Abstract
Context:
Intracranial atherosclerosis is a common cause of stroke in India. Transcranial Doppler (TCD) provides a noninvasive way to study basal intracranial blood vessels. The Oxfordshire Community Stroke Project (OCSP) classification is a simple clinical stroke classification system that has prognostic significance and has been associated with size and location of the infarct.
Aim:
This study was undertaken to identify patterns of TCD abnormalities in our stroke population particularly in relation to the OCSP classification.
Setting and Design:
A cross-sectional study was conducted at a tertiary care center in South India.
Methods:
Recent nondisabling ischemic stroke patients were studied. TCD was used to insonate bilateral middle cerebral, bilateral anterior cerebral, bilateral vertebral, and basilar arteries. Mean flow velocity was used to define normal or abnormal flow as per standard criteria.
Statistical Methods:
Association between abnormal flow velocities and OCSP classification was studied using Chi-square tests. Univariate and multivariate analysis was performed to determine factors associated with abnormal flow velocities.
Results:
Of the 59 participants studied, 42 (71%; 95% confidence interval [CI]: 57.3-84.7%) had abnormal flow velocities in one or more vessels and this was significantly associated with smoking (odds ratio = 5; 95% CI: 1.2–21.8). All abnormal flow velocities were blunted flow velocities. Anterior circulation flow velocity abnormalities were seen among all OCSP stroke subtypes, but posterior circulation flow abnormalities were associated with posterior circulation infarcts (P = 0.03).
Conclusion:
Intracranial flow velocity abnormalities are frequent among Indian stroke population. Further studies are needed to characterize these abnormalities fully.
Keywords: Blunted flow velocities, Oxfordshire Community Stroke Project classification, transcranial Doppler
INTRODUCTION
Stroke subtypes in the East have been shown to be different from the West. A review of South Asian strokes by Wasay et al. reported that among Indians, the most common etiology of stroke was large vessel stroke accounting for 41%.[1] However, unlike western populations where extracranial atherosclerosis is common, among South Asians, intracranial atherosclerosis is more common, with reported prevalence of 30%.[1,2]
Transcranial Doppler (TCD) is an important and noninvasive tool to study the flow characteristics of the intracranial basal cerebral blood vessels. It has a sensitivity of 70%–90% and specificity of 90%–95% for detection of intracranial stenosis in the anterior circulation and sensitivity of 50%–80% and specificity of 80%–96% for posterior circulation.[3]
The Oxfordshire Community Stroke Project (OCSP) classification is a clinical stroke classification system which was derived by Bamford et al.[4] This uses clinical information to categorize computed tomography-proven ischemic strokes as total anterior circulation infarcts (TACIs) (hemiparesis/hemisensory loss with higher cortical disturbances and hemianopia), partial anterior circulation infarcts (PACIs) (any two of the three or pure higher cortical disturbance), lacunar infarcts (LACIs) (pure motor or pure sensory or ataxic hemiparesis or clumsy hand-dysarthria), and posterior circulation infarcts (POCIs) (bilateral signs, crossed signs, and isolated homonymous hemianopia). It has been shown to be associated with size and location of the infarct. It has also been used to prognosticate.[5] Previous studies have shown that distinct vascular abnormalities maybe associated with different stroke subtypes.[6] If these findings are true, then OCSP classification can be used to identify patients for specific therapies or investigations for underlying vascular pathology.
We undertook this study to identify the common patterns of intracranial flow abnormalities in our stroke patients and their association with different stroke subtypes.
METHODS
The study was a retrospective analysis of data collected for a trial of yoga in stroke between December 2012 and September 2014. It was conducted in the stroke outpatient department of a tertiary care institute in the south of India. Patients with recent ischemic stroke (between 1 and 6 months of the ictus) with modified Rankin score ≤3 and insonable temporal and occipital windows were included in this study. Patients with serious comorbid cardiovascular or respiratory illness or aphasia were excluded from the study. Baseline data including demographic information, details of comorbid illness, and investigations performed as part of stroke workup including echo and extracranial duplex studies were collected. OCSP classification was performed using clinical and imaging data by a neurologist. TCD studies were performed on all the included patients using 2MHz probe on Rimed, Israeli, four-view software by a trained neurovascular technician using standard techniques. Bilateral middle cerebral arteries (MCAs), anterior cerebral arteries (ACAs), vertebral arteries (VAs), and the basilar artery (BA) were insonated and mean flow velocities documented. Assuming a prevalence of 30%,[1] estimating the proportion of those with intracranial atherosclerosis using 59 participants and p = 0.05, we were able to achieve a precision of 12%.
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial laid down criteria for detection of >50% intracranial stenosis of MCA and vertebrobasilar arteries.[7] Zhao et al. further refined these criteria.[8] These have been used to classify velocities [Table 1] of participants in our trial as normal or abnormal.[9,10] Association between abnormal flow velocities and OCSP classification was studied using Chi-square tests. Univariate and multivariate analysis was performed to determine factors associated with abnormal flow velocities. All statistical analyses were carried out using STATA ver. 14.2, StataCorp, Texas, USA.
Table 1.
Criteria for abnormal velocity

RESULTS
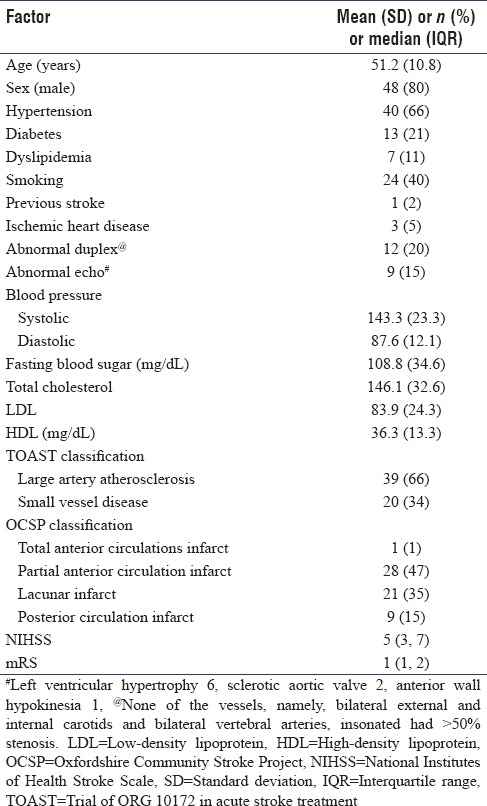
A total of 59 participants were part of the study. Most (80%) were male. Anterior circulation strokes (OCSP classification of PACI (47%) and LACI (35%)) were the most common type of stroke occurring in 50 participants (85%). Other baseline characteristics are in Table 2.
Table 2.
Baseline characteristics
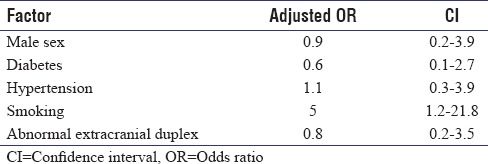
The flow velocities in both MCA and BA were classified as abnormal according to criteria by Zhao et al. Forty-two participants (71%; 95% confidence interval [CI]: 57.3%–84.7%) had abnormal flow velocity in one or more vessels while 10 (17%) participants had abnormal flows in all three vessels. All abnormal velocities were blunted. Multivariate analysis using logistic regression [Table 3] revealed that only smoking was significantly associated with abnormal flow velocities.
Table 3.
Multivariate analysis for factors associated with abnormal flow velocities
Comparisons of the mean velocity of MCA, BA, and VA between the different types of strokes according to OCSP yielded significantly lower mean velocity in BA in posterior circulation strokes as compared to anterior circulation strokes (P = 0.03) [Figure 1]. The other velocities were not significantly different between the groups.
Figure 1.
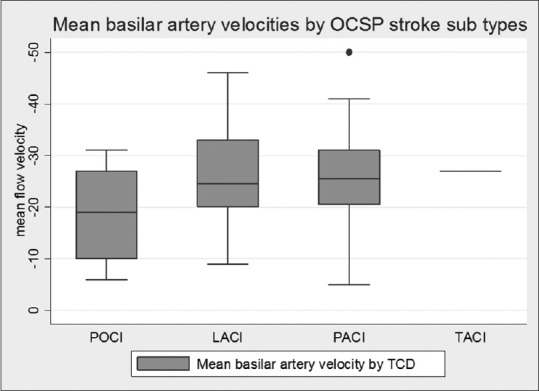
Mean basilar artery flow velocities#.#P value for mean flow velocity in posterior circulation infarcts versus others = 0.03
DISCUSSION
In our study of recent ischemic strokes, we found that TCD velocity of basal cerebral vessels were abnormal in 72% (95% CI: 57.3%–84.7%) of participants tested and all abnormal velocities were blunted velocities. These blunted flow velocities were significantly associated with smoking. While anterior circulation flow abnormalities had no specific distribution, posterior circulation flow abnormalities were more frequent among patients with posterior circulation strokes.
Sada et al. studied 374 asymptomatic participants with vascular risk factors using TCD. They defined middle cerebral stenosis by velocity criteria given by Feldman et al. from SONIA.[7] They found a prevalence of 7.2% for intracranial MCA stenosis in the study population.[11] They, however, did not comment on blunted flow velocities. Our study shows that using velocity criteria, 71% (95% CI: 57.3–84.7) of our cohort of recent nondisabling ischemic strokes had evidence of abnormal flow in at least one of the vessels studied. Among patients with stroke and extracranial carotid disease, Ghosh et al. reported a prevalence of 52.7% for intracranial atherosclerosis using magnetic resonance angiogram (MRA).[12] Although we found a comparable prevalence of intracranial atherosclerosis, none of our patients had significant extracranial carotid or vertebral stenosis. All our abnormal flow velocities were blunted velocities. The significance of blunted flow velocities is not completely understood. Blunted velocities could signify diffuse atherosclerosis, long-segment stenosis, or multiple distal obstructions. Wijnhoud et al. studied 489 strokes or TIA patients followed over 2 years. They found that compared to the second decile of mean flow velocities, the first decile (mean flow velocity of 33.7 cm/s) was associated with a hazard ratio of 10.9 (95% CI: 1.3–88) for recurrent strokes or TIA.[13] This suggests that blunted flow velocities are not benign.
We found the abnormal velocities to be significantly associated with smoking. Icikay et al. showed that mean flow velocity falls with hematocrit and smoking produces rise in hematocrit.[14] This may explain the association. Another possibility is smoking which is often associated with a diffuse vascular process[15] and this may also result in blunted flow velocities.
Some previous studies have attempted to explore the relationship between OCSP subtypes and TCD abnormalities. Among 30 patients with stroke, Naylor et al. found MCA occlusion or severe stenosis in all 14 with TACI and 2 with PACI. Distal branch occlusions were seen in 6 of 13 patients with PACI while no abnormalities were seen in patients with LACI.[16] Mead et al. conducted a study to explore patterns of vascular abnormalities by OCSP subtypes in Edinburgh, UK. They studied 476 patients of whom 36% had PACI while LACI and POCI accounted for 25% and 24%, respectively. They found anterior circulation abnormalities (MCA and ACA) to be significantly more common in patients with TACI and PACI as against POCI and LACI. Similarly, posterior circulation abnormalities were more frequently associated with POCI. They concluded that intracranial abnormalities were significantly related to OCSP subtypes.[6] Among 699 Chinese patients, Li et al. found abnormalities in MCA and vertebrobasilar velocities among all four OCSP subtypes, with no significant differences between the groups.[17] In a slightly more diverse South Asian population from Singapore, TCD and MRA were used by De Silva et al. to find that intracranial arterial flow abnormalities were equally distributed among all the OCSP subtypes.[18]
Our study of recent nondisabling ischemic strokes had a distribution of stroke patients more like Mead et al. with 47% of participants with PACI, rather than the Asian studies both of which had predominant LACI subtype. We did not find anterior circulation abnormalities to be associated with any stroke subtype, like the two Asian studies. However, posterior circulation abnormalities were associated with POCI like the study by Mead et al. and unlike the Asian studies. We used only mean flow velocities to determine normal or abnormal blood flows while other studies have variably included other parameters including pulsatility index and spectral patterns. While these differences may contribute to these disparate findings, we postulate that the pattern of abnormalities we found reflects the unique Asian Indian stroke phenotype.
Our study has certain limitations. We only included patients if they had nondisabling strokes. Hence, we could include only one TACI. Most of our participants were male probably reflecting the referral bias of a hospital-based population. TCD is a subjective measure, but all the velocities were recorded by a single neurosonographer minimizing interobserver error. We did not look at other TCD parameters such as pulsatility index and spectral patterns, in this study.
CONCLUSION
TCD velocity abnormalities are frequent among Asian Indian stroke patients, with blunted flow velocities being the most common abnormality. More studies are needed with inclusion of wider variety of patients, including other TCD parameters to fully characterize the nature of intracranial vascular disease among Indian stroke patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev Neurol. 2014;10:135–43. doi: 10.1038/nrneurol.2014.13. [DOI] [PubMed] [Google Scholar]
- 2.Sethi S, Solanki R, Gupta H. Color and duplex doppler imaging evaluation of extracranial carotid artery in patients presenting with transient ischemic attack and stroke – A clinical and radiological correlation. Indian J Radiol Imag. 2005;15:7. [Google Scholar]
- 3.Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, et al. Assessment: Transcranial doppler ultrasonography: Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2004;62:1468–81. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 4.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 5.Paci M, Nannetti L, D'Ippolito P, Lombardi B. Outcomes from ischemic stroke subtypes classified by the Oxfordshire community stroke project: A systematic review. Eur J Phys Rehabil Med. 2011;47:19–23. [PubMed] [Google Scholar]
- 6.Mead GE, Wardlaw JM, Dennis MS, Lewis SC, Warlow CP. Relationship between pattern of intracranial artery abnormalities on transcranial Doppler and Oxfordshire community stroke project clinical classification of ischemic stroke. Stroke. 2000;31:714–9. doi: 10.1161/01.str.31.3.714. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Barlinn K, Sharma VK, Tsivgoulis G, Cava LF, Vasdekis SN, et al. Velocity criteria for intracranial stenosis revisited: An international multicenter study of transcranial Doppler and digital subtraction angiography. Stroke. 2011;42:3429–34. doi: 10.1161/STROKEAHA.111.621235. [DOI] [PubMed] [Google Scholar]
- 9.Topcuoglu MA. Transcranial Doppler ultrasound in neurovascular diseases: Diagnostic and therapeutic aspects. J Neurochem. 2012;123(Suppl 2):39–51. doi: 10.1111/j.1471-4159.2012.07942.x. [DOI] [PubMed] [Google Scholar]
- 10.Alexandrov AV. Neurovascular Examination in the Rapid Evaluation of Stroke Patients using Ultrasound Waveform Interpretation. West Sussex: Wiley-Blackwell; 2013. [Google Scholar]
- 11.Sada S, Reddy Y, Rao S, Alladi S, Kaul S. Prevalence of middle cerebral artery stenosis in asymptomatic subjects of more than 40 years age group: A transcranial doppler study. Neurol India. 2014;62:510–5. doi: 10.4103/0028-3886.144443. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh M, Ghosh K, Chatterjee A, Bhattacharya A, Acharya A, Chakraborty S, et al. Correlation of intracranial atherosclerosis with carotid stenosis in ischemic stroke patients. Ann Indian Acad Neurol. 2015;18:412–4. doi: 10.4103/0972-2327.165473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijnhoud AD, Koudstaal PJ, Dippel DW. The prognostic value of pulsatility index, flow velocity, and their ratio, measured with TCD ultrasound, in patients with a recent TIA or ischemic stroke. Acta Neurol Scand. 2011;124:238–44. doi: 10.1111/j.1600-0404.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- 14.Isikay CT, Uzuner N, Gücüyener D, Ozdemir G. The effects of hematocrit and age on transcranial Doppler measurements in patients with recent ischemic stroke. Neurol India. 2005;53:51–4. doi: 10.4103/0028-3886.15055. [DOI] [PubMed] [Google Scholar]
- 15.Edjoc RK, Reid RD, Sharma M. The effectiveness of smoking cessation interventions in smokers with cerebrovascular disease: A systematic review. BMJ Open. 2012;2:pii: e002022. doi: 10.1136/bmjopen-2012-002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naylor AR, Sandercock PA, Sellar RJ, Warlow CP. Patterns of vascular pathology in acute, first-ever cerebral infarction. Scott Med J. 1993;38:41–4. doi: 10.1177/003693309303800203. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Wong KS, Kay R. Relationship between the oxfordshire community stroke project classification and vascular abnormalities in patients with predominantly intracranial atherosclerosis. J Neurol Sci. 2003;207:65–9. doi: 10.1016/s0022-510x(02)00397-0. [DOI] [PubMed] [Google Scholar]
- 18.De Silva DA, Woon FP, Pin LM, Chen CP, Chang HM, Wong MC, et al. Intracranial large artery disease among OCSP subtypes in ethnic South Asian ischemic stroke patients. J Neurol Sci. 2007;260:147–9. doi: 10.1016/j.jns.2007.04.020. [DOI] [PubMed] [Google Scholar]


