Abstract
Imatinib mesylate, a tyrosine kinase inhibitor, is commonly used in the treatment of chronic myeloid leukemia. IgA pemphigus is a rare bullous dermatosis caused by IgA autoantibodies. Clinical manifestations include localized or generalized itchy blisters and pustules. Drug-induced IgA pemphigus has been rarely reported in the literature till date. Here, we report one such rare case caused by imatinib which responded poorly to oral prednisolone but showed excellent response to tablet dapsone.
Keywords: IgA, imatinib, subcorneal pustular dermatosis type
Introduction
Imatinib mesylate is a tyrosine kinase inhibitor (TKi) approved for the treatment of chronic myeloid leukemia (CML) and gastrointestinal stromal tumors.[1]
Cutaneous adverse reactions caused by imatinib have been reported to occur in 11–67% of the patients.[2] Nonspecific maculopapular eruptions and edema are the most common cutaneous side effects, however, other cutaneous reactions such as lichenoid-like reaction, acute generalized exanthematous pustulosis, exacerbation of psoriasis, cutaneous hypopigmentation, epidermal necrolysis, and pityriasis-like reactions have also been observed.[2,3,4] However, we were unable to find any previous report of imatinib-induced IgA pemphigus in the literature.
Case Report
A 45-year-old lady, a recently diagnosed case of CML, was started on Tab. imatinib 400 mg per day. Six weeks after starting the drug, she presented with extensive red raised skin rash over her entire body. She gave a history of burning sensation at the site of the lesions. Itching was moderate but did not disturb her sleep. There was no history of fever, joint pain, oral, conjunctival, or genital ulcerations. She denied any exposure to chemical agents and contact allergens. She was only taking tablet multivitamin along with tablet imatinib.
General and systemic examination was essentially normal. Dermatological examination revealed involvement of the face, neck, trunk, and all four extremities in the form of annular erythematous plaques with surface studded with micropustules [Figures 1 and 2]. At places the micropustules had burst and coalesced with adjoining micropustules to form thin white scales. Diffuse superficial desquamation was noted at places. No tense blisters were seen, and the Nikolsky sign was negative. Examination of the mucosal areas, nails, palms, and soles was normal.
Figure 1.
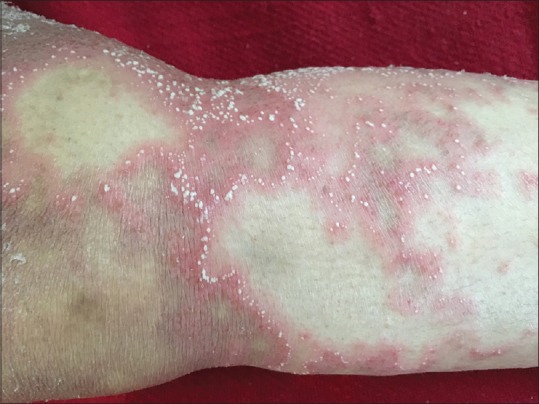
Annular erythematous plaques studded with micropustules present over lower extremities
Figure 2.
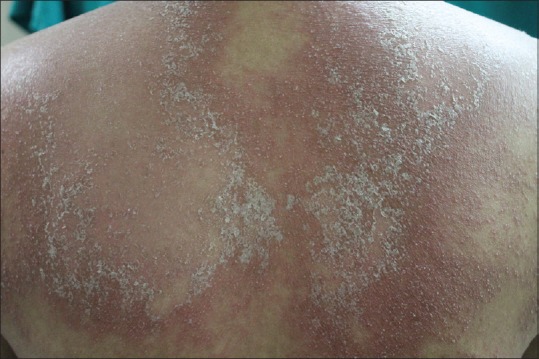
Annular erythematous plaques studded with micropustules over back with areas showing superficial desquamation
Routine hematological and biochemical parameters did not reveal any abnormality. Skin biopsy revealed the epidermis to have moderate spongiosis with multiple intraepithelial neutrophilic bullae predominantly subcorneal and intraspinous. The bullae contained neutrophils and plasma cells with no acantholytic cells. Superficial reticular dermis showed perivascular edema with a perivascular predominantly neutrophilic infiltrate with scattered lymphoplasmacytic cells seen in deeper reticular dermis [Figure 3]. Direct immunofluorescence was positive for intercellular IgA in the epidermis [Figure 4].
Figure 3.
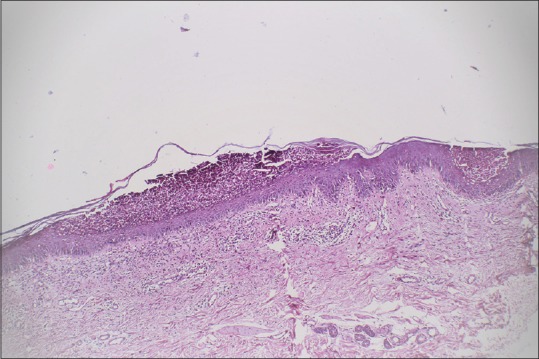
(H and E, ×200) Multiple subcorneal bullae seen. Bullae contain collections of abundant neutrophils
Figure 4.
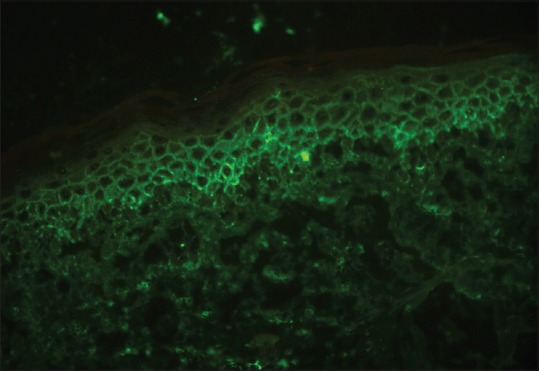
Direct immunofluorescence revealed IgA deposits in the intercellular space of the upper layers of epidermis
Based on the above clinical, histopathological, and direct immunoflorescence findings, a diagnosis of likely imatinib-induced IgA pemphigus (subcorneal pustular dermatosis type) was made and tab. imatinib was immediately stopped. The patient was started on oral prednisolone at a dose of 1mg/kg body weight along with antihistamines and topical emollients. Over the next 7 days, the patient showed no improvement, hence, Tab. dapsone was introduced while tapering off prednisolone. Within 48 hrs of starting dapsone, the lesions started regressing and complete resolution of lesions took 4 weeks [Figure 5]. Imatinib was substituted by Tab. hydroxyurea. Patient was followed up for 10 months with no fresh recurrence of skin lesions.
Figure 5.
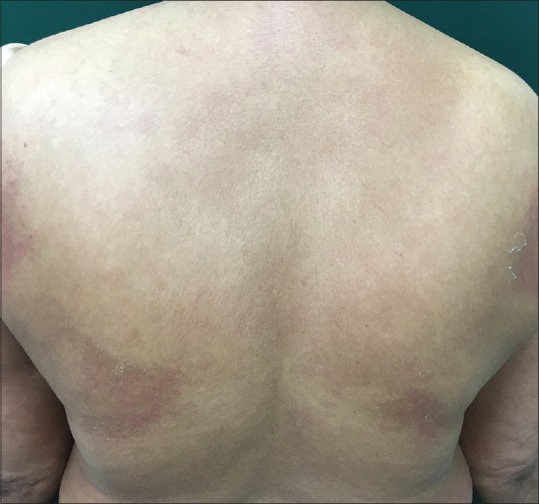
Complete regression of skin lesions after 4 weeks of treatment with dapsone
Discussion
Similar to other multikinase inhibitors (MKI), imatinib has been related to varied cutaneous adverse reactions. Majority of these side-effects such as alopecia, dyschromia, erythema, pruritus, hand foot skin reaction, papulopustular rash, stomatitis, xerosis, and exfoliative dermatitis are likely caused by the inhibition of the physiologic function of cutaneous protein kinases and are dose-dependent. Second mechanism of skin toxicity is via immune-mediated hypersensitivity cutaneous reactions such as urticaria, angioedema, maculopapular rash, acute generalized exanthematous pustulosis, Stevens–Johnson syndrome, neutrophilic dermatitis, and mucositis.[5]
IgA pemphigus was first described by Wallach, Foldes, and Cottenot in 1982 under the name subcorneal pustular dermatosis and monoclonal IgA. Similar cases have been published under various names such as intraepidermal neutrophilic IgA dermatosis, intercellular IgA dermatosis, IgA pemphigus foliaceus, IgA herpetiform pemphigus, intraepidermal IgA pustulosis, or intercellular IgA vesiculopustular dermatosis.[6] “IgA pemphigus” was first proposed by Hodak et al. in 1990 and has become the most common entity.[6] It is a rare entity among autoimmune intraepidermal blistering diseases presenting with a vesiculopustular eruption, neutrophil infiltration, acantholysis, and tissue-bound and circulating IgA antibodies targeting desmosomal or nondesmosomal cell surface components in the epidermis. Two distinct types of IgA pemphigus are the subcorneal pustular dermatosis (SPD) type and the intraepidermal neutrophilic (IEN) type depending on the pathological and DIF findings.[7] Initial manifestations of IgA pemphigus are flaccid vesicles and pustules on erythematous bases with a predilection for flexural areas. Blisters and pustules rupture quickly to form crusted annular or circinate papuloplaques with confluent tendency. Nikolsky sign is negative with no mucosal involvement. Acantholysis in IgA pemphigus is significantly milder than that seen in classic pemphigus. Pathophysiology entails a IgA reaction to the keratinocyte cell surfaces. Antigen implicated for SPD is desmocollin-1, whereas the antigen of the IEN type is still unknown. Mechanism by which IgA autoantibodies produce characteristic skin lesions in IgA pemphigus is still speculated. IgA autoantibodies might bind to the Fc receptor CD89 on monocytes and granulocytes, resulting in the accumulation of neutrophils and subsequent proteolytic cleavage of the keratinocyte cell–cell junction.[7]
Associations of IgA pemphigus include malignancies such as IgA gammopathy, rheumatoid arthritis, Sjögren syndrome, ulcerative colitis, Crohn's disease, and HIV infection.[8]
Morphological differentials of IgA pemphigus include acute generalized exanthematous pustulosis (AGEP), annular and circinate psoriasis, and amicrobial pustulosis of folds. AGEP was ruled out in our case as onset of rash was after 6 weeks of starting imatinib, and the patient had no features of systemic involvement and response to oral prednisolone was poor. Annular and circinate patterns of psoriasis were ruled out as no history of psoriasis or current psoriasis was present, and this variant of psoriasis tends to be more common in childhood. Amicrobial pustulosis of folds is a very rare condition seen in young females with autoimmune abnormalities, particularly systemic lupus erythematosus. Our patient had no features of connective tissue disorder and the lesions were generalized and not restricted to cutaneous folds or periorificial regions, hence, this differential diagnosis was also excluded.
Literature search shows a paucity of reports on drug-induced subcorneal pustular dermatoses type IgA pemphigus. Kishimoto et al. described a case of SCPD-type IgA pemphigus caused by thiol drugs whereas Saunder et al. reported a case of subcorneal pustular dermatosis following adalimumab therapy for rheumatoid arthritis.[9,10] The Naranjo causality score in our case was five. Imatinib-induced subcorneal pustular dermatosis has not been reported before; the rarity of this case prompted us to report it.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Savage DG, Antman KH. Imatinib mesylate – A new oral targeted therapy. N Engl J Med. 2002;346:683–93. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 2.Valeyrie L, Bastuji-Garin S, Revuz J, Bachot N, Wechsler J, Berthaud P, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: A prospective study of 54 patients. J Am Acad Dermatol. 2003;48:201–6. doi: 10.1067/mjd.2003.44. [DOI] [PubMed] [Google Scholar]
- 3.Woo SM, Huh CH, Park KC, Youn SW. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007;34:724–6. doi: 10.1111/j.1346-8138.2007.00369.x. [DOI] [PubMed] [Google Scholar]
- 4.Brazzelli V, Prestinari F, Roveda E, Barbagallo T, Bellani E, Vassallo C, et al. Pityriasis rosea like eruption during treatment with imatinib mesylate: Description of 3 cases. J Am Acad Dermatol. 2005;53:240–3. doi: 10.1016/j.jaad.2004.10.888. [DOI] [PubMed] [Google Scholar]
- 5.Paolo CD, Minetti S, Mineni M, Inverardi S, Rizzini FL. Cutaneous Adverse Reactions to Imatinib: A Case Report of a Successful Slow Protocol for Induction of Drug Tolerance. J Allergy Ther. 2015;6:203. [Google Scholar]
- 6.Hashimoto T, Yasumoto S, Nagata Y, Okamoto T, Fujita S. Clinical, histopathological and immunological distinction in two cases of IgA pemphigus. Clin Exp Dermatol. 2002;27:636–40. doi: 10.1046/j.1365-2230.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda H, Kobayashi H, Hashimoto T, Itoh K, Yamane M, Nakamura J. Subcorneal pustular dermatosis type of IgA pemphigus: Demonstration of autoantibodies to desmocollin-1 and clinical review. Br J Dermatol. 2000;143:144–8. doi: 10.1046/j.1365-2133.2000.03604.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa T, Hashimoto T. Dermatoses with intraepidermal IgA deposits. Clin Dermatol. 2000;18:315–8. doi: 10.1016/s0738-081x(99)00122-4. [DOI] [PubMed] [Google Scholar]
- 9.Kishimoto K, Iwatsuki K, Akiba H, Motoki Y, Kaneko F. Subcorneal pustular dermatosis- type IgA pemphigus induced by thiol drugs. Eur J Dermatol. 2001;11:41–4. [PubMed] [Google Scholar]
- 10.Saunder MB, Glassman SJ. Palmoplantar subcorneal pustular dermatosis following adalimumab therapy for rheumatoid arthritis. Int J Dermatol. 2013;52:624–8. doi: 10.1111/j.1365-4632.2012.05707.x. [DOI] [PubMed] [Google Scholar]


