Abstract
Rationale: Large airway dimensions on computed tomography (CT) have been associated with lung function, symptoms, and exacerbations in chronic obstructive pulmonary disease (COPD), as well as with symptoms in smokers with preserved spirometry. Their prognostic significance in persons without lung disease remains undefined.
Objectives: To examine associations between large airway dimensions on CT and respiratory outcomes in a population-based cohort of adults without prevalent lung disease.
Methods: The Multi-Ethnic Study of Atherosclerosis recruited participants ages 45–84 years without cardiovascular disease in 2000–2002; we excluded participants with prevalent chronic lower respiratory disease (CLRD). Spirometry was measured in 2004–2006 and 2010–2012. CLRD hospitalizations and deaths were classified by validated criteria through 2014. The average wall thickness for a hypothetical airway of 10-mm lumen perimeter on CT (Pi10) was calculated using measures of airway wall thickness and lumen diameter. Models were adjusted for age, sex, principal components of ancestry, body mass index, smoking, pack-years, scanner, percent emphysema, genetic risk score, and initial forced expiratory volume in 1 second (FEV1) percent predicted.
Results: Greater Pi10 was associated with 9% faster FEV1 decline (95% confidence interval [CI], 2 to 15%; P = 0.012) and increased incident COPD (odds ratio, 2.22; 95% CI, 1.43–3.45; P = 0.0004) per standard deviation among 1,830 participants. Over 78,147 person-years, higher Pi10 was associated with a 57% higher risk of first CLRD hospitalization or mortality (P = 0.0496) per standard deviation. Of Pi10’s component measures, both greater airway wall thickness and narrower lumen predicted incident COPD and CLRD clinical events.
Conclusions: In adults without CLRD, large airway dimensions on CT were prospectively associated with accelerated lung function decline and increased risks of COPD and CLRD hospitalization and mortality.
Keywords: chronic obstructive pulmonary disease, computed tomography, lung function, risk stratification
Chronic lower respiratory diseases (CLRDs) are a leading cause of hospitalization and the fourth leading cause of death in the United States (1, 2), despite large reductions in cigarette smoking and other risk factors (3). The Centers for Disease Control and Prevention defines CLRD to include chronic obstructive pulmonary disease (COPD), chronic bronchitis, emphysema, and asthma (2)—diseases characterized by chronic or intermittent airflow limitation that result, to varying extents, from alterations in the airways.
Whereas spirometry provides a global measure of airway physiology and is the gold standard diagnostic test for COPD, computed tomography (CT) can precisely characterize the dimensions of the proximal airways. Alterations in large airway structure on CT correlate with pathological changes of smooth muscle hypertrophy, inflammation, and subepithelial fibrosis (4). Pi10, the average wall thickness for a hypothetical airway of 10-mm lumen perimeter on CT, has been associated in patients with COPD with chronic bronchitis (5), greater symptoms (6), increased exacerbations, and increased mortality in the context of higher emphysema (7–9). Pi10 has also been associated with respiratory symptoms among smokers with preserved lung function (10). Pi10 increases across GOLD (Global Initiative for Chronic Obstructive Lung Disease) stages in COPD, and spatial matching of airways suggests that this is due to decreased airway wall area coupled with even greater decrements in airway lumen size, resulting in greater wall area percentage and Pi10 in COPD (11).
In healthy adults without CLRD, the prognostic significance of large airway dimensions on CT remains undefined. We therefore examined whether Pi10—and secondarily its component measures of airway wall thickness (AWT) and lumen size—were associated with accelerated lung function decline, incident spirometrically defined COPD, and risk of first hospitalization or mortality due to CLRD, in a population-based study cohort free of clinical CLRD at baseline. Some results of this study were previously published in the form of an abstract (12).
Methods
Design and Sample
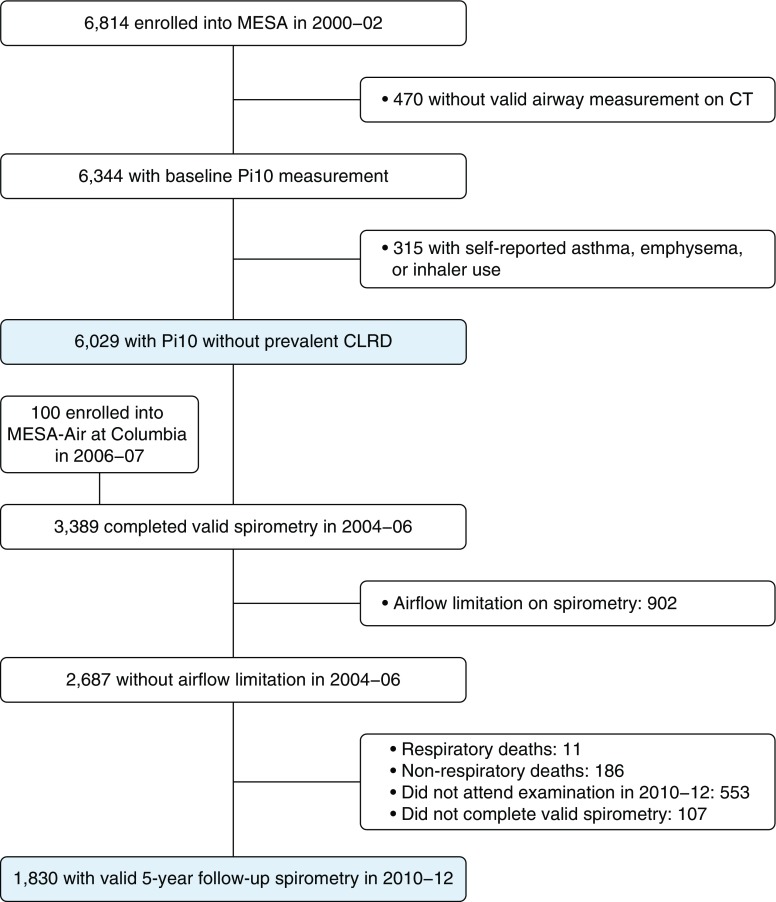
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled 6,814 participants ages 45–84 years who self-reported white, African American, Hispanic, or Asian race/ethnicity in 2000–2002 (Figure 1). Exclusion criteria were history of clinical cardiovascular disease, weight greater than 136 kg, and impediments to long-term participation. Participants were recruited from Forsyth County, North Carolina; northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles, California (13). The MESA Air Study recruited 257 additional participants in 2006–2007 using identical inclusion criteria (14). For the present study, we excluded participants with prevalent clinical CLRD, defined as self-report of a prior physician diagnosis of CLRD, or current use of inhaled corticosteroids or bronchodilators.
Figure 1.
Flowchart of Multi-Ethnic Study of Atherosclerosis (MESA) participants included in the present study. CLRD = chronic lower respiratory disease. CT = computed tomography; Pi10 = average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography.
Large Airway Dimensions on CT
All participants underwent low-dose cardiac CT at baseline in 2000–2002 on one of three types of scanner: the Imatron electron beam computed tomography (EBT) scanner, the Siemens multidetector computed tomography (MDCT) scanner, or the GE Healthcare Life Sciences MDCT scanner (15). For each participant, two cardiac-gated computed tomographic scans were obtained following a standardized protocol at full inspiration that reduced cardiac-related motion artifact in the left lower lobe. These scans included approximately 66% of the lung from carina to lung bases (16) and were reconstructed at approximately 3-mm increments. Of paired scans, the scan with the greater volume of lung air was used, except in cases of discordant quality scores, when the higher-quality scan was used.
Airway dimensions were assessed in the lower lobes using a simple algorithm applicable to clinical low-dose chest computed tomographic scans, which are typically reconstructed at 0.7- to 2.5-mm increments (17). Airways scanned perpendicular to the airway long axis (i.e., airway lumen was approximately circular) were measured in two dimensions using a modified full-width half-maximum principle with dedicated software (PASS; NCSS, Inc.), as previously described (18), to define outer and inner airway wall borders.
Pi10 was calculated by creating individual regression plots for each participant of the square root of the wall area against the corresponding internal perimeter for each measured airway in the lower lobes (19–22). Airways with internal perimeter less than or equal to 6 mm were excluded from Pi10 calculations; furthermore, Pi10 was not calculated in participants with fewer than six eligible airway measures (18). Among participants with valid Pi10, the distribution for the number of airways measured per participant was rightward skewed (average, 8.6; range, 6–41).
In addition to deriving Pi10, direct measurements of airway lumen diameter and AWT were undertaken in a single segmental lower lobe (LB10 or RB10) airway for each participant, an approach that was validated in a subset of MESA participants (18). Scan–rescan reproducibility was high for Pi10 (intraclass correlation coefficient [ICC] 0.86 within reader, 0.77 between readers) and lumen diameter (ICC 0.94 within reader, 0.87 between readers) and good to adequate for AWT (ICC 0.68 within reader, 0.55 between readers) (18).
Lung Function
Spirometry was performed in 2004–2006 in 3,965 participants of 4,483 randomly selected from among those consenting to genetic analyses (99%), with baseline endothelial measures (89%), and examination at that time (91%), plus 100 MESA Air Study participants. Asian Americans were oversampled (23). All these participants were selected for spirometry at 5-year follow-up examination in 2010–2012 using the same protocol and equipment, when those with prebronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) less than 0.70 or less than the lower limit of normal were selected for post-bronchodilator measurements. Spirometry was conducted using an automated dry rolling seal spirometer according to American Thoracic Society guidelines; examinations with less than two acceptable measures repeatable within 200 ml were excluded (24).
For lung function analyses, the sample was restricted to participants with valid spirometry at both examinations and without airflow limitation on initial spirometry, defined as prebronchodilator FEV1/FVC less than 0.70 (25). Incident COPD was defined as post-bronchodilator FEV1/FVC less than 0.70 in 2010–2012 (25). For sensitivity analyses, a lower limit of normal threshold (26) was used to define incident prebronchodilator airflow limitation at 2010–2012 examination.
Incident CLRD Events
Interviewers contacted participants or family members every 9–12 months from study baseline through December 2014 to ascertain all hospitalizations and deaths. The National Death Index was reviewed to ensure complete follow-up for mortality.
Hospitalization or mortality due to CLRD were defined following algorithms previously validated in this cohort (27, 28) as events with one of the following International Classification of Diseases (ICD) codes listed as the primary discharge diagnosis or as the underlying cause of death: asthma (ICD-9 code 493, ICD-10 codes J45–J46), COPD (ICD-9 code 496, ICD-10 code J44), chronic bronchitis (ICD-9 codes 490–491, ICD-10 codes J40–J42), or emphysema (ICD-9 code 492, ICD-10 code J43). COPD hospitalization/mortality were defined by ICD codes for COPD, chronic bronchitis, or emphysema.
Covariates
Age, sex, race/ethnicity, education, secondhand smoke, and tobacco use were self-reported at baseline. Never smokers were defined by lifetime smoking less than 100 cigarettes, and current smokers were defined by cigarette use within the past 30 days. Urinary cotinine was measured at baseline, and 78 participants (2%) who denied current smoking but had cotinine greater than 100 ng/ml were reclassified as current smokers. Pack-years were calculated as (cigarettes per day/20) × years smoked. Height and weight were measured using standard techniques. Percent emphysema was defined as (lung voxels less than −950 Hounsfield units/total imaged lung voxels × 100) on cardiac CT, as previously described (16). Long-term outdoor concentrations of particulate matter less than or equal to 2.5 μm in aerodynamic diameter were estimated at each participant’s home for the 2 years preceding baseline examination using spatiotemporal models (29).
Genotyping was performed using the Affymetrix Genome-Wide Human SNP Array 6.0 (Thermo Fisher Scientific), with 897,981 single-nucleotide polymorphisms (SNPs) passing study-specific quality control, and imputation of an additional approximately 2 million SNPs via 1000 Genomes imputation (phase 3, version 5, imputationserver.sph.umich.edu/index. html) and the Haplotype Reference Consortium (http://www.haplotype-reference-consortium.org/). Genotype data were used to estimate principal components of genetic ancestry (30, 31) and weighted genetic risk scores for COPD (32).
Statistical Approach
Baseline participant characteristics were tabulated by Pi10 quartile. Unadjusted comparisons were tested by one-way analysis of variance or Fisher’s exact test.
For regression analyses, Pi10 was logarithmically transformed for normality. Measures of association were reported per standard deviation (SD) of logarithmically transformed Pi10. Associations with change in FEV1 were tested in random intercept mixed models (33). Logistic regression was used to test associations with incident COPD at 5-year follow-up. Cox proportional hazards models were used to calculate hazard ratios (HRs) for incident CLRD hospitalization/mortality, with time to event defined as days between baseline examination and event date or last follow-up. The proportional hazards assumption was checked via residual plots. Analyses were repeated for hospitalization/mortality due to asthma versus COPD. Linearity of associations between untransformed Pi10 and CLRD hospitalization/mortality was tested in fully adjusted generalized additive models. Competing risks regression was performed as a sensitivity analysis (34).
Models were adjusted for a priori confounders and precision variables. The partially adjusted model included age, sex, race/ethnicity, and body mass index. The fully adjusted model also included smoking status, pack-years, CT scanner type, voxel size, and percent emphysema. Extended models, which were limited to participants with genotyping, added adjustment for principal components of genetic ancestry in place of self-reported race/ethnicity, COPD genetic risk score, and initial FEV1 percent predicted (26). The impact of further adjustment for education, secondhand smoke, and particulate matter less than or equal to 2.5 μm in aerodynamic diameter was tested in sensitivity analyses. Effect measure modification was assessed via multiplicative interaction terms and stratified models. AWT and lumen diameter were logarithmically transformed, and their associations with the outcomes were tested in fully adjusted models including both AWT and lumen diameter. Analyses were conducted by using SAS version 9.3 software (SAS Institute) or R software (R Foundation for Statistical Computing).
Results
Baseline Characteristics
Of 6,814 total MESA participants, 470 (6.9%) were missing valid airway measurements, and an additional 315 (4.6%) had clinical CLRD at baseline (Figure 1). The mean age of the remaining 6,029 participants was 62 years at enrollment. Forty-eight percent were male, 39% were white, 28% were African American, 22% were Hispanic/Latino American, and 12% were Asian American. Forty-six percent were never smokers, 40% were former smokers, and 14% were current smokers; among smokers, 42% reported a history of less than 10 pack-years. Higher Pi10 quartiles demonstrated greater proportions of men, current smokers, Hispanic/Latino Americans, and Asian Americans, and participants in these quartiles were more likely to have been examined on EBT scanners (Table 1).
Table 1.
Characteristics of Multi-Ethnic Study of Atherosclerosis Lung Study participants without prevalent clinical chronic lower respiratory disease, by quartile of Pi10
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| n* | 1,508 | 1,508 | 1,509 | 1,508 |
| Age, yr, mean (SD) | 62.2 (9.8) | 62.0 (10.3) | 62.1 (10.3) | 62.6 (10.3) |
| Male sex, n (%) | 660 (43.8) | 655 (43.4) | 752 (49.8) | 840 (55.7) |
| Race/ethnicity, n (%) | ||||
| White | 707 (46.9) | 565 (37.5) | 536 (35.5) | 498 (33.0) |
| African American | 537 (35.6) | 379 (25.1) | 394 (26.1) | 340 (22.6) |
| Hispanic/Latino American | 159 (10.5) | 365 (24.2) | 358 (23.7) | 454 (30.1) |
| Asian American | 105 (7.0) | 199 (13.2) | 221 (14.7) | 216 (14.3) |
| BMI, kg/m2, mean (SD) | 27.7 (5.3) | 27.5 (5.2) | 28.2 (5.3) | 29.2 (5.3) |
| Smoking status, n (%) | ||||
| Never | 682 (45.2) | 705 (46.8) | 717 (47.6) | 664 (44.1) |
| Former | 628 (41.6) | 620 (41.2) | 569 (37.7) | 601 (39.9) |
| Current | 198 (13.1) | 181 (12.0) | 222 (14.7) | 242 (16.1) |
| Pack-years, median (IQR)† | 15.0 (3.5–31.0) | 12.0 (2.0–30.0) | 12.8 (3.2–29.1) | 17.2 (4.0–35.2) |
| CT measures | ||||
| Pi10, mm, median (IQR) | 4.32 (4.20–4.40) | 4.55 (4.51–4.59) | 4.70 (4.66. 4.75) | 4.92 (4.85–5.06) |
| Wall thickness, mm, median (IQR) | 1.33 (1.24–1.42) | 1.44 (1.34–1.50) | 1.49 (1.40–1.57) | 1.56 (1.45–1.67) |
| Lumen diameter, mm, median (IQR) | 3.39 (2.55–4.32) | 3.28 (2.54–4.17) | 3.15 (2.48–4.06) | 3.03 (2.42–4.00) |
| Percent emphysema, median (IQR) | 2.70 (1.13–5.70) | 3.19 (1.60–5.77) | 3.04 (1.36–5.45) | 2.17 (0.79–4.92) |
| CT scanner type, n (%)‡ | ||||
| EBT/Imatron | 510 (33.8) | 861 (57.1) | 856 (56.7) | 939 (62.3) |
| MDCT/Siemens | 183 (12.1) | 547 (36.3) | 626 (41.5) | 550 (36.5) |
| MDCT/GE | 815 (54.1) | 100 (6.6) | 27 (1.8) | 19 (1.3) |
Definition of abbreviations: BMI = body mass index; CT = computed tomography; EBT = electron beam computed tomography scanner; IQR = interquartile range; MDCT = multidetector computed tomography scanner; Pi10 = average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography; SD = standard deviation.
Numbers reported as mean (SD) or number (percent) unless otherwise noted.
Excludes all participants with prevalent chronic lower respiratory disease, defined as self-reported asthma or emphysema and/or inhaler use at study baseline.
Among ever smokers.
Imatron EBT was used at three sites (Columbia, Northwestern University, and University of California, Los Angeles). Siemens MDCT was used at two sites (Johns Hopkins and University of Minnesota). GE Healthcare Life Sciences MDCT was used at one site (Wake Forest University).
Lung Function Decline and Incident COPD
Among 6,029 participants without prevalent clinical CLRD, a subset of 3,389 (55.3%) was selected for and completed valid prebronchodilator spirometry in 2004–2006 (Figure 1). Among 2,687 (73%) participants without initial airflow limitation, 1,830 (68.1%) had valid prebronchodilator spirometry in 2010–2012. Average change in FEV1 was 27.6 ml/yr, and 40 participants (2.2%) demonstrated incident COPD at 5-year follow-up.
Participants in higher Pi10 quartiles had lesser initial FEV1 percent predicted, greater FEV1 decline, and higher incidence of COPD at follow-up (Table 2). In fully adjusted analyses, per SD increment in Pi10, there was, on average, 2.47 ml greater decline in FEV1 per year (95% confidence interval [CI], 0.66, 4.28; P = 0.008) (Table 3). This was equivalent to a 9% greater decline compared with the overall average and comparable to the effect estimate for former smoking (4.40 ml/yr). Per SD of Pi10, the adjusted odds of incident spirometry-defined COPD were 1.87-fold higher (95% CI, 1.26, 2.78; P = 0.002) (Table 3).
Table 2.
Unadjusted comparisons of lung function measures and incidence of first hospitalization or mortality due to chronic lower respiratory disease, according to Pi10 quartile, among Multi-Ethnic Study of Atherosclerosis Lung Study participants without prevalent clinical chronic lower respiratory disease
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P Value | |
|---|---|---|---|---|---|
| Lung function | |||||
| No. with complete spirometry follow-up* | 457 | 458 | 458 | 457 | |
| FEV1, % predicted, mean (SD) | 100.5 (15.5) | 98.1 (14.4) | 97.2 (14.6) | 96.5 (15.2) | 0.0003 |
| FEV1/FVC, percent, mean (SD) | 0.79 (0.05) | 0.78 (0.05) | 0.79 (0.05) | 0.78 (0.05) | 0.694 |
| Change in FEV1†, ml/yr, mean (SD) | −25.6 (1.8) | −25.9 (1.7) | −29.0 (2.2) | −31.9 (1.9) | 0.0090 |
| Incident COPD‡, n (%) | 2 (0.5%) | 8 (1.9%) | 15 (3.5%) | 15 (3.5%) | 0.007 |
| Events | |||||
| Number with complete events follow-up§ | 1,508 | 1,508 | 1,509 | 1,508 | |
| Events follow-up, n (IDR) | |||||
| CLRD event | 10 (5.1) | 20 (10.2) | 21 (10.7) | 33 (17.1) | 0.005 |
| COPD event | 8 (4.1) | 15 (7.6) | 13 (6.6) | 25 (12.9) | 0.021 |
| Asthma event | 2 (1.0) | 5 (2.5) | 8 (4.1) | 8 (4.1) | 0.185 |
| All-cause mortality | 227 (115.4) | 223 (113.0) | 268 (136.2) | 283 (145.3) | 0.005 |
Definition of abbreviations: CLRD = chronic lower respiratory disease; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IDR = incidence density rate; Pi10 = average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography; SD = standard deviation.
Excludes participants without valid spirometry in both 2004–2006 and 2010–2012 as well as those with valid spirometry demonstrating airflow limitation in 2004–2006, defined as FEV1/FVC less than 0.70, and those with prevalent CLRD, defined as self-reported asthma or emphysema and/or inhaler use at study baseline.
Estimated from a mixed model including only time as a predictor. P value was estimated from the multiplicative interaction term in a model including average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography (Pi10) quartile, time, and Pi10 quartile × time.
Incident COPD was defined as FEV1/FVC less than 0.70 on post-bronchodilator spirometry in 2010–2012.
Excludes all participants with prevalent CLRD, defined as self-reported asthma or emphysema and/or inhaler use at study baseline. Events are defined as hospitalizations/mortality assigned the primary discharge diagnosis or underlying cause of asthma (International Classification of Diseases, 9th Revision [ICD-9], code 493; International Classification of Diseases, 10th Revision [ICD-10], code J45-6), COPD (ICD-9 code 496, ICD-10 code J44), chronic bronchitis (ICD-9 code 490-1, ICD-10 code J40-2), or emphysema (ICD-9 code 492, ICD-10 code J43). COPD events are defined as those coded as COPD, chronic bronchitis, or emphysema.
Table 3.
Associations between large airway dimensions on computed tomography, longitudinal lung function, and incidence of first hospitalization or mortality due to chronic lower respiratory disease, among Multi-Ethnic Study of Atherosclerosis Lung Study participants without prevalent clinical chronic lower respiratory disease
| Annual Change in FEV1* (ml) (n = 1,830) | Incident COPD† (n = 1,717) Cases (n = 40 [2.3%]) | CLRD Hospitalization/Mortality‡ (n = 6,029) Events (n = 84 [1.4%]) | ||||
|---|---|---|---|---|---|---|
| Model | B per SD§ (95% CI) | P Value | OR per SD§ (95% CI) | P Value | HR per SD§ (95% CI) | P Value |
| Pi10, sequential adjustment║ | ||||||
| Unadjusted | −2.46 (−4.32, 0.60) | 0.010 | 1.49 (1.11, 2.01) | 0.009 | 1.33 (1.17, 1.52) | <0.001 |
| Partially adjusted | −2.45 (−4.26, −0.64) | 0.008 | 1.71 (1.24, 2.35) | 0.001 | 1.30 (1.13, 1.50) | <0.001 |
| Fully adjusted | −2.47 (−4.28, −0.66) | 0.008 | 1.87 (1.26, 2.78) | 0.002 | 1.38 (1.20, 1.60) | <0.001 |
| Genetic risk adjusted | −2.66 (−4.54, −0.79) | 0.005 | 2.21 (1.47, 3.33) | <0.001 | 1.41 (1.22, 1.63) | <0.001 |
| FEV1 adjusted | −2.45 (−4.36, −0.55) | 0.0118 | 2.22 (1.43, 3.45) | <0.001 | 1.57 (1.00, 2.45) | 0.0496 |
| Directly measured airway dimensions, fully adjusted║ | ||||||
| Lumen diameter | −0.58 (−2.64, 1.48) | 0.582 | 0.37 (0.25, 0.56) | <0.001 | 0.75 (0.59, 0.96) | 0.023 |
| AWT | −1.95 (−3.98, 0.07) | 0.059 | 1.97 (1.25, 3.10) | 0.004 | 1.34 (1.01, 1.78) | 0.040 |
Definition of abbreviations: AWT = airway wall thickness; B = β-estimate is milliliters per year per standard deviation increment of average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography; CI = confidence interval; CLRD = chronic lower respiratory disease; COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume in 1 second; HR = hazard ratio; OR = odds ratio; Pi10 = average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography; SD = standard deviation.
FEV1 was measured by prebronchodilator spirometry in 2004–2006 and 2010–2012. Annual change in FEV1 was analyzed by random intercept mixed models. Models exclude participants with FEV1/FVC less than 0.70 at the initial spirometry examination as well as participants with prevalent CLRD, defined as self-reported asthma or emphysema and/or inhaler use at study baseline.
Incident COPD was defined as FEV1/FVC less than 0.70 by post-bronchodilator spirometry in 2010–2012, which was present in 40 participants of 1,717 with full post-bronchodilator data (2.3%), and analyzed using logistic regression models. Models exclude participants with FEV1/FVC less than 0.70 at the initial spirometry examination as well as participants with prevalent CLRD, defined as self-reported asthma or emphysema and/or inhaler use at study baseline.
Events are defined as hospitalizations/mortality assigned the primary discharge diagnosis or underlying cause of asthma (International Classification of Diseases, 9th Revision [ICD-9], code 493; International Classification of Diseases, 10th Revision [ICD-10], codes J45–J46), COPD (ICD-9 code 496, ICD-10 code J44), chronic bronchitis (ICD-9 codes 490–491, ICD-10 codes J40–J42), or emphysema (ICD-9 code 492, ICD-10 code J43). Models exclude participants with prevalent CLRD, defined as self-reported asthma or emphysema and/or inhaler use at study baseline.
Results are per SD of log-transformed airway dimensions. For Pi10, these were equivalent on the logarithmic scale to 0.057 and 0.064 in the lung function and events analyses, respectively. For directly measured airway dimensions, these were equivalent to 0.37 and 0.13 for lumen diameter and AWT, respectively.
Models were adjusted for age, sex, race/ethnicity, height, and weight (partially adjusted), as well as for smoking status, pack-years, voxel size, computed tomography scanner type, and percent emphysema (fully adjusted). Height, weight, smoking status, and pack-years were time-varying covariates in the mixed models. The genetic risk–adjusted model is also adjusted for principal components of genetic ancestry (in the place of self-reported race/ethnicity) and COPD genetic risk score. The FEV1-adjusted model was additionally adjusted for the FEV1 percent predicted at the initial spirometry examination. For analyses of annual change in FEV1, models adjusted for the genetic risk score and the initial FEV1 were limited to 1,762. For analyses of incident COPD, models adjusted for the genetic risk score and the initial FEV1 were limited to 1,652 (cases = 39). For analyses of CLRD hospitalization/mortality, models adjusted for the genetic risk score were limited to 5,631 (events = 76); models adjusted for the initial FEV1 were further limited to 3,322 (events = 41). Directly measured airway dimensions were available in only a subset of participants with events follow-up, limiting the model to 5,626 (events = 72). Models using directly measured airway dimensions included both lumen diameter and AWT; results modeling these exposures separately yielded similar results.
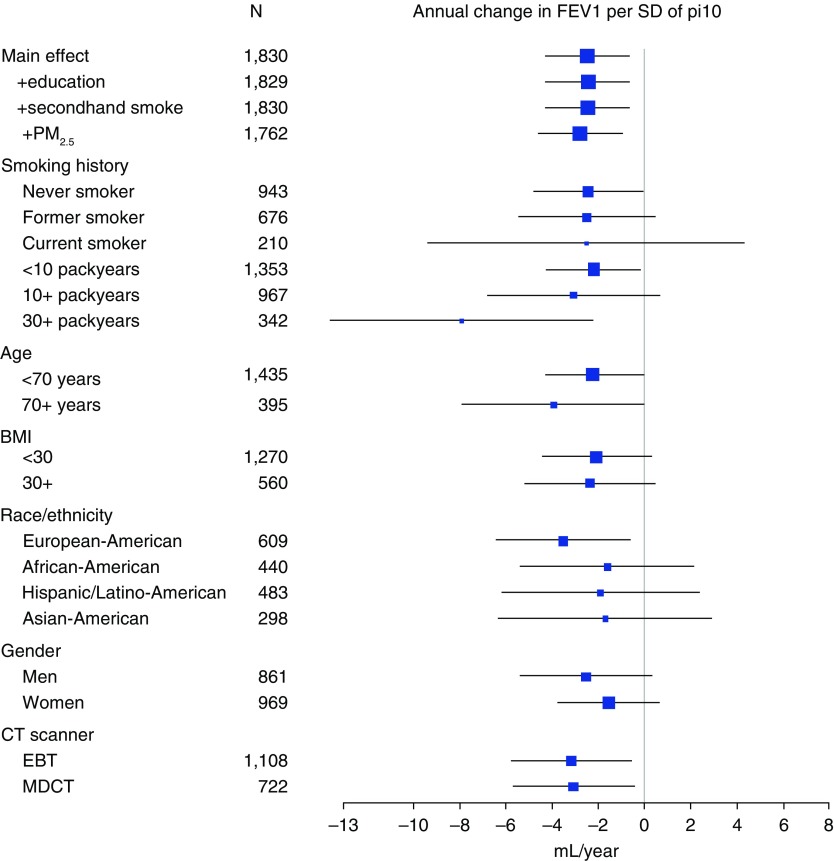
Statistical evidence for effect modification by smoking status was absent (P = 0.93), although in stratified analyses, the magnitude of the effect estimates increased with pack-years of smoking, and it was markedly higher in participants with 30 or more pack-years, who could qualify for lung cancer screening CT (−7.92 ml/SD; 95% CI, −13.59, −2.25). Associations were consistent across strata of other potential effect modifiers, including sex and race/ethnicity, and scanner type (Figure 2); however, site-stratified analyses showed inconsistent associations in the small number of participants scanned on a GE Healthcare Life Sciences MDCT scanner (see Figure E2 in the online supplement).
Figure 2.
Stratified associations between average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography (Pi10) and annual change in forced expiratory volume in 1 second (FEV1) over five years of follow-up, with 95% confidence intervals, in participants without initial airflow limitation or prevalent clinical chronic lower respiratory disease. Fully adjusted models include age, sex, race/ethnicity, body mass index (BMI), smoking status, pack-years, percent emphysema, voxel size, and computed tomography (CT) scanner type. EBT = electron beam computed tomography; MDCT = multidetector computed tomography; PM2.5 = particulate matter less than or equal to 2.5 μm in aerodynamic diameter; SD = standard deviation.
Results were of similar or greater magnitude after adjusting for principal components of genetic ancestry and genetic risk score, initial FEV1 percent predicted, and air pollution and other covariates (Table 3, Figure 2). Similar associations were demonstrated for incident airflow limitation, as defined by the prebronchodilator lower limit of normal (Table E1). Effect estimates were also consistent when those participants with initial lung function measurements but missing follow-up measures were also included (Table E2).
With respect to Pi10’s component measures, greater AWT was positively correlated with Pi10 (r = 0.46; P < 0.001), showed a trend toward association with change in FEV1, and was significantly associated with incident COPD (Table 3). Lumen diameter was negatively correlated with Pi10 (r = −0.06; P < 0.001), and greater lumen diameter was strongly protective with respect to incident COPD.
Incident CLRD Hospitalizations and Mortality
All 6,029 participants without prevalent CLRD were followed for hospitalization or mortality due to CLRD for a median of 14.0 years and 78,147 person-years. By the end of follow-up, 84 participants had experienced a first hospitalization due or died of CLRD (1.4% cumulative incidence; incidence density rate, 10.75/10,000 person-years).
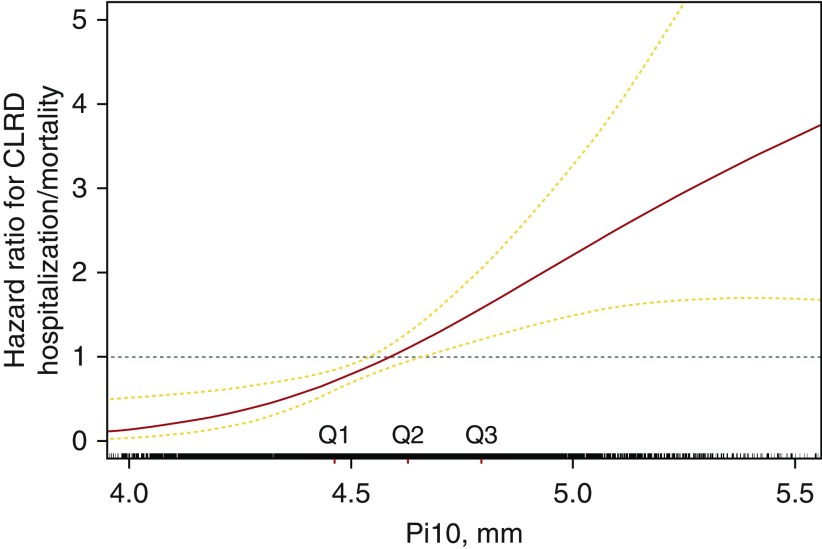
In fully adjusted models (Table 3), each SD increment of Pi10 was associated with a 1.38-fold greater rate of first hospitalization or mortality due to CLRD (95% CI, 1.20–1.60; P < 0.001). The association between Pi10 and CLRD events appeared linear (Figure 3) (P value for nonlinearity, 0.37).
Figure 3.
Generalized additive model of associations between average wall thickness for a hypothetical airway of 10-mm lumen perimeter on computed tomography (Pi10) and risk of first hospitalization or mortality due to chronic lower respiratory disease (CLRD) over fourteen years of follow-up in participants without prevalent clinical CLRD. The model is fully adjusted for age, sex, race/ethnicity, body mass index, smoking status, pack-years, percent emphysema, voxel size, and computed tomography scanner type.
Of first hospitalizations and mortality due to CLRD, 61 were attributed to COPD and 23 were attributed to asthma. In fully adjusted models, Pi10 was associated with COPD events (HR, 1.48/SD; 95% CI, 1.24–1.77; P < 0.001) but not asthma events (HR, 1.14/SD; P = 0.38).
Results were similar after adjusting for genetic risk and initial FEV1 (Table 3). There was no significant evidence for effect modification by smoking status, sociodemographics, or body habitus (P > 0.10 for multiplicative interaction terms (Figure E1). In contrast to EBT scanners, associations were nonsignificant for participants scanned by MDCT, particularly Siemens scanners (Figure E2). Results were similar in competing risks regression (Table E3). Both greater AWT and narrower lumen diameter were significantly associated with incident CLRD events (Table 3).
Discussion
Large airway dimensions on computed tomographic scans were associated with accelerated lung function decline and increased risk of incident spirometry-defined COPD and CLRD hospitalization and mortality, independent of initial lung function, in a large, prospective, population-based sample free of lung disease at baseline. These prospective findings add to the growing literature which suggests that early changes in airway morphology predict risk of CLRD and that Pi10 and other CT measures of large airway dimensions may be suitable for the identification and characterization of individuals at increased risk of developing CLRD, particularly COPD.
This is the first study, to our knowledge, to demonstrate associations of large airway wall dimensions with lung function decline and incident COPD and CLRD events in a general population-based sample of adults without clinical lung disease. Prior cross-sectional work using high-risk smoking cohorts has linked increased Pi10 with increased respiratory symptoms (5, 6, 35, 36), greater airflow limitation (37, 38), greater bronchodilator responsiveness (39), and decreased diffusing capacity (40), in addition to greater exacerbation rate in the prior year (7). Greater Pi10 was also found to modify associations between percent emphysema and all-cause mortality in smokers (9). Elevated Pi10 has recently been associated with a “symptomatic smoker” phenotype characterized by preserved lung function and an increased risk of exacerbations (10). Extending this finding, we observed an association in smokers between increased Pi10 and risk of first hospitalization or mortality due to CLRD.
Pi10 is a composite measure of AWT and lumen diameter, which vary differentially in CLRD (11, 18). Increased Pi10 may indicate greater AWT, narrowed lumina, or both. Indeed, in prior cross-sectional work controlling for level within the airway tree hierarchy, we found that smokers with COPD demonstrated decreased AWT but even greater decreases in lumen; hence, Pi10 was higher (11). These results were consistent with micro-CT results of explanted lungs in patients with COPD which suggested that small airway narrowing and disappearance were precursors to pathologic emphysema and airflow obstruction (41). A recent study further supported this pathophysiology by showing that total airway count (TAC) on CT was decreased in COPD and associated with FEV1 decline, as well as that “parent” airways proximal to missing “daughter” branches have both narrower lumina and thinner walls (42). Although TAC was not available in our study, the strong associations in the present study between narrower airway lumina and increased risks of incident COPD and CLRD events are highly consistent with this literature. Furthermore, similar to recent findings associating TAC with lung function in never smokers (42), our findings in healthy never and light smokers suggest the possibility that large airway measures may index developmental or early-life factors relevant to development of COPD (43, 44).
By contrast, we note that our findings associating increased AWT with incident COPD and CLRD are discordant with recent evidence for thinner airway walls in COPD (11). There are several possible explanations. First, increased AWT was seen in smokers (45) and may represent early injuries due to inflammation, remodeling, and/or chronic mucus hypersecretion; these early injuries may precede airway shrinkage and loss (46) along the length of the airway tree (11, 47) as clinical COPD develops and progresses. Indeed, increased AWT has been associated with self-reported chronic mucus hypersecretion (48), which has been prospectively associated with accelerated lung function decline, incident COPD, and poor clinical outcomes (49). Second, even if AWT is decreased in the majority of patients with smoking-related COPD, greater AWT may be associated with alternative CLRD subphenotypes, such as “symptomatic smokers” (10) and individuals with asthma (50). Third, we were not able to spatially match the airway measures in this study, owing to the scanning protocol; hence, it remains possible that the findings for AWT may arise from differential sampling within the airway tree hierarchy (11). We believe this sampling bias is unlikely in our study participants without clinical disease, though if present it would suggest that differential airway loss (41, 42) occurs in preclinical disease and can be detected using measures such as Pi10 derived from standard clinical scanners.
Strengths of the present work include a prospective design over 14 years of follow-up; a new approach to analysis of incident CLRD via clinical events in addition to spirometry-defined lung function; a clinically applicable, reliable measure of large airway dimensions on computed tomographic scans; adjustment for COPD genetic risk; and use of a highly characterized, population-based, epidemiologic cohort that included a large number of light smokers and never smokers—subgroups systematically excluded from most prior studies of COPD. Nonetheless, several limitations must be considered.
We used two-dimensional measures on images acquired on older low-dose CT scanners, which are less precise than three-dimensional airway reconstructions on contemporary research CT scanners. Nonetheless, scan–rescan reproducibility for Pi10 on MESA baseline CT (ICC, 0.77–0.86) was similar to or better than that obtained with gold standard measures in SPIROMICS (Subpopulations and Intermediatae Outcomes in COPD Study; ICC, 0.79–0.82) (51), and, because three-dimensional airway reconstructions may not be feasible on most clinical scans, the current approach may have greater potential clinical applicability. Upper lobe airways were not included, although MESA baseline computed tomographic scans included all lower lobe airways. Measures of hyperinflation were not available (52), nor were calculations of functional small airway disease, which require coregistration of inspiratory and expiratory computed tomographic scans (53), but these are also not currently applicable to clinical scans.
Our results were consistent on EBT scanners, whereas subgroup analyses stratified by MDCT manufacturer yielded more heterogeneous results. Pi10 measurements derived from GE Healthcare Life Sciences MDCT scanners, which were lower on average, were not significantly associated with lung function, although they did associate with events. The converse—associations with lung function but not events—was observed for Siemens MDCT scanners. These findings could represent random error in the setting of small samples, given that they did not consistently support systematic measurement error or effect modification by scanner type or manufacturer, although these possibilities could not be excluded.
Spirometry was available for only a subset and was acquired after baseline, preventing us from excluding participants with subclinical airflow limitation at the time of CT. A substantial proportion of participants were excluded from lung function analyses because of incomplete follow-up; however, because higher Pi10 was associated with higher rates of censoring events (i.e., CLRD mortality), lung function results may be biased conservatively.
Clinical events, including mortality and exacerbations, were defined by administrative coding, which has potential for both over- and underdiagnosis (54). Nevertheless, our event definition was based on algorithms developed and validated in MESA (27, 28). We defined events due to any CLRD as our primary outcome, because misclassification among CLRD is common and the degree of biological and clinical overlap between asthma and COPD remains controversial. Stronger associations with incident COPD versus asthma events were observed, but this may simply reflect a greater burden of incident COPD than of asthma in the middle-aged and older adults we studied.
Restricting our study to participants without clinical CLRD at baseline avoided biases related to potential medication effects and reverse causation, and it allowed us to interpret our events endpoint as an indicator of incident clinical disease. However, this limited the number of events, raising concerns regarding statistical power and potential overadjustment. Nonetheless, our results were similar and statistically significant in crude and sequentially adjusted analyses and were consistent with lung function results.
Incidence of airflow limitation and CLRD events was low in the participants included in this analysis, who were initially without airflow limitation and/or prevalent clinical CLRD. Notably, among MESA participants with prevalent CLRD, the 5-year incidence of spirometry-defined COPD was 7%, which is not dissimilar to that in prior occupational and geriatric studies (55–57), and the cumulative incidence of CLRD hospitalization/mortality was 10%. MESA also excluded participants with clinical cardiovascular disease at baseline and is therefore healthier than the U.S. population average. MESA nevertheless represents the largest available resource to study associations between CT findings and CLRD in a healthy U.S. population-based sample.
In conclusion, greater Pi10 was associated with accelerated lung function decline, incident spirometry-defined COPD, and CLRD hospitalization and mortality in a general population-based sample without clinical lung disease. This suggests that airway dimensions on CT may index environmental and developmental determinants of COPD or their interactions (44) beyond established risk factors. Although associations were relatively modest and may not be readily applicable to current clinical care, the question whether these measures could improve personalized risk assessment for CLRD and potentially identify and characterize early, preclinical COPD warrants further consideration.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at www.mesa-nhlbi.org.
Footnotes
The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study is funded by National Institutes of Health (NIH) grants R01-HL077612 and R01-HL075476. Additional funding is provided by NIH grants R21-HL129924 (E.C.O.), K23-HL130627 (E.C.O.), R01-HL130506 (B.M.S.), and R01-HL093081 (R.G.B.). MESA also was supported by NIH contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-RR-025005 from the National Center for Research Resources. This publication was also developed under a STAR research assistance agreement (RD831697 [MESA Air]) awarded by the U.S. Environmental Protection Agency (EPA). It was not formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
Author Contributions: E.C.O.: literature search, funding, study design, data analysis, data interpretation, drafting of the manuscript, and preparation of tables and figures; B.M.S., R.K., K.M.D., and J.E.S.: study design, data interpretation, and manuscript review; E.A.H.: study design, data collection, data interpretation, and manuscript review; J.D.K., A.W.M., J.I.R., E.D.M., D.R.J., G.L.B., A.R.F., and K.W.: study design, data collection, data interpretation, and manuscript review; J.N.N.: data collection, data interpretation, and manuscript review; and R.G.B.: study design, funding, data collection, data interpretation, and drafting and review of the manuscript. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors meet the International Committee of Medical Journal Editors criteria for authorship, all having made substantial contributions to study conception, design, and/or data collection; interpretation of data; drafting and/or critical revision of the manuscript; and final manuscript review. All coauthors agree to be accountable for all aspects of the work and will ensure that any questions regarding the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012;379:1341–1351. doi: 10.1016/S0140-6736(11)60968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochanek KD, Murphy S, Xu J, Arias E. Mortailty in the United States, 2016. NCHS Data Brief. 2017;293:1–8. [PubMed] [Google Scholar]
- 3.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med. 2013;368:351–364. doi: 10.1056/NEJMsa1211127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias JA, Zhu Z, Chupp G, Homer RJ. Airway remodeling in asthma. J Clin Invest. 1999;104:1001–1006. doi: 10.1172/JCI8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim V, Han MK, Vance GB, Make BJ, Newell JD, Hokanson JE, et al. The chronic bronchitic phenotype of COPD: an analysis of the COPDGene Study. Chest. 2011;140:626–633. doi: 10.1378/chest.10-2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez CH, Chen YH, Westgate PM, Liu LX, Murray S, Curtis JL, et al. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67:399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JH, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, Coxson H, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 10.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith BM, Hoffman EA, Rabinowitz D, Bleecker E, Christenson S, Couper D, et al. Comparison of spatially matched airways reveals thinner airway walls in COPD: the Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:987–996. doi: 10.1136/thoraxjnl-2014-205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelsner EC, Hoffman EA, Smith BM, Michos E, Jacobs DR, Jr, Kalhan R, et al. Prognostic significance of large airway measures on computed tomography (CT) in the general population: the MESA-Lung Study. Eur Respir J. 2016;48(Suppl 60):OA4999. [Google Scholar]
- 13.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 14.Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and validity of lung density measures from cardiac CT Scans—the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiles C. Lung cancer screening with low-dose computed tomography. Radiol Clin North Am. 2014;52:27–46. doi: 10.1016/j.rcl.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donohue KM, Hoffman EA, Baumhauer H, Guo J, Ahmed FS, Lovasi GS, et al. Asthma and lung structure on computed tomography: the Multi-Ethnic Study of Atherosclerosis Lung Study. J Allergy Clin Immunol. 2013;131:361–368.e11. doi: 10.1016/j.jaci.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saba OI, Hoffman EA, Reinhardt JM. Maximizing quantitative accuracy of lung airway lumen and wall measures obtained from X-ray CT imaging. J Appl Physiol (1985) 2003;95:1063–1075. doi: 10.1152/japplphysiol.00962.2002. [DOI] [PubMed] [Google Scholar]
- 20.Reinhardt JM, D’Souza ND, Hoffman EA. Accurate measurement of intrathoracic airways. IEEE Trans Med Imaging. 1997;16:820–827. doi: 10.1109/42.650878. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:519–532. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coxson HO, Mayo JR, Behzad H, Moore BJ, Verburgt LM, Staples CA, et al. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol (1985) 1995;79:1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 26.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 27.Oelsner EC, Carr JJ, Enright PL, Hoffman EA, Folsom AR, Kawut SM, et al. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71:624–632. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oelsner EC, Loehr LR, Henderson AG, Donohue KM, Enright PL, Kalhan R, et al. Classifying chronic lower respiratory disease events in epidemiologic cohort studies. Ann Am Thorac Soc. 2016;13:1057–1066. doi: 10.1513/AnnalsATS.201601-063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adar SD, Klein R, Klein BE, Szpiro AA, Cotch MF, Wong TY, et al. Air pollution and the microvasculature: a cross-sectional assessment of in vivo retinal images in the population-based Multi-Ethnic Study of Atherosclerosis (MESA) PLoS Med. 2010;7:e1000372. doi: 10.1371/journal.pmed.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell R, Davidson D, Divers J, Manichaikul A, Carr JJ, Detrano R, et al. Genetic ancestry and the relationship of cigarette smoking to lung function and per cent emphysema in four race/ethnic groups: a cross-sectional study. Thorax. 2013;68:634–642. doi: 10.1136/thoraxjnl-2012-202116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manichaikul A, Hoffman EA, Smolonska J, Gao W, Cho MH, Baumhauer H, et al. Genome-wide study of percent emphysema on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189:408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wain LV, Shrine N, Artigas MS, Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017;49:416–425. doi: 10.1038/ng.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aaron CP, Schwartz JE, Bielinski SJ, Hoffman EA, Austin JH, Oelsner EC, et al. Intercellular adhesion molecule 1 and progression of percent emphysema: the MESA Lung Study. Respir Med. 2015;109:255–264. doi: 10.1016/j.rmed.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 35.Xie X, Dijkstra AE, Vonk JM, Oudkerk M, Vliegenthart R, Groen HJ. Chronic respiratory symptoms associated with airway wall thickening measured by thin-slice low-dose CT. AJR Am J Roentgenol. 2014;203:W383–W390. doi: 10.2214/AJR.13.11536. [DOI] [PubMed] [Google Scholar]
- 36.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181:353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed Hoesein FA, de Jong PA, Lammers JW, Mali WP, Schmidt M, de Koning HJ, et al. Computed tomography structural lung changes in discordant airflow limitation. PLoS One. 2013;8:e65177. doi: 10.1371/journal.pone.0065177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers: correlation with lung function. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 39.Kim V, Desai P, Newell JD, Make BJ, Washko GR, Silverman EK, et al. Airway wall thickness is increased in COPD patients with bronchodilator responsiveness. Respir Res. 2014;15:84. doi: 10.1186/s12931-014-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grydeland TB, Thorsen E, Dirksen A, Jensen R, Coxson HO, Pillai SG, et al. Quantitative CT measures of emphysema and airway wall thickness are related to DLCO. Respir Med. 2011;105:343–351. doi: 10.1016/j.rmed.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 41.McDonough JE, Yuan R, Suzuki M, Seyednejad N, Elliott WM, Sanchez PG, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365:1567–1575. doi: 10.1056/NEJMoa1106955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat M, Hague CJ, et al. CanCOLD Collaborative Research Group; Canadian Respiratory Research Network. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression: findings from a population-based study. Am J Respir Crit Care Med. 2018;197:56–65. doi: 10.1164/rccm.201704-0692OC. [DOI] [PubMed] [Google Scholar]
- 43.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 44.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med. 2017;196:1021–1030. doi: 10.1164/rccm.201703-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donohue KM, Hoffman EA, Baumhauer H, Guo J, Budoff M, Austin JH, et al. Cigarette smoking and airway wall thickness on CT scan in a multi-ethnic cohort: the MESA Lung Study. Respir Med. 2012;106:1655–1664. doi: 10.1016/j.rmed.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 47.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 48.Dijkstra AE, Postma DS, ten Hacken N, Vonk JM, Oudkerk M, van Ooijen PM, et al. Low-dose CT measurements of airway dimensions and emphysema associated with airflow limitation in heavy smokers: a cross sectional study. Respir Res. 2013;14:11. doi: 10.1186/1465-9921-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193:662–672. doi: 10.1164/rccm.201511-2210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography: relation to clinical indices. Am J Respir Crit Care Med. 2000;162:1518–1523. doi: 10.1164/ajrccm.162.4.9909044. [DOI] [PubMed] [Google Scholar]
- 51.Motahari A, Newell JD, Han MK, Bleecker ER, Carretta EE, Couper DJ, et al. Short term repeatability of CT-derived pulmonary airway and density measures: role of quality control measures in assuring measurement reliability: SPIROMICS [abstract] Am J Respir Crit Care Med. 2017;195:A6506. [Google Scholar]
- 52.Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, Pinto-Plata V, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:591–597. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 53.Galban CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omori H, Nagano M, Funakoshi Y, Onoue A, Mihara S, Marubayashi T, et al. Twelve-year cumulative incidence of airflow obstruction among Japanese males. Intern Med. 2011;50:1537–1544. doi: 10.2169/internalmedicine.50.4412. [DOI] [PubMed] [Google Scholar]
- 56.Luoto JA, Elmstahl S, Wollmer P, Pihlsgard M. Incidence of airflow limitation in subjects 65–100 years of age. Eur Respir J. 2016;47:461–472. doi: 10.1183/13993003.00635-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soyseth V, Johnsen HL, Bugge MD, Hetland SM, Kongerud J. Prevalence of airflow limitation among employees in Norwegian smelters: a longitudinal study. Occup Environ Med. 2011;68:24–29. doi: 10.1136/oem.2009.049452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.