Abstract
The electrophoretic fractionation represents one of the most reliable methods for the identification of blood proteins in ruminants. The aim of this study was to evaluate the serum proteinogram of sheep with acute ruminal lactic acidosis (ARA) using the SDS-PAGE electrophoresis technique. Ten Santa Inês ewes were used and blood was collected to establish the basal values for induction of ARA. Sucrose was administered orally in a single dose of 15 g/kg body mass. After the administration, blood samples were obtained at the following moments: 4, 8, 12, 16, 20, 24, 28, 32, 36, 48, 72, 96, 120 and 144 h. Subsequently, samples were obtained every seven days for three further weeks, until complete one month. The total of 13 proteins were identified: immunoglobulins A and G, ceruloplasmin, transferrin, albumin, α1-antitrypsin, haptoglobin, α1-acid glycoprotein, proteins of molecular weight 95, 46, 36 and 31 kDa. The increase of haptoglobin from 08 h coincides with the ruminal pH decrease, possibly due to the death of Gram negative bacteria and also the inflammatory process on the rumen. Fibrinogen was presented on highest mean at 48 h and returned to normal with 144 h. We can conclude that changes in serum levels of acute phase proteins can assist the clinical evaluation and diagnosis of ARA in sheep.
Keywords: Electrophoresis, Haptoglobin, Proteins, Inflammation, Santa Inês, Sheep, Ruminal lactic acidosis
1. Introduction
According to metabolic function, the serum proteins can be divided into categories such as the acute phase (APP), immunoproteins, complement, coagulation and transport proteins. The APP alter the concentration in animals subject to challenges such as infection, inflammation, surgical trauma and stress. Its activities contribute to host defense by neutralizing inflammatory agents, reducing the extent of tissue damage and acting on tissue repair, and these proteins may change their serum concentrations up to 25% in response to stimulation of pro-inflammatory cytokines [1]. In cattle, the major knows APP are serum amyloid A (SAA), haptoglobin (Hp) and fibrinogen (Fb) [2], [3], [4].
Tissue injury, infection or exposure to pro-inflammatory molecules such as lipopolysaccharide (LPS) results in an immediate defense mechanism that is not specific, named acute phase response (APR). The APR involves both local and systemic components and a complex mechanism of many cell types and organs as well as other mediators and cytokines [5].
The electrophoretic fractionation is one of the most reliable blood protein identification method. The technique of sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) has been widely used to evaluate the APP of inflammatory response, contributing to the diagnosis and prognosis of several diseases, such as equine laminitis [6], equine acute abdomen [7], pneumonia in calves [8] and mastitis in ewes [9].
Acute ruminal lactic acidosis (ARA), is a ruminal fermentative disorder with acute indigestion resulting from the sudden digestion of foods containing easily fermentable carbohydrates, such as barley, wheat, sugar beet and sugar in high quantities in cattle and sheep [10], [11], [12]. The systemic acidosis influences on cellular and humoral immunity [13], [14] and it is known that ruminal acidosis is particularly present in dairy cows during the transition period, a period in which other metabolic disorders such as ketosis and hypocalcemia are prevalent [15], [16]. Dairy cows are susceptible to the occurrence of subacute ruminal acidosis (SARA) or ARA, besides the occurrence of metabolic acidosis due to the high concentrations of carbohydrate diet used in these animals. Several studies have shown that SARA leads to increases in serum concentrations of SAA and Hp in this species [17], [18], [19], [20].
The serum concentration of APP can provide useful quantitative information for study and diagnosis of metabolic diseases, and monitoring the therapy response [21]. Studies relating the electrophoretic profile of serum proteins in ruminants and the occurrence of ARA have been described on literature [22], [23] however, we found no similar study in sheep. The number of sheep in Brazil is constantly growing, and intensive production models are used in order to obtain short-term weight gain goals due to the increase of animal confined and susceptible to various diseases. This study aimed to evaluate the serum proteinogram including the acute phase response in sheep with experimentally induced ARA.
2. Materials and methods
2.1. Study design
For induction of ARA, 10 adult, healthy, non-pregnant Santa Inês ewes, fed exclusively with grass, were used. All animals were dewormed and fed in a basal diet with coast cross hay (Cynodon dactylon), they also had free access to drinking water and mineral salt mixture. The adaptation period lasted 30 days. At the beginning of the experiment (Day [D] -15, -7 and 0), blood was collected from all animals to establish the basal values for serum proteinogram analysis, and the mean values were calculated. The ARA induction was preconized conformed as described by Sabes [24]. After the sucrose administration, blood samples were obtained at the following intervals: 4, 8, 12, 16, 20, 24, 28, 32, 36, 48, 72, 96, 120 and 144 h. Subsequently, samples were obtained every seven days for three further weeks until complete one month, and frozen at −20 °C.
2.2. SDS-PAGE electrophoresis analysis
Blood samples were obtained by puncture of the jugular vein after using vacuum system (BD Vacutainer™) with multiple collection needle 25 × 8 mm and sterile plastic tube without anticoagulant and 10 mL capacity. The samples were centrifuged at 2500g for 10 min to obtain the serum fraction. Serum proteins were fractionated by means of SDS-PAGE gel electrophoresis [25]. Concentration of protein fractions was determined by use of computerized densitometry (Shimadzu CS 9301, Shimadzu Corporation, Tokyo, Japan). Proteins were identified by means of reference markers (Sigma Chemical Company, St Louis, MO, USA) with molecular weights of 24,000 Da, 29,000 Da, 36,000 Da, 45,000 Da, 55,000 Da, 66,000 Da, 97,000 Da, 116,000 Da and 205,000 Da. Serum protein concentrations (mg/dL) were determined by multiplying the percentage of each fraction by the total serum protein (TP) concentration obtained by semi-automatic spectrophotometer (Labquest, Labtest, Brazil) using the biuret method.
Fibrinogen was determined by heat precipitation [26] and measured using a refractometer (Atago T2-NE Clinical, Atago Co. Ltd., Japan). These analyses were performed at the Research Support Laboratory, Department of Veterinary Clinic and Surgery, School of Agrarian and Veterinary Sciences, São Paulo State University, Jaboticabal, Brazil. This study received approval from the Ethics Comittee of FCAV/Unesp, Jaboticabal, São Paulo, Brazil (Protocol n°. 03888/14).
2.3. Data analysis
The data were submitted to variance analysis with repeated measures in which the factor tested was the harvest (18 levels for serum protein fractions values and ten levels for fibrinogen values), with the animals constituting the 10 blocks. A regression analysis (linear, quadratic and cubic) was performed using the computer program General Linear Models Procedure (GLM). The means were subjected to analysis by Tukey test at 5% significance.
3. Results
Thirteen proteins were identified using the SDS-PAGE method: Immunoglobulin A (IgA) and G (IgG – light (IgGl) and heavy (IgGh) chains), ceruloplasmin (Cp), transferrin (Tf), albumin (Ab), α1-antitrypsin (α1-AT), haptoglobin (Hp), α1-acid glycoprotein (α1-GA), and proteins of molecular weight 95 (MWP95), 46 (MWP46), 36 (MWP36) and 31 (MWP31) kDa.
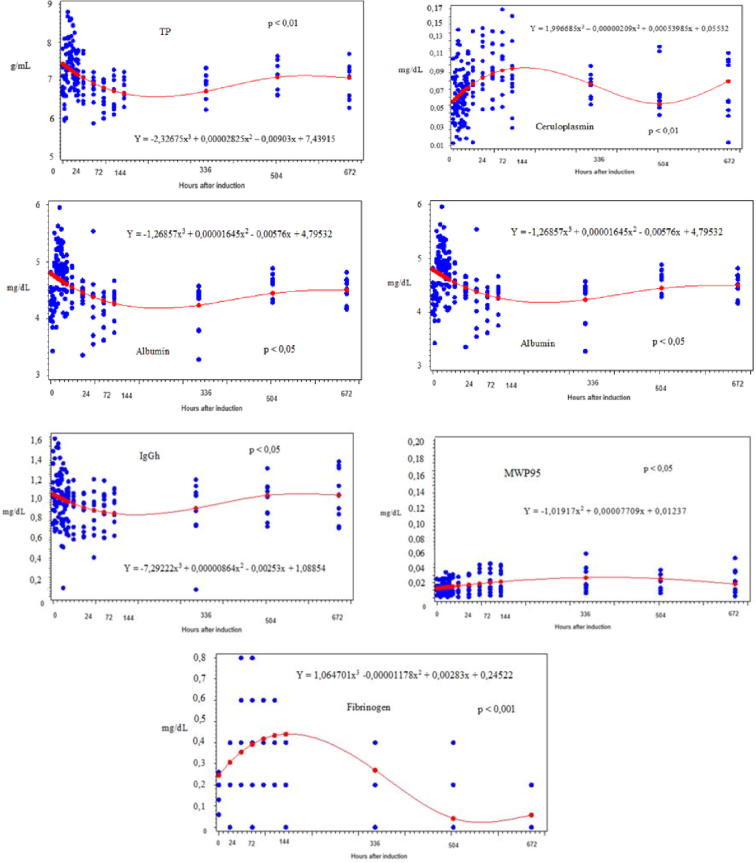
The following proteins were not associated with time (p > 0.05): IgA, IgGl, Tf, α1-AT, α1-GA, MWP46, MWP36 and MWP31. Time showed cubic regression with the values of Cp (on the Tukey test the means showed a significant difference at 72, 96 and 120 h), Al (significant difference was observed with 12, 16, 20 and 24 h on Tukey Test); Hp (only with 72 h there was significant difference by Tukey test); IgGl (no significant difference was observed at Tukey Test), MWP95 (Tukey Test showed no significant difference), TP (difference was observed at 12, 16, 20 and 24 h) and Fb (significant difference observed at 48, 72, 96 and 120 h). The mean, standard deviation and coefficient of variation (CV) of the serum proteinogram are shown on Table 1, Table 2. The graphical representations of the regression analysis of the proteins with statistical significance were presented at Fig. 1.
Table 1.
Mean ± standard deviation and coefficient of variation (CV) of serum protein concentrations (mg/dL) by SDS – PAGE, fibrinogen - Fib (mg/dL) obtained by heat precipitation and total serum protein - TP (g/dL) obtained by biuret method of sheep with ARA. Harvest moments: control*, 4 hs, 8 hs, 12 hs, 16 hs, 20 hs, 24 hs 28 hs, 32 hs, 36 hs, 48 hs, 72 hs, 96 hs, 120 hs, 144 hs, 2nd, 3rd and 4th weeks after oral sucrose administration.
| TP (g/dL) |
IgA (mg/dL) |
Ceruloplasmin (mg/dL) |
Transferrin (mg/dL) |
Albumin (mg/dL) |
Haptoglobin (mg/dL) | Fib (mg/dL) |
|
|---|---|---|---|---|---|---|---|
| Control* | 7,06 ± 0,13 | 0,016 ± 0,007 | 0,056 ± 0,010 | 0,387 ± 0,021 | 4,414 ± 0,102 | 0,012 ± 0,002 | 0,15 ± 0,02 |
| 04 h | 6,60 ± 0,19 | 0,008 ± 0,002 | 0,054 ± 0,007 | 0,356 ± 0,032 | 4,168 ± 0,112 | 0,014 ± 0,002 | – |
| 08 h | 7,19 ± 0,18 | 0,016 ± 0,005 | 0,060 ± 0,009 | 0,368 ± 0,041 | 4,621 ± 0,141 | 0,022 ± 0,005 | – |
| 12 h | 7,85 ± 0,18 | 0,016 ± 0,005 | 0,080 ± 0,013 | 0,418 ± 0,026 | 4,808 ± 0,151 | 0,039 ± 0,020 | – |
| 16 h | 7,69 ± 0,15 | 0,011 ± 0,003 | 0,057 ± 0,011 | 0,375 ± 0,043 | 4,987 ± 0,135 | 0,020 ± 0,004 | – |
| 20 h | 7,70 ± 0,15 | 0,019 ± 0,008 | 0,059 ± 0,008 | 0,344 ± 0,060 | 5,152 ± 0,128 | 0,023 ± 0,005 | – |
| 24 h | 7,66 ± 0,16 | 0,011 ± 0,003 | 0,050 ± 0,007 | 0,384 ± 0,047 | 5,084 ± 0,108 | 0,033 ± 0,007 | 0,22 ± 0,04 |
| 28 h | 7,24 ± 0,16 | 0,013 ± 0,005 | 0,059 ± 0,006 | 0,376 ± 0,042 | 4,750 ± 0,105 | 0,034 ± 0,007 | – |
| 32 h | 7,12 ± 0,11 | 0,010 ± 0,004 | 0,064 ± 0,008 | 0,345 ± 0,049 | 4,681 ± 0,096 | 0,039 ± 0,007 | – |
| 36 h | 7,15 ± 0,15 | 0,027 ± 0,013 | 0,079 ± 0,009 | 0,391 ± 0,038 | 4,646 ± 0,110 | 0,028 ± 0,005 | – |
| 48 h | 6,99 ± 0,14 | 0,008 ± 0,004 | 0,077 ± 0,011 | 0,433 ± 0,049 | 4,567 ± 0,075 | 0,065 ± 0,017 | 0,54 ± 0,06 |
| 72 h | 6,76 ± 0,12 | 0,016 ± 0,006 | 0,100 ± 0,010 | 0,403 ± 0,028 | 4,353 ± 0,118 | 0,093 ± 0,030 | 0,48 ± 0,07 |
| 96 h | 6,61 ± 0,11 | 0,028 ± 0,008 | 0,097 ± 0,009 | 0,416 ± 0,024 | 4,327 ± 0,172 | 0,063 ± 0,028 | 0,44 ± 0,04 |
| 120 h | 6,59 ± 0,13 | 0,009 ± 0,003 | 0,103 ± 0,011 | 0,429 ± 0,025 | 4,100 ± 0,096 | 0,051 ± 0,020 | 0,44 ± 0,05 |
| 144 h | 6,77 ± 0,09 | 0,019 ± 0,010 | 0,083 ± 0,012 | 0,433 ± 0,033 | 4,303 ± 0,083 | 0,033 ± 0,015 | 0,32 ± 0,04 |
| 2nd week | 6,72 ± 0,20 | 0,012 ± 0,005 | 0,063 ± 0,008 | 0,410 ± 0,034 | 4,193 ± 0,133 | 0,053 ± 0,041 | 0,22 ± 0,05 |
| 3rd week | 7,10 ± 0,12 | 0,008 ± 0,003 | 0,062 ± 0,010 | 0,379 ± 0,025 | 4,519 ± 0,115 | 0,014 ± 0,002 | 0,10 ± 0,04 |
| 4th week | 7,06 ± 0,14 | 0,017 ± 0,007 | 0,077 ± 0,014 | 0,392 ± 0,019 | 4,478 ± 0,072 | 0,015 ± 0,001 | 0,04 ± 0,02 |
| CV | 4,68 | 117,24 | 34,18 | 21,17 | 6,44 | 115,77 | 45,62 |
Control: mean of the harvests -15, -7 days and immediately before the sucrose administration.
Table 2.
Mean ± standard deviation and coefficient of variation (CV) of serum protein concentrations (mg/dL) by SDS – PAGE of sheep with ARA. Harvest moments: control*, 4 h, 8 h, 12 h, 16 h, 20 h, 24 h 28 h, 32 h, 36 h, 48 h, 72 h, 96 h, 120 h, 144 h, 2nd, 3rd and 4th weeks after oral sucrose administration.
| α1AT (mg/dL) |
α1 GA (mg/dL) |
IgGl (mg/dL) |
IgGh (mg/mL) |
MWP95 (mg/dL) |
MWP46 (mg/dL) |
MWP36 (mg/dL) |
MWP31 (mg/dL) |
|
|---|---|---|---|---|---|---|---|---|
| Control* | 0,165 ± 0,011 | 0,031 ± 0,003 | 0,461 ± 0,055 | 1,100 ± 0,250 | 0,012 ± 0,002 | 0,003 ± 0,008 | 0,005 ± 0,009 | 0,007 ± 0,001 |
| 04 h | 0,175 ± 0,012 | 0,034 ± 0,004 | 0,474 ± 0,064 | 1,045 ± 0,100 | 0,009 ± 0,001 | 0,005 ± 0,001 | 0,006 ± 0,001 | 0,013 ± 0,002 |
| 08 h | 0,187 ± 0,008 | 0,034 ± 0,005 | 0,454 ± 0,056 | 1,105 ± 0,082 | 0,010 ± 0,002 | 0,004 ± 0,001 | 0,005 ± 0,009 | 0,012 ± 0,002 |
| 12 h | 0,199 ± 0,011 | 0,044 ± 0,005 | 0,541 ± 0,076 | 1,243 ± 0,075 | 0,012 ± 0,002 | 0,004 ± 0,001 | 0,006 ± 0,009 | 0,014 ± 0,003 |
| 16 h | 0,209 ± 0,128 | 0,036 ± 0,004 | 0,553 ± 0,076 | 1,104 ± 0,073 | 0,012 ± 0,002 | 0,003 ± 0,007 | 0,005 ± 0,001 | 0,019 ± 0,004 |
| 20 h | 0,217 ± 0,018 | 0,045 ± 0,005 | 0,499 ± 0,079 | 1,028 ± 0,087 | 0,013 ± 0,004 | 0,005 ± 0,001 | 0,005 ± 0,007 | 0,015 ± 0,003 |
| 24 h | 0,223 ± 0,012 | 0,041 ± 0,005 | 0,546 ± 0,067 | 0,893 ± 0,123 | 0,011 ± 0,002 | 0,002 ± 0,005 | 0,005 ± 0,008 | 0,013 ± 0,002 |
| 28 h | 0,211 ± 0,009 | 0,062 ± 0,027 | 0,489 ± 0,057 | 0,997 ± 0,053 | 0,011 ± 0,002 | 0,003 ± 0,007 | 0,004 ± 0,005 | 0,013 ± 0,003 |
| 32 h | 0,207 ± 0,016 | 0,038 ± 0,004 | 0,482 ± 0,040 | 0,982 ± 0,065 | 0,012 ± 0,003 | 0,006 ± 0,003 | 0,005 ± 0,002 | 0,011 ± 0,002 |
| 36 h | 0,192 ± 0,011 | 0,028 ± 0,005 | 0,520 ± 0,060 | 0,996 ± 0,061 | 0,034 ± 0,018 | 0,003 ± 0,009 | 0,002 ± 0,003 | 0,025 ± 0,018 |
| 48 h | 0,196 ± 0,014 | 0,036 ± 0,006 | 0,480 ± 0,045 | 0,941 ± 0,064 | 0,012 ± 0,002 | 0,004 ± 0,001 | 0,003 ± 0,006 | 0,010 ± 0,001 |
| 72 h | 0,188 ± 0,013 | 0,040 ± 0,005 | 0,462 ± 0,043 | 0,872 ± 0,057 | 0,012 ± 0,003 | 0,007 ± 0,002 | 0,005 ± 0,001 | 0,014 ± 0,003 |
| 96 h | 0,196 ± 0,011 | 0,026 ± 0,003 | 0,425 ± 0,055 | 0,885 ± 0,076 | 0,018 ± 0,004 | 0,005 ± 0,001 | 0,002 ± 0,004 | 0,014 ± 0,003 |
| 120 h | 0,187 ± 0,011 | 0,032 ± 0,006 | 0,420 ± 0,054 | 0,936 ± 0,059 | 0,025 ± 0,005 | 0,004 ± 0,001 | 0,002 ± 0,006 | 0,014 ± 0,001 |
| 144 h | 0,195 ± 0,016 | 0,032 ± 0,006 | 0,440 ± 0,044 | 0,959 ± 0,050 | 0,024 ± 0,005 | 0,003 ± 0,009 | 0,004 ± 0,005 | 0,012 ± 0,002 |
| 2nd week | 0,186 ± 0,013 | 0,031 ± 0,005 | 0,495 ± 0,053 | 0,926 ± 0,108 | 0,024 ± 0,005 | 0,004 ± 0,005 | 0,004 ± 0,007 | 0,012 ± 0,002 |
| 3rd week | 0,203 ± 0,018 | 0,039 ± 0,005 | 0,496 ± 0,056 | 1,068 ± 0,078 | 0,021 ± 0,004 | 0,004 ± 0,001 | 0,007 ± 0,001 | 0,011 ± 0,002 |
| 4th week | 0,194 ± 0,027 | 0,034 ± 0,005 | 0,486 ± 0,047 | 1,082 ± 0,082 | 0,020 ± 0,005 | 0,007 ± 0,002 | 0,006 ± 0,009 | 0,019 ± 0,002 |
| CV | 14,77 | 59,94 | 21,41 | 18,59 | 102,19 | 95,60 | 63,54 | 110,21 |
Control: mean of the harvests -15, -7 days and immediately before the sucrose administration.
Fig. 1.
Graphical representations of the regression analysis of the proteins with statistical significance in sheep with acute ruminal lactic acidosis.
4. Discussion
All animals showed clinical signs of ARA and also metabolic acidosis as previously described by Sabes [24] such as apathy, appetite loss, bilateral abdominal distension, ruminal atony, dehydration, tachycardia, tachypnea and five animals developed acute laminitis clinically noticed. Other analyses were performed such as arterial blood hemogasometry, evaluation of urinary pH and lactate blood levels during the experimental period to confirm the ruminal and metabolic acidosis. Although the literature says that mortality cases are extremely common in animals with ARA and metabolic acidosis [27], no animal was treated during the period and no animal died in this experiment.
The inflammation resulting from ARA and some clinical signs can be noticed through alterations beyond serum protein concentrations. The TP indicates changes from 08 h, with the hightest value at 12 h, and at the same moment the animals showed signs of dehydration and hemoconcentration, resulting from the fluid influx from the blood stream into the rumen. At 36 h, the TP values returned to normal, remaining until the end of the observation period. These data do not corroborate with the study conducted by Vieira et al. [28] in which the TP variations were not observed until 48 h after induction of ARA in small ruminants.
Some studies have shown that lactic acid presented in high levels on the rumen and on systemic circulation is the key factor on triggering the inflammatory response in cases of ARA [29], [30], [31], [32]. The ruminitis, presence of LPS and other pro inflammatory substances on systemic circulation can lead to the increase on the expression of APP. Jacobsen et al. [31] reported that LPS is a potent inducer of APP and it is known that LPS concentrations on the ruminal fluid increase during lactic acidosis, and also may occur the migration from the rumen to the systemic circulation, stimulating the hepatic synthesis of APP [33], [34], [35]. Furthermore, the acid pH and certain organic acids can activate components of humoral and cellular response, improving the inflammatory process [29], [30], [32] as observed after ARA induction in heifers using oligofructose.
It is known that the APR in ruminants is different in relation to other species, and the Hp is one of the most important APP. In healthy bovine serum, the Hp concentration is less than 20 mg/L, but it may increase to values higher than 2 g/L in two days in cases of infections [36]. In cattle, the Hp is effective on the diagnosis and prognosis of diseases such as mastitis, enteritis, peritonitis, pneumonia, endocarditis and endometritis [37]. On the present study, the Hp values increased from 08 h, remaining changed until the second week and then returning to values similar to the control moment. The increase in Hp values was associated with the ruminal acid ambient [38], which increased the lysis of Gram-negative bacteria, LPS release and its translocation into the systemic circulation, resulting in inflammatory response [17], [39]. However Huo et al. [40] showed that only LPS cannot be the sole inducer of inflammatory response and subsequently increasing the concentration of Hp. Skinner and Roberts [41] confirmed that Hp was a better indicator of bacterial infection than the white blood cell count. Danscher et al. [23] compared the APR in heifers with ARA induced with silage, indicating that the inflammatory response also depends on pre-existing conditions in the rumen, which can affect the degree of ruminitis, changing the bacterial population and the amount of lactic acid produced.
The α1-AT showed an increase from 08 h, with the highest mean obtained at 24 h. This protein has anti-proteinase activity, and the values increase can be explained by their characteristic to inhibit proteases released by phagocytes or pathogens, limiting the tissue damage at the site of inflammation [42].
The use of Cp as a marker of inflammation is not as common as the use of other APP as Hp [36]. In the present study, this APP presented a different dynamic of other proteins, and its mean showed an increase from 32 h, with the higher mean founded at 120 h. This increase is considered late. On the compiled literature there was no study describing the Cp alterations in animals with ARA. Pfeffer and Rogers [43] observed an increase on concentration of Cp in sheep with pneumonia and bronchial obstruction, and this increase was detected on early days. Pereira et al. [44] and Costa et al. [9] studied lambs with copper poisoning and induced acute mastitis in sheep, they also reported an increase in this protein. The study of Cp is little explored, however Murata et al. [36] believed that this ferroxidase can be used as an indicator of infection in cattle.
Albumin is classified as a negative APP because the need of amino acids for the APR synthesis of positive proteins is greatly increased, making it necessary to readjust the synthesis of hepatic proteins [42]. The highest Ab mean was obtained at 20 h, same period that the animals showed clinical signs of dehydration, returning to values similar to the control with 144 h. Costa et al. [9] induced acute mastitis in sheep and described a slight increase in Ab at 96 h after infection and then values returned to normal, coinciding with the clinical improvement of animals. Another protein that is also classified as negative is Tf, which has the characteristic of bind iron molecules, inhibiting the multiplication of some virus, bacteria and fungi and limiting their access to iron [42]. The increase in serum concentration during the inflammatory process can be used as a non-specific immune response against pathogenic microorganisms [36].
The IgA values did not change significantly, but it is believed that this immunoglobulin can protect surfaces against infections [9]. IgG also showed no significant difference, but in a study conducted by Costa et al. [9], this immunoglobulin is considered one of the responsible for the control of various infections. In cattle, the α1-GA is known to be an important anti-inflammatory protein and capable of modulating monocytes and polymorphonuclear cells in the inflammatory response, including chemotaxins, free reactive oxygen radicals and degranulation of other cells [45]. Eckersall [21] suggested that this protein might be used as a marker for chronic diseases in sheep, and also described an increase in its concentration in cases of caseous lymphadenitis. In cattle this APP shows an increase at 24 to 72 h following inflammatory conditions [46], but in dairy cattle with traumatic reticuloperitonitis this APP showed high values in animals with acute inflammation compared to chronic [47]. In this study, the highest value was found with 28 h, returning to normal with 120 h. This protein may modulate the recruitment and platelet aggregation, reducing local inflammation and preventing changes on the injured tissue [48].
Fibrinogen is classified as a minor/moderate APP in cattle, and in response to inflammation and infection can increase their values in two to three times [37]. The plasma concentration rises in the stimulatory action of interleukins (IL-1 and 6) of the necrotic factor released during the inflammatory process [49]. According to Schalm et al. [50] during the acute inflammatory process, the plasma concentration of this substance increases, reaching the highest values between the fifth and seventh day. The stimulation of Fb synthesis according to Jain [51] occurs in six to eight hours after injury, and in this study the maximum values were obtained after 48 h of induction; returning to normal after 144 h. The obtained data showed that Fb can be used as a marker of acute inflammatory response in sheep as described by Pfeffer and Rogers [43]; Pfeffer et al. [52], Costa et al [9], Gonzalez et al. [22], Danscher et al. [23]. But authors like Vieira et al. [28] stated that Fb values do not change after induction of acidosis through sucrose or diets with high concentrate values [20], [22], [38]. Kirbas et al. [12] stated that the Fb concentration is preferable as a marker to APR as compared to the leukogram in evaluating traumatic inflammatory processes in cattle, such as traumatic reticuloperitonitis.
On the compiled literature there is no mention about MWP95, MWP46, MWP36 and MWP31. Therefore their functions are unknown, however, several of them show potential for use as APPs. A variety of new APPs and their mediators can be introduced to veterinary science due to a continuous request for those substances and intensive research about their applications, thus accelerating the understanding of innate immune mechanisms and pathophysiology of infection and inflammation [36].
5. Conclusions
Changes in serum levels of total protein, haptoglobin, ceruloplasmin, fibrinogen and acute negative phase proteins can assist the clinical evaluation and diagnosis of acute ruminal lactic acidosis in sheep. The SDS-PAGE electrophoresis technique allows the identification of a variety of serum proteins, some of them with unknown functions.
Competing interests
The authors declare that they have no competing interests.
Acknowledgement
The authors acknowledge the São Paulo Research Foundation (Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP) grant number 2014/00317-1 for financial assistance.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Evans G.O. 2nd ed. CRC Press; 2009. Animal Clinical Chemistry – a practical guide for toxicologists and biomedical researchers. [Google Scholar]
- 2.Eckersall P.D., Conner J.G. Bovine and canine acute phase proteins. Vet Res Comm. 1988;12:169–178. doi: 10.1007/BF00362798. [DOI] [PubMed] [Google Scholar]
- 3.Gruys E., Obwolo M.J., Toussaint M.J.M. Diagnostic significance of the major acute phase proteins in veterinary clinical chemistry: a review. Vet Bull. 1994;64:1009–1018. [Google Scholar]
- 4.Eckersall P.D. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev Méd Vét. 2000;151:577–584. [Google Scholar]
- 5.Baumann H., Gauldie J. The acute phase response. Imm Tod. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. 1016/0167-5699(94)90137–6. [DOI] [PubMed] [Google Scholar]
- 6.Fagliari J.J., McClenahan D., Evanson O.A., Weiss D.J. Changes in plasma protein concentrations in poneis with experimentally induced alimentary laminitis. Amer J Vet Res. 1988;59:1234–1237. [PubMed] [Google Scholar]
- 7.Fagliari J.J., Silva S.L. Hemogram and plasma proteins of healthy horses and horses with acute abdomen before and after laparotomy. Arq Bras Med Vet Zoot. 2002;54:559–567. [Google Scholar]
- 8.Fagliari J.J., Weiss D.J., McClenahan D., Evanson O.A. Serum protein concentrations in calves with experimentally induced pneumonic pasteurellosis. Arq Bras Med Vet Zoot. 2003;55:383–387. [Google Scholar]
- 9.Costa N.A., Simão L.C.V., Santos R.A., Afonso J.A.B., Fagliari J.J., Cardoso E.C. Proteinogram and serum concentrations of copper, iron and zinc in Santa Inês ewes with Staphylococcus aureus experimentally induced mastitis. Pesq Vet Bras. 2010;30:435–442. [Google Scholar]
- 10.Braun U., Rihs T., Schefer U. Ruminal lactic acidosis in sheep and goats. Vet Rec. 1992;130:343–349. doi: 10.1136/vr.130.16.343. [DOI] [PubMed] [Google Scholar]
- 11.Garry FB. Indigestion in ruminants. In: Large Animal Internal Medicine. 3rd ed. Mosby, Missouri. 2002.
- 12.Kirbas A., Baydar E., Kandemir F.M., Dorman E., Kizil O., Yildirim B.A. Evaluation of serum cardiac troponin-I concentration in sheep with acute ruminal lactic acidosis. Vet Arhiv. 2014;84:355–364. [Google Scholar]
- 13.Hofirek B., Slosarkova S., Ondrova J. Effect of chronic metabolic acidosis on migration activity of polymorphonuclear leukocytes in sheep. Vet Med. 1995;40:171–175. [PubMed] [Google Scholar]
- 14.Plaizier J.C., Krause D.O., Gozho G.N., Mcbride B.W. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet J. 2008;176:21–31. doi: 10.1016/j.tvjl.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Kleen J.L., Hooijer G.A., Rehage J., Noordhuizen J.P.T.M. Subacute ruminal acidosis (SARA): a review. J Vet Med. 2003;50:406–414. doi: 10.1046/j.1439-0442.2003.00569.x. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan F.J., Doherty M.L. Production diseases of the transition cow. Vet J. 2008;176:3–9. doi: 10.1016/j.tvjl.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Gozho G.N., Plaizier J.C., Krause D.O., Kennedy A.D., Wittenberg K.M. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J Dairy Sci. 2005;88:1399–1403. doi: 10.3168/jds.S0022-0302(05)72807-1. [DOI] [PubMed] [Google Scholar]
- 18.Gozho G.N., Krause D.O., Plaizier J.C. Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. J Dairy Sci. 2006;89:4404–4413. doi: 10.3168/jds.S0022-0302(06)72487-0. [DOI] [PubMed] [Google Scholar]
- 19.Khafipoor E., Plaizier J.C., Krause D.O. Influence of grain induced subacute ruminal acidosis (SARA) on lipopolysaccharide endotoxin (LPS), and acute phase proteins. Can J Anim Sci. 2006;86:577–580. [Google Scholar]
- 20.Gozho G.N., Krause D.O., Plaizier J.C. Ruminal lipopolysaccharide concentration and inflammatory response during grain-induced subacute ruminal acidosis in dairy cows. J Dairy Sci. 2007;90:856–866. doi: 10.3168/jds.S0022-0302(07)71569-2. [DOI] [PubMed] [Google Scholar]
- 21.Eckersall P.D. Acute phase protein: biomarkers of disease in cattle and sheep. Catt Prac. 2007;15:240–243. [Google Scholar]
- 22.González F.H.D., Ruipérez F.H., Sánchez J.M., Souza J.C., Martinez-Subiela S., Cerón J.J. Haptoglobin and serum amyloid A in subacute ruminal acidosis in goats. Rev Med Vet Zoot. 2010;57:159–167. [Google Scholar]
- 23.Danscher A.M., Thoefner M.B., Heegaard P.M.H., Ekstrøm C.T., Jacobsen S. Acute phase protein response during acute ruminal acidosis in cattle. Livest Sci. 2011;135:62–69. [Google Scholar]
- 24.Sabes AF. Acidose láctica ruminal aguda induzida experimentalmente em ovinos: estudo clínico e laboratorial, 87p. Dissertação (mestrado) - Universidade Estadual Paulista Júlio de Mesquita Filho, Faculdade de Ciências Agrárias e Veterinárias de Jaboticabal, 2015. Avaiable at: http://hdl.handle.net/11449/136729
- 25.Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 26.Allison R.W. Avaliação laboratorial das proteínas do plasma e do soro sanguíneo. In: Thrall M.A., Weiser G., Allison R.W., Campbell T.W., editors. Hematologia e bioquímica clínica veterinária. Roca; Brazil: 2015. pp. 398–411. [Google Scholar]
- 27.Underwood W.J. Rumen lactic acidosis. Part 1 - epidemiology and pathophisiology. Compend Contin Educ Pract Vet. 1992;14:1127–1133. [Google Scholar]
- 28.Vieira A.C., Câmara A.C., Mendonça C.L., Afonso A.B. Hematological and biochemical profile of sheep supplemented with salinomycin and submitted to experimental lactic ruminal acidosis. Cien Anim Bras. 2012;13:259–271. [Google Scholar]
- 29.Fishelson Z., Horstmann R.D., Mullereberhard H.J. Regulation of the alternative pathway of complement by pH. J Immun. 1987;138:3392–3395. [PubMed] [Google Scholar]
- 30.Trevani A.S., Andonegui G., Giordano M., Lopez D.H., Gamberale R., Minucci F. Extracellular acidification induces human neutrophil activation. J Immun. 1999;162:4849–4857. [PubMed] [Google Scholar]
- 31.Jacobsen S., Andersen P.H., Toelboel T., Heegaard P.M.H. Dose dependency and individual variability of the lipopolysaccharide-induced bovine acute phase protein response. J Dairy Sci. 2004;87:3330–3339. doi: 10.3168/jds.S0022-0302(04)73469-4. [DOI] [PubMed] [Google Scholar]
- 32.Kellum J.A., Song M.C., Li J.Y. Science review: extracellular acidosis and the immune response: clinical and physiologic implications. Crit Care. 2004;8:331–336. doi: 10.1186/cc2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dougherty R.W., Coburn K.S., Cook H.M., Allison M.J. Preliminary study of appearance of endotoxin in circulatory system of sheep and cattle after induced grain engorgement. Am J Vet Res. 1975;36:831–832. [PubMed] [Google Scholar]
- 34.Boosman R., Németh F., Gruys E. Bovine laminitis. Histopathologic and arteriographic aspects and its relation to endotoxemia. Vet Quart. 1990;13:163–171. [Google Scholar]
- 35.Andersen P.H., Hesseholt M., Jarløv N. Endotoxin and arachidonic acid metabolites in portal, hepatic and arterial blood of cattle with acute ruminal acidosis. Acta Vet Scand. 1994;35:223–234. doi: 10.1186/BF03548327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murata H., Shimada N., Yoshioka M. Current research on acute phase proteins in veterinary diagnosis. An overview. Vet J. 2004;168:28–40. doi: 10.1016/S1090-0233(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 37.Petersen H.H., Nielsen J.P., Heegaard P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 2004;35:163–187. doi: 10.1051/vetres:2004002. [DOI] [PubMed] [Google Scholar]
- 38.Mohebbi M., Sajedianfard J., Nazifi S., Samimi A.S. Changes of serum amyloid A, haptoglobin, ceruloplasmin, fibrinogen and lipid-associated sialic acid in sheep fed high grain rations with altered digestive functions. Comp Clin Path. 2010;19:541–546. [Google Scholar]
- 39.Khafipour E., Krause D.O., Plaizier J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci. 2009;92:1060–1070. doi: 10.3168/jds.2008-1389. [DOI] [PubMed] [Google Scholar]
- 40.Huo W., Zhu W., Mao S. Effects of feeding increasing proportions of corn grain on concentration of lipopolysaccharide in the rumen fluid and the subsequent alterations in immune responses in goats. J Anim Sci. 2010;26:1437–1445. doi: 10.5713/ajas.2013.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skinner J.G., Roberts L. Bovine haptoglobin response in clinically defined field conditions. Vet Rec. 1991;128:147–149. doi: 10.1136/vr.128.7.147. [DOI] [PubMed] [Google Scholar]
- 42.Tothova C., Nagy O., Kovac G. Acute phase proteins and their use in the diagnosis of diseases in ruminants. A review. J Vet Med. 2014;59:163–180. [Google Scholar]
- 43.Pfeffer A., Rogers K.M. Acute phase response of sheep. Changes in the concentrations of ceruloplasmin, fibrinogen, haptoglobin and the major blood cell types associated with pulmonar damage. Res Vet Sci. 1989;46:118–124. [PubMed] [Google Scholar]
- 44.Pereira W.A.B., Faria J.L.M., Silva P.C., Hatayde M.R., Fagliari J.J. Serum proteinogram in copper-poisoning sheep. Cien Anim Bras. 2009;1:354–359. [Google Scholar]
- 45.Ceciliani F., Ceron J.J., Eckersall P.D., Sauerwein H. Acute phase proteins in ruminants. J Prot. 2012;5:4207–4231. doi: 10.1016/j.jprot.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Jones M.L., Allison R.W. Evaluation of the ruminant complete blood cell count. Vet Clin North Am Food Anim Pract. 2007;23:377–402. doi: 10.1016/j.cvfa.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Kirbas A., Ozkanlar Y., Aktas M.S., Ozkanlar S., Ulas N., Erol H.S. Acute phase biomarkers for inflammatory response in dairy cows with traumatic reticuloperitonitis. Israel J Vet Med. 2015;70 23-2. [Google Scholar]
- 48.Hochepied T., Berger F.G., Baumann H., Ibert C. α1-acid glycoprotein. An acute phase protein with inflammatory and immunomodulating properties. Cytok Growth Fact Rev. 2003;14:25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 49.Andrews D.A., Reagan W.J., Denicola D.B. Plasma fibrinogen in recognizing equine inflammatory disease. Comp Cont Educ Pract Vet. 1994;16:1349–1357. [Google Scholar]
- 50.Schalm O.W., Smith R., Kaneko J.J. Plasma protein: fibrinogen ration in dogs, cattle and horses - Part I. Influence of age on normal values and explanation of use in disease. Calif Vet. 1975;24 09-10. [Google Scholar]
- 51.Jain N.C. The plasma proteins, dysproteinemias and immune deficiency disorders. Essent Vet Hemat. 1993;21:349–380. [Google Scholar]
- 52.Pfeffer A., Rogers K.M., O’Keeffe L., Osborn P.J. Acute phase protein response, food intake, live weight change and lesions following intrathoracic injection of yeast in sheep. Res Vet Sci. 1993;55:360–366. doi: 10.1016/0034-5288(93)90108-r. [DOI] [PubMed] [Google Scholar]