergent-compatible protein assay kit, 4–20% SDS-PAGE gels, and 2X Laemmli sample buffer were purchased from Bio-Rad (Hercules, CA, USA). PVDF membranes were from Millipore (Billerica, MA, USA). Enhanced chemiluminescence was from GE Healthcare (Pittsburgh, PA, USA).
Human podocyte culture and irradiation
Human podocytes were cultured and differentiated in RPMI 1640 culture medium containing 10% FBS and 1% penicillin/streptomycin as previously described (17). In brief, immortalized normal podocytes were propagated at 33°C and then thermoshifted for differentiation for 14 d at 37°C in a collagen-coated T75 flask. A single dose of irradiation was delivered from an RS2000 X-ray irradiator (225 kV) according to the manufacturer’s specifications (Rad Source Technologies, Suwanee, GA, USA). The dose rate was adjusted by using a movable shelf and set at 195 cGy/min. For RTX treatment (Genentech, Inc., South San Francisco, CA, USA), cells were pretreated with RTX (100 µg/ml) for 30 min before irradiation (8 Gy) (14). A nonspecific IgG (100 µg/ml) was used as control for RTX treatment. For S1P treatment, cells were irradiated (8 Gy) and after 2 h were treated with S1P (100 nM) or vehicle for 5 min.
Immunofluorescence
Immunofluorescence and laser-scanning confocal microscopy experiments were carried out as previously described with minor modifications (18). Human podocytes were differentiated at 37°C on collagen type-1-coated 35-mm confocal dishes (MatTek Corp., Ashland, MA, USA). Cells were fixed with 4% paraformaldehyde for 15 min at 37°C and permeabilized with 0.1% Triton X-100 on ice for 15 min. Cells were blocked with 2% FBS and incubated with primary antibodies (1:200) in 2% FBS overnight. Fluorescence detection was performed using Alexa 488 anti-rabbit secondary antibody (Thermo Fisher Scientific) as per the manufacturer’s instructions. F-actin was visualized by rhodamine phalloidin, and nuclei were stained with DAPI. Samples were examined, and images were collected using a Zeiss confocal microscope (LSM710 Meta; Carl Zeiss, Inc., Thornwood, NY, USA) via a ×40 objective. To quantify γ-H2AX foci formation for each data point, 200 nuclei were evaluated. Data were analyzed using free downloadable LSM Image Browser Software.
Quantitative RT-PCR
Cells were washed with ice-cold PBS and directly lysed with the lysis buffer provided in the RNA minieasy kit (Qiagen, Hilden, Germany). RNA extraction was carried out as per the manufacturer’s protocol. RNA was quantified by NanoDrop (Thermo Fisher Scientific) and converted to cDNA using a SuperScript III First-Strand Synthesis kit (Thermo Fisher Scientific). The cDNA was diluted (1:15), and 1 µl was used per 25-µl reaction. Using the Perfecta SYBR Green FastMix (Quantabio), the reaction was executed in a real-time PCR system (Applied Biosystems, Foster City, CA, USA) as previously described (14). Standard and real-time quantitative PCR assays (Applied Biosystems) were performed for SMPDL3b and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following primer sequences were used: SMPDL3b, 5′–CTATACCAGCAATGCGCTGA and 3′–GAGAAGACGCAAAACAAGGC; GAPDH, 5′–GTCAGTGGTGGACCTGACCT and 3′–GTCAACGGTACATCTGGGGA. Relative quantification of different samples was determined as 2−ΔΔCt (ΔΔCt = ΔCt affected sample, ΔCt unaffected sample). The values were normalized and expressed as fold changes in gene expression over GAPDH.
Protein extraction and Western blotting
Podocytes were homogenized in cold RIPA buffer supplemented with protease and phosphatase inhibitor cocktails. Protein concentration was quantitated using a detergent-compatible protein assay kit (Bio-Rad). Samples were prepared in 2x Laemmli sample buffer. An equal amount (20 µg) of protein was loaded onto 4–20% SDS-PAGE gels (Bio-Rad) that were transferred onto PVDF membranes. Immunoblotting was performed by blocking the membranes with 5% skim milk diluted in Tris-buffered saline. The following primary antibodies were used for Western blotting: rabbit polyclonal anti-SMPDL3b (1:1000) (Genway Biotech, Inc., San Diego, CA, USA), mouse monoclonal anti-actin (1:1000) (Abcam), and rabbit anti-caspase-3 (1:1000). After washing 3 times with Tris-buffered saline with 0.1% Tween 20, the PVDF membranes were incubated with the following HRP-conjugated secondary antibodies: HRP-conjugated anti-rabbit IgG (1:1500), HRP-conjugated anti-mouse IgG (1:1500). The blots were developed by enhanced chemiluminescence (GE Healthcare). Blots were scanned using a ChemiDocTouch Gel and Western Blot Imaging System (Bio-Rad). Densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Liquid chromatography–mass spectrometry analysis
Cell pellets containing 1 × 106 cells per sample were subjected to liquid extraction. Liquid chromatography–mass spectrometry (LC–MS) analysis of sphingolipids was performed at the Lipidomics core facility at the Medical University of South Carolina using electrospray ionization/tandem mass spectrometry on a mass spectrometer (Quantum; Thermo Fisher Scientific) as previously described (19).
Anoikis assay
Anoikis assay was performed as previously described (20) in regular tissue culture grade plates or in ultralow attachment plates (ULAPs) (Corning). Podocytes were suspended in RPMI 1640 with 10% FBS at a level of 1 × 105 cells/ml, and 2 ml of cell suspension was added to each well and incubated in the microplates for 24 h in a humidified (37°C and 5% CO2) incubator. Cells were irradiated at 8 Gy and incubated for 24 h before the MTT assay.
Ceramidase enzymatic assays
Ceramidase (CDase) enzymatic activities were performed according to previously described methods (21, 22) using NBD-C12 ceramide. The reaction mixtures were incubated at 37°C for 1 h, and reactions were stopped by adding methanol/chloroform (1:1, v/v). After 5 min, the mixture was centrifuged, and the organic phase was taken and dried using a Savant SpeedVac evaporator (Thermo Fisher Scientific). Lipids were dissolved in 25 µl of chloroform/methanol (2:1, v/v), and 15 µl was spotted on to thin-layer chromatography (TLC) plates. The product was separated from the substrate by developing the plate in chloroform/methanol/25% ammonium hydroxide (90:30:0.5, by volume) solvent system. The TLC plate was scanned using Storm 860 Phosphoimager (GE Healthcare) system set to fluorescence mode. The NBD-dodecanoic acid product was analyzed using ImageQuant software (GE Healthcare).
Animal experiments and X-ray irradiation
We used wild-type (WT) female C57BL/6 mice purchased from The Jackson Laboratory (Bar Harbor, ME, USA). All animal studies have been approved by the University of Miami Institutional Animal Care and Use Committee. Animals (age 10–14 wk) were irradiated with a single dose of X-ray of 14 Gy using an image-guided small animal arc radiation treatment system (iSMAART). iSMAART was developed to achieve highly precise radiation targeting through the use of onboard cone beam computed tomography guidance (23). Both kidneys were clearly delineated in the contrast cone beam computed tomography and irradiated with a 14 Gy dose by a single X-ray beam from the posterior-to-anterior direction sequentially. Mice were killed at 11 wk after irradiation by cervical dislocation, and kidneys were either snap frozen in liquid nitrogen for preparation of frozen sections and protein isolation or fixed in formalin and processed for paraffin embedding. Primary antibodies were: anti-goat polyclonal anti-WT-1 (1:300) (Santa Cruz Biotechnology), rabbit polyclonal anti-SMPDL3b (Genway Biotech, Inc.). Fluorescence detection was performed using Alexa Fluor 488 anti-rabbit and Alexa Fluor 568 secondary antibody as per the manufacturer’s instructions. Nuclei were stained with DAPI (blue). Confocal images were taken using a Zeiss LSM 700 Meta confocal microscope.
Statistical analysis
Results are expressed as the means ± se. One-way ANOVA was used to compare the groups, and statistically significant results were also compared using Student’s t test with Tukey’s correction for multiple comparisons (Graph Pad Prism software; La Jolla, CA, USA). Statistical significance was set at P < 0.05.
RESULTS
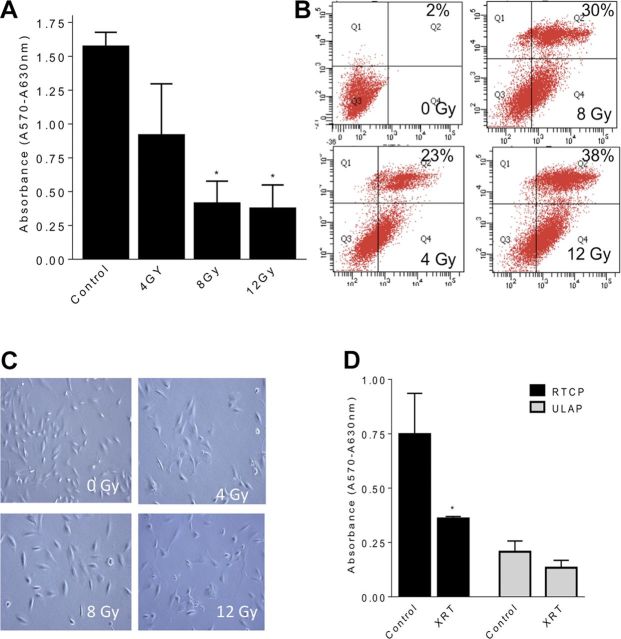
In order to study radiation-induced changes, podocytes were exposed to various radiation doses. Cell viability was assessed by MTT assay and flow cytometry sorting for positive annexin V/PI staining after 24 h. Ionizing radiation induced a dose-dependent drop in podocyte viability (Fig. 1A). Flow cytometry revealed an increase in annexin V/PI-positive cells by 23, 30, and 38% at 4, 8, and 12 Gy, respectively (Fig. 1B). Marked morphological changes consisting of loss of filopodia were also noted (Fig. 1C). Given the importance of cell anchorage in podocyte survival, we investigated podocyte death by anoikis after irradiation. Podocytes cultured on ULAPs did not demonstrate significant changes in cell viability (Fig. 1D), suggesting a minimal role for anoikis. However, ULAP conditions had a significant impact on cell viability.
Figure 1.
Effect of ionizing radiation on viability of human renal podocytes. Metabolic activity was assessed by MTT assay, and results are indicated relative to control cells (no radiation). A) Differentiated podocytes were irradiated at 0, 4, 8, or 12 Gy, and MTT assay was carried after 24 h. B) Apoptosis was quantified by annexin V/propidium iodide (PI) staining followed by flow cytometry. Annexin V and PI values for each treatment were plotted on the x and y axis, respectively. Shown are the percentage cells of each treatment group in top right quadrant (annexin V+, PI+). C) Podocytes were grown on 10-cm dishes and irradiated at 0, 4, 8, or 12 Gy. Cell morphology was examined under an inverted light microscope equipped with a CCD camera using an ×20 objective. Representative images are shown. D) Podocytes were irradiated (8 Gy) and incubated for 24 h at 37°C into wells of 6-well regular tissue culture plates (RTCPs) or ULAPs. Results shown are the mean values of 3 different experiments each performed in triplicate, with error bars indicating sem from multiple experiments. *P < 0.05.
Irradiated podocytes displayed marked morphological changes consisting of loss of cellular processes as filopodia (Fig. 1C). Using phalloidin staining, we observed marked loss of cortical actin staining within 2 h of radiation injury. Furthermore, the actin binding protein ezrin, which stabilizes the association of actin filaments to the plasma membrane, was relocated from cell periphery to a more cytosolic location (Fig. 2A). Quantitation of these results revealed cytoskeletal changes in 75% of irradiated cells (Fig. 2B).
Figure 2.
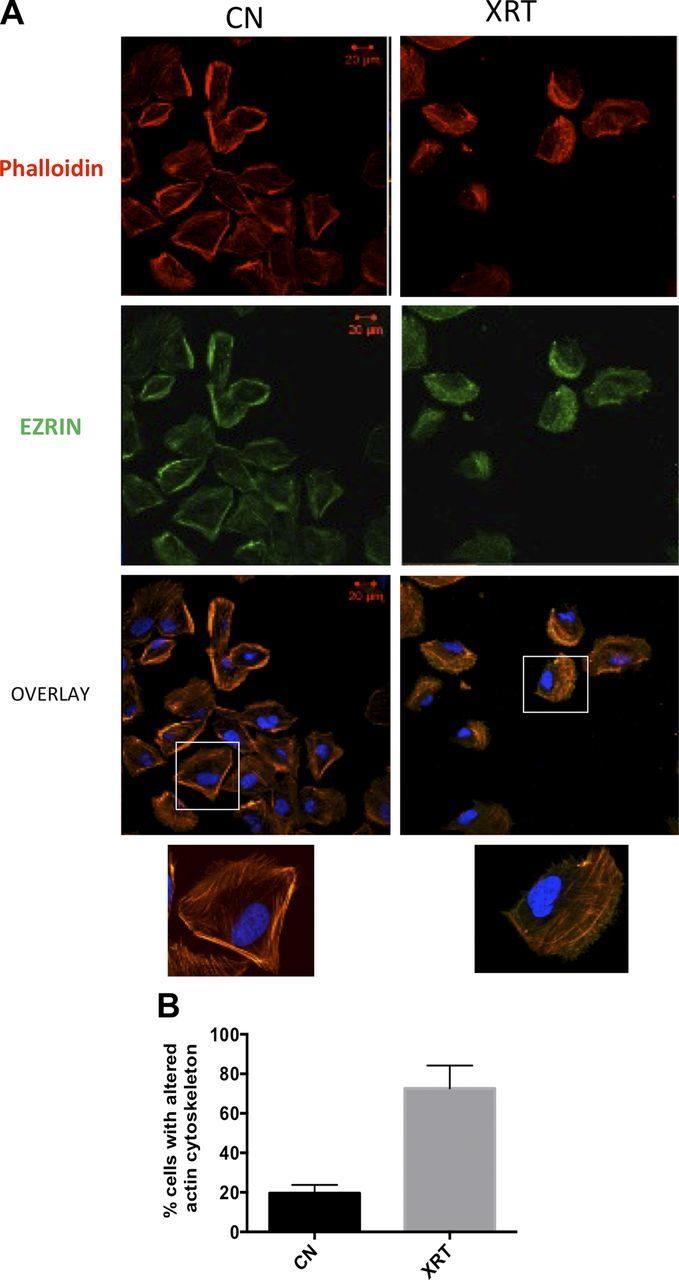
Effect of irradiation on podocyte cytoskeleton. A) Podocytes were irradiated at 0 Gy [control (cn)] or 8 Gy and morphological changes were studied by laser scanning confocal microscopy after 2 h. F-actin is labeled with rhodamine phalloidin (red), and actin-binding protein ezrin is labeled by FITC-conjugated antibody (green) and nuclei with DAPI (blue). Original magnification, ×40. Scale bars, 20 µM. Representative areas are enlarged in the insets. B) The percentage of cells with cytoskeletal remodeling was obtained by averaging cells with altered actin and ezrin staining per 100 cells. Results are representative of at least 3 independent experiments.
Prior work from our group and others has shown a key role for bioactive sphingolipids in mediating responses to stress agents such as cisplatin and ionizing radiation (24, 25). Therefore, it became important to evaluate whether the observed morphological changes are mediated by alterations in sphingolipid metabolism. To that end, podocytes were irradiated by a single dose of 8 Gy, and cellular lipids were extracted. Mass spectrometric measurements revealed a marked elevation in ceramide levels. This change was mainly due to an increase in ceramide species with C16, C24:00, and C24:1 acyl chains (Fig. 3A). In parallel with changes in ceramide levels, there was a significant drop in sphingosine and sphingosine-1-phosphate (S1P) levels (Fig. 3B, C).
Figure 3.
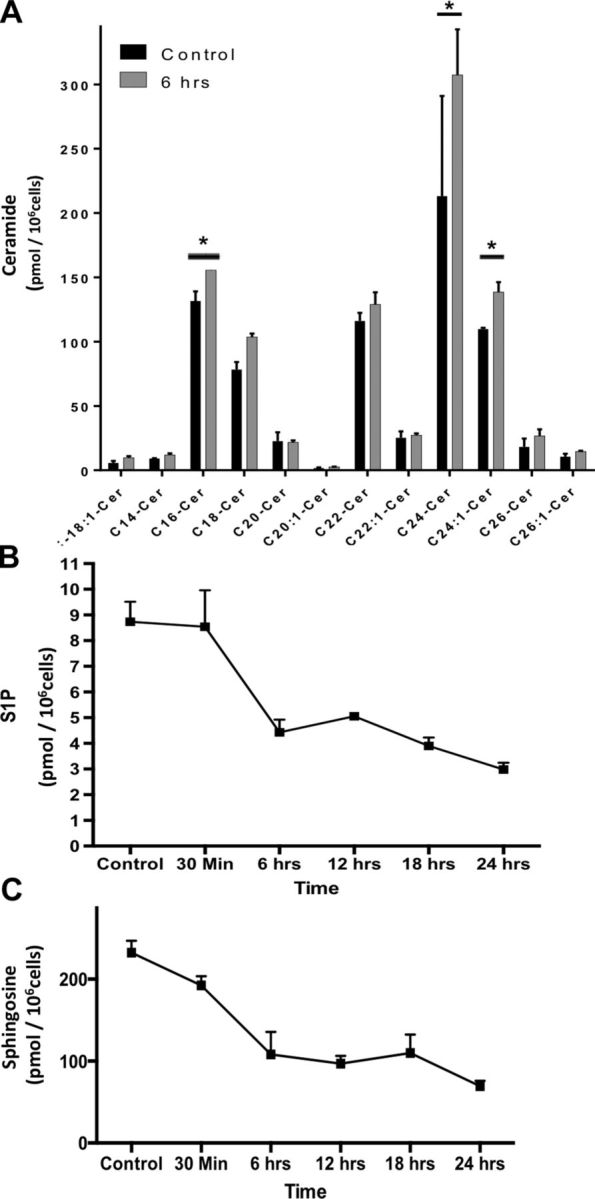
LC-MS analysis of radiation effects on sphingolipids. Cells were irradiated with 0 Gy (control [cn]) or 8 Gy, and pellets containing 1 × 106 cells per sample were collected at 0.5, 6, 12, 18, and 24 h. After Bligh Dyer extraction of lipids, the different treatment groups were subjected to mass spectrometric analysis to determine levels of ceramide subspecies (A), s1P (B), and sphingosine (C). Results represent averages ± se from 3 independent experiments. *P < 0.05.
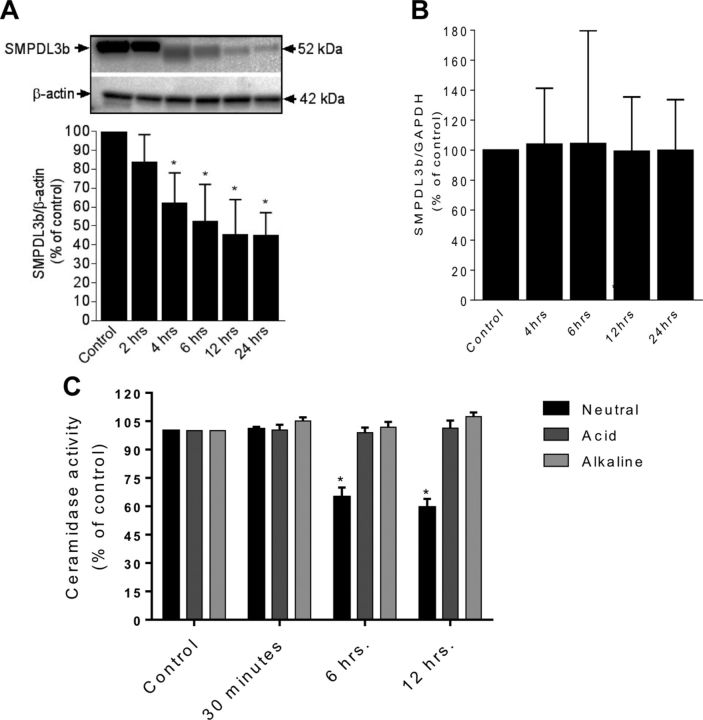
This sphingolipidomic profile suggested the operation of a ceramidase. To that end, in vitro ceramidase assays using NBD-C12 ceramide were conducted. A significant drop in neutral ceramidase (NCDase) activity was detected at 6 and 12 h after radiation (Fig. 4C). However, acid and alkaline ceramidase activities were not significantly altered along the same time frame. Prior work from our group identified a key role for SMPDL3b in podocytopathy (13, 14, 26). To investigate its role, SMPDL3b was detected via Western blotting. Radiation injury induced a time-dependent drop in SMPDL3b protein levels starting at 4 h (Fig. 4A). These changes are unlikely to be mediated by a transcriptional event because SMPDL3b mRNA levels remained unchanged (Fig. 4B).
Figure 4.
Modulation of SMPDL3b levels and CDase activity by radiation. Cells plated on 10-cm dishes were irradiated at 8 Gy, and pellets were collected at various time points. A) SMPDL3b protein expression by Western blotting. Results were quantitated by densitometry using ImageJ software. B) Transcriptional analysis of podocytes SMPDL3b expression after radiation (8 Gy) for different time points after irradiation. C) Changes in neutral ceramidase, acid ceramidase, and alkaline ceramidase were measured by TLC after radiation at the 8 Gy using NBD-labeled C12 ceramide. Results shown are the mean values of 3 different experiments each performed in triplicate, with error bars indicating sem from multiple experiments. *P < 0.05.
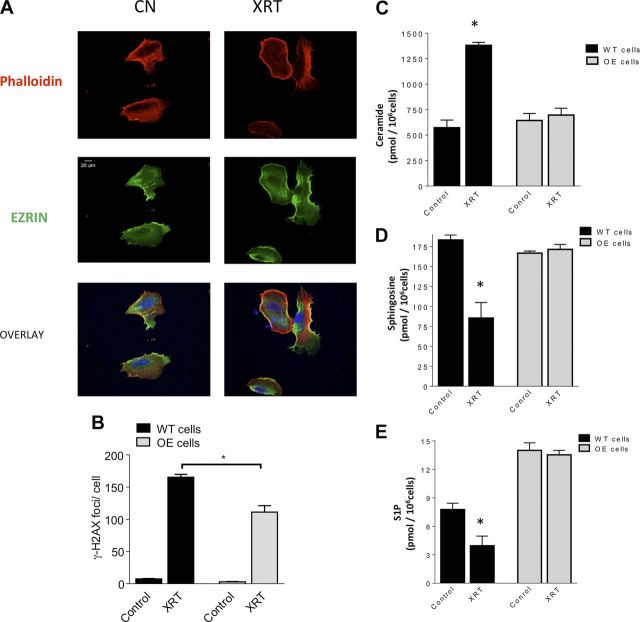
Given the above results, we investigated the effect of radiation on podocytes stably overexpressing SMPDL3b [SMPDL3b overexpressers (OEs)]. Microscopically, SMPDL3b OEs retained cortical actin and membrane-associated ezrin localization after radiation (Fig. 5A). The extent of DNA damage was further analyzed by quantitating γ-H2AX foci per nucleus at 2 h after irradiation. SMPDL3b OE podocytes displayed reduction in γ-H2AX foci by approximately one third compared with WT cells (Fig. 5B). Importantly, SMPDL3b OE podocytes had no significant changes in total ceramide, sphingosine, or S1P levels after irradiation (Fig. 5C–E). Notably, SMPDL3b OE cells had higher basal levels of S1P as compared with WT podocytes (Fig. 5E). These results suggest a role for SMPDL3b in mediating radiation effects on renal podocytes.
Figure 5.
Overexpression of SMPDL3b protects podocytes from radiation-induced cytoskeletal changes and DNA damage. A) Immunofluorescence staining of SMPDL3b-overexpressing podocytes after 0 Gy (control [cn]) and 8 Gy irradiation. F-actin is labeled with rhodamine phalloidin (red), actin binding protein with ezrin (green), and nuclei with DAPI (blue). Original magnification, ×40. Scale bar, 20 µM. B) Quantitation of γ-H2AX foci in WT and SMPDL3b-overexpressing podocytes at baseline and 2 h after exposure to a single dose of 8 Gy. Mean γ-H2AX foci was obtained after evaluating 200 nuclei counterstained with DAPI for γ-H2AX foci. Asterisks indicate significant differences as compared with mock-treated cells. *P < 0.001. Sphingolipid metabolites were analyzed by LC–MS in WT and SMPDL3b-overexpressing podocytes 6 h after radiation at 8 Gy. Shown are normalized results for ceramide levels (C), sphingosine levels (D), and S1P levels (E). Results represent averages ± se from 3 independent experiments. *P < 0.05.
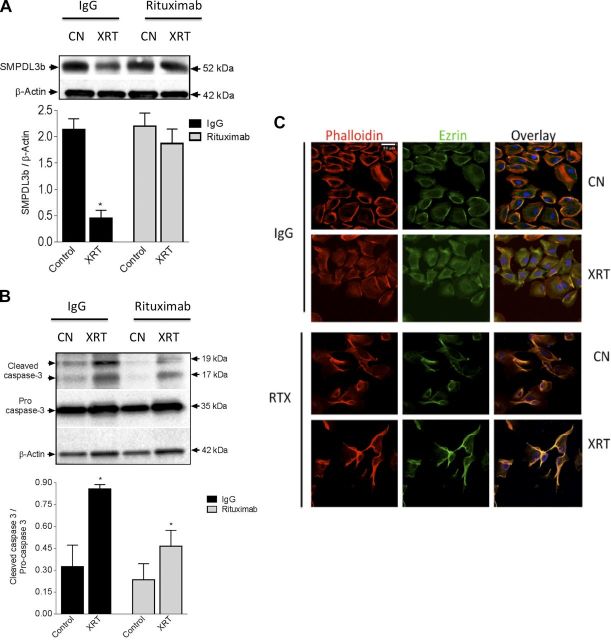
The monoclonal antibody RTX has been shown to bind to SMPDL3b through a yeast library screening approach (15). Specifically, in podocytes RTX has been shown to stabilize SMPDL3b at the plasma membrane in adverse conditions as FSGS (14). Pretreatment of podocytes with RTX (100 μg/ml) mitigated SMPDL3b loss (Fig. 6A) and attenuated caspase 3 cleavage (Fig. 6B) after radiation. Furthermore, cells pretreated with RTX retained cellular filopodia, cortical actin, and membrane-associated ezrin by immunofluorescence (Fig. 6C). Moreover, treatment of irradiated podocytes with S1P (100 nM) for 5 min rescued irradiated podocytes from cytoskeletal remodeling (Fig. 7). Together, these results point to a central role for SMPDL3b in radiation-induced podocyte injury.
Figure 6.
RTX pretreatment mitigates radiation-induced podocytopathy. A) Podocytes were irradiated (8 Gy) in the presence of RTX (100 µg/ml) or IgG (100 µg/ml). B) SMPDL3b protein expression and cleaved caspase-3 were assayed by immunoblotting. Values represent means ± sem. *P < 0.05. C) Immunofluorescence staining of podocytes after 8 Gy radiation in the presence of RTX (100 µg/ml) or IgG (100 µg/ml) antibody. Actin fibers are labeled with rhodamine phalloidin (red), actin binding protein ezrin with FITC-conjugated antibody (green), and nuclei with DAPI (blue). Original magnification, ×40. Scale bar, 50 µM.
Figure 7.
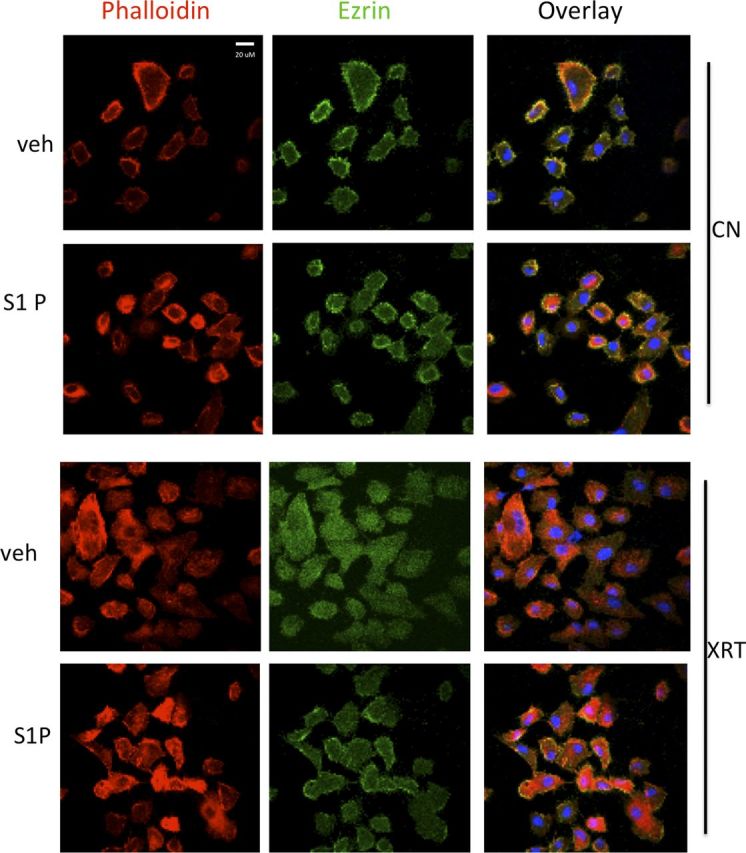
Exogenous S1P rescues podocytes from radiation-induced cytoskeletal remodeling. Podocytes were irradiated with 0 Gy [control (cn)] or 8 Gy and incubated for 2 h before treatment with S1P (100 nM) or vehicle (2% dodecane/98% ethanol). Actin fibers are labeled with rhodamine phalloidin (red), actin binding protein ezrin with FITC-conjugated antibody (green), and nuclei with DAPI (blue). Original magnification, ×40. Scale bar, 20 µM.
We further tested the effect of ionizing radiation on SMPDL3b using a mouse model. To that end, C57BL/6 mice were subjected to bilateral kidney irradiation using a previously described small animal image-guided radiation system (iSMAART) (23). After 11 wk, we noted a marked drop in SMPDL3b and the podocyte marker WT-1 by immunofluorescence. In parallel, whole kidney homogenates displayed a 50% drop in SMPDL3b protein levels (Fig. 8). These results confirm down-regulation of SMPDL3b by ionizing radiation in vivo.
Figure 8.
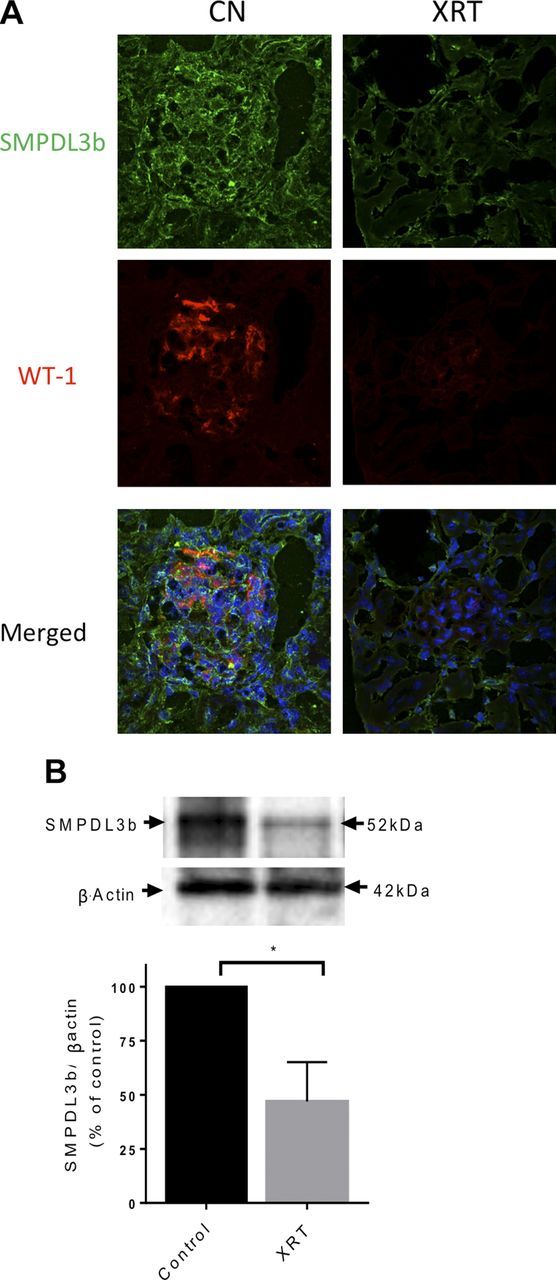
Down-regulation of SMPDL3b in irradiated murine kidneys. WT C57BL6 mice were exposed to focal bilateral kidney irradiation (14 Gy). After 11 wk, kidneys were extracted and frozen. A) Immunofluorescence staining of paraffin-embedded renal tissues with SMPDL3b (FITC, green), WT-1 (red), and nuclei (DAPI) after 0 Gy [control (cn)] and 14 Gy irradiation. B) Isolates from irradiated and control kidneys were tested for SMPDL3b protein levels via Western blotting. Statistically significant differences were determined using Student's t test. A value of P < 0.05 was considered significant.
DISCUSSION
The current study identifies a novel lipid-based pathway mediating radiation-induced podocyte injury. Ionizing radiation triggered a time-dependent loss of SMPDL3b at the protein level. This was paralleled by a drop in NCDase activity, elevation of ceramide levels, and drop in sphingosine and S1P levels. Morphologically, irradiated podocytes displayed marked actin remodeling and relocation of ezrin to the cytosol. These microscopic changes could be reversed, at least in part, by SMPDL3b overexpression, pretreatment with RTX, or administration of exogenous S1P. Collectively, these results point to a central role for sphingolipids in radiation-induced podocytopathy.
Over the past 2 decades, work from our group and others has identified a key role for sphingolipids in mediating cellular stress responses, including radiation damage (24, 27–30). The sphingolipid metabolic pathway consists of a network of anabolic and catabolic enzymes distributed throughout different cellular compartments. At the heart of sphingolipid metabolism is ceramide, the most studied sphingolipid and the precursor of complex sphingolipids. De novo ceramide synthesis starts with the condensation of serine and palmitoyl-CoA, eventually leading to ceramide formation on the cytosolic surface of the endoplasmic reticulum. Alternatively, ceramide can be released from degradation of complex sphingolipids such as sphingomyelin through the action of sphingomyelinases. In turn, ceramide can be degraded by ceramidase reaction, an obligate step for generation of S1P. Several studies have implicated specific sphingolipid-metabolizing enzymes in mediating radiation responses. For instance, apoptosis of tumor endothelial cells after high radiation doses has been shown to be mediated by acid sphingomyelinase in animal models (31). On the other hand, overexpression of acid ceramidase was recently shown to mediate radioresistance of prostate cancer cells (32, 33). The current project builds on these prior reports to further investigate the role of a novel sphingomyelinase (SMPDL3b) in radiation-induced podocyte injury.
The results from the current study extend our understanding of the bioactive role of SMPDL3b. Recently, work by Heinz et al. (34) showed that SMPDL3b co-purifies with TLRs via a mass spectrometry approach. The authors elegantly describe how SMPDL3b negatively regulates TLR function. Early studies by Fornoni et al. (14) showed that treatment of cultured podocytes with serum derived from patients with recurrent FSGS induced down-regulation of SMPDL3b. Interestingly, preservation of podocyte SMPDL3b by RTX mitigates recurrent FSGS in high-risk patients. On the other hand, in diabetic kidney disease high SMPLDL3b expression in combination with increased soluble urokinase plasminogen activator receptor leads to RhoA activation and podocyte death (26). The current work defines a key role for SMPDL3b loss in renal podocytes after radiation injury.
Renal dysfunction remains an important complication of cancer therapy. Glomerular dysfunction has been reported in up to 60% of patients with cancer receiving abdominal radiation therapy. Radiation oncologists are often challenged by scenarios whereby administration of tumoricidal radiation doses exceeds the known renal tolerance dose. Apart from limited success with angiotensin-converting enzyme inhibitors and corticosteroids (6, 7), management of radiation nephropathy poses a clinical challenge. The current project identifies a role for SMPDL3b in radiation-induced podocytopathy and paves the way for investigating potential novel renal radioprotective agents such as RTX and S1P.
To our knowledge, this is the first study to examine the impact of sphingolipid metabolism on radiation-induced renal injury. To date, there has been limited success with agents aimed at renal radioprotection in patients with cancer who are at risk for developing radiation nephropathy. The current study demonstrates how alteration of the sphingolipid-metabolizing enzyme SMPDL3b affects renal podocytes. The study further explores the use of RTX, which has been shown to bind SMPDL3b, as a radioprotective antibody for renal podocytes. The results show that radiation-induced loss of SMPDL3b alters podocyte sphingolipid homeostasis, resulting in podocyte dysfunction. However, the mechanism of SMPDL3b loss remains to be investigated. The study also demonstrates how targeting SMDPL3b by RTX could provide a venue for protecting renal podocytes from radiation stress. Recent technological advances afforded radiation oncologists tumoricidal doses of radiation therapy (also known as SBRT or SABR) (35). However, the proximity of kidneys to the tumor target, particularly in abdominal malignancies, poses a therapeutic challenge. Therefore, identification of novel renal radioprotective agents will help deliver ablative radiation doses while minimizing the risk of nephrotoxicity.
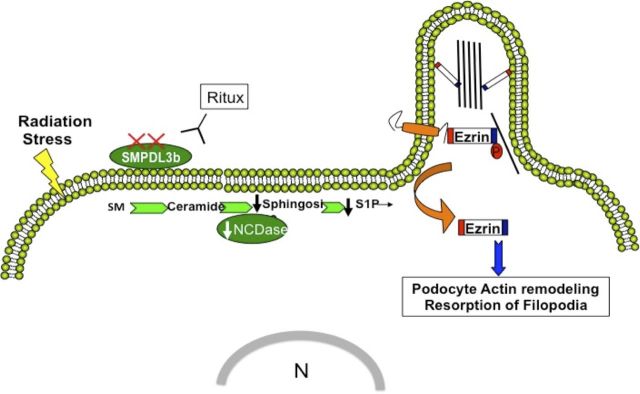
In summary, the results in the current study highlight the importance of sphingolipid metabolism in radiation-induced podocyte injury. The study identifies a novel mechanism by which loss of SMPDL3b mediates cytoskeletal remodeling in renal podocytes. Based on these findings, a hypothetical model is illustrated in Fig. 9.
Figure 9.
Model of radiation-induced podocytopathy. The model depicts loss of SMPDL3b as a key event after radiation injury of renal podocytes. This triggers changes in sphingolipid metabolism, including a drop in NCDase activity and a downstream decrease in S1P levels resulting in cytosolic relocation of ezrin and remodeling of the actin cytoskeleton. SM, sphingomyelin.
ACKNOWLEDGMENTS
A.F. is an inventor of pending patents aimed to diagnose or treat proteinuric renal diseases, and stands to gain royalties from their future commercialization. A.F. is also a consultant for Hoffman-La Roche, Genentech, Jannsen, Mesoblast, Abbvie, Boehringer Ingelheim, Alexion, and Bristol-Myers Squibb. A.F. is funded by U.S. National Institutes of Health Grants DK104753 and DK090316.
Glossary
- FBS
fetal bovine serum
- FSGS
focal segmental glomerulosclerosis
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HRP
horseradish peroxidase
- LC–MS
liquid chromatography–mass spectrometry
- OE
overexpresser
- RTX
rituximab
- S1P
sphingosine-1-phosphate
- SMPDL3b
sphingomyelin phosphodiesterase acid-like 3B
- TLC
thin-layer chromatography
- ULAP
ultralow attachment plate
- WT
wild-type
AUTHOR CONTRIBUTIONS
Y. Zeidan, A. Fornoni, and B. Marples designed the research; A. Ahmad, Y. Zeidan, and B. Marples analyzed the data; A. Ahmad, A. Mitrofanova, J. Bielawski, and Y. Yang performed the research; A. Ahmad and Y. Zeidan wrote the paper; and all authors contributed to editing the paper.
REFERENCES
- 1.Cassady J. R. (1995) Clinical radiation nephropathy. Int. J. Radiat. Oncol. Biol. Phys. , 1249–1256 [DOI] [PubMed] [Google Scholar]
- 2.Dawson L. A., Kavanagh B. D., Paulino A. C., Das S. K., Miften M., Li X. A., Pan C., Ten Haken R. K., Schultheiss T. E. (2010) Radiation-associated kidney injury. Int. J. Radiat. Oncol. Biol. Phys. (3, Suppl)S108–S115 [DOI] [PubMed] [Google Scholar]
- 3.Cohen E. P., Robbins M. E. C. (2003) Radiation nephropathy. Semin. Nephrol. , 486–499 [DOI] [PubMed] [Google Scholar]
- 4.Luxton R. W. (1961) Radiation nephritis: a long-term study of 54 patients. Lancet , 1221–1224 [DOI] [PubMed] [Google Scholar]
- 5.Yang G. Y., May K. S., Iyer R. V., Chandrasekhar R., Wilding G. E., McCloskey S. A., Khushalani N. I., Yendamuri S. S., Gibbs J. F., Fakih M., Thomas C. R. Jr (2010) Renal atrophy secondary to chemoradiotherapy of abdominal malignancies. Int. J. Radiat. Oncol. Biol. Phys. , 539–546 [DOI] [PubMed] [Google Scholar]
- 6.Sera N., Hida A., Imaizumi M., Nakashima E., Akahoshi M. (2013) The association between chronic kidney disease and cardiovascular disease risk factors in atomic bomb survivors. Radiat. Res. , 46–52 [DOI] [PubMed] [Google Scholar]
- 7.Cheng J. C., Schultheiss T. E., Wong J. Y. C. (2008) Impact of drug therapy, radiation dose, and dose rate on renal toxicity following bone marrow transplantation. Int. J. Radiat. Oncol. Biol. Phys. , 1436–1443 [DOI] [PubMed] [Google Scholar]
- 8.Stephens L. C., Robbins M. E., Johnston D. A., Thames H. D., Price R. E., Peters L. J., Ang K. K. (1995) Radiation nephropathy in the rhesus monkey: morphometric analysis of glomerular and tubular alterations. Int. J. Radiat. Oncol. Biol. Phys. , 865–873 [DOI] [PubMed] [Google Scholar]
- 9.Ruotsalainen V., Ljungberg P., Wartiovaara J., Lenkkeri U., Kestilä M., Jalanko H., Holmberg C., Tryggvason K. (1999) Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA , 7962–7967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oikawa T., Freeman M., Lo W., Vaughan D. E., Fogo A. (1997) Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of renin-angiotensin inhibition. Kidney Int. , 164–172 [DOI] [PubMed] [Google Scholar]
- 11.Lennon R., Pons D., Sabin M. A., Wei C., Shield J. P., Coward R. J., Tavaré J. M., Mathieson P. W., Saleem M. A., Welsh G. I. (2009) Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol. Dial. Transplant. , 3288–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basic-Jukic N., Kes P., Coric M., Basic-Kes V. (2013) Renal complications of Fabry disease. Curr. Pharm. Des. , 6046–6050 [DOI] [PubMed] [Google Scholar]
- 13.Merscher S., Fornoni A. (2014) Podocyte pathology and nephropathy: sphingolipids in glomerular diseases. Front. Endocrinol. (Lausanne) , 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fornoni A., Sageshima J., Wei C., Merscher-Gomez S., Aguillon-Prada R., Jauregui A. N., Li J., Mattiazzi A., Ciancio G., Chen L., Zilleruelo G., Abitbol C., Chandar J., Seeherunvong W., Ricordi C., Ikehata M., Rastaldi M. P., Reiser J., Burke G. W. III (2011) Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl. Med. , 85ra46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perosa F., Favoino E., Caragnano M. A., Dammacco F. (2006) Generation of biologically active linear and cyclic peptides has revealed a unique fine specificity of rituximab and its possible cross-reactivity with acid sphingomyelinase-like phosphodiesterase 3b precursor. Blood , 1070–1077 [DOI] [PubMed] [Google Scholar]
- 16.Tasaki M., Shimizu A., Hanekamp I., Torabi R., Villani V., Yamada K. (2014) Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation. J. Am. Soc. Nephrol. , 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleem M. A., O’Hare M. J., Reiser J., Coward R. J., Inward C. D., Farren T., Xing C. Y., Ni L., Mathieson P. W., Mundel P. (2002) A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. , 630–638 [DOI] [PubMed] [Google Scholar]
- 18.Gandy K. A., Canals D., Adada M., Wada M., Roddy P., Snider A. J., Hannun Y. A., Obeid L. M. (2013) Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. Biochem. J. , 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bielawski J., Szulc Z. M., Hannun Y. A., Bielawska A. (2006) Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods , 82–91 [DOI] [PubMed] [Google Scholar]
- 20.Uehara N., Matsuoka Y., Tsubura A. (2008) Mesothelin promotes anchorage-independent growth and prevents anoikis via extracellular signal-regulated kinase signaling pathway in human breast cancer cells. Mol. Cancer Res. , 186–193 [DOI] [PubMed] [Google Scholar]
- 21.Geoffroy K., Wiernsperger N., Lagarde M., El Bawab S. (2004) Bimodal effect of advanced glycation end products on mesangial cell proliferation is mediated by neutral ceramidase regulation and endogenous sphingolipids. J. Biol. Chem. , 34343–34352 [DOI] [PubMed] [Google Scholar]
- 22.Wu B. X., Snook C. F., Tani M., Büllesbach E. E., Hannun Y. A. (2007) Large-scale purification and characterization of recombinant Pseudomonas ceramidase: regulation by calcium. J. Lipid Res. , 600–608 [DOI] [PubMed] [Google Scholar]
- 23.Sha H., Udayakumar T. S., Johnson P. B., Dogan N., Pollack A., Yang Y. (2016) An image guided small animal stereotactic radiotherapy system. Oncotarget , 18825–18836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeidan Y. H., Jenkins R. W., Hannun Y. A. (2008) Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J. Cell Biol. , 335–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacour S., Hammann A., Grazide S., Lagadic-Gossmann D., Athias A., Sergent O., Laurent G., Gambert P., Solary E., Dimanche-Boitrel M. T. (2004) Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. , 3593–3598 [DOI] [PubMed] [Google Scholar]
- 26.Yoo T. H., Pedigo C. E., Guzman J., Correa-Medina M., Wei C., Villarreal R., Mitrofanova A., Leclercq F., Faul C., Li J., Kretzler M., Nelson R. G., Lehto M., Forsblom C., Groop P. H., Reiser J., Burke G. W., Fornoni A., Merscher S. (2015) Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J. Am. Soc. Nephrol. , 133–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeidan Y. H., Jenkins R. W., Korman J. B., Liu X., Obeid L. M., Norris J. S., Hannun Y. A. (2008) Molecular targeting of acid ceramidase: implications to cancer therapy. Curr. Drug Targets , 653–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeidan Y. H., Hannun Y. A. (2010) The acid sphingomyelinase/ceramide pathway: biomedical significance and mechanisms of regulation. Curr. Mol. Med. , 454–466 [DOI] [PubMed] [Google Scholar]
- 29.Zeidan Y. H., Hannun Y. A. (2007) Translational aspects of sphingolipid metabolism. Trends Mol. Med. , 327–336 [DOI] [PubMed] [Google Scholar]
- 30.Aureli M., Murdica V., Loberto N., Samarani M., Prinetti A., Bassi R., Sonnino S. (2014) Exploring the link between ceramide and ionizing radiation. Glycoconj. J. , 449–459 [DOI] [PubMed] [Google Scholar]
- 31.Truman J. P., García-Barros M., Kaag M., Hambardzumyan D., Stancevic B., Chan M., Fuks Z., Kolesnick R., Haimovitz-Friedman A. (2010) Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One , e12310[Erratum] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahdy A. E., Cheng J. C., Li J., Elojeimy S., Meacham W. D., Turner L. S., Bai A., Gault C. R., McPherson A. S., Garcia N., Beckham T. H., Saad A., Bielawska A., Bielawski J., Hannun Y. A., Keane T. E., Taha M. I., Hammouda H. M., Norris J. S., Liu X. (2009) Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol. Ther. , 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng J. C., Bai A., Beckham T. H., Marrison S. T., Yount C. L., Young K., Lu P., Bartlett A. M., Wu B. X., Keane B. J., Armeson K. E., Marshall D. T., Keane T. E., Smith M. T., Jones E. E., Drake R. R. Jr., Bielawska A., Norris J. S., Liu X. (2013) Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J. Clin. Invest. , 4344–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinz L. X., Baumann C. L., Köberlin M. S., Snijder B., Gawish R., Shui G., Sharif O., Aspalter I. M., Müller A. C., Kandasamy R. K., Breitwieser F. P., Pichlmair A., Bruckner M., Rebsamen M., Blüml S., Karonitsch T., Fauster A., Colinge J., Bennett K. L., Knapp S., Wenk M. R., Superti-Furga G. (2015) The lipid-modifying enzyme SMPDL3B negatively regulates innate immunity. Cell Reports , 1919–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeng J., Baik C., Bhatia S., Mayr N., Rengan R. (2014) Combination of stereotactic ablative body radiation with targeted therapies. Lancet Oncol. , e426–e434 [DOI] [PubMed] [Google Scholar]