Abstract
Rationale: Respiratory muscle weakness is common in critically ill patients; the role of targeted inspiratory muscle training (IMT) in intensive care unit rehabilitation strategies remains poorly defined.
Objectives: The primary objective of the present study was to describe the range and tolerability of published methods for IMT. The secondary objectives were to determine whether IMT improves respiratory muscle strength and clinical outcomes in critically ill patients.
Methods: We conducted a systematic review to identify randomized and nonrandomized studies of physical rehabilitation interventions intended to strengthen the respiratory muscles in critically ill adults. We searched the MEDLINE, Embase, HealthSTAR, CINAHL, and CENTRAL databases (inception to September Week 3, 2017) and conference proceedings (2012 to 2017). Data were independently extracted by two authors and collected on a standardized report form.
Results: A total of 28 studies (N = 1,185 patients) were included. IMT was initiated during early mechanical ventilation (8 studies), after patients proved difficult to wean (14 studies), or after extubation (3 studies), and 3 other studies did not report exact timing. Threshold loading was the most common technique; 13 studies employed strength training regimens, 11 studies employed endurance training regimens, and 4 could not be classified. IMT was feasible, and there were few adverse events during IMT sessions (nine studies; median, 0%; interquartile range, 0–0%). In randomized trials (n = 20), IMT improved maximal inspiratory pressure compared with control (15 trials; mean increase, 6 cm H2O; 95% confidence interval [CI], 5–8 cm H2O; pooled relative ratio of means, 1.19; 95% CI, 1.14–1.25) and maximal expiratory pressure (4 trials; mean increase, 9 cm H2O; 95% CI, 5–14 cm H2O). IMT was associated with a shorter duration of ventilation (nine trials; mean difference, 4.1 d; 95% CI, 0.8–7.4 d) and a shorter duration of weaning (eight trials; mean difference, 2.3 d; 95% CI, 0.7–4.0 d), but confidence in these pooled estimates was low owing to methodological limitations, including substantial statistical and methodological heterogeneity.
Conclusions: Most studies of IMT in critically ill patients have employed inspiratory threshold loading. IMT is feasible and well tolerated in critically ill patients and improves both inspiratory and expiratory muscle strength. The impact of IMT on clinical outcomes requires future confirmation.
Keywords: artificial respiration, weaning, respiratory muscles, physical therapy, inspiratory muscle training
A majority of mechanically ventilated patients develop diaphragmatic weakness during critical illness (1), predisposing them to prolonged ventilation, readmission to the intensive care unit (ICU), and death (1–4); in addition, survivors have poor long-term clinical outcomes (5). Many factors impair diaphragmatic function in these patients (6), including mechanical ventilation (7) and sepsis (8).
Physical rehabilitation strategies are employed to accelerate liberation from ventilation and improve clinical outcomes, possibly by preventing or mitigating ICU-acquired weakness (9). Rehabilitation efforts in the ICU have been focused largely on peripheral muscle dysfunction (10–13), whereas respiratory muscle rehabilitation has received relatively less attention. Inspiratory muscle training (IMT) targets the diaphragm and accessory inspiratory muscles with the goal of improving muscle strength and endurance. IMT techniques include threshold loading, resistive loading, and whole-body mobilization (see Figure 1). IMT regimens may vary widely in load, frequency, and duration. IMT has been shown to improve inspiratory muscle strength, exercise performance, or quality of life in patients with chronic obstructive pulmonary disease (COPD) (14), chronic heart failure (15), asthma (16), or cystic fibrosis (17), as well as in athletes (18). Previous systematic reviews have suggested that in ventilated patients, IMT may improve inspiratory muscle strength and potentially reduce the duration of ICU stay (19, 20). However, these reviews were focused on specific IMT techniques; did not report on functional status or quality of life; did not incorporate recent important studies; and did not examine specific potential determinants of treatment effect, such as timing, type of loading, or baseline muscle strength.
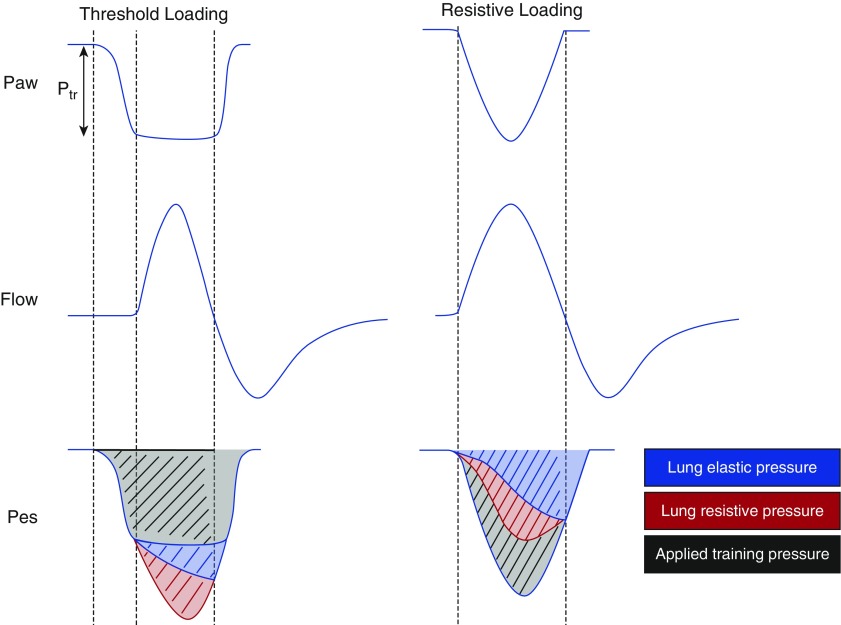
Figure 1.
Inspiratory muscle training techniques. Two main types of inspiratory muscle training techniques have been applied in critically ill patients: resistive loading and threshold loading. Resistive loading involves the application of a resistor to the airway (right). The resistor increases the pressure required for the respiratory muscles to generate a given flow (the area shaded black). The required pressure is the product of resistance and flow and therefore depends on the inspiratory flow that the patient attempts to generate. Accordingly, the total training effect varies with the patient’s respiratory mechanics and respiratory drive, making it difficult to standardize. Threshold loading involves the application of a threshold valve to the airway (left). The valve is designed so that a certain level of training pressure (Ptr) must be generated by the patient’s respiratory muscles before it opens to permit inspiratory flow (analogous to the effect of intrinsic positive end-expiratory pressure). Consequently, the pressure required to maintain at least some inspiratory flow is independent of the flow and volume generated by the patient or ventilator. The total training effect (shaded in black) will therefore be independent of mechanics and respiratory drive and is easier to standardize. A threshold load may also be applied directly on the ventilator by setting a pressure trigger at a desired threshold pressure level. Threshold loading is typically performed by applying a Ptr anywhere between 20 and 50% of the patient’s maximal inspiratory pressure for a relatively brief period of time (a few repetitions or a few minutes) at regular intervals. Note: chest wall elastance and resistance are ignored on the diagram for simplicity. Paw = airway pressure; Pes = esophageal pressure.
IMT has received relatively little attention in ICU rehabilitation programs, and the most recent ICU rehabilitation guidelines do not mention IMT (9). We conducted a systematic review to establish the feasibility and tolerability of IMT in critically ill adult patients. We also aimed to characterize the range of techniques employed for IMT and to determine whether IMT improves respiratory muscle strength and clinical outcomes in critically ill patients relative to routine care.
Methods
Study Selection and Literature Review
We systematically searched the literature for observational studies and clinical trials of IMT (which we defined as “any physical rehabilitation intervention employed with the goal of improving inspiratory muscle strength”) in adults admitted to the ICU. To delineate studies of interventions intended to rehabilitate the inspiratory muscles, we included only those studies reporting measurement of maximal inspiratory pressure (MIP). Single case reports were excluded. Studies were identified by searching the MEDLINE, CINAHL, CENTRAL, Embase, and HealthSTAR databases (inception to September Week 3, 2017) for randomized and nonrandomized studies using search terms that included “respiratory muscle training,” “inspiratory muscle training,” and “physiotherapy.” (See the online supplement for complete details of the search strategy.) Two investigators independently reviewed the abstracts retrieved by this search, and any studies identified as potentially eligible by either reviewer underwent full-text review. These full-text papers were reviewed independently and in duplicate to determine eligibility. Bibliographies of all papers were reviewed for additional abstracts. The search was initially conducted in winter 2014 by two investigators (M.P., D.B.), updated in spring 2016 (S.V., C.U.) and November 2016 (U.S., S.A.-M.), and finally updated in September 2017 (S.V., M.B.). Agreement between investigators on eligibility assessment of all downloaded papers was quantified by Cohen’s kappa. Disagreements about eligibility were resolved by consensus and, when necessary, by another investigator (E.C.G.). No review protocol was separately published. We present the results according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendations (21).
Data Abstraction
Data were abstracted independently and in duplicate to record characteristics of study participants and study design, IMT technique (specific exercises, duration, frequency, intensity), IMT protocol adherence and tolerability, physiological endpoints (MIP and maximal expiratory pressure [MEP]) (22), and clinical outcomes (duration of ventilation, duration of weaning, mortality, ICU and hospital lengths of stay, adverse events). Studies were classified according to whether the IMT regimen was strength training (≤200 repetitions per session with higher load), endurance training (>200 repetitions per session with lower load), or unclear, according to accepted criteria (23).
Assessment of Methodological Quality
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) framework was employed to evaluate the methodological quality of randomized clinical trials included in this review (24). The risk of bias was evaluated for each trial according to GRADE criteria (25). Observational studies were evaluated using the Newcastle-Ottawa Scale (26). MIP measurement technique was also assessed according to published methodological standards (27, 28). We judged the risk of bias due to lack of blinding to be “unclear” for physiological and clinical endpoints because these measurements are less likely to be influenced by patient or assessor perception. Each outcome was evaluated for serious risk of bias, imprecision, inconsistency, indirectness, and publication bias (29).
Data Analysis and Synthesis
The main outcomes were the protocol adherence rate (proportion of patients who completed the study protocol) and the rate of serious adverse events during IMT (frequency of IMT sessions aborted for any reason), both quantified by the median (interquartile range) of rates across studies. The effect of IMT on MIP, MEP, and clinical endpoints was assessed by random effects meta-analysis with inverse-variance weighting. Only randomized trials were included in meta-analyses. The effect of treatment on MIP and MEP was quantified in terms of the mean difference between groups in the change from baseline to post-treatment values, the post-treatment value alone, or the percentage change from baseline to post-treatment value (quantified as the relative ratio of means). Computation of standard errors took into account the correlated nature of the data (see the online supplement for details). The results of meta-analysis of binary outcomes are presented as risk ratios. All estimates are presented with 95% confidence intervals (CIs).
Where necessary, median and interquartile range were transformed to mean and standard deviation using validated computational methods (30). Heterogeneity between trials was quantified using the I2 measure, with I2 > 75% deemed to denote substantial heterogeneity (31). Publication bias was assessed using a funnel plot and testing for funnel plot asymmetry (32). We conducted sensitivity analyses excluding studies reported in abstract form only, studies deemed to be at high risk of bias, and studies that did not employ a high-quality MIP measurement technique (defined as measurements that were obtained by coaching patients and using a one-way valve). The effects of timing and technique of IMT were evaluated in exploratory sensitivity analyses by tests of interaction. Meta-analyses and metaregression were conducted using the “meta” package in R version 3.0.2 software (www.r-project.org).
Results
Search Results
In our literature search, we identified 9,051 studies; of these, 95 papers were downloaded for full-text review. Twenty-two papers met our eligibility criteria. In a bibliographic search, we identified two additional eligible studies, and four conference abstracts met our inclusion criteria (see Figure E1 in the online supplement). Agreement on study eligibility was strong (κ = 0.89; 95% CI, 0.78–0.99).
Study Characteristics
Twenty eight studies (N = 1,185 patients) were included (details of the study population and design are provided in Table E1), of which 20 (n = 765) were randomized controlled trials (33–52), 2 were nonrandomized studies with a comparison group (53, 54), 4 were prospective studies without a comparison group (55–58), and 2 were retrospective studies without a comparison group (59, 60). Mean baseline MIP ranged between 15 and 54 cm H2O; mean baseline MEP ranged between 23 and 46 cm H2O. The demographic characteristics of most studies were generally representative of adult ICU patients (mean age range, 35–82 years; proportion of female patients ranged from 15 to 57%; mean Acute Physiology and Chronic Health Evaluation II score ranged from 17 to 34 points).
Methodological Quality
Many randomized controlled trials had important potential sources of bias (Figure E2). Most randomized trials did not report whether allocation was concealed, and outcome assessors were not blinded to randomization in most trials. The methodological quality of observational studies was also limited (Table E2). Studies frequently did not describe some relevant aspects of MIP measurement technique (Table E3).
IMT Techniques, Tolerability, and Feasibility
A variety of techniques were employed for IMT (Table E1). The most common technique was to employ an external threshold loading device to apply varying levels of threshold loading using an external device (19 studies) or by adjusting the ventilator trigger threshold (2 studies) (35, 54). Other approaches included flow resistive training (one study) (55), diaphragmatic breathing exercises (two studies) (48, 53), and biofeedback on respiratory pattern (one study) (39). More general physical therapy regimens were also studied for their effect on inspiratory muscle strength, including mobilization (48, 56, 58), postural training (36, 42), and upper arm exercise (44). Some studies employed combinations of these techniques. Eleven studies employed an endurance training IMT regimen (35, 36, 39, 42–44, 48, 54–56, 59), 13 studies employed a strength training IMT regimen (33, 34, 37, 38, 40, 41, 45, 46, 49, 50, 52, 57, 60), and 4 studies could not be classified (47, 51, 53, 58).
The timing of IMT initiation varied between studies. IMT was applied after 24 hours of mechanical ventilation (1 study) (35), once patients were awake and cooperative (1 study) (48), once the patient was transitioned to partially assisted ventilation (1 study) (34), once patients met readiness-to-wean criteria (5 studies) (37, 38, 41, 49, 50), once patients failed attempted weaning (12 studies) (39, 40, 45–47, 52–55, 57, 59, 60), or after liberation from ventilation (3 studies) (33, 43, 44). Four studies did not specify the timing of intervention (36, 51, 56, 58). The duration of IMT varied widely between studies, ranging from 3 days to up to 6 weeks of therapy (Table 1). Most studies compared IMT with usual care.
Table 1.
GRADE summary of findings for the impact of inspiratory muscle training in critically ill patients
| Outcome | Impact Effect (95% CI) | No. of Participants (RCTs) | Quality of the Evidence (GRADE) |
|---|---|---|---|
| Change in maximal inspiratory pressure from baseline after IMT | Mean difference in change 6 (5 to 8) cm H2O higher in IMT group than in control group | 647 (15 RCTs) | ⊕◯◯◯ Very low*,†,‡ |
| Pooled ratio of means for change in MIP relative to baseline MIP, 1.21 (1.16 to 1.26) | |||
| Change in maximal inspiratory pressure from baseline after IMT (sensitivity analysis excluding studies at high risk of bias) | Mean difference 9 (7 to 12) cm H2O higher in IMT group than in control group | 175 (3 RCTs) | ⊕⊕⊕⊕ High |
| Maximal inspiratory pressure after IMT | Mean difference 7 (6 to 8) cm H2O higher in IMT group than in control group | 575 (15 RCTs) | ⊕⊕◯◯ Low*,‡ |
| Change in maximal expiratory pressure from baseline after IMT | Mean difference in change 9 (5 to 14) cm H2O higher in IMT group than in control group | 153 (4 RCTs) | ⊕⊕⊕◯ Moderate* |
| Pooled ratio of means for change in MEP relative to baseline MEP, 1.39 (1.27 to 1.54) | |||
| Change in maximal expiratory pressure from baseline after IMT (sensitivity analysis excluding studies at high risk of bias) | Mean difference in change 9 (5 to 14) cm H2O higher in IMT group than in control group | 106 (2 RCTs) | ⊕⊕⊕⊕ High |
| Duration of ventilation | Pooled duration of ventilation was 4.1 (0.8 to 7.4) d shorter in IMT group than in control group | 325 (9 RCTs) | ⊕◯◯◯ Very low*,†,‡,§ |
| Duration of ventilation (sensitivity analysis excluding studies at high risk of bias) | Pooled duration of ventilation was 4.6 (−1.0 to 10.1) d shorter in IMT group than in control group | 220 (4 RCTs) | ⊕⊕◯◯ Low†,§ |
| Duration of weaning from mechanical ventilation | Pooled duration of weaning from mechanical ventilation was 2.3 (0.7 to 3.9) d shorter in IMT group than in control group | 257 (8 RCTs) | ⊕◯◯◯ Very low*,†,§ |
| Duration of weaning (sensitivity analysis excluding studies at high risk of bias) | Pooled duration of weaning from mechanical ventilation was 3.2 (0.6 to 5.8) d shorter in IMT group than in control group | 209 (5 RCTs) | ⊕⊕◯◯ Low†,§ |
| ICU length of stay | Length of stay in ICU was 3.1 (−1.0 to 7.1) d shorter in IMT group than in control group | 28 (2 RCTs) | ⊕◯◯◯ Very low*,‡,§ |
| Mortality in ICU | Pooled relative risk of death in ICU was 0.67 (0.20 to 2.20) in IMT group compared with control group | 197 (3 RCTs) | ⊕⊕◯◯ Low‡,§ |
| GRADE Working Group grades of evidence | |||
| High quality: We are very confident that the true effect lies close to that of the estimate of the effect. | |||
| Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. | |||
| Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. | |||
| Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||
Definition of abbreviations: CI = confidence interval; GRADE = Grading of Recommendations, Assessment, Development, and Evaluation; ICU = intensive care unit; IMT = inspiratory muscle training; MEP = maximal expiratory pressure; MIP = maximal inspiratory pressure; RCT = randomized controlled trial.
Multiple studies were at high risk of bias.
Inconsistency: statistical heterogeneity was substantial (I2 > 75% and P < 0.05), and confidence intervals show no or minimal overlap.
Studies employed cointerventions.
Imprecision: confidence intervals did not exclude statistically significant benefit or harm.
All included studies reported that IMT was feasible in critically ill patients. Protocol adherence rates were generally high (11 studies; median, 95%; interquartile range, 77 to 100%), and researchers in 2 trials reported that there were no differences in adherence rates between experimental and sham IMT (40, 44). Adverse events were infrequent during IMT sessions: seven studies reported no adverse events during IMT (33, 34, 37, 40, 47, 52, 57). In a study of 34 patients receiving prolonged flow resistive IMT, investigators reported one serious adverse event during IMT (severe bradycardia and syncope) (55). In another study employing prolonged threshold loading (5–30 min) for IMT (35), researchers reported that 14% of IMT sessions were aborted because of paradoxical breathing (5%), tachypnea (4%), desaturation (3%), or hemodynamic instability (2%).
Impact on Respiratory Muscle Strength
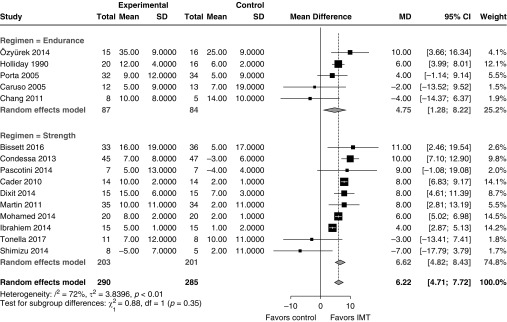
In randomized trials, IMT was associated with a significant increase in MIP from baseline compared with control (Figure 2; 15 studies; pooled mean difference, 6 cm H2O; 95% CI, 5–8 cm H2O; I2 = 72%). Relative to baseline MIP, MIP increased on average by 40% with IMT compared with 18% with control (Figure E3; 15 trials; pooled relative ratio of means, 1.19; 95% CI, 1.14–1.25; I2 = 86%). In one additional study, investigators reported an increase in MIP of 22% with IMT versus 8% with control (absolute values were not provided, so those data could not be pooled) (51). The change in MIP could not be computed using the data provided in four other trials (42, 45, 47, 48). There was no evidence of publication bias (Figure E4). The post-treatment MIP was also higher in patients undergoing IMT than among control individuals (Figure E5; 15 trials; mean difference, 7 cm H2O, 95% CI, 6–8 cm H2O; I2 = 33%). Similar effects were observed in a sensitivity analysis limited to trials with rigorous MIP measurement technique and without serious risk of bias (Figure E6; three trials; mean difference, 9 cm H2O; 95% CI, 7–12 cm H2O; I2 = 0%).
Figure 2.
Effect of inspiratory muscle training (IMT) on the change in maximal inspiratory pressure from baseline to the completion of the treatment course. The effect of IMT did not significantly differ with strength training versus endurance training regimens. Weight refers to the contribution of each study to the meta-analysis estimate of effect. CI = confidence interval; MD = mean difference; SD = standard deviation.
IMT was also associated with a significant increase in MEP from baseline compared with control (Figure E7; four studies; mean difference, 9 cm H2O; 95% CI, 5–14 cm H2O; I2 = 40%). Relative to baseline MEP, MEP increased on average by 63% with IMT compared with 17% with control (Figure E8; four studies; pooled relative ratio of means, 1.39; 95% CI, 1.25–1.55; I2 = 43%). Similar effects on MEP were observed in a sensitivity analysis limited to studies without serious risk of bias (Figure E9; two studies; mean difference, 9 cm H2O; 95% CI, 5–14 cm H2O; I2 = 34%).
The effect of IMT on MIP tended to be greater with strength training than with endurance training, but the subgroup difference was small and did not reach significance (Figure 2; interaction P = 0.35). Similarly, the effect of IMT on MIP tended to be greater with threshold loading than with other techniques (mobilization to chair, upper extremity exercise), but the subgroup difference did not reach significance (Figure E10; interaction P = 0.27). The effect of IMT on MIP was not affected by the timing of IMT initiation (Figure E11; P = 0.51) or by the mean baseline MIP in each study (Figure E12; R2 = 0%; P = 0.11).
Impact on Clinical Outcomes
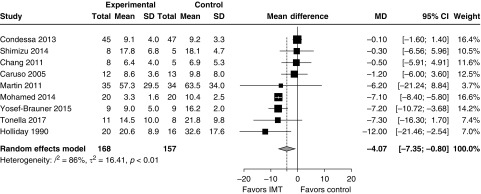
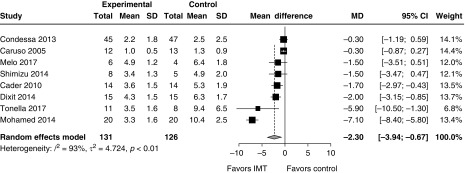
IMT was associated with a reduced duration of ventilation (Figure 3; nine trials; pooled mean difference, 4.1 d; 95% CI, 0.8–7.4 days; I2 = 86%). After excluding studies at serious risk of bias, the difference in duration of ventilation was not significant (Figure E13; four trials; pooled mean difference, 4.6 d; 95% CI, −1.0 to 10.1 d; I2 = 94%). IMT was associated with reduced duration of weaning from ventilation (Figure 4; 8 trials; pooled mean difference, 2.3 d; 95% CI, 0.7–4.0 d; I2 = 93%). After excluding studies at serious risk of bias, the reduction in the duration of weaning remained significant (Figure E14; five trials; pooled mean difference, 3.2 d; 95% CI, 0.6–5.8 d; I2 = 95%). Only one study examined time from randomization to liberation from ventilation (IMT vs. control, 3.3 vs. 10.4 d; P < 0.001) (41). ICU length of stay was not significantly reduced with IMT (Figure E15; two trials; pooled mean difference, 3.1 d; 95% CI, −1.0 to 7.1 d; I2 = 59%). IMT was not associated with an increased risk of death in the ICU (Figure E16; four studies; RR, 0.65; 95% CI, 0.24–1.76; I2 = 15%) or hospital (Figure E17; two studies; relative risk, 2.16; 95% CI, 0.23–20.38; I2 = 58%), but CIs around these estimates could not exclude clinically important benefit or harm. One trial reported a trend toward higher mortality with IMT; the causes of death in that study did not appear to be directly related to IMT (33). One trial reported improved quality of life but not functional status after 2 weeks of IMT (33). Pooled effects are integrated with assessment of methodological quality according to the GRADE framework in Table 1.
Figure 3.
Impact of inspiratory muscle training (IMT) on the duration of ventilation in mechanically ventilated patients. After exclusion of studies at serious risk of bias, the treatment effect was no longer significant (mean difference, 4.6 d; 95% CI, −1.0 to 10.1 d; I2 = 94%). Weight refers to the contribution of each study to the meta-analysis estimate of effect. CI = confidence interval; MD = mean difference; SD = standard deviation.
Figure 4.
The impact of inspiratory muscle training (IMT) on the duration of weaning from mechanical ventilation. After exclusion of studies at serious risk of bias, the effect remained significant (3.2 d; 95% CI, 0.6–5.8 d; I2 = 95%). Weight refers to the contribution of each study to the meta-analysis estimate of effect. CI = confidence interval; MD = mean difference; SD = standard deviation.
Discussion
In this systematic review, we found that IMT is feasible and well tolerated during critical illness. The vast majority of studies employed inspiratory threshold loading and flow resistive loading for IMT, although other methods were also reported. We also found that IMT can achieve a modest but potentially meaningful improvement in respiratory muscle strength. IMT might potentially shorten the duration of ventilation by accelerating weaning, but the impact on these outcomes remains uncertain owing to methodological limitations of the included trials.
Previous reviews restricted IMT to techniques that use threshold loading devices or adjust ventilator trigger sensitivity (19, 20). Our review was designed primarily to characterize the range of techniques for inspiratory muscle rehabilitation in the ICU. Compared with the most recent review (21), the present review includes 18 additional studies (10 additional trials), including nonrandomized studies and studies in which researchers enrolled nonventilated ICU patients. This larger number of studies enhances our confidence that IMT is feasible and well tolerated and highlights the range of strategies that have been employed to rehabilitate the respiratory muscles. This review is also the first to systematically evaluate the effect of IMT on expiratory muscle strength and to incorporate patient-centered outcomes such as functional status and quality of life, now recognized as centrally important in the field (61). It is worth noting that only one study evaluated the impact of IMT on patient-centered post-ICU outcomes—these outcomes require further attention in future trials.
We explored a range of factors that might determine the effectiveness of IMT. The effect of IMT on inspiratory muscle strength did not vary significantly with the timing of IMT or the baseline severity of diaphragmatic weakness. We found that the increase in respiratory muscle strength tended to be greater when using threshold loading strategies than with other techniques. Unlike other techniques, threshold loading enables accurate control and titration of respiratory muscle loading independent of inspiratory flow or patient respiratory mechanics (see Figure 1). Threshold loading devices are easily applied to the airway briefly, and strength training regimens (in contrast to endurance training regimens) require only a few minutes on a daily basis, lending themselves to use in busy clinical environments.
As noted in previous reviews, we found that IMT can produce a modest improvement in inspiratory muscle strength compared with control. This finding was confirmed in a sensitivity analysis of trials of sufficient methodological quality to permit strong confidence in effect estimates. IMT was also associated with a modest improvement in maximal expiratory strength in our review. The clinical significance of this treatment effect is uncertain. The patient’s ability to tolerate respiratory loading is quantified by the respiratory muscle tension–time index (TTI), which is the quotient of inspiratory pressure during tidal breathing and MIP multiplied by the quotient of inspiratory time and total respiratory cycle time (62, 63). TTI thus represents the relative load and capacity balance of the respiratory system and strongly predicts time to task failure of the respiratory system (64). Once TTI exceeds 0.13–0.15, the development of respiratory failure is inevitable; values range between 0.12 and 0.20 in patients who are difficult to wean (62, 63). Increasing MIP lowers TTI and increases the time to task failure. We found that IMT can increase MIP relative to baseline by approximately 20% above and beyond the effect of control, yielding a potentially substantial reduction in TTI and in the capacity to tolerate respiratory loading. TTI may be further lowered by improvements in diaphragmatic efficiency related to enhanced abdominal muscle function with IMT (increased MEP). The abdominal muscles play a critical role in the cough reflex and in optimizing neuromuscular coupling of the diaphragm by enhancing the length–tension relationship of the diaphragm before inspiration, effectively enhancing the load–capacity balance of the muscle (65). Consequently, improvements in both inspiratory and expiratory muscle strength resulting from IMT may synergistically enhance the chances of successful liberation from ventilation (66). Notably, because MIP is in the denominator of TTI, patients with lower baseline MIP may have the greatest benefit from IMT (in terms of percentage reduction in TTI), even if the absolute increase in MIP with IMT is unrelated to baseline MIP.
As reported in a previous review (20), IMT was associated with a shorter duration of ventilation and weaning in pooled analyses. Confidence in these findings is limited by concerns about methodological quality, including frequent cointervention with general physical therapy strategies, risk of bias due to nonrandom sequence allocation in some controlled studies, significant statistical heterogeneity, and lack of precision in effect estimates. The duration of weaning was reduced with IMT, even after excluding trials at significant risk of bias. However, the definition of weaning varied somewhat between studies, limiting generalizability and contributing to heterogeneity in treatment effect between studies. Consequently, we cannot confidently confirm or refute the hypothesis that IMT accelerates liberation from mechanical ventilation.
A key limitation in this systematic review is the heterogeneity between studies in the timing, technique, control comparison, and duration of IMT. This limitation was expected because of the broad definition of IMT used to permit a description of the full range of techniques. To address this limitation, we conducted several sensitivity analyses and restricted all meta-analyses to randomized trials.
The optimal approach to IMT remains uncertain. In our review, there was no significant difference between strength and endurance training regimens, though strength training regimens require less training time and may therefore be more feasible. Importantly, resting the muscles adequately may be an important component of effective IMT regimens. One study included IMT as part of multiple brief daily T-tube trials (46); prolonged or frequent loading may explain the apparent reduction in MIP observed in that study. Load-induced injury to the diaphragm is well documented in animal models (67, 68) and humans (69, 70), and it is particularly likely to occur in the context of acute respiratory failure (71) and sepsis (72). Caution about excessive or prolonged loading is therefore warranted when designing IMT regimens. Furthermore, it is probably prudent to defer IMT until patients are stable and sepsis and systemic inflammation are resolving.
Future trials should be conducted to evaluate the effect of IMT on weaning from mechanical ventilation and on the long-term experience of ICU survivors in large, generalizable studies sufficient to confirm or refute clinically important benefits in terms of patient-centered outcomes. Given the feasibility, standardization, and consistent evidence of physiological effectiveness of a strength training strategy employing threshold loading, we propose that this would be the preferred strategy to evaluate. We suggest that a large multicenter randomized trial powered to detect meaningful changes in patient-important outcomes (i.e., the risk of prolonged mechanical ventilation and quality of life and functional status in ICU survivors) is required to determine whether IMT should be routinely incorporated into the care of critically ill patients. Such a trial should employ rigorously defined consensus definitions for weaning duration and other endpoints to make the results readily interpretable for clinical practice.
Implications for Clinical Practice
On the basis of currently available evidence, we suggest that IMT is feasible and safe in mechanically ventilated patients. If clinicians wish to provide IMT, a strength training regimen using threshold loading, such as that studied by Martin and colleagues (40), would be a reasonable approach based on its tolerability and relatively minimal time requirement. On the basis of the foregoing considerations regarding TTI, clinicians may consider using IMT to improve diaphragmatic strength in patients who prove difficult to liberate from ventilation because of diaphragmatic weakness.
Conclusions
IMT in critically ill adults is feasible and well tolerated and can achieve a modest but potentially meaningful improvement in respiratory muscle strength. The potential impact of IMT on clinical outcomes as well as long-term functional status and quality of life requires future confirmation.
Supplementary Material
Footnotes
Supported by a Canadian Institutes of Health Research postdoctoral fellowship (E.C.G.).
Author Contributions: M.P., N.D.F., and E.C.G. conceived and designed the study. S.V., U.S., S.A.-M., C.U., D.B., M.P., and E.C.G. collected the data. E.C.G. conducted the analysis. S.V. drafted the manuscript. All authors critically reviewed the manuscript for important intellectual content.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dres M, Dubé BP, Mayaux J, Delemazure J, Reuter D, Brochard L, et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195:57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 2.Supinski GS, Callahan LA. Diaphragm weakness in mechanically ventilated critically ill patients. Crit Care. 2013;17:R120. doi: 10.1186/cc12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim WY, Suh HJ, Hong SB, Koh Y, Lim CM. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med. 2011;39:2627–2630. doi: 10.1097/CCM.0b013e3182266408. [DOI] [PubMed] [Google Scholar]
- 4.Adler D, Dupuis-Lozeron E, Richard JC, Janssens JP, Brochard L. Does inspiratory muscle dysfunction predict readmission after intensive care unit discharge? Am J Respir Crit Care Med. 2014;190:347–350. doi: 10.1164/rccm.201404-0655LE. [DOI] [PubMed] [Google Scholar]
- 5.Damuth E, Mitchell JA, Bartock JL, Roberts BW, Trzeciak S. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:544–553. doi: 10.1016/S2213-2600(15)00150-2. [DOI] [PubMed] [Google Scholar]
- 6.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 7.Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator-induced diaphragmatic dysfunction – human studies confirm animal model findings! Crit Care. 2011;15:206. doi: 10.1186/cc10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demoule A, Jung B, Prodanovic H, Molinari N, Chanques G, Coirault C, et al. Diaphragm dysfunction on admission to the intensive care unit: prevalence, risk factors, and prognostic impact-a prospective study. Am J Respir Crit Care Med. 2013;188:213–219. doi: 10.1164/rccm.201209-1668OC. [DOI] [PubMed] [Google Scholar]
- 9.Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. ATS/CHEST Ad Hoc Committee on Liberation from Mechanical Ventilation in Adults. An Official American Thoracic Society/American College of Chest Physicians clinical practice guideline: Liberation from mechanical ventilation in critically ill adults. Rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med. 2017;195:120–133. doi: 10.1164/rccm.201610-2075ST. [DOI] [PubMed] [Google Scholar]
- 10.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss M, Nordon-Craft A, Malone D, Van Pelt D, Frankel SK, Warner ML, et al. A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. Am J Respir Crit Care Med. 2016;193:1101–1110. doi: 10.1164/rccm.201505-1039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA. 2016;315:2694–2702. doi: 10.1001/jama.2016.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 14.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 15.Montemezzo D, Fregonezi GA, Pereira DA, Britto RR, Reid WD. Influence of inspiratory muscle weakness on inspiratory muscle training responses in chronic heart failure patients: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1398–1407. doi: 10.1016/j.apmr.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Silva IS, Fregonezi GAF, Dias FAL, Ribeiro CTD, Guerra RO, Ferreira GMH. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;(9):CD003792. doi: 10.1002/14651858.CD003792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid WD, Geddes EL, O’Brien K, Brooks D, Crowe J. Effects of inspiratory muscle training in cystic fibrosis: a systematic review. Clin Rehabil. 2008;22:1003–1013. doi: 10.1177/0269215508090619. [DOI] [PubMed] [Google Scholar]
- 18.HajGhanbari B, Yamabayashi C, Buna TR, Coelho JD, Freedman KD, Morton TA, et al. Effects of respiratory muscle training on performance in athletes: a systematic review with meta-analyses. J Strength Cond Res. 2013;27:1643–1663. doi: 10.1519/JSC.0b013e318269f73f. [DOI] [PubMed] [Google Scholar]
- 19.Moodie L, Reeve J, Elkins M. Inspiratory muscle training increases inspiratory muscle strength in patients weaning from mechanical ventilation: a systematic review. J Physiother. 2011;57:213–221. doi: 10.1016/S1836-9553(11)70051-0. [DOI] [PubMed] [Google Scholar]
- 20.Elkins M, Dentice R. Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: a systematic review. J Physiother. 2015;61:125–134. doi: 10.1016/j.jphys.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures in adults. Respir Care. 2009;54:1348–1359. [PubMed] [Google Scholar]
- 23.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 24.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Accessed 2017 Mar 15]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 27.Truwit JD, Marini JJ. Validation of a technique to assess maximal inspiratory pressure in poorly cooperative patients. Chest. 1992;102:1216–1219. doi: 10.1378/chest.102.4.1216. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 29.Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bissett BM, Leditschke IA, Neeman T, Boots R, Paratz J. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax. 2016;71:812–819. doi: 10.1136/thoraxjnl-2016-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cader SA, Souza de Vale RG, Castro JC, Bacelar SC, Biehl C, Gomes MCV, et al. Inspiratory muscle training improves maximal inspiratory pressure and may assist weaning in older intubated patients: a randomised trial. J Physiother. 2010;56:171–177. doi: 10.1016/s1836-9553(10)70022-9. [DOI] [PubMed] [Google Scholar]
- 35.Caruso P, Denari SDC, Ruiz SAL, Bernal KG, Manfrin GM, Friedrich C, et al. Inspiratory muscle training is ineffective in mechanically ventilated critically ill patients. Clinics (São Paulo) 2005;60:479–484. doi: 10.1590/s1807-59322005000600009. [DOI] [PubMed] [Google Scholar]
- 36.Chang MY, Chang LY, Huang YC, Lin KM, Cheng CH. Chair-sitting exercise intervention does not improve respiratory muscle function in mechanically ventilated intensive care unit patients. Respir Care. 2011;56:1533–1538. doi: 10.4187/respcare.00938. [DOI] [PubMed] [Google Scholar]
- 37.Condessa RL, Brauner JS, Saul AL, Baptista M, Silva ACT, Vieira SR. Inspiratory muscle training did not accelerate weaning from mechanical ventilation but did improve tidal volume and maximal respiratory pressures: a randomised trial. J Physiother. 2013;59:101–107. doi: 10.1016/S1836-9553(13)70162-0. [DOI] [PubMed] [Google Scholar]
- 38.Dixit A, Prakash S. Effects of threshold inspiratory muscle training versus conventional physiotherapy on the weaning period of mechanically ventilated patients: a comparative study. Int J Physiother Res. 2014;2:424–428. [Google Scholar]
- 39.Holliday JE, Hyers TM. The reduction of weaning time from mechanical ventilation using tidal volume and relaxation biofeedback. Am Rev Respir Dis. 1990;141:1214–1220. doi: 10.1164/ajrccm/141.5_Pt_1.1214. [DOI] [PubMed] [Google Scholar]
- 40.Martin AD, Smith BK, Davenport PD, Harman E, Gonzalez-Rothi RJ, Baz M, et al. Inspiratory muscle strength training improves weaning outcome in failure to wean patients: a randomized trial. Crit Care. 2011;15:R84. doi: 10.1186/cc10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohamed AR, Basiouny HMS, Salem NM. Response of mechanically ventilated respiratory failure patients to respiratory muscles training. Med J Cairo Univ. 2014;82:19–24. [Google Scholar]
- 42.Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854. doi: 10.1016/s0003-9993(98)90369-0. [DOI] [PubMed] [Google Scholar]
- 43.Özyürek S, Malkoç M, Günerli A, Koca U, Egeli T. Inpatient inspiratory muscle training after upper abdominal surgery [abstract] Eur Respir J. 2014;44(Suppl 58):P3663. [Google Scholar]
- 44.Porta R, Vitacca M, Gilè LS, Clini E, Bianchi L, Zanotti E, et al. Supported arm training in patients recently weaned from mechanical ventilation. Chest. 2005;128:2511–2520. doi: 10.1378/chest.128.4.2511. [DOI] [PubMed] [Google Scholar]
- 45.Saad IAB, Tonella R, Roceto LS, Delazari LEB, Castilho L, Falcão ALE, et al. A new device for inspiratory muscle training in patients with tracheostomy tube in ICU: a randomized trial [abstract] Eur Respir J. 2014;44(Suppl 58):P4297. [Google Scholar]
- 46.Shimizu JM, Manzano RM, Quitério RJ, Tenório da Costa Alegria V, Junqueira TT, El-Fakhouri S, et al. Determinant factors for mortality of patients receiving mechanical ventilation and effects of a protocol muscle training in weaning. Man Ther Posturol Rehabil J. 2014;12:180–187. [Google Scholar]
- 47.Shrestha BK, Qutob HF, Files DC, Berry M, Dhar S, Bowton DL, et al. Feasibility and safety of inspiratory muscle training in critically ill intubated patients [abstract] Am J Respir Crit Care Med. 2014;189:A3883. [Google Scholar]
- 48.Yosef-Brauner O, Adi N, Ben Shahar T, Yehezkel E, Carmeli E. Effect of physical therapy on muscle strength, respiratory muscles and functional parameters in patients with intensive care unit-acquired weakness. Clin Respir J. 2015;9:1–6. doi: 10.1111/crj.12091. [DOI] [PubMed] [Google Scholar]
- 49.dos Santos Pascotini F, Denardi C, Nunes GO, Trvisan ME, da Pieve Antunes V. Treinamento muscular respiratório em pacientes em desmame da ventilação mecânica. ABCS Health Sci. 2014;39:12–16. [Google Scholar]
- 50.Ibrahiem AAA, Mohamed AR, Elbasiouny HS. Effect of respiratory muscles training in addition to standard chest physiotherapy on mechanically ventilated patients. J Med Res Pract. 2014;3:52–58. [Google Scholar]
- 51.Melo PF, Da Silva V, Vieira L, Lima L, Lira A, Silva PE, et al. High intensity inspiratory muscle training in patients with traumatic brain injury under mechanical ventilation: preliminary results of a randomized controlled trial [abstract] Am J Respir Crit Care Med. 2017;195:A2749. [Google Scholar]
- 52.Tonella RM, Ratti LDSR, Delazari LEB, Junior CF, Da Silva PL, Herran ARDS, et al. Inspiratory muscle training in the intensive care unit: a new perspective. J Clin Med Res. 2017;9:929–934. doi: 10.14740/jocmr3169w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281. doi: 10.2522/ptj.20050036. [DOI] [PubMed] [Google Scholar]
- 54.Elbouhy MS, AbdelHalim HA, Hashem AMA. Effect of respiratory muscles training in weaning of mechanically ventilated COPD patients. Egypt J Chest Dis Tuberc. 2014;63:679–687. [Google Scholar]
- 55.Aldrich TK, Karpel JP, Uhrlass RM, Sparapani MA, Eramo D, Ferranti R. Weaning from mechanical ventilation: adjunctive use of inspiratory muscle resistive training. Crit Care Med. 1989;17:143–147. [PubMed] [Google Scholar]
- 56.Barros C, Lima A, Vilaca AF, Correia RF, Goncalves TF, Silva RMO, et al. Impact of standardized mobilization in mechanically ventilated patients on respiratory muscular strength [abstract] Eur Respir J. 2015;46(Suppl 59):PA2171. [Google Scholar]
- 57.Martin AD, Davenport PD, Franceschi AC, Harman E. Use of inspiratory muscle strength training to facilitate ventilator weaning: a series of 10 consecutive patients. Chest. 2002;122:192–196. doi: 10.1378/chest.122.1.192. [DOI] [PubMed] [Google Scholar]
- 58.Supinski GS, Netzel PF, Valentine EN, Callahan LA. Effect of physical therapy on respiratory parameters in mechanically ventilated patients [abstract] Am J Respir Crit Care Med. 2017;195:A2740. [Google Scholar]
- 59.Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole-body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265. doi: 10.1097/01.ccm.0000181730.02238.9b. [DOI] [PubMed] [Google Scholar]
- 60.Sprague SS, Hopkins PD. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys Ther. 2003;83:171–181. [PubMed] [Google Scholar]
- 61.Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, III, et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi consensus study. Am J Respir Crit Care Med. 2017;196:1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vassilakopoulos T, Zakynthinos S, Roussos C. The tension–time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998;158:378–385. doi: 10.1164/ajrccm.158.2.9710084. [DOI] [PubMed] [Google Scholar]
- 63.Harikumar G, Egberongbe Y, Nadel S, Wheatley E, Moxham J, Greenough A, et al. Tension–time index as a predictor of extubation outcome in ventilated children. Am J Respir Crit Care Med. 2009;180:982–988. doi: 10.1164/rccm.200811-1725OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bellemare F, Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol Respir Environ Exerc Physiol. 1982;53:1190–1195. doi: 10.1152/jappl.1982.53.5.1190. [DOI] [PubMed] [Google Scholar]
- 65.Laghi F, Shaikh HS, Morales D, Sinderby C, Jubran A, Tobin MJ. Diaphragmatic neuromechanical coupling and mechanisms of hypercapnia during inspiratory loading. Respir Physiol Neurobiol. 2014;198:32–41. doi: 10.1016/j.resp.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 66.McCaughey EJ, Berry HR, McLean AN, Allan DB, Gollee H. Abdominal functional electrical stimulation to assist ventilator weaning in acute tetraplegia: a cohort study. PLoS One. 2015;10:e0128589. doi: 10.1371/journal.pone.0128589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reid WD, Huang J, Bryson S, Walker DC, Belcastro AN. Diaphragm injury and myofibrillar structure induced by resistive loading. J Appl Physiol (1985) 1994;76:176–184. doi: 10.1152/jappl.1994.76.1.176. [DOI] [PubMed] [Google Scholar]
- 68.Reid WD, Clarke TJ, Wallace AM. Respiratory muscle injury: evidence to date and potential mechanisms. Can J Appl Physiol. 2001;26:356–387. doi: 10.1139/h01-023. [DOI] [PubMed] [Google Scholar]
- 69.Orozco-Levi M, Lloreta J, Minguella J, Serrano S, Broquetas JM, Gea J. Injury of the human diaphragm associated with exertion and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1734–1739. doi: 10.1164/ajrccm.164.9.2011150. [DOI] [PubMed] [Google Scholar]
- 70.Laghi F, D’Alfonso N, Tobin MJ. Pattern of recovery from diaphragmatic fatigue over 24 hours. J Appl Physiol (1985) 1995;79:539–546. doi: 10.1152/jappl.1995.79.2.539. [DOI] [PubMed] [Google Scholar]
- 71.Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation: impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 72.Ebihara S, Hussain SNA, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165:221–228. doi: 10.1164/ajrccm.165.2.2108041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.