Abstract
Exercise training enhances physical performance and confers health benefits, largely through adaptations in skeletal muscle. Mitochondrial adaptation, encompassing coordinated improvements in quantity (content) and quality (structure and function), is increasingly recognized as a key factor in the beneficial outcomes of exercise training. Exercise training has long been known to promote mitochondrial biogenesis, but recent work has demonstrated that it has a profound impact on mitochondrial dynamics (fusion and fission) and clearance (mitophagy), as well. In this review, we discuss the various mechanisms through which exercise training promotes mitochondrial quantity and quality in skeletal muscle.—Drake, J. C., Wilson, R. J., Yan, Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle.
Keywords: PGC-1α, biogenesis, remodeling, mitophagy
Exercise training is a potent and reproducible means of improving physical performance and overall health, thus preventing multiple chronic diseases, largely through induced adaptations in skeletal muscle (1). Skeletal muscle makes up approximately 40% of total body mass and is one of the most metabolically active tissues in the human body (2). Mitochondria, which oxidize nutrient substrates to generate ATP, are primarily responsible for meeting the energy demands of prolonged exercise. The efficiency by which mitochondria meet these demands, and thus fuel skeletal muscle function and overall physical performance, is determined by their overall function (i.e., the culmination of content, structure, and respiration) (3, 4). Exercise training elicits pronounced improvements in overall mitochondrial quantity and quality through several highly coordinated adaptive processes, including synthesis and incorporation of new proteins and mitochondrial DNA (mtDNA) in the existing reticulum (i.e., biogenesis) (5) and structural separation (i.e., fission) and joining (i.e., fusion) (6), as well as degradation of damaged and dysfunctional mitochondria (i.e., mitophagy) (7) (Fig. 1). These exercise-inducible processes underlie enhanced physical performance, as well as other health benefits, as a result of exercise training. In this review, we focus on the molecular mechanisms, including upstream regulation and downstream actions that lead to improved mitochondrial function in skeletal muscle with exercise training.
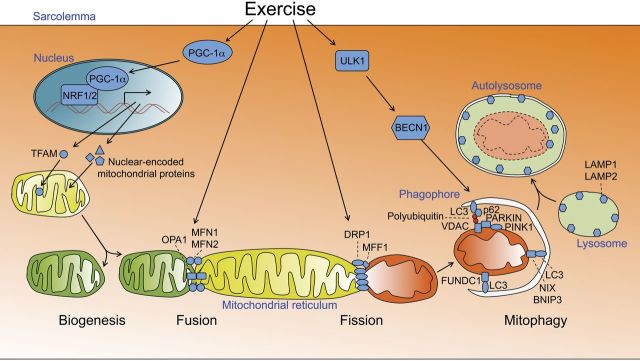
Figure 1.
Exercise training–induced mitochondrial adaptation in skeletal muscle. Exercise stimulates PGC-1α activity and expression, which promotes mitochondrial biogenesis, mainly by coordinating the transcriptions in the nuclear genome (through interaction with NRF1/2) and the mitochondrial genome (through transcription of the TFAM gene). The new, healthy mitochondria (green) likely fuse with (mitochondrial fusion) and the old, damaged mitochondria (red) likely separate from (mitochondrial fission) the mitochondrial reticulum. Exercise promotes both fusion (through OPA1 and MFN1/2) and fission (through MFF1 and DRP1) of IMM, OMM, and content. Damaged mitochondria, presumably due to the loss of mitochondrial membrane potential, can be recognized by autophagy machinery through the autophagy proteins MAP1LC3, PINK/PARKIN/p62, NIX/BNIP3, and FUNDC1 acting alone or in combination. At the same time, exercise leads to sequential activation of ULK1 and BECLIN1 and their downstream effectors to promote phagophore formation, which engulfs the damaged mitochondria to form an autophagosome. Fusion of the autophagosome with the lysosome completes the mitochondrial life cycle. Exercise training improves mitochondrial quantity through mitochondrial biogenesis and mitochondrial quality through a combination of mitochondrial biogenesis, dynamics, and mitophagy. Mitochondrial color change denotes quality from green, indicative of good quality, to red, indicative poor quality.
MITOCHONDRIAL BIOGENESIS
In skeletal muscle, mitochondria form a reticulated network with interconnected mitochondria clustered underneath the sarcoplasm (subsarcolemmal mitochondria) and individually laid between myofibrils on each side of the z-line (intermyofibrillar mitochondria) (8). The making of new mitochondria (i.e., biogenesis) requires synthesis and assembly of lipids, proteins, and mtDNA expanding from pre-existing mitochondria, rather than through de novo generation (9). In 1967, John Holloszy (10) published the first direct evidence that exercise training promotes mitochondrial biogenesis in skeletal muscle. This seminal study showed that a strenuous program of treadmill running in rats led to significant increases in mitochondrial protein and enzyme activity in recruited skeletal muscles. Later, the use of stable isotope techniques provided definitive evidence that both acute exercise and exercise training increase the synthesis of mitochondrial proteins (indicative of biogenesis) (5) in human skeletal muscle (11, 12). Extensive research, particularly with the recent advances in molecular genetics in animal models of physiologic exercise, has led to our current understanding that exercise training–induced mitochondrial biogenesis is a highly coordinated process involving both the nuclear and mitochondrial genomes.
Transcriptional control of mitochondrial biogenesis
The nuclear genome encodes most of the genes for the mitochondria, whereas mitochondria possess an independent genome consisting of 37 genes, 13 of which encode for essential proteins in the electron transport chain. Thus, mitochondrial biogenesis requires fine-tuned coordination between transcription of nuclear and mitochondrial genome–encoded genes. The transcription of mitochondrial genes is primarily regulated through the transcriptional coactivator peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) (13). PGC-1α has been implicated in the regulation of skeletal muscle mitochondrial biogenesis as a result of exercise training, and hence is a commonly used marker of skeletal muscle adaptation to exercise training (14). Global genetic deletion of the Pgc-1a gene in mice blunted endurance exercise training–induced mitochondrial biogenesis in skeletal muscle (15). Furthermore, muscle-specific deletion of Pgc-1a showed compromised exercise performance, along with a reduction in several markers for mitochondrial biogenesis (16, 17), as well as blunted mitochondrial biogenesis after exercise training (18). On the contrary, transgenic mice with muscle-specific overexpression of Pgc-1a displayed a significantly enhanced oxidative phenotype in skeletal muscle, along with significantly increased mitochondrial proteins in otherwise fast-twitch, glycolytic muscle fibers (19), indicative of increased mitochondrial biogenesis. Taken together, these findings strongly support that transcriptional regulation through PGC-1α is vital for skeletal muscle mitochondrial adaptation in response to endurance exercise training.
Although PGC-1α has profound influence on mitochondrial biogenesis through its control of gene transcription, PGC-1α does not act alone. As a transcriptional coactivator, PGC-1α relies on transcription factors to promote gene transcription in the process of mitochondrial biogenesis. Once activated in response to exercise, PGC-1α works cooperatively with estrogen-related receptor (ERR)-α and nuclear respiratory factor (NRF)-1 to increase nuclear transcription of most mitochondrial genes (20, 21). PGC-1α interacts with ERRα through LLKYL, a leucine-rich nuclear receptor interacting motif (22), inducing transcription of nuclear genome–encoded mitochondrial genes that are involved in a myriad of mitochondrial functions [reviewed in Giguère (23)], as well as ERRα itself (24). In recent work, Cho et al. (25) showed that PGC-1α/ERRα transcriptional activity in C2C12 myotubes is dependent on another associated protein: PGC-1– and ERR-induced regulator in muscle (Perm)-1. Knockdown of Perm1 in cultured cells reduces the expression of Pgc-1α/Errα target genes and O2 consumption, but the role of Perm1 in skeletal muscle in vivo in the context of exercise is not yet known.
PGC-1α interacts with NRF1 at aa 103–403, which partially overlaps the region that interacts with peroxisome proliferator-activated receptor (PPAR)-γ (20). Exercise increases the interaction of PGC-1α/NRF1 in the nucleus and increases the transcription of nuclear genome–encoded genes for electron transport chain proteins in the mitochondria (20, 21). PGC-1α/NRF1 also increases the transcription of nuclear genome–encoded transcription factor A, mitochondrial (Tfam), a key mitochondrial transcription factor (20). In addition, PGC-1α translocates to the mitochondria in response to exercise, where it forms a complex with Tfam on the D-loop of mtDNA, promoting the transcription of mitochondrial genome–encoded genes and mtDNA replication in mitochondrial biogenesis (20, 21, 26). Therefore, functional interactions of PGC-1α with ERRα and NRF1 and its translocation to mitochondria allow for orchestrated regulation of two independent genomes to meet the transcriptional requirements of exercise-induced mitochondrial biogenesis.
Upstream regulators of PGC-1α
Both the transcription of PGC-1a mRNA and subcellular translocation of PGC-1α protein in inducing the transcription of nuclear- and mitochondrial genome–encoded mitochondrial mRNAs are regulated by multiple upstream factors under the condition of exercise (27). Current evidence suggests that PGC-1α is regulated by factors that are sensitive to the mechanical and energy-related cues imposed by prolonged exercise (21, 28–30). As such, these interconnected signaling pathways represent major targets for future development of translational interventions and for determining the efficacy of a given exercise stimulus.
p38 MAPK
The stress-inducible p38 MAPK has long been suggested to regulate exercise-related PGC-1α activity and expression. Although p38 MAPK is activated by various types of exercise (27, 31), the precise mechanism of exercise-induced activation and the isoform specificity remain to be fully elucidated. One potential mechanism of exercise-induced p38 MAPK activation is through the elevation of cytosolic Ca2+ during muscle contraction. Wright et al. (32) showed that caffeine-stimulated Ca2+ release in skeletal muscle ex vivo leads to activation of p38 MAPK and phosphorylation of the downstream target, activating transcription factor (ATF)-2. This treatment increases Pgc-1a mRNA expression, which can be blocked by a pharmacological inhibitor specific to the α and β isoforms of p38.KN93, a specific blocker for Ca2+/calmodulin-dependent protein kinase II (CaMKII), also blocked ATF2 activation, suggesting that activation of p38 MAPK is dependent on CaMKII activity. It is worth noting that activation of p38 MAPK by inflammatory cytokines is capable of phosphorylating PGC-1α at Thr262, Ser265, and Thr298, causing PGC-1α stabilization and activation (28). However, the relevance of this inflammatory cytokine-mediated regulation of PGC-1α activity in exercise has not been investigated.
In addition to p38 MAPK-induced ATF2 activation with endurance exercise, it has been demonstrated in vitro that p38 MAPK also phosphorylates and activates myocyte enhancer factor (MEF)-2 (33). In vivo studies using bioluminescence imaging showed that MEF2- and ATF2-binding sites of the Pgc-1a promoter are critical for muscle contraction–induced Pgc-1a promoter activity, possibly underscoring enhanced Pgc-1a mRNA expression in response to exercise (29). This finding strongly suggests a role for p38 MAPK in regulating PGC-1α gene expression in skeletal muscle. More convincing evidence for the functional importance of p38 MAPK in exercise-induced mitochondrial biogenesis in skeletal muscle has been obtained in mouse molecular genetic models. Mice with muscle-specific deletions of the p38g gene, but not those of the p38a or p38b gene, showed blunted increases of Pgc-1a mRNA expression and protein markers of mitochondrial biogenesis in skeletal muscle in response to exercise (34). This phenotypic feature is very similar to that in mice with muscle-specific Pgc-1a gene deletion (18). Consistent with these findings, Boppart et al. (35) have shown that p38γ, not p38α, is activated in skeletal muscle after an acute bout of exercise. Therefore, the current understanding is that activation of p38 MAPK, specifically the p38γ isoform, promotes mitochondrial biogenesis in response to exercise, mainly through transcriptional regulation of the Pgc-1a gene.
5′-AMP-activated protein kinase
Prolonged exercise requires a large amount of ATP, which may result in at least a transient and local accumulation of ADP and AMP in the heavily recruited fibers. The energy-sensitive 5′ AMP-activated protein kinase (AMPK) is activated primarily by the metabolic cue of increased AMP and, to a lesser degree, ADP to restore energy homeostasis (36, 37). The current understanding is that elevated AMP and/or ADP binds to the AMPKγ subunit (38), resulting in a conformational change that exposes the amino-terminal kinase domain in the catalytic α subunit. The α subunit can then be phosphorylated at Thr172 by activating kinases, such as CaMK kinase (CaMKK) and liver kinase B (LKB)-1, resulting in AMPK activation (39, 40). Muscle-specific knockout of both α isoforms of AMPK results in impaired exercise capacity and dysfunctional mitochondria (41), strongly suggesting a functional link between AMPK and mitochondrial quality. In rat skeletal muscle, exercise-induced AMPK activation is accompanied by an increase in PGC-1α protein, and the AMP mimetic 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) is capable of replicating this effect in vitro (42). Active AMPK promotes PGC-1α transcriptional activity, presumably through phosphorylation at Thr177 and Ser538 (43). In addition, exercise-induced PGC-1α translocation to the nucleus and mitochondria appears to be dependent on AMPK activation (21, 30), but it is unclear by what mechanism AMPK facilitates PGC-1α translocation. Therefore, AMPK may act in concert with p38 MAPK to transcriptionally regulate mitochondrial biogenesis through PGC-1α in response to the energetic stress of exercise training.
p53
p53, a well-known tumor suppressor gene, has been linked recently to exercise-induced mitochondrial biogenesis in skeletal muscle (44). In particular, global genetic deletion of the p53 gene in mice led to a dose-dependent reduction in exercise capacity, along with a reduced oxidative phenotype in skeletal muscle (44). One proposed mechanism of p53 function in this context is the lack of transcriptional activation of the Tfam gene and, hence, mtDNA transcription and replication (44). There is evidence of exercise-induced translocation of p53 to the mitochondria, where it interacts with Tfam at the D-loop of mtDNA, promoting mitochondrial biogenesis (45). It is unclear whether AMPK-dependent PGC-1α translocation and interaction with Tfam at the D-loop of mtDNA is a redundant process or is additive to p53 mitochondrial translocation.
Further support for a potent role of p53 in mitochondrial adaptation is the finding that p53 is essential for exercise-induced activation of multiple signaling pathways related to mitochondrial biogenesis, as shown by attenuated or delayed p38 MAPK, AMPK, and CaMKII responses to acute exercise, along with impaired induction of Pgc-1a mRNA and protein in p53 knockout mice (46). These findings support a novel role of p53 as an inducer of mitochondrial biogenesis, acting upstream of Pgc-1α signaling in mitochondrial gene transcription and mtDNA replication. A recent study showed that humans with Li-Fraumeni syndrome, a germline mutation of the p53 gene resulting in an inhibited DNA damage response and early onset of sarcoma and carcinomas, have enhanced capacity of oxidative phosphorylation along with elevated Tfam and cytochrome c oxidase 2 (SCO2) in skeletal muscle (47). Therefore, the physiologic function of p53 in exercise training–induced mitochondrial adaptation remains an unresolved question.
Epigenetic regulation of the Pgc-1α gene
Epigenetic regulation of the Pgc-1a gene has broad implications in exercise-induced adaptions. Hypermethylation of the Pgc-1a promoter suppresses its expression, along with reduced target gene expression (48), highlighting the functional importance of epigenetic regulation of the Pgc-1a gene. Particularly relevant to exercise training are the findings that exercise induces expression of PGC-1α, pyruvate dehydrogenase lipoamide kinase (PDK)-4, and PPAR-δ, concurrent with hypomethylation of their respective promoters. These findings were replicated in ex vivo skeletal muscle contractions and by caffeine stimulation in cultured myotubes (49). Recent epigenetic studies in human skeletal muscle are also consistent with these findings (50). Hypermethylation of the PGC-1a promoter, including CpG-260, in human primary muscle cells treated with palmitate and in skeletal muscle of patients with type 2 diabetes negatively correlates with PGC-1a and its target gene expression (48). Laker et al. (51) found that obesity during pregnancy induces Pgc-1a hypermethylation at CpG-260 in the offspring skeletal muscle with reduced mRNA expression, which is completely prevented by maternal exercise during pregnancy. Most important, amelioration of Pgc-1a hypermethylation in the offspring skeletal muscle prevents early onset of age-dependent insulin resistance. These findings strongly suggest the importance of epigenetic regulation of the Pgc-1a gene in conferring the health benefits of maternal exercise to the offspring.
Posttranslational modifications of PGC-1α
We have discussed how phosphorylation of PGC-1α protein by AMPK influences its cotranscriptional activity, but other posttranslational modifications also regulate PGC-1α in skeletal muscle. For example, Cantó et al. (37) showed that AMPK enhances the NAD-dependent deacetylase Sirtuin (SIRT)-1 activity by increasing cellular level of NAD+, resulting in the deacetylation of SIRT1 targets, including PGC-1α, and modulation of their activities. Consistent with these findings, knockdown of SIRT1 in skeletal muscle results in decreased mitochondrial content and respiration and reduced exercise capacity (52), whereas overexpression of SIRT1 increases the expression of multiple nuclear and mitochondrial genome–encoded electron transport chain proteins through deacetylation of PGC-1α protein (53). Therefore, PGC-1α acetylation is another vital part of exercise training–induced mitochondrial biogenesis in skeletal muscle. It is noteworthy that PGC-1α protein can also be regulated by methylation at multiple arginine sites by protein arginine methyltransferase 1 (PRMT1), resulting in increased PGC-1α activity (54). However, it is not yet clear whether this methylation-mediated activation is relevant to exercise training.
Translational control of mitochondrial biogenesis
It is clear that acute exercise and exercise training increases PGC-1α activity and its upstream regulators to promote the transcription of mitochondrial genes, but transcribed mRNA must be translated into functional proteins to achieve mitochondrial biogenesis. The main regulator of translation and protein synthesis in skeletal muscle is the mechanistic target of rapamycin complex (mTORC)-1, which promotes translation primarily through its downstream substrate’s eukaryotic initiation factor 4E-binding protein (4E-BP)-1 and p70 ribosomal S6 kinase (S6K)-1 [reviewed in Laplante and Sabatini (55)]. When nonphosphorylated, 4E-BP1 inhibits translation by binding to the scaffold protein eukaryotic initiation factor (eIF)-4E on the 5′ end of nascent mRNA, but dissociates from eIF4E upon phosphorylation by mTORC1 at Thr37 and -46. This dissociation allows other initiation factors to bind, promoting translation (55, 56). Phosphorylation of S6K1 at Thr389 by mTORC1 also facilitates concurrent initiation factor binding to mRNAs to promote translation (56).
AMPK, when activated, inhibits mTORC1, presumably to conserve energy by inhibiting translation and protein synthesis [reviewed in Laplante and Sabatini (55)]. It is notable that S6K1 and 4E-BP1 phosphorylation has been shown to be maintained during sustained muscle contraction ex vivo (57), even though protein synthesis rates are decreased by as much as 75% by electrical stimulation in epitrochlearis muscle (58). It is possible that phosphorylation of S6K1 and 4E-BP1 is maintained through an mTORC1-independent mechanism, such as p38 MAPK-dependent 90 kDa ribosomal S6K (RSK). RSK phosphorylation is increased with sustained muscle contraction (59) and can phosphorylate S6K1 and 4E-BP1 at the same sites as mTORC1 (60, 61). However, this hypothesis does not explain how translation and protein synthesis remain stalled during ex vivo muscle contraction. One possibility is the inhibition of eukaryotic elongation factor (eEF)-2 soon after exercise commences (60, 62), which promotes the energy-costly elongation step of translation (63). In this case, the maintenance of S6K1 and 4E-BP1 phosphorylation during prolonged exercise may act to keep the system “primed” (57) so that translation can resume as soon as exercise ceases, and eEF2 is activated once sufficient ATP again becomes available. This mechanism could help facilitate the translation of mitochondrial genes after their exercise-induced expression through p38 MAPK, AMPK, or p53-dependent mechanisms.
There is evidence to support an alternate theory that enhanced mitochondrial biogenesis with exercise begins early during the onset of exercise, before transcriptional activation of the Pgc-1a gene (64, 65), perhaps because of translation of pre-existing mitochondrial gene mRNA. Because translation may be inhibited through eEF2 as early as 1 min into a bout of endurance exercise (62), it is possible that exercise-induced translation of mitochondrial genes is regulated by mechanisms other than the canonical regulation through mTORC1. For example, inhibition of mTORC1 by dietary restriction in Drosophila induces expression of its target, the translational repressor 4E-BP1 (66). The induction of 4E-BP1 does not hinder the translation of mitochondrial genes, but instead induces mitochondrial protein expression (66). Furthermore, long-term inhibition of mTORC1 with rapamycin does not affect mitochondrial protein synthesis (67) or respiration (68) in skeletal muscle, nor does it affect exercise capacity in mice (68). Thus, translation of mitochondrial genes may not be dependent on mTORC1, or there may be redundant, unappreciated mechanisms that compensate when mTORC1 is inhibited for prolonged periods, such as during sustained exercise, to ensure energy homeostasis. Clearly, much remains to be understood with regard to how transcription, translation, and mitochondrial biogenesis are regulated during exercise in real time.
MITOCHONDRIAL REMODELING
In addition to mitochondrial biogenesis, dynamic remodeling of the mitochondrial reticulum assists in promoting mitochondrial quality. The mitochondrial reticulum presumably undergoes constant fusion (the joining of separate mitochondria leading to the formation of continuous networks) and fission (the division of mitochondria into individual units) to maintain mitochondrial content and structural homeostasis and, thereby, mitochondrial quality. Deletions of various components involved in mitochondrial fusion or fission are embryonically lethal, highlighting the functional importance of mitochondrial dynamics (6), but the role of mitochondrial remodeling in skeletal muscle in the context of exercise is still emerging.
Mitochondrial fusion
Mitochondrial fusion is a process involving the joining of the inner mitochondrial membrane (IMM) and outer mitochondrial membrane (OMM) of either two distinct mitochondria or different regions of the mitochondrial reticulum, which results in the mixing of membranes as well as content within the intermembrane space and the matrix (69). Mitochondrial fusion clearly plays an important role in maintaining mitochondrial integrity, as its disruption in skeletal muscle results in accumulation of mtDNA mutations and mitochondrial dysfunction, with poor mitochondrial quality (70). Mitochondrial fusion is dependent on the transmembrane GTPases mitofusion (MFN)-1 and -2 and optic atrophy (OPA)-1. MFN1 and -2 are functionally redundant transmembrane proteins embedded in the OMM that coordinate fusion of OMMs between regions of the reticulum (71). On the one hand, in vitro data indicate that PGC-1α/ERRα promotes MFN2 transcription by binding to the cis-elements on the promoter (72). On the other hand, OPA1 is associated with and essential for mitochondrial fusion of the inner mitochondrial membrane (IMM). Deletion of the Opa1 gene prevents IMM fusion, whereas deletion of the MFfn1/2 genes results in loss of both OMM and IMM fusion (73), significantly impairing mitochondrial function (74). It is generally accepted that mitochondrial fusion mixes mitochondrial components, which may be critical for mitochondrial quality control.
Mitochondrial fission
Mitochondrial fusion is opposed by mitochondrial fission, which promotes the fragmentation of mitochondria. Where fusion requires intact mitochondrial membrane potential (6), fission is triggered in part by a loss of membrane potential and separates damaged mitochondria from the healthy reticulum. Therefore, fission is also critical for maintaining overall mitochondrial quality. It is well accepted that GTPase dynamin-related protein (DRP)-1 is crucial for fission of the OMM (75). Depolarization of mitochondria recruits the soluble N-terminal GTPase DRP1 from the cytosol to assemble on the damaged mitochondria. Early studies suggest that DRP1-GTPase–mediated mitochondrial fission is dependent on mitochondrial fission (FIS)-1 and the FIS1 adapter mitochondrial division (MDV)-1 protein in recruiting DRP1 to the mitochondria (76, 77). FIS1 is anchored to the outer membrane, where it forms a 6-helix bundle with tandem tetratricopeptide repeat motifs that serve as an interface between MDV1 and DRP1 (78). More recent evidence suggests that FIS1 is dispensable for mitochondrial fission, and instead mitochondrial fission factor (MFF)-1, a novel tail-anchored membrane protein, is essential for the recruitment of DRP1 to the damaged mitochondria (79). DRP1 forms a spiral around the OMM that, after the hydrolysis of bound GTP, causes the budding off of the damaged portion of the mitochondria. Once isolated, the damaged mitochondria are then degraded by mitophagy and their components recycled (discussed in subsequent sections).
Mitochondrial fusion and fission in exercise
The sequence of mitochondrial fission and fusion events in skeletal muscle in response to exercise training is not well understood. As mentioned, mitochondrial fusion is dominant in metabolically active cells. It allows the spreading of metabolites, enzymes, and mitochondrial gene products throughout the entire mitochondrial reticulum (80). The hypothesis that fusion promotes electrical coupling suggests that membrane potential equilibrates from oxygen-rich areas along mitochondrial filaments to remote, low-oxygen areas, driving ATP synthesis (81). This complementation of electrical potential would be advantageous in skeletal muscle because of the high energy demand of exercise (2, 6). However, acute exercise in rats resulted in reduced MFN1/2 protein expression in skeletal muscle up to 24 h after exercise (72, 82), although Mfn1 and -2 mRNA was elevated as early as 3 and 12 h after exercise, respectively (82). It is noteworthy that acute exercise induced Fis1 protein expression in skeletal muscle after exercise. In mice, Drp1 activation in skeletal muscle was increased during exercise at 1 h and remained elevated at exhaustion (83). Together, these data appear to support the notion that acute exercise favors mitochondrial fission in skeletal muscle, consistent with a model where acute exercise may stimulate the clearance of damaged mitochondria to make way for the incorporation, through fusion, of newly synthesized mitochondria during recovery, facilitating improvement of mitochondrial quality (Fig. 1). However, one should not simply extrapolate these acute exercise findings to form a conclusion about exercise training. In addition, it should be noted that the aforementioned data in skeletal muscle are limited to protein and mRNA expression of a few known fusion and fission regulators. The development of more dynamic methods to visualize mitochondrial fusion and fission in skeletal muscle, along with the use of molecular markers with specificity to mitochondrial health status, would be of great benefit to the field.
MITOCHONDRIAL BREAKDOWN
The sequestering of damaged or dysfunctional mitochondria through fission is followed by breakdown. This process is emerging as a vital component of mitochondrial quality control that is sensitive to exercise training (Fig. 1). Autophagy is a catabolic process by which organelles, protein aggregates, and pathogens are degraded to promote survival. Autophagy can be remarkably specific, targeting only particular organelles, such as mitochondria—termed mitophagy [reviewed in Miller et al. (7)]. After fission, damaged or dysfunctional mitochondria are sequestered into a double-membrane structure, called an autophagosome. The formation of the autophagosomes is triggered by a cascade of signaling events, beginning with the activation of uncoordinated (UNC)-51-like kinase 1 (ULK1), which is in complex with autophagy-related (ATG)-13 gene and focal adhesion kinase family interacting protein of 200 kDa (FIP200) (84). Activation of the ULK1/ATG13/FIP200 complex leads to the generation of the autophagosome membrane through ATG6 (also known as Beclin1). The elongation and closure of the autophagosome around the target mitochondria is dependent in part on microtubule-associated protein 1A/1B-light chain (MAP1LC)-3, which docks the autophagosome to the mitochondria through mitochondria-associated proteins containing MAP1LC3-interacting regions (LIRs) [e.g., BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3)/Nix and p62] (85). Upon maturation, the autophagosome fuses with a lysosome, resulting in the degradation of the content to basic amino acids (86), presumably providing substrates for the synthesis of new protein. Mitophagy acts as an intracellular recycling mechanism that is critical for the maintenance of mitochondrial quality.
Exercise-induced regulation of mitophagy
Our laboratory, along with others, has demonstrated that both acute exercise and exercise training improve mitochondrial quality in skeletal muscle, in part, through enhanced autophagy and mitophagy (83, 87–89). Many of the mechanisms underlying this important adaptive process in skeletal muscle remain unclear. Evidence in lower organisms and in vitro models suggests that mitophagy is regulated by AMPK. For example, isolated hepatocytes deficient in AMPK have increased levels of mitochondrial proteins and the cargo receptor p62/sequestosome (SQSTM)-1, as well as abnormal mitochondrial structure and disrupted mitochondrial membrane potential (90). Therefore, inhibition of AMPK may impair mitophagy, thus resulting in accumulation of mitochondria of poor quality. AMPK is proposed to promote mitophagy through a 2-pronged mechanism. First, by inhibiting mTORC1 from localizing to the lysosome. When activated, mTORC1 is shuttled to the lysosome by the Rag-GTPase complex, Ragulator, where mTORC1 accumulates and renders the lysosome inactive (91). mTORC1 also inhibits the activity of ULK1 by joining the ULK1/Atg13/FIP200 complex and phosphorylating ULK1 at Ser757 (55). These actions of mTORC1 normally inhibit autophagy and, presumably, mitophagy as well. AMPK promotes autophagy and mitophagy by inhibiting mTORC1 through phosphorylating the upstream inhibitor, tuberous sclerosis complex (TSC)-2 tumor suppressor, and the mTORC1 component raptor (55), thus hindering the localization of mTORC1 to the lysosome. Second, AMPK is proposed to regulate mitophagy through direct interaction with ULK1. Among 4 consensus AMPK phosphorylation sites, 3 (Ser555, Thr574, and Ser637) were phosphorylated after AMPK activation by phenformin treatment (90). Mutation of Ser555 to alanine resulted in the most dramatic reduction of phosphorylation of ULK1, suggesting that Ser555 is the main AMPK phosphorylation site. In murine C2C12 myoblasts, AMPK interacts with ULK1, as measured by immunoprecipitation, but not with other autophagy-related proteins (92). A known AMPK agonist, AICAR, increases phosphorylation of ULK1 at Ser555 and disrupts the interaction of mTORC1 with the ULK1/Atg13/FIP200 complex in vitro. Taken together, these findings show that activation of AMPK may promote mitophagy by inhibition of mTORC1 localization to the lysosome and simultaneous activation of ULK1 by phosphorylation at Ser555 (Fig. 1).
Exercise may induce mitophagy in skeletal muscle through the AMPK–ULK1 axis, but this hypothesis has not been reconciled with how damaged mitochondria are recognized by the mitophagy machinery in response to exercise. One possible way by which damaged mitochondria are targeted for degradation through mitophagy is through the phosphatase and tensin homolog (PTEN)-induced putative kinase (PINK)/Parkin cascade. PINK1 is a mitochondria-targeted serine/threonine kinase that is constantly imported into the mitochondria, where it is cleaved and subsequently degraded by mitochondrial proteases under basal conditions (93). When mitochondrial membrane potential is lost, such as under the condition of mitochondrial damage, PINK1 is not imported into the mitochondria, but accumulates on the translocase of the OMM (TOM) complexes (93). This leads to recruitment of the E3 ubiquitin ligase Parkin to the damaged mitochondria, where it interacts with PINK1 and the voltage-dependent anion channel (VDAC). Once recruited to mitochondria, Parkin mediates the polyubiquitination of several mitochondrial proteins, including MFN1/2 (94). Ubiquitination of MFN1/2 inhibits its function, thus facilitating the fragmentation of mitochondria through fission. Further, Parkin polyubiquitinates VDAC, resulting in the accumulation of LIR-containing p62, which binds to MAP1LC3, thus recruiting the autophagosome to the damaged mitochondria for degradation through mitophagy (95). Absence of either PINK1 or Parkin results in an accumulation of dysfunctional and fragmented mitochondria (96), making the PINK/Parkin pathway an important one for the development of interventions that target damaged mitochondria.
Although the PINK/Parkin avenue of mitochondrial breakdown through mitophagy has been intensely examined in various disease models, it remains poorly understood in the context of exercise, with scattered findings. For example, 6 wk of exercise training increases PINK1 mRNA expression in human skeletal muscle (97), and Parkin protein decreases in the fasted state after acute exercise, which may suggest an increase in mitochondrial clearance (98). However, more precise, mechanistic studies are needed to determine the roles of PINK1/Parkin in skeletal muscle mitophagy with exercise.
Another LIR-containing protein, the BH3-only family protein BNIP3, and the analogous protein Nix, may mediate the recognition of damaged mitochondria with exercise. BNIP3 and Nix localize to the OMM where they may interact with MAP1LC3 for the docking of the autophagosome (99). Deletion of the amino terminus of Nix, which contains its LIR, severely reduced the clearance of damaged mitochondria in cultured reticulocytes (99), highlighting the importance of BNIP and Nix in promoting mitophagy. In skeletal muscle, BNIP3 expression was increased in response to acute exercise (98). Our laboratory has demonstrated that BNIP3 expression in skeletal muscle is increased after exercise training (87). Together, these findings illustrate that BNIP3 and Nix are sensitive to both acute exercise and exercise training and thus may be involved in the targeting of damaged mitochondria for degradation through mitophagy in skeletal muscle.
Another factor by which damaged mitochondria are recognized is Fun14 domain-containing protein (FUNDC)-1, recently identified as binding specifically to the OMM to promote mitophagy (100). Basally, FUNDC1 is inhibited through phosphorylation at Tyr18 by Src kinases. However, during hypoxia, Src is inactivated, resulting in the dephosphorylation of FUNDC1 at Tyr18. During hypoxia, ULK1 is simultaneously activated and translocated to the mitochondria where it activates FUNDC1 by phosphorylation at Ser17, increasing the association of ULK1 with MAP1LC3 and the elongating autophagosome and thus facilitating mitochondrial breakdown through mitophagy (101). FUNDC1 has not been investigated in skeletal muscle but could provide an interesting direction for future research.
It is important to reiterate that mitochondria in skeletal muscle exist as an interconnected reticulum (8). In a nonpathologic state, mitochondria that are targeted for degradation through mitophagy in skeletal muscle likely represent only a small subset of the mitochondrial reticulum at any given time. During exercise training, this number presumably increases transiently after each repetitive bout of exercise. As a consequence, mitochondrial quality improves, and the transient increase in mitophagy decreases over time. Therefore, mitophagy in skeletal muscle may be confined to particular locales within the mitochondrial reticulum. Regardless of the mechanism by which damaged mitochondria are recognized in skeletal muscle in response to exercise, gross assessments of mitophagy may not fully represent the effect of exercise on mitochondrial quality through mitophagy.
Basal autophagy and autophagy capacity with exercise
Although the capacity of autophagy and mitophagy in response to a given stress, such as a single bout of exercise, is important in improving mitochondrial quality, basal mitophagy may also be necessary to maintain mitochondrial quality after exercise training. Basal autophagy refers to the continuous degradation of damaged cellular components (including mitochondria) to maintain fundamental cellular functions, whereas autophagy capacity refers to the capacity to increase autophagy flux in response to stress. In supporting the importance of basal autophagy, we have found that exercise training significantly increased expression of autophagy-related genes (indicative of increased autophagy capacity) and basal autophagy, as measured by p62 and MAP1LC3 (87). These features of exercise training were replicated by muscle-specific overexpression of PGC-1α. On the contrary, genetic deletion of the Atg6 (Beclin1) gene in mice led to reduced basal autophagy in skeletal muscle and blunted mitochondrial adaptations after exercise training. Therefore, exercise training may have cumulative effects that result in both a greater capacity to induce autophagy and mitophagy in response to exercise and greater clearance at rest. Theoretically, this state of increased basal autophagy/mitophagy and capacity in skeletal muscle ultimately results in the improvement and maintenance of mitochondrial quality and could be an integral part of how exercise training promotes health and increases physical performance.
PERSPECTIVE AND CONCLUSIONS
Mitochondria are highly dynamic organelles that are capable of meeting the ever-changing energy demands of exercise through multiple interconnected processes. The improvements of mitochondrial quantity and quality are paramount in improving skeletal muscle contractile and metabolic functions. This adaptive process requires a balance between the opposing processes of mitochondrial biogenesis and mitophagy, along with extremely versatile mitochondrial dynamics, which ultimately contribute to the improved physical performance and health benefits of exercise training (Fig. 1). We have highlighted the major molecular regulators of these processes in skeletal muscle in the context of exercise. Better understanding of the mechanisms of mitochondrial quality control will facilitate the discovery and development of targeted therapies.
Glossary
- 4E-BP
eukaryotic translation initiation factor 4E-binding protein
- AICAR
5-aminoimidazole-4-carboxamide ribonucleoside
- AMPK
5′ AMP-activated protein kinase
- ATF
activating transcription factor
- ATG
autophagy-related
- BNIP
BCL2/adenovirus E1B 19 kDa interacting protein
- CaMK
Ca2+/calmodulin-dependent protein kinase
- CaMKK
CaMK kinase
- DRP
dynamin-related protein
- eEF
eukaryotic elongation factor
- eIF
eukaryotic initiation factor
- ERR
estrogen-related receptor
- FIP200
focal adhesion kinase family interacting protein of 200 kDa
- FIS
mitochondrial fission
- IMM
inner mitochondrial membrane
- MAP1LC
microtubule-associated protein 1A/1B-light chain
- MDV
mitochondrial division
- MEF
myocyte enhancer factor
- MFN
mitofusin
- OPA
optic atrophy
- mtDNA
mitochondrial DNA
- mTORC
mechanistic target of rapamycin complex
- NRF
nuclear respiratory factor
- OMM
outer mitochondrial membrane
- PERM
PGC- and ERR-induced regulator in muscle
- PGC-1α
peroxisome proliferator-activated receptor γ coactivtor 1α
- PINK
phosphatase and tensin homolog (PTEN)-induced putative kinase
- PPAR
peroxisome proliferator-activator receptor
- RSK
90 kDa ribosomal S6 kinase
- S6K
S6 kinase
- SIRT
sirtuin
- TFAM
transcription factor A, mitochondrial
- ULK1
uncoordinated (UNC)-51-like autophagy-activating kinase 1
- VDAC
voltage-dependent anion channel
REFERENCES
- 1.Noakes T., Spedding M. (2012) Olympics: run for your life. Nature , 295–296 [DOI] [PubMed] [Google Scholar]
- 2.Janssen, I., Heymsfield, S. B., Baumgartner, R. N., and Ross, R. (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 89, 465–471 [DOI] [PubMed]
- 3.Crane J. D., Abadi A., Hettinga B. P., Ogborn D. I., MacNeil L. G., Steinberg G. R., Tarnopolsky M. A. (2013) Elevated mitochondrial oxidative stress impairs metabolic adaptations to exercise in skeletal muscle. PLoS One , e81879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson M. L., Irving B. A., Lanza I. R., Vendelbo M. H., Konopka A. R., Robinson M. M., Henderson G. C., Klaus K. A., Morse D. M., Heppelmann C., Bergen H. R. III, Dasari S., Schimke J. M., Jakaitis D. R., Nair K. S. (2014) Differential effect of endurance training on mitochondrial protein damage, degradation, and acetylation in the context of aging. [E-pub ahead of print] J. Gerontol. A Biol. Sci. Med. Sci. 10.1093/gerona/glu221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller B. F., Hamilton K. L. (2012) A perspective on the determination of mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. , E496–E499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youle R. J., van der Bliek A. M. (2012) Mitochondrial fission, fusion, and stress. Science , 1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campello S., Strappazzon F., Cecconi F. (2014) Mitochondrial dismissal in mammals, from protein degradation to mitophagy. Biochim. Biophys. Acta , 451–460 [DOI] [PubMed] [Google Scholar]
- 8.Kirkwood S. P., Munn E. A., Brooks G. A. (1986) Mitochondrial reticulum in limb skeletal muscle. Am. J. Physiol. , C395–C402 [DOI] [PubMed] [Google Scholar]
- 9.Ryan M. T., Hoogenraad N. J. (2007) Mitochondrial-nuclear communications. Annu. Rev. Biochem. , 701–722 [DOI] [PubMed] [Google Scholar]
- 10.Holloszy J. O. (1967) Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. , 2278–2282 [PubMed] [Google Scholar]
- 11.Wilkinson S. B., Phillips S. M., Atherton P. J., Patel R., Yarasheski K. E., Tarnopolsky M. A., Rennie M. J. (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. , 3701–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scalzo R. L., Peltonen G. L., Binns S. E., Shankaran M., Giordano G. R., Hartley D. A., Klochak A. L., Lonac M. C., Paris H. L., Szallar S. E., Wood L. M., Peelor F. F. III, Holmes W. E., Hellerstein M. K., Bell C., Hamilton K. L., Miller B. F. (2014) Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J. , 2705–2714 [DOI] [PubMed] [Google Scholar]
- 13.Vega R. B., Huss J. M., Kelly D. P. (2000) The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. , 1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilegaard H., Saltin B., Neufer P. D. (2003) Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J. Physiol. , 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leick L., Lyngby S. S., Wojtaszewski J. F., Pilegaard H. (2010) PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp. Gerontol. , 336–342 [DOI] [PubMed] [Google Scholar]
- 16.Handschin C., Chin S., Li P., Liu F., Maratos-Flier E., Lebrasseur N. K., Yan Z., Spiegelman B. M. (2007) Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J. Biol. Chem. , 30014–30021 [DOI] [PubMed] [Google Scholar]
- 17.Zechner C., Lai L., Zechner J. F., Geng T., Yan Z., Rumsey J. W., Collia D., Chen Z., Wozniak D. F., Leone T. C., Kelly D. P. (2010) Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. , 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geng T., Li P., Okutsu M., Yin X., Kwek J., Zhang M., Yan Z. (2010) PGC-1alpha plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am. J. Physiol. Cell Physiol. , C572–C579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J., Wu H., Tarr P. T., Zhang C. Y., Wu Z., Boss O., Michael L. F., Puigserver P., Isotani E., Olson E. N., Lowell B. B., Bassel-Duby R., Spiegelman B. M. (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature , 797–801 [DOI] [PubMed] [Google Scholar]
- 20.Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., Spiegelman B. M. (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell , 115–124 [DOI] [PubMed] [Google Scholar]
- 21.Safdar A., Little J. P., Stokl A. J., Hettinga B. P., Akhtar M., Tarnopolsky M. A. (2011) Exercise increases mitochondrial PGC-1alpha content and promotes nuclear-mitochondrial cross-talk to coordinate mitochondrial biogenesis. J. Biol. Chem. , 10605–10617 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Ichida M., Nemoto S., Finkel T. (2002) Identification of a specific molecular repressor of the peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1alpha). J. Biol. Chem. , 50991–50995 [DOI] [PubMed] [Google Scholar]
- 23.Giguère V. (2008) Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr. Rev. , 677–696 [DOI] [PubMed] [Google Scholar]
- 24.Schreiber S. N., Knutti D., Brogli K., Uhlmann T., Kralli A. (2003) The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J. Biol. Chem. , 9013–9018 [DOI] [PubMed] [Google Scholar]
- 25.Cho Y., Hazen B. C., Russell A. P., Kralli A. (2013) Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1)- and estrogen-related receptor (ERR)-induced regulator in muscle 1 (Perm1) is a tissue-specific regulator of oxidative capacity in skeletal muscle cells. J. Biol. Chem. , 25207–25218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon, J. W., Rungi, A. A., Inagaki, H., and Hood, D. A. (2001) Effects of contractile activity on mitochondrial transcription factor A expression in skeletal muscle. J. Appl. Physiol. 90, 389-396 [DOI] [PubMed]
- 27.Zhang Y., Uguccioni G., Ljubicic V., Irrcher I., Iqbal S., Singh K., Ding S., Hood D. A. (2014) Multiple signaling pathways regulate contractile activity-mediated PGC-1α gene expression and activity in skeletal muscle cells. Physiol. Rep. , e12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puigserver P., Rhee J., Lin J., Wu Z., Yoon J. C., Zhang C. Y., Krauss S., Mootha V. K., Lowell B. B., Spiegelman B. M. (2001) Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol. Cell , 971–982 [DOI] [PubMed] [Google Scholar]
- 29.Akimoto T., Li P., Yan Z. (2008) Functional interaction of regulatory factors with the Pgc-1alpha promoter in response to exercise by in vivo imaging. Am. J. Physiol. Cell Physiol. , C288–C292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith B. K., Mukai K., Lally J. S., Maher A. C., Gurd B. J., Heigenhauser G. J., Spriet L. L., Holloway G. P. (2013) AMP-activated protein kinase is required for exercise-induced peroxisome proliferator-activated receptor co-activator 1 translocation to subsarcolemmal mitochondria in skeletal muscle. J. Physiol. , 1551–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akimoto T., Pohnert S. C., Li P., Zhang M., Gumbs C., Rosenberg P. B., Williams R. S., Yan Z. (2005) Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. , 19587–19593 [DOI] [PubMed] [Google Scholar]
- 32.Wright D. C., Geiger P. C., Han D. H., Jones T. E., Holloszy J. O. (2007) Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J. Biol. Chem. , 18793–18799 [DOI] [PubMed] [Google Scholar]
- 33.Zhao M., New L., Kravchenko V. V., Kato Y., Gram H., di Padova F., Olson E. N., Ulevitch R. J., Han J. (1999) Regulation of the MEF2 family of transcription factors by p38. Mol. Cell. Biol. , 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogozelski A. R., Geng T., Li P., Yin X., Lira V. A., Zhang M., Chi J. T., Yan Z. (2009) p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PLoS One , e7934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boppart M. D., Asp S., Wojtaszewski J. F., Fielding R. A., Mohr T., Goodyear L. J. (2000) Marathon running transiently increases c-Jun NH2-terminal kinase and p38 activities in human skeletal muscle. J. Physiol. , 663–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mounier R., Théret M., Lantier L., Foretz M., Viollet B. (2015) Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol. Metab. , 275–286 [DOI] [PubMed] [Google Scholar]
- 37.Cantó C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., Auwerx J. (2009) AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature , 1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao B., Heath R., Saiu P., Leiper F. C., Leone P., Jing C., Walker P. A., Haire L., Eccleston J. F., Davis C. T., Martin S. R., Carling D., Gamblin S. J. (2007) Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature , 496–500 [DOI] [PubMed] [Google Scholar]
- 39.Davies S. P., Helps N. R., Cohen P. T., Hardie D. G. (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase: studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. , 421–425 [DOI] [PubMed] [Google Scholar]
- 40.Thomson D. M., Porter B. B., Tall J. H., Kim H. J., Barrow J. R., Winder W. W. (2007) Skeletal muscle and heart LKB1 deficiency causes decreased voluntary running and reduced muscle mitochondrial marker enzyme expression in mice. Am. J. Physiol. Endocrinol. Metab. , E196–E202 [DOI] [PubMed] [Google Scholar]
- 41.Lantier L., Fentz J., Mounier R., Leclerc J., Treebak J. T., Pehmøller C., Sanz N., Sakakibara I., Saint-Amand E., Rimbaud S., Maire P., Marette A., Ventura-Clapier R., Ferry A., Wojtaszewski J. F., Foretz M., Viollet B. (2014) AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. , 3211–3224 [DOI] [PubMed] [Google Scholar]
- 42.Irrcher I., Adhihetty P. J., Sheehan T., Joseph A. M., Hood D. A. (2003) PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am. J. Physiol. Cell Physiol. , C1669–C1677 [DOI] [PubMed] [Google Scholar]
- 43.Jäger S., Handschin C., St-Pierre J., Spiegelman B. M. (2007) AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA , 12017–12022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park, J. Y., Wang, P. Y., Matsumoto, T., Sung, H. J., Ma, W., Choi, J. W., Anderson, S. A., Leary, S. C., Balaban, R. S., Kang, J. G., and Hwang, P. M. (2009) p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ. Res. 105, 705–712, 711 p following 712 [DOI] [PMC free article] [PubMed]
- 45.Saleem A., Hood D. A. (2013) Acute exercise induces tumour suppressor protein p53 translocation to the mitochondria and promotes a p53-Tfam-mitochondrial DNA complex in skeletal muscle. J. Physiol. , 3625–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saleem A., Carter H. N., Hood D. A. (2014) p53 is necessary for the adaptive changes in cellular milieu subsequent to an acute bout of endurance exercise. Am. J. Physiol. Cell Physiol. , C241–C249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P. Y., Ma W., Park J. Y., Celi F. S., Arena R., Choi J. W., Ali Q. A., Tripodi D. J., Zhuang J., Lago C. U., Strong L. C., Talagala S. L., Balaban R. S., Kang J. G., Hwang P. M. (2013) Increased oxidative metabolism in the Li-Fraumeni syndrome. N. Engl. J. Med. , 1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrès R., Osler M. E., Yan J., Rune A., Fritz T., Caidahl K., Krook A., Zierath J. R. (2009) Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. , 189–198 [DOI] [PubMed] [Google Scholar]
- 49.Barrès R., Yan J., Egan B., Treebak J. T., Rasmussen M., Fritz T., Caidahl K., Krook A., O’Gorman D. J., Zierath J. R. (2012) Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. , 405–411 [DOI] [PubMed] [Google Scholar]
- 50.Lindholm, M. E., Marabita, F., Gomez-Cabrero, D., Rundqvist, H., Ekstrom, T. J., Tegner, J., and Sundberg, C. J. (2014) An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training. Epigenetics 9, 1557-1569 [DOI] [PMC free article] [PubMed]
- 51.Laker R. C., Lillard T. S., Okutsu M., Zhang M., Hoehn K. L., Connelly J. J., Yan Z. (2014) Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes , 1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menzies K. J., Singh K., Saleem A., Hood D. A. (2013) Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. , 6968–6979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chalkiadaki A., Igarashi M., Nasamu A. S., Knezevic J., Guarente L. (2014) Muscle-specific SIRT1 gain-of-function increases slow-twitch fibers and ameliorates pathophysiology in a mouse model of Duchenne muscular dystrophy. PLoS Genet. , e1004490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teyssier C., Ma H., Emter R., Kralli A., Stallcup M. R. (2005) Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev. , 1466–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laplante M., Sabatini D. M. (2012) mTOR signaling in growth control and disease. Cell , 274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell , 569–580 [DOI] [PubMed] [Google Scholar]
- 57.Liu Y., Vertommen D., Rider M. H., Lai Y. C. (2013) Mammalian target of rapamycin-independent S6K1 and 4E-BP1 phosphorylation during contraction in rat skeletal muscle. Cell. Signal. , 1877–1886 [DOI] [PubMed] [Google Scholar]
- 58.Miranda L., Horman S., De Potter I., Hue L., Jensen J., Rider M. H. (2008) Effects of contraction and insulin on protein synthesis, AMP-activated protein kinase and phosphorylation state of translation factors in rat skeletal muscle. Pflugers Arch. , 1129–1140 [DOI] [PubMed] [Google Scholar]
- 59.Ryder J. W., Fahlman R., Wallberg-Henriksson H., Alessi D. R., Krook A., Zierath J. R. (2000) Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle; involvement of the mitogen- and stress-activated protein kinase 1. J. Biol. Chem. , 1457–1462 [DOI] [PubMed] [Google Scholar]
- 60.Kroczynska B., Joshi S., Eklund E. A., Verma A., Kotenko S. V., Fish E. N., Platanias L. C. (2011) Regulatory effects of ribosomal S6 kinase 1 (RSK1) in IFNλ signaling. J. Biol. Chem. , 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roux P. P., Shahbazian D., Vu H., Holz M. K., Cohen M. S., Taunton J., Sonenberg N., Blenis J. (2007) RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. , 14056–14064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rose A. J., Broholm C., Kiillerich K., Finn S. G., Proud C. G., Rider M. H., Richter E. A., Kiens B. (2005) Exercise rapidly increases eukaryotic elongation factor 2 phosphorylation in skeletal muscle of men. J. Physiol. , 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryazanov A. G., Davydova E. K. (1989) Mechanism of elongation factor 2 (EF-2) inactivation upon phosphorylation: phosphorylated EF-2 is unable to catalyze translocation. FEBS Lett. , 187–190 [DOI] [PubMed] [Google Scholar]
- 64.Wright D. C., Han D. H., Garcia-Roves P. M., Geiger P. C., Jones T. E., Holloszy J. O. (2007) Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J. Biol. Chem. , 194–199 [DOI] [PubMed] [Google Scholar]
- 65.Perry C. G., Lally J., Holloway G. P., Heigenhauser G. J., Bonen A., Spriet L. L. (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. , 4795–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zid B. M., Rogers A. N., Katewa S. D., Vargas M. A., Kolipinski M. C., Lu T. A., Benzer S., Kapahi P. (2009) 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell , 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drake J. C., Peelor F. F. III, Biela L. M., Watkins M. K., Miller R. A., Hamilton K. L., Miller B. F. (2013) Assessment of mitochondrial biogenesis and mTORC1 signaling during chronic rapamycin feeding in male and female mice. J. Gerontol. A Biol. Sci. Med. Sci. , 1493–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ye L., Widlund A. L., Sims C. A., Lamming D. W., Guan Y., Davis J. G., Sabatini D. M., Harrison D. E., Vang O., Baur J. A. (2013) Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany, N.Y.) , 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karbowski M., Arnoult D., Chen H., Chan D. C., Smith C. L., Youle R. J. (2004) Quantitation of mitochondrial dynamics by photolabeling of individual organelles shows that mitochondrial fusion is blocked during the Bax activation phase of apoptosis. J. Cell Biol. , 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eisner V., Lenaers G., Hajnóczky G. (2014) Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J. Cell Biol. , 179–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H., Chomyn A., Chan D. C. (2005) Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. , 26185–26192 [DOI] [PubMed] [Google Scholar]
- 72.Cartoni R., Léger B., Hock M. B., Praz M., Crettenand A., Pich S., Ziltener J. L., Luthi F., Dériaz O., Zorzano A., Gobelet C., Kralli A., Russell A. P. (2005) Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J. Physiol. , 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Song Z., Ghochani M., McCaffery J. M., Frey T. G., Chan D. C. (2009) Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell , 3525–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caffin F., Prola A., Piquereau J., Novotova M., David D. J., Garnier A., Fortin D., Alavi M. V., Veksler V., Ventura-Clapier R., Joubert F. (2013) Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1-deficient mice. J. Physiol. , 6017–6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smirnova E., Shurland D. L., Ryazantsev S. N., van der Bliek A. M. (1998) A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. , 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.James D. I., Parone P. A., Mattenberger Y., Martinou J. C. (2003) hFis1, a novel component of the mammalian mitochondrial fission machinery. J. Biol. Chem. , 36373–36379 [DOI] [PubMed] [Google Scholar]
- 77.Mozdy A. D., McCaffery J. M., Shaw J. M. (2000) Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. , 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Chan N. C., Ngo H. B., Gristick H., Chan D. C. (2012) Crystal structure of mitochondrial fission complex reveals scaffolding function for mitochondrial division 1 (Mdv1) coiled coil. J. Biol. Chem. , 9855–9861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Otera H., Wang C., Cleland M. M., Setoguchi K., Yokota S., Youle R. J., Mihara K. (2010) Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. , 1141–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Westermann B. (2012) Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta , 1833–1838 [DOI] [PubMed] [Google Scholar]
- 81.Skulachev V. P. (2001) Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem. Sci. , 23–29 [DOI] [PubMed] [Google Scholar]
- 82.Ding H., Jiang N., Liu H., Liu X., Liu D., Zhao F., Wen L., Liu S., Ji L. L., Zhang Y. (2010) Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim. Biophys. Acta , 250–256 [DOI] [PubMed] [Google Scholar]
- 83.Pagano A. F., Py G., Bernardi H., Candau R. B., Sanchez A. M. (2014) Autophagy and protein turnover signaling in slow-twitch muscle during exercise. Med. Sci. Sports Exerc. , 1314–1325 [DOI] [PubMed] [Google Scholar]
- 84.Hosokawa N., Hara T., Kaizuka T., Kishi C., Takamura A., Miura Y., Iemura S., Natsume T., Takehana K., Yamada N., Guan J. L., Oshiro N., Mizushima N. (2009) Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol. Biol. Cell , 1981–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Birgisdottir A. B., Lamark T., Johansen T. (2013) The LIR motif: crucial for selective autophagy. J. Cell Sci. , 3237–3247 [DOI] [PubMed] [Google Scholar]
- 86.Yu X., Long Y. C. (2014) Autophagy modulates amino acid signaling network in myotubes: differential effects on mTORC1 pathway and the integrated stress response. FASEB J. 29, 394–407 [DOI] [PubMed] [Google Scholar]
- 87.Lira V. A., Okutsu M., Zhang M., Greene N. P., Laker R. C., Breen D. S., Hoehn K. L., Yan Z. (2013) Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. , 4184–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Laker R. C., Xu P., Ryall K. A., Sujkowski A., Kenwood B. M., Chain K. H., Zhang M., Royal M. A., Hoehn K. L., Driscoll M., Adler P. N., Wessells R. J., Saucerman J. J., Yan Z. (2014) A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J. Biol. Chem. , 12005–12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He C., Bassik M. C., Moresi V., Sun K., Wei Y., Zou Z., An Z., Loh J., Fisher J., Sun Q., Korsmeyer S., Packer M., May H. I., Hill J. A., Virgin H. W., Gilpin C., Xiao G., Bassel-Duby R., Scherer P. E., Levine B. (2012) Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature , 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R., Asara J. M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R. J. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science , 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sancak Y., Bar-Peled L., Zoncu R., Markhard A. L., Nada S., Sabatini D. M. (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell , 290–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanchez A. M., Csibi A., Raibon A., Cornille K., Gay S., Bernardi H., Candau R. (2012) AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J. Cell. Biochem. , 695–710 [DOI] [PubMed] [Google Scholar]
- 93.Jin S. M., Lazarou M., Wang C., Kane L. A., Narendra D. P., Youle R. J. (2010) Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. , 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen Y., Dorn G. W. II (2013) PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science , 471–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Geisler S., Holmström K. M., Skujat D., Fiesel F. C., Rothfuss O. C., Kahle P. J., Springer W. (2010) PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. , 119–131 [DOI] [PubMed] [Google Scholar]
- 96.Büeler H. (2010) Mitochondrial dynamics, cell death and the pathogenesis of Parkinson’s disease. Apoptosis , 1336–1353 [DOI] [PubMed] [Google Scholar]
- 97.Scheele C., Petrovic N., Faghihi M. A., Lassmann T., Fredriksson K., Rooyackers O., Wahlestedt C., Good L., Timmons J. A. (2007) The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC Genomics , 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jamart C., Naslain D., Gilson H., Francaux M. (2013) Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am. J. Physiol. Endocrinol. Metab. , E964–E974 [DOI] [PubMed] [Google Scholar]
- 99.Novak I., Kirkin V., McEwan D. G., Zhang J., Wild P., Rozenknop A., Rogov V., Löhr F., Popovic D., Occhipinti A., Reichert A. S., Terzic J., Dötsch V., Ney P. A., Dikic I. (2010) Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. , 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W., Huang L., Xue P., Li B., Wang X., Jin H., Wang J., Yang F., Liu P., Zhu Y., Sui S., Chen Q. (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. , 177–185 [DOI] [PubMed] [Google Scholar]
- 101.Wu W., Tian W., Hu Z., Chen G., Huang L., Li W., Zhang X., Xue P., Zhou C., Liu L., Zhu Y., Zhang X., Li L., Zhang L., Sui S., Zhao B., Feng D. (2014) ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. , 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]