Abstract
The U.S. National Institutes of Health (NIH) announced last May that steps will be taken to address the over-reliance on male cells and animals in preclinical research. To further address this announcement, in September 2014, scientists with varying perspectives came together at Georgetown University to discuss the following questions. (1) What metrics should the NIH use to assess tangible progress on policy changes designed to address the over-reliance on male cells and animals in preclinical research? (2) How effective can education be in reducing the over-reliance on male cells and animals in preclinical research and what educational initiatives sponsored by the NIH would most likely effect change? (3) What criteria should the NIH use to determine rigorously defined exceptions to the future proposal requirement of a balance of male and female cells and animals in preclinical studies? (4) What additional strategies in addition to proposal requirements should NIH use to reduce the overreliance of male cells and animals in preclinical research? The resulting consensus presented herein includes input from researchers not only from diverse disciplines of basic and translational science including biology, cell and molecular biology, biochemistry, physiology, pharmacology, neuroscience, cardiology, endocrinology, nephrology, psychiatry, and obstetrics and gynecology, but also from recognized experts in publishing, industry, advocacy, science policy, clinical medicine, and population health. We offer our recommendations to aid the NIH as it selects, implements, monitors, and optimizes strategies to correct the over-reliance on male cells and animals in preclinical research.—Sandberg, K., Umans, J. G., Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research.
Keywords: gender, sexual dimorphism, sex differences, sex bias, over-reliance
The over-reliance on male animals and cells in preclinical research obscures key sex differences that could guide clinical studies…. The NIH is now developing policies that require applicants to report their plans for the balance of male and female cells and animals in preclinical studies in all future applications, unless sex-specific inclusion is unwarranted, based on rigorously defined exceptions. These policies will be rolled out in phases beginning in October 2014, with parallel changes in review activities and requirements (1).
Last May, Francis S. Collins, Director of the U.S. National Institutes of Health (NIH), and Janine A. Clayton, Director of the Office of Research on Women’s Health at the NIH, stated in the journal Nature that preclinical research overly relies on male cells and animals (1). In September, a multidisciplinary group of scientists, from across North America and drawn not only from diverse disciplines of basic and translational science but also bringing insights from publishing, industry, advocacy, science policy, clinical medicine, and population health, came together at Georgetown University to form a Work Group to develop and articulate specific recommendations to assist the NIH in formulating new strategies. Our recommendations along with our collective rationales are discussed in this article.
The NIH, which is the largest funder of basic science in the world, invests heavily in basic science research because the mission of the agency is “to seek fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to enhance health, lengthen life, and reduce illness and disability” (http://www.nih.gov/about/mission.htm). To ensure that the application of basic science knowledge optimally and equitably benefits human health, the NIH announcement in May assured that the over-reliance of male cells and animals in preclinical research would be addressed through a multipronged approach, including policy changes and educational initiatives beginning in October 2014 (1).
This call to action followed growing recognition of the unintended and serious consequences of under-representing female cells and animals in preclinical research. Since its inception in 1990, the NIH Office of Research on Women's Health has been a strong voice for the inclusion of females in preclinical and clinical research throughout the scientific community (http://orwh.od.nih.gov/about/index.asp). This message has been staunchly supported by the Society for Women's Health Research (http://www.womenshealthresearch.org) and other advocacy groups. Within the scientific community, there has been a growing awareness of the impact of one's sex and gender in biology, physiology, and pathophysiology, and new scientific societies have arisen that focus exclusively on the influence of sex and gender, including the Organization for the Study of Sex Differences (http://www.ossd.wildapricot.org) in 2006 and the International Society for Gender Medicine (http://www.isogem.com/) in 2007. Furthermore, journals have been launched including Gender Medicine in 2004 and the Biology of Sex Differences in 2010 that focus primarily on the influence of biologic sex and gender in biology and disease. Sex and gender are defined in terms of their biologic and social constructs, respectively. Although they can be independent or synergistic determinants of outcomes (2), here we focus on sex-linked biology.
Several papers and systematic reviews have recently highlighted the fact that the majority of preclinical studies are conducted in male cells and animals. Analysis of journal articles for 2009 across 10 biologic fields found that the majority of research reports using mammals used only 1 sex and that sex was male (3). The preferred use of male rodents was most evident in neuroscience and immunology, where single sex studies of male animals outnumbered those of females by >5:1. A similar trend was observed in research articles published in the American Journal of Physiology, Regulatory, Integrative and Comparative Physiology between 2012 and the first 6 mo of 2014, in which case, the ratio of single sex studies conducted in males to females was 4:1 (4). Furthermore, the ratio of research conducted on male to female animal models has not changed over the last 20 y, because this imbalance today is similar to ratios ascertained in the early 1990s (5–7).
Sex is a fundamental biologic variable with profound consequences. Under-representing female cells and animals in preclinical research has resulted in a poorer understanding of the biologic, physiologic, and pathophysiological mechanisms in the female compared with the male. Without data in females, it is impossible to determine whether results obtained in male cells and animals also applies to female cells and animals. Basic science serves as the pipeline for drug discovery and new therapeutics. Therefore, findings of sex differences in pathophysiologic mechanisms in animal models of disease impact the translation of these preclinical findings to the health of men and women. In 2001, the U.S. General Accounting Office reported (GAO-01-286R) that 8 of the 10 prescription drugs withdrawn from the U.S. market by the Food and Drug Administration (FDA) posed greater health risks for women than for men, and 3 of these were due to a potentially fatal ventricular arrhythmia (Torsades de Pointes) (http://www.gao.gov/new.items/d01286r.pdf). Although a disparity in prescribing may have contributed to the disproportionate burden of adverse events in women, a sex difference in potassium channel biology is also a likely culprit. Indeed, when this clinical observation went from “bedside to bench,” preclinical research suggested that women would be more susceptible to adverse arrhythmias than men because of sex differences in QT intervals (i.e., a measure of ventricular electrical depolarization and repolarization). Female ventricular cardiomyocytes, both in laboratory animals and in humans, have a longer QT interval and lower repolarizing potassium current densities than male ventricular cardiomyocytes (8). Furthermore, drugs that affect potassium channels including certain antihistamines (terfenadine, astemizole), antibiotics (erythromycin), antimalarials (halofantrine), and antiarrhythmics (quinidine, d-sotalol) are more able to dangerously prolong the QT interval and thereby induce Torsades de Pointes in women compared with men, in part because of protective effects of testosterone on drug-induced QT prolongation. Thus, realizing sex differences exist in potassium channel biology in cardiomyocytes early in the research pipeline for drug discovery may have prevented the disproportionate burden of ventricular arrhythmias observed in women.
Not considering biologic sex as a factor may contribute to irreproducibility in scientific studies. As a funding agency, the NIH is deeply concerned about the high irreproducibility rates recently reported in preclinical studies (9). To illustrate this point, consider a science citation classic published in 2007 by Guzik et al. (10), which demonstrated that “T cells play an important role in the genesis of hypertension”; nowhere in this paper was the sex of the animals reported. If investigators attempting to reproduce these findings were to use female mice, they would not be able to replicate this study. After spending significant time and resources, these scientists might conclude erroneously that the work published by Guzik et al. wasn't reproducible. However, when researchers considered the variable of sex, they replicated Guzik et al.'s finding that male T cells contribute to the genesis of angiotensin II-dependent hypertension in the male host (11); however, they also discovered that male T cells fail to drive hypertension in the female host, whereas female T cells protect the male host from angiotensin II-dependent hypertension (12, 13).
This example not only demonstrates that major sex differences exist in mechanisms of immune modulation of hypertension, but it also highlights the critical importance of reporting the sex of animals used in studies. As of 2012, all the journals of the American Physiological Society require reporting the sex of experimental animals and material derived from experimental animals. Journals of the Endocrine Society have gone a step further, now requiring that the sex of the animal be included in the title (14). Although the NIH does not establish policy for individual journals, the agency could promote the reporting of sex in journal articles by requiring that principal investigators designate the sex of their cells and animals in all publications reporting NIH-supported research, in addition to their other requirements such as acknowledgment of grant support.
(1) RECOMMENDED METRICS TO ASSESS TANGIBLE PROGRESS ON POLICY CHANGES DESIGNED TO ADDRESS THE OVER-RELIANCE OF MALE CELLS AND ANIMALS IN PRECLINICAL RESEARCH
To determine the effectiveness of new NIH policies and initiatives, it will be essential to prospectively establish metrics to assess progress. Otherwise, the NIH will be unable to determine whether their strategies are working or whether they need to be modified to accomplish the agency's goal of correcting the over-reliance of male cells and animals in preclinical research. Because there would be a substantial time delay between changes in policy impacting publications, clinical trials, and health outcomes, we recommend the NIH establish short-, mid-, and long-term metrics to gauge progress.
Recommended short-term metrics for studies on cells and animals include the following:
The number of applications and grant awards proposing single sex studies in males and those in females;
The number of applications and grant awards proposing dual sex studies;
Among applications and grant awards, the percentage conducted in only one sex;
Of single sex studies, the ratio of those in males to those in females among applications and grant awards; and
Of dual sex studies, the ratio of males to females proposed per application and per grant award.
We recommend that all of the above metrics be reported both by NIH study section (e.g., Hypertension and Microcirculation) and by award mechanism (e.g., R21 and R01) across the Center for Scientific Review. For funded grants, these metrics should be reported by institute [e.g., the U.S. National Heart Lung and Blood Institute (NHLBI)], by division within each institute (e.g., the Division of Cardiovascular Sciences in NHLBI), and by program within each division (e.g., the Program in Basic and Early Translational Research, Division of Cardiovascular Sciences, NHLBI).
Tangible progress could be claimed if the average ratio of male to female single sex studies in cells and animals and the average proposed males to females per application and per grant awarded in dual sex studies reaches equivalence, in aggregate, across the entire NIH portfolio within 5 years. We do not recommend that equivalence be a goal for every project, program, or division because some projects and programs will predominantly focus on a single sex for appropriate reasons (see recommendations for exception criteria 3). Progress also would be reflected by an increase in the number of applications and grant awards that proposed using both sexes compared to the current number of proposed dual sex studies.
Although not yet available in cells or invertebrates, information needed to capture and report these metrics in vertebrates is already available because the NIH requires identification of the sex and number of animals proposed in point 1 of the 5 mandatory points on the vertebrate animal section on all grant applications (http://grants.nih.gov/grants/olaw/vaschecklist.pdf). In fact, the NIH states that “failure to address the five points may result in the application being designated as incomplete and will be grounds for the Public Health Service to defer the application from the peer review round. Alternatively, the application's impact/priority score may be negatively affected” (http://grants.nih.gov/grants/olaw/vaschecklist.pdf). Thus, these metrics, at least in vertebrates, can be determined now for 2014 to serve as a baseline from which to measure progress going forward.
Recommended midterm metrics for studies on cells and animals citing NIH support include the following:
The number of published papers on a single sex study conducted solely in male or solely in female cells or animals;
The number of published papers using both sexes;
The ratio of published papers on dual compared to single sex studies;
Of single sex papers, the average ratio of male to female studies; and
Of dual sex papers either in the paper itself or in the online supplement, the number of those reporting a sex difference or the number demonstrating the absence of a difference after using a meaningfully powered comparative analysis.
We recommend these metrics be reported on all original research articles citing NIH support as the NIH rolls out new policies and initiatives; that these metrics be reported annually by institute, division, and program; and that this be compared using metrics calculated for 2014 as a baseline.
If tangible progress is made, the ratio of male to female studies would move toward 1:1 over time among all publications citing NIH support that are conducted in cells or animals of a single sex. Approaching equivalence would not be a necessary goal for every program or division within the NIH, but it is an appropriate goal for the NIH portfolio in aggregate.
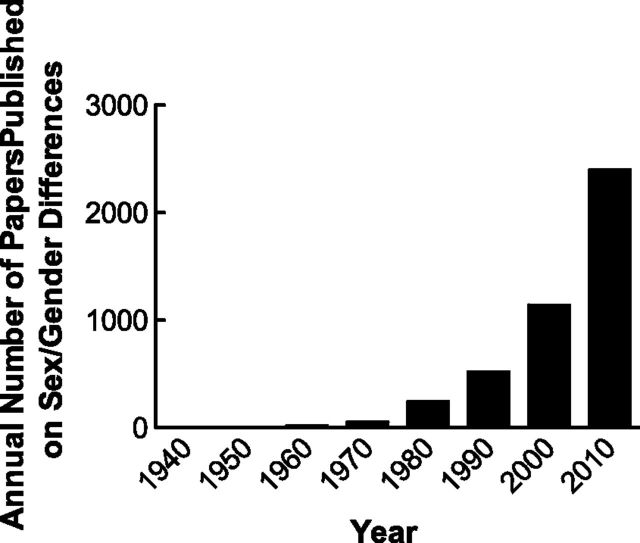
Although the annual number of publications reporting on sex or gender differences in PubMed is increasing over time (Fig. 1), the annual number in 2010 represented <2% of the total number of papers cited in PubMed that year. We predict the number and rate of increase will rise markedly as investigators begin to compare the sexes and discover differences in biology, physiology, and pathophysiology within their model cell and animal systems.
Figure 1.
Annual number of papers cited in PubMed using sex differences, gender differences, or sexual dimorphism in the title, abstract, or subject or as a keyword or unique identifier as a function of each decade year.
These publication metrics would require no new information because the NIH “public access policy” already requires scientists to submit final peer-reviewed journal manuscripts that arise from NIH funds to the NIH-controlled PubMed Central immediately on acceptance for publication (http://publicaccess.nih.gov/). To increase efficiency and decrease the burden to both investigators and the agency, we recommend that the NIH modify the PubMed Central online submission process to capture these data automatically as articles from principal investigators are uploaded (e.g., include drop down menus for male-only and female-only cell, animal and human subject studies). Oversight of the PubMed Central submission process could be embedded in the administrative review of annual progress reports and by an educated peer-review panel should the progress report be part of a competitive renewal application.
Recommended long-term metrics include the following:
The number of human subject studies and clinical trials in which the design explicitly addresses sex-specific hypotheses informed by preclinical research including biomarker and medical device development and early phase efficacy and safety trials; and,
Increased information on sex-specific risks for adverse events identified before commercialization.
If the NIH successfully addresses the over-reliance of male cells and animals in preclinical research, resulting discoveries would likely drive change in the design of human subject research that ultimately benefits diagnosis, treatment, prevention, and human health. Even before seeing these long term benefits, we would expect to see changes in clinical trial design, which could be captured efficiently via explicit presentation on ClinicalTrials.gov. We also expect that new therapeutics and medical devices will derive from clues revealed by relative resilience in one sex and susceptibility in the other. For example, the onset of cardiovascular disease occurs several years earlier in men than women while many autoimmune diseases are far more prevalent in women than men.
The FDA rarely requires that labeled drug dosing be described in a sex-specific manner; however, in May 2013, the FDA approved new labeling that lowered the dose of zolpidem products in women (http://www.fda.gov/drugs/drugsafety/ucm352085.htm). With less over-reliance on male cells and animals in preclinical studies, we might expect an increase in the frequency of sex-specific labeling of drugs by the FDA, hopefully at the time of approval rather than following postmarketing reports of sex-specific adverse events. In addition, preclinical studies may suggest sex-specific efficacy and/or safety of drugs that ultimately leads to clinical trials resulting in major consensus statements recommending sex-specific therapeutic strategies for treating certain disorders and diseases. For example, a single-blind randomized crossover study in East African men and women with hypertension showed that the angiotensin-converting enzyme inhibitor lisinopril was more effective at lowering blood pressure in men than the diuretic hydrochlorothiazide, whereas both classes of antihypertensives were equally effective in women (15). Although women are still under-represented in early phase clinical trials (16, 17) and in medical device clinical studies approved by the FDA (Guidance for Industry and Food and Drug Administration Staff, issued August 22, 2014), there have been great strides made by NIH since 1993 in the inclusion of women in clinical trials, and now women make up nearly half of the subjects of phase 3 clinical trials (16, 17). It is time for preclinical research to catch up because understanding the biologic basis for these sex differences could lead to the discovery of new therapeutics, regimens, and medical devices that will benefit both men and women. A greater understanding of sex differences in cellular biology and animal physiology would also inform the field of preclinical toxicology prior to large in vivo studies, which is another critical component of the drug discovery and new therapeutics pipeline.
(2) RECOMMENDED EDUCATIONAL INITIATIVES THAT WOULD MOST LIKELY EFFECT CHANGE AND LEAD TO A REDUCTION IN THE OVER-RELIANCE OF MALE CELLS AND ANIMALS IN PRECLINICAL RESEARCH
Education will be key to long-term culture evolution to ensure that changes in policy are not superficial and “gamed” in practice. The first step is to educate the scientific community why we need to rectify the imbalance in research on male and female cells and animals. Scientists need to understand the nature and magnitude of the over-reliance on male cells and animals and the missed opportunities and adverse consequences to science and human health if this balance is not achieved. Education should be geared to providing a general knowledge of the mechanisms underlying sex differences including the distinction between sex chromosome and gonadal steroid effects, how to conduct sex difference research, and the implications for experimental design and analysis. These initiatives will serve to educate scientists on the gaps in knowledge regarding the impact of biologic sex in biology and disease and dispel fallacious assumptions such as the female is the variable compared with the male or that measured parameters are always more variable in the female compared with the male due to the estrous cycle (see fuller discussion of this latter point under recommendations for exception criteria 3).
Educational vehicles should include the following: a core (modular) curriculum targeting the scientific community at large with a blueprint for content, and its development and delivery developed via a peer-reviewed process such as through request for applications or for proposals that results in online training materials that are specifically tailored by discipline and career level. Scientific societies should then be engaged to develop discipline-specific content, itself tailored to investigators and trainees at every level, and required training with clear checkpoints, similar to the Responsible Conduct of Research (http://grants1.nih.gov/grants/guide/notice-files/NOT-OD-10-019.html), Humane Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/olaw.htm), and Health and Safety training (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-14-127.html) that are required for “all trainees, fellows, participants, and scholars receiving support through any NIH training, career development award (individual or institutional), research education grant, and dissertation research grant.”
Core curriculum will target the scientific community at large. Societies should then be engaged to develop discipline-specific content, itself tailored to investigators and trainees at every level. Training should be tiered, targeting every NIH-supported training, career development, and mentorship mechanism, with inclusion in individual development plans and as a requirement and explicit review criterion for all research grant mechanisms. To ensure rapid cultural change and enforcement of these new training requirements, additional special training should be required for mentors in training programs, study section members, program and review staff, and members of Institutional Animal Care and Use Committees. Hopefully, one day this training will become unnecessary and obsolete once the culture of the scientific community embraces the importance of sex as a biologic variable.
(3) WHAT CRITERIA SHOULD THE NIH USE TO DETERMINE “RIGOROUSLY DEFINED EXCEPTIONS” TO THE FUTURE PROPOSAL REQUIREMENT OF A “BALANCE OF MALE AND FEMALE CELLS AND ANIMALS IN PRECLINICAL STUDIES”?
One way the NIH proposes to address the over-reliance on male cells and animals in preclinical research is to require grant applicants “to report their plans for the balance of male and female cells and animals in preclinical studies in all future applications, unless sex-specific inclusion is unwarranted, based on rigorously defined exceptions” (1). We recommend that inclusion of both sexes be considered a critical and scored part of the research strategy on all grant applications in preclinical research. For example, it should no longer be accepted as good science to propose generating a gene knockout or transgenic animal and then phenotype it in only one sex.
Exceptions should be just that, exceptions; the rationale for their use should be well justified and could include the following:
A disease, model, process, or pathway that is specific to one sex; for example, studies of preeclampsia or testicular cancer;
Availability and feasibility issues in nonhuman primate (NHP) research; studies involving NHPs are singularly useful because of the ease with which the results of well-designed investigations can be translated to human conditions. Availability, however, is a major limitation because vendors and breeders will make young males available for research (especially for testing in the pharmaceutical industry) while retaining the females as future breeders. Furthermore, NHPs are expensive. Financial limitations often prevent obtaining males and females in sufficient numbers to make statistically meaningful comparisons feasible. Thus, researchers will typically study only one sex to limit the variables studied. The sex of choice is almost always males because of their aforementioned greater availability from vendors or breeding colonies;
Sex-predominant disease, model, process, or pathway; for example, systemic lupus erythematosus occurs 10 times more frequently in women than men;
Sex differences in mechanisms that warrant sex-specific studies; for example, studies on a signal transduction pathway that is activated in one sex but not the other;
Studies in which the experimental model results in a marked sex imbalance in lifespan, thus making it impossible to separate the factor of age from the factor of sex;
Development of a sex-specific model when appropriate models in that sex are lacking; for example, there are few female rodent models of chronic kidney disease; and
Unique cell line without comparison line for the other sex; however, the investigator should determine (if possible), the sex of that cell line.
We recognize that these exceptions are not of equivalent weight and that over-interpretation and use of these exceptions may slow progress toward balancing studies of males and females in preclinical research, unless coupled with specific additional NIH policies. For example, when a biologic or disease model exists in only one sex, there should be strong NIH support and appreciation for investigators who propose to develop a parallel model in the understudied sex. The exception due to sex predominance is complex, as sex differences in incidence, prevalence, or severity may not parallel one another. We suggest that specific presentation and review of this exception should lead to iterative reevaluation as suggested recently by McCullough et al. (18) so that well-educated reviewers retain the flexibility to determine whether the claimed exclusion remains justified in each specific instance.
One common criticism voiced over this new NIH initiative is that “adding females to the experimental design will more than double animal numbers since the female must be studied as a function of the estrus cycle.” The validity of this assumption, however, has been refuted recently (3, 19, 20). The influence of estrous cycle stage is not inherently indicative of larger observed variance, and for many traits, females can be studied irrespective of the estrous cycle stage without a substantial increase in outcome variance (20). A recent meta-analysis of 293 articles compared female mice at random stages of the estrous cycles with male mice on nearly 10,000 trait measurements that included behavioral, neuroanatomical, and immunologic variables (19). Using the coefficient of variation (the ratio of the standard deviation to the mean) as a measure of variability, the authors reported that unstaged females were no more variable than males across a variety of anatomical, behavior, and physiologic traits. Moreover, the distribution of trait variability was significantly broader in males than in females, perhaps because of group housing and the position of the male mouse in the dominance hierarchy in its home cage (19, 20).
Studies that use males and females should analyze data with sex as an independent variable. If sex turns out not to be a significant factor for a specific variable, then combining male and female data together will serve to increase the sample size (3); however, in the absence of information about sex difference or the existence of equivocal data surrounding a specific parameter, both males and females should be used in numbers sufficient to permit valid, sufficiently powered statistically analysis (3). Two caveats should be noted: the genetic background of female mice can impact the extent of variability because of estrous cyclicity (20), and it is difficult to unequivocally prove the absence of a sex difference in a mechanism given the possibility that sex differences could exist at multiple points along a signaling cascade that resulted in no differences in the final measured parameter if these sex differences canceled each other out (20).
(4) WHAT ALTERNATIVES TO PROPOSAL REQUIREMENTS SHOULD THE NIH USE TO REDUCE THE “OVER-RELIANCE OF MALE CELLS AND ANIMALS IN PRECLINICAL RESEARCH”?
It is unlikely to be feasible or necessary to perform every experiment with equal numbers of male and female cells and animals. Instead, resources should be targeted to develop new tools and support research that will most efficiently reveal sex differences that are important to health outcomes. We propose 5 specific funding mechanisms to develop these models, insights, and the investigators who will use them:
Request for applications to develop and study animal models in the understudied sex when diseases or disorders are known to differ between the sexes and the available models of the disease are inadequate in the understudied sex;
Expansion of the administrative supplement program (PA-15-034), which supports “research highlighting the impact of sex/gender differences (or similarities) and/or sex and gender factors in human health and illness, including basic, preclinical, clinical and behavioral studies” and in particular, “studies relevant to understanding of the significance of biological sex on cells; comparative studies of male and female tissues, organ systems and physiological systems; sex based comparisons of pathophysiology, biomarkers, gene expression, clinical presentation and prevention and treatment of diseases”;
New peer-reviewed funding mechanism by which funded grants receive a 1- to 2-y cost extension to interrogate newly identified sex differences at a mechanistic level;
Expansion of the Building Interdisciplinary Research Careers in Women's Health mentored career development program (RFA-OD-15-001), which “connects junior faculty, known as BIRCWH Scholars, to senior faculty with shared research interest in women’s health and sex differences research”; and
Establish institutional (e.g., T32) and individual (e.g., F31 and F32) training grants that are specifically designated to support predoctoral students and postdoctoral fellows who will investigate the impact of sex and/or gender on cell biology, gene expression, tissues, and organ systems under physiologic and pathophysiologic conditions.
CONCLUSIONS
This year marks the 50th anniversary of the U.S. Surgeon General's report on Smoking and Health (http://www.surgeongeneral.gov/library/reports/50-years-of-progress/consumer-guide.pdf). Educational initiatives, escalating financial disincentives, and substantial changes in regulations concerning advertising and restricted locations where individuals can smoke have had a major impact on reducing smoking in this country, and now, 50 y later, there has been a 57% reduction in the number of American adults who smoke. Likewise, we expect that the decision by the Director of the NIH to use a multipronged approach including regulation, educational initiatives, and incentives will be effective in improving the balance in the use of male and female cells and animals in preclinical research not only in this country but throughout the world, because NIH policies are frequently adopted by other funding agencies including international institutions. However, the early implementation of metrics to measure the effectiveness of these strategies is essential if we are to know when alternative strategies must be considered and to ensure that progress is made in a timely manner. The opportunities for drug discovery, new and improved therapeutics, and regimens and medical devices arising from research on the impact of biologic sex in physiology and pathophysiology are vast. We cannot afford to delay their discovery another day. Furthermore, raising awareness in researchers that sex is a critical biologic variable that must be considered in the research design will enrich and improve the field of science as a whole.
Glossary
- FDA
U.S. Food and Drug Administration
- NHLBI
U.S. National Heart Lung and Blood Institute
- NHP
nonhuman primate
- NIH
U.S. National Institutes of Health
REFERENCES
- 1.Clayton J. A., Collins F. S. (2014) Policy: NIH to balance sex in cell and animal studies. Nature , 282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger N. (2003) Genders, sexes, and health: what are the connections—and why does it matter? Int. J. Epidemiol. , 652–657 [DOI] [PubMed] [Google Scholar]
- 3.Beery A. K., Zucker I. (2011) Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. , 565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandberg K., Verbalis J. G., Yosten G. L., Samson W. K. (2014) Sex and basic science. A Title IX position. Am. J. Physiol. Regul. Integr. Comp. Physiol. , R361–R365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkley K. J. (1992) Vive la différence! Trends Neurosci. , 331–332 [DOI] [PubMed] [Google Scholar]
- 6.Blanchard D. C., Griebel G., Blanchard R. J. (1995) Gender bias in the preclinical psychopharmacology of anxiety: male models for (predominantly) female disorders. J. Psychopharmacol. (Oxford) , 79–82 [DOI] [PubMed] [Google Scholar]
- 7.Sechzer J. A., Rabinowitz V. C., Denmark F. L., McGinn M. F., Weeks B. M., Wilkens C. L. (1994) Sex and gender bias in animal research and in clinical studies of cancer, cardiovascular disease, and depression. Ann. N. Y. Acad. Sci. , 21–48 [DOI] [PubMed] [Google Scholar]
- 8.Ebert S. N., Liu X. K., Woosley R. L. (1998) Female gender as a risk factor for drug-induced cardiac arrhythmias: evaluation of clinical and experimental evidence. J. Womens Health , 547–557 [DOI] [PubMed] [Google Scholar]
- 9.Wadman M. (2013) NIH mulls rules for validating key results. Nature , 14–16 [DOI] [PubMed] [Google Scholar]
- 10.Guzik T. J., Hoch N. E., Brown K. A., McCann L. A., Rahman A., Dikalov S., Goronzy J., Weyand C., Harrison D. G. (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. , 2449–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollow D. P., Uhrlaub J., Romero-Aleshire M. J., Sandberg K., Nikolich-Zugich J., Brooks H. L., Hay M. (2014) Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension , 384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji H., Zheng W., Li X., Liu J., Wu X., Zhang M. A., Umans J. G., Hay M., Speth R. C., Dunn S. E., Sandberg K. (2014) Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension , 573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandberg K., Ji H., Hay M. (2014) Sex-specific immune modulation of primary hypertension. Cell. Immunol. pii: S0008-8749(14)00178-6. doi: 10.1016/j.cellimm.2014.12.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaustein J. D. (2012) Animals have a sex, and so should titles and methods sections of articles in Endocrinology. Endocrinology , 2539–2540 [DOI] [PubMed] [Google Scholar]
- 15.Falconnet C., Bochud M., Bovet P., Maillard M., Burnier M. (2004) Gender difference in the response to an angiotensin-converting enzyme inhibitor and a diuretic in hypertensive patients of African descent. J. Hypertens. , 1213–1220 [DOI] [PubMed] [Google Scholar]
- 16.Pinnow E., Sharma P., Parekh A., Gevorkian N., Uhl K. (2009) Increasing participation of women in early phase clinical trials approved by the FDA. Womens Health Issues , 89–93 [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Carlin A. S., Faustino P. J., Motta M. I., Hamad M. L., He R., Watanuki Y., Pinnow E. E., Khan M. A. (2009) Participation of women in clinical trials for new drugs approved by the food and drug administration in 2000-2002. J. Womens Health (Larchmt) , 303–310 [DOI] [PubMed] [Google Scholar]
- 18.McCullough L. D., de Vries G. J., Miller V. M., Becker J. B., Sandberg K., McCarthy M. M. (2014) NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol. Sex Diff. , 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prendergast B. J., Onishi K. G., Zucker I. (2014) Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. , 1–5 [DOI] [PubMed] [Google Scholar]
- 20.Mogil J. S., Chanda M. L. (2005) The case for the inclusion of female subjects in basic science studies of pain. Pain , 1–5 [DOI] [PubMed] [Google Scholar]