Summary
Leptosphaeria maculans, the causal agent of blackleg disease in canola (Brassica napus), secretes an array of effectors into the host to overcome host defense. Here we present evidence that the L. maculans effector protein AvrLm1 functions as a virulence factor by interacting with the B. napus mitogen-activated protein (MAP) kinase 9 (BnMPK9), resulting in increased accumulation and enhanced phosphorylation of the host protein. Transient expression of BnMPK9 in Nicotiana benthamiana induces cell death, and this phenotype is enhanced in the presence of AvrLm1, suggesting that induction of cell death due to enhanced accumulation and phosphorylation of BnMPK9 by AvrLm1 supports the initiation of necrotrophic phase of L. maculans infection. Stable expression of BnMPK9 in B. napus perturbs hormone signaling, notably salicylic acid response genes, to facilitate L. maculans infection. Our findings provide evidence that a MAP kinase is directly targeted by a fungal effector to modulate plant immunity.
Subject Areas: Parasitology, Plant Biology, Interaction of Plants with Organisms
Graphical Abstract

Highlights
-
•
Leptosphaeria maculans effector AvrLm1 interacts with the Brassica napus MPK9 (BnMPK9)
-
•
AvrLm1 increases the accumulation and enhances the phosphorylation of BnMPK9
-
•
AvrLm1 enhances BnMPK9-dependent cell death in Nicotiana benthamiana
-
•
Stable expression of BnMPK9 in B. napus facilitates L. maculans infection
Parasitology; Plant Biology; Interaction of Plants with Organisms
Introduction
Fungal pathogens of crop plants cause significant yield losses and affect global food security, particularly in the face of increased demand due to a growing world population (Fisher et al., 2012). Diverse lifestyle, genome plasticity and evolution, prolific reproduction, and longevity of spores under harsh environment impede the efficient control of fungal diseases. Like bacterial and oomycete plant pathogens, fungi also secrete small proteins, known as effectors, which counter plant defense and modulate plant physiology to promote pathogen growth and reproduction (Presti et al., 2015, Stergiopoulos and de Wit, 2009). Accumulating evidences show that the secreted effectors are key players in suppressing pathogen-associated molecular patterns (PAMPs)-triggered immunity (PTI) induced upon fungal recognition by plant (Presti et al., 2015). As an example, the lysine motif (LysM) effector ECP6 secreted by the tomato pathogen Cladosporium fulvum suppresses chitin triggered immunity. ECP6 binds chitin via the intramolecular chitin-binding groove formed by the LysM domains, resulting in the sequestering of chitin released from the cell walls of invading hyphae (de Jonge et al., 2010, Sánchez-Vallet et al., 2013). Plants, on the other hand, have evolved disease resistance (R) genes encoding receptor proteins that detect and respond to pathogen effector proteins, leading to the activation of effector-triggered immunity (ETI) (Jones and Dangl, 2006). Effectors that trigger ETI are referred to as avirulence (Avr) genes. Discovery of the host targets of effectors is essential for our understanding of effector activity and molecular mechanisms of plant defense (Win et al., 2012). There are many examples of plant proteins that are targeted by bacterial effectors secreted by the type III secretion system (Block and Alfano, 2011, Deslandes and Rivas, 2012). However, despite the economic importance of diseases caused by the fungal plant pathogens, for most fungal effectors, the host targets and underlying molecular mechanisms remain unknown.
Mitogen-activated protein (MAP) kinase cascades are highly conserved signaling modules in eukaryotes and have a central role in plant immunity against pathogen attack (Meng and Zhang, 2013, Pitzschke et al., 2009). A MAP kinase cascade is commonly composed of three types of kinases including a MAP kinase kinase kinase (MAPKKK), a MAP kinase kinase (MKK), and a MAP kinase (MPK). In Arabidopsis, pathogen-responsive MPK signaling has been reported to be involved in both PTI and ETI, in which the best characterized MPKs are MPK3, MPK4, and MPK6 (Asai et al., 2002, Rasmussen et al., 2012). PAMPs, such as flg22 (a conserved 22-amino acid flagellin peptide) and elf18 (elongation factor-Tu peptide), activate the kinase signaling cascades involving MPK3, MPK4, and MPK6 (Felix et al., 1999, Gao et al., 2008, Zipfel et al., 2006). In contrast, plant pathogens have evolved mechanisms to target the MPK signaling pathways to enhance plant susceptibility. For example, Pseudomonas syringae HopAI1 targets MPK3 and MPK6 and inactivates their kinase function to suppress plant defense responses (Zhang et al., 2007). P. syringae HopF2 targets MKK5 and can inactivate MKK5 via ADP-ribosylation of the C terminus of MKK5 in vitro (Wang et al., 2010). AvrB is a P. syringae effector that interacts with MPK4 to perturb hormone signaling and promote infection (Cui et al., 2010). Phytophthora infestans RXLR effector PexRD2 as a virulence factor interacts with the kinase domain of the host MAPKKKε to suppress MAPKKKε-dependent phosphorylation of MPKs to modulate plant immunity (King et al., 2014). These findings highlight the importance of MPK pathways in plant immunity and as targets of bacterial and oomycete pathogen effectors. However, to date no MAPKs have been identified as targets of effectors from fungal plant pathogens.
Plant MPKs can be classified into four groups (A, B, C, D) based on the conserved amino acid sequences of the TxY motif present in the activation loop of MPKs (Ichimura et al., 2002). Among the best studied MPKs, MPK3 and MPK6 are in group A and MPK4 belongs to group B. However, MPK9 belongs to the D group with the plant-specific TDY phosphorylation motif and is distinct from the A, B, and C groups. In Arabidopsis, previous studies have reported that AtMPK9, together with AtMPK12, is involved in stomatal closure (Lee et al., 2016). Both abscisic acid (ABA)- and methyl jasmonate-induced stomatal closure are impaired in Arabidopsis double mpk9/mpk12 mutants, but not in mpk9 or mpk12 single mutants, indicating a functional redundancy (Jammes et al., 2009, Khokon et al., 2015). The mpk9/mpk12 double mutant is highly susceptible to P. syringae pv. tomato (Pst) DC3000 infection (Jammes et al., 2011). However, Montillet et al. (2013) reported that ABA signaling, including open stomata 1 (OST1) protein kinase, AtMPK9, or AtMPK12, plays a limited role in response to Pst early infection. This is based on the observations that there is no difference between double mpk9/mpk12 mutants and wild-type Arabidopsis Col in response to flg22-induced stomatal closure and expression of ABA-specific marker genes is not affected by Pst treatment (Montillet et al., 2013). Thus it is unclear whether MPK9 acts in ABA-mediated guard cell immune signaling in response to biotic stresses. More recently, a study by Nagy et al. (2015) revealed that AtMPK9 is activated through intramolecular autophosphorylation independent of any upstream MAPKKs, which is similar to MAPKK-independent activation mechanisms reported for the mammalian atypical MAPKs, such as extracellular signal-regulated kinase (ERK)7/8 (Klevernic et al., 2006, Nagy et al., 2015).
Leptosphaeria maculans, the fungal agent of blackleg disease (phoma stem canker), causes major yield loss on Brassica napus (canola/rapeseed) crops worldwide (West et al., 2001). During infection it remains extracellular and exhibits a range of modes of infection from biotrophy to necrotrophy on Brassica hosts. Genome-wide transcriptomic analyses in the B. napus-L. maculans pathosystem revealed that the majority of known and predicted effectors had no expression during in vitro culture but were highly up-regulated during infection, supporting their roles as virulence factors (Haddadi et al., 2016). Resistance against L. maculans at the cotyledon stage is race specific. So far, 19 race-specific resistance (R) genes have been reported from Brassica species (Larkan et al., 2016), but only two R genes, LepR3 and Rlm2, have been cloned, both encoding membrane-localized receptor-like proteins (RLPs) (Larkan et al., 2013, Larkan et al., 2015). LepR3 perceives L. maculans AvrLm1 and triggers Brassica defense, leading to hypersensitive response (HR) at the site of infection (Larkan et al., 2013). Transient expression of both LepR3 and AvrLm1 induces HR in Nicotiana benthamiana leaves, and SOBIR1 and BAK1 receptor-like kinases (RLK), two components of LepR3 complex, are required for the perception of AvrLm1 (Ma and Borhan, 2015). To date, seven Avr genes (AvrLm1, 2, 3, 4–7, 5–9, 6, and 11) have been cloned from L. maculans, all encoding cysteine-rich proteins, except for AvrLm1, which contains only one cysteine residue (Gout et al., 2006). Cysteine enrichment is a feature found in most of the effectors of apoplastic fungi such as L. maculans and is believed to protect the effector protein, by formation of disulfide bonds, from plant proteases released into the apoplastic space during pathogen invasion (van den Burg et al., 2003). Lack of cysteine enrichment in the AvrLm1 protein could indicate that AvrLm1 is translocated inside the plant host cells. Given that LepR3 is a cell surface receptor, recognition of AvrLm1 by LepR3 likely occurs in the apoplast (Gout et al., 2006, Larkan et al., 2013, Ma and Borhan, 2015). However, it is still possible that AvrLm1 is translocated inside the plant cells to modulate host immunity. Owing to technical challenges, host cellular location for a vast majority of effector proteins of fungal and oomycete plant pathogens has not been determined and localization of pathogen effectors in the host plant is often inferred from their structure and their plant target proteins.
Although AvrLm1 was cloned a decade ago, the host target and molecular mechanism underlying AvrLm1 virulence have remained unknown. In this study, we report the identification of MPK 9 as a novel target of L. maculans effector AvrLm1. Stable expression of BnMPK9 in B. napus enhances the growth of L. maculans by suppressing the salicylic acid (SA) pathway, suggesting that BnMPK9 negatively regulates plant defense. Our findings indicate that AvrLm1 as a virulence factor functions by stabilizing BnMPK9 and inducing its phosphorylation, leading to enhanced cell death and plant susceptibility to the hemibiotrophic pathogen L. maculans.
Results
AvrLm1 as a Virulence Factor Modulates Plant Hormone Signaling
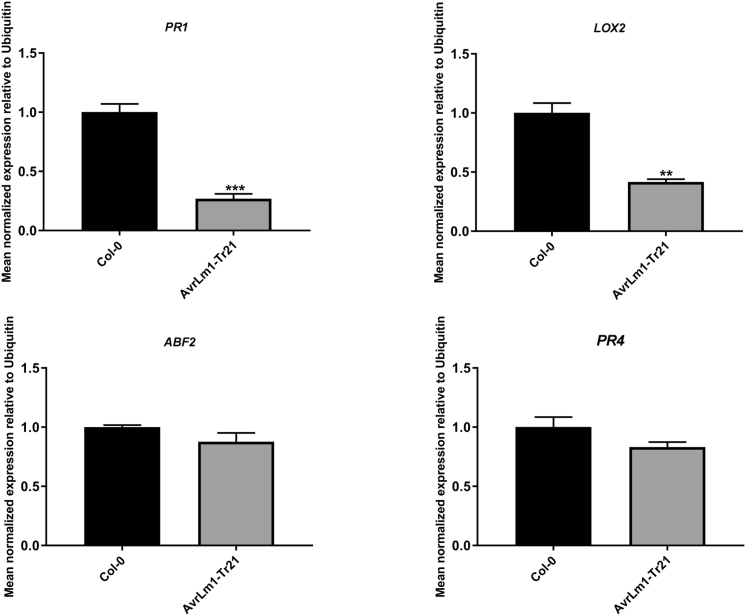
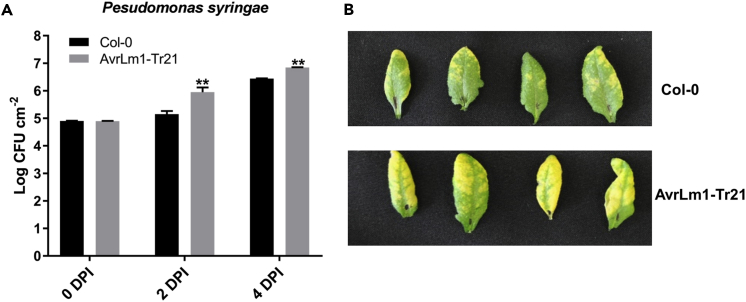
Effectors contribute to fungal virulence by protection of fungal structures and modulation of host metabolism. Comparison of near-isogenic L. maculans isolates with and without the effector AvrLm1 suggested that isolates carrying AvrLm1 were more aggressive on susceptible B. napus cultivars (Huang et al., 2009). To further examine the virulence function of AvrLm1, we generated transgenic Arabidopsis Columbia-0 (Col-0) lines carrying AvrLm1 in which expression of the full-length AvrLm1 gene was driven by the constitutive CaMV 35S promoter. A homozygous single-insertion line was generated (Col-AvrLm1), and expression of AvrLm1 was confirmed by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) (See Figure S1). Expression of SA, jasmonic acid (JA), ethylene (ET), and ABA signaling response marker genes (PR1, LOX-2, PR4, and ABF2) in Col-AvrLm1 and Col-0 plants were compared. Expressions of PR1 and LOX2 were significantly decreased in Col-AvrLm1, whereas expressions of ABF2 and PR4 were the same as in Col-0, indicating that AvrLm1 suppress SA and JA signaling but does not affect the ABA and ET pathways (Figure 1). Col-AvrLm1 plants were challenged with the bacterial pathogen Pst DC3000. Col-0 was also inoculated with Pst-DC3000 as a control. As shown in Figure 2, Col-AvrLm1 showed enhanced disease symptoms (severe chlorosis) and higher bacterial growth compared with the Col-0 control. These findings indicate that AvrLm1 functions as a virulence factor by suppressing plant defense.
Figure 1.
Relative Expression of Salicylic Acid (SA), Ethylene (ET), Jasmonic Acid (JA) and Abscisic Acid (ABA) Marker Genes in AvrLm1-Expressing Arabidopsis Lines
Leaves of 4-week-old plants were collected for RNA extraction. Expressions of Arabidopsis defense marker genes including PR1, LOX2, ABF2, and PR4 were determined by quantitative reverse-transcriptase polymerase chain reaction and normalized to Arabidopsis ubiquitin gene. Values represent means ± standard error (SE) from three independent experiments. ** and *** represent the significant differences (p < 0.01, and p < 0.001, respectively, a two-tailed Student's t test).
Figure 2.
AvrLm1-Expressing Arabidopsis Is More Susceptible to Pseudomonas syringae
(A) Bioassay was performed on Col-0 and one Col-0 35S::ΔspAvrLm1 (AvrLm1-Tr21) transformant. Bacterial growth of P. syringae was scored at 0, 2, and 4 dpi. Bars indicate standard deviation of eight replicates; ** indicates significant difference between the wild-type Col-0 and the AvrLm1-Tr21 in a two-tailed Student's t test (p = 0.01).
(B) Photographs were taken from representative leaves at 4 dpi.
AvrLm1 Is Secreted into the Apoplast of Brassica upon L. maculans Infection
L. maculans resides in the plant extracellular space during Brassica infection. To monitor AvrLm1 during L. maculans infection, AvrLm1 driven by its native promoter was tagged with the fluorescent protein mCherry and transferred to L. maculans isolate 3R11 (3R11:AvrLm1-mCherry). An additional transgenic isolate carrying the fluorescent gene YFP driven by a fungal constitutive promoter was generated to serve as a control. To ensure that addition of mCherry tag to AvrLm1 did not disrupt its Avr function (being recognized by LepR3) or the overall fitness of 3R11 isolate, the 3R11:AvrLm1-mCherry was inoculated on the susceptible B. napus cultivar Topas DH16516 and the Topas:LepR3 transgenic line NLA8-2 (Larkan et al., 2013). The 3R11:AvrLm1-mCherry was virulent on Topas DH16516 (see Figure S2). As anticipated, the transgenic strain 3R11:AvrLm1-mCherry was avirulent on the LepR3-transformed NLA8-2 line, confirming that addition of mCherry tag did not interfere with the function of AvrLm1 (Figure S2). Subsequently, 3R11:AvrLm1-mCherry was inoculated on the host Topas DH16516 plants for the localization study. Infected cotyledons of Topas DH16516 were examined by confocal microscopy, and red fluorescent signals of the tagged AvrLm1 were detected along the cell wall of the hyphae. Intense focal fluorescent spots were observed where intercellular hyphae were in contact with the host mesophyll cells. In contrast, yellow fluorescent signals in the control 3R11-YFP were more uniformly observed throughout the fungal hyphae (Figure 3). We were not able to detect AvrLm1-mCherry inside the cell or apoplastic space of the host infected tissue. However, accumulation of mCherry signals along the hyphal cell wall and particularly focal accumulation where hyphae were in the vicinity of host cells could be an indication of localized AvrLm1 secretion into the apoplast or accumulation within the cytoplasm of the host plant cells.
Figure 3.
Localization of AvrLm1 upon L. maculans Infection of Brassica
One-week-old Topas DH16516 seedlings were inoculated with spore suspension and cotyledons were used for microscopic observation at 4 days post-inoculation. Transgenic L. maculans 3R11 carrying AvrLm1-mCherry, whose expression was driven by the native AvrLm1 promoter or carrying YFP under constitutive promoter, was visualized using confocal microscopy. Arrows indicate the local accumulation of AvrLm1-mCherry and secreted AvrLm1-mCherry aligning the fungal cell wall. No mCherry signal could be detected within the mycelium of L. maculans 3R11 expressing only YFP. Scale bars represent 25 μm.
To further investigate if AvrLm1-mCherry protein accumulates in the apoplast, we extracted the apoplastic fluids from L. maculans-infected cotyledons at 4 days post-inoculation (dpi). Apoplastic fluid was probed with anti-mCherry antibody, and AvrLm1-mCherry fusion protein was detected from the apoplastic fluids isolated from the infected plants (Figure S3). In conclusion, the localization of AvrLm1-mCherry on the surface of the fungal hyphae and detection of secreted AvrLm1-mCherry proteins in the apoplastic fluids indicate that AvrLm1-mCherry is very likely secreted into the apoplast.
AvrLm1 Physically Associates with BnMPK9
To investigate the molecular mechanism underlying the AvrLm1 virulence function, yeast two-hybrid screening was performed using AvrLm1 without signal peptide (ΔspAvrLm1) as a bait against a prey cDNA library prepared from young Arabidopsis seedlings. Four AvrLm1-interacting candidate proteins from Arabidopsis were identified including EBF1 (EIN3-binding F box protein 1), MPK17, MPK9, and T21P5.2 (hypothetical protein) (see Table S1). To validate the interaction for the candidate proteins, ΔspAvrLm1 was used as the bait against each of the protein product of Arabidopsis AtEBF1, AtMPK17, AtMPK9, and AtT21P5.2 with full-length open reading frames as the respective preys. Among the four candidates only full-length AtMPK9 (AT3G18040) was validated as interacting with AvrLm1 (Figure S4). BLAST search against the genome of B. napus Darmor-bzh (Chalhoub et al., 2014) identified two Brassica homologs of AtMPK9, BnaA05g22170D and BnaCnng17720D, residing in the A and C genomes of B. napus, respectively. Protein sequence alignment (Figure S5) identified that both shared the same level of homology with AtMPK9. Therefore only one gene, BnaCnng17720D (hereafter referred to as BnMPK9), was chosen for further analysis. Interaction between AvrLm1 and BnMPK9 was confirmed by yeast two-hybrid assay (Figure 4A). We next confirmed this interaction with bimolecular fluorescence complementation (BiFC) in planta. To generate the BiFC constructs, pENTR/Zeo:BnMPK9 and pENTR/Zeo:ΔspAvrLm1 were recombined into the binary vectors pDEST-GW-VYCE and pDEST-GW-VYNE. BiFC assay was performed by transiently co-expressing ΔspAvrLm1-VYNE or BnMPK9-VYNE with BnMPK9-VYCE in N. benthamiana leaves. As shown in Figure 4B, a strong yellow fluorescent signal was observed in cells co-expressing AvrLm1-VYCE and BnMPK9-VYNE, but not with the negative control (BnMPK9VYNE and BnMPK9VYCE). To further validate this association in vivo, the co-immunoprecipitation assay was performed upon transient expression of HA-BnMPK9 and AvrLm1-GFP constructs in N. benthamiana. Hemagglutinin (HA)-tagged BnMPK9 co-immunoprecipitated with green fluorescence protein (GFP)-tagged AvrLm1, and vice versa (Figures 4C and S6). Taken together, these observations support that BnMPK9 is the host target for AvrLm1.
Figure 4.
AvrLm1 Associates with BnMPK9
(A) Interaction of AvrLm1 and BnMPK9 was detected by Y2H. Yeasts expressing the indicated combinations of bait (B) and prey (P) were spotted on the synthetic dropout medium without leucine and tryptophan (SD-WL) and SD medium without leucine, tryptophan, histidine, and adenine supplemented with X-α-Gal and Aureobasidin A (SD-HAWL + X-α-Gal). Only those co-expressing AvrLm1 and BnMPK9 grew on SD-HAWL plates and developed blue coloration, indicative of an interaction between both proteins.
(B) BiFC confirmed the interaction between AvrLm1 and BnMPK9. VYCE-fused BnMPK9 and VYNE-fused AvrLm1 were transiently co-expressed in N. benthamiana. Co-infiltration of VYNE-fused BnMPK9 and VYCE-fused BnMPK9 was used as negative control. Yellow florescent protein (YFP) signal was visualized by confocal microscopy. Yellow fluorescence confirms protein-protein interaction due to complementation of the split YFP protein.
(C) Co-immunoprecipitation of AvrLm1 and BnMPK9 from the total plant protein extracts. GFP-tagged AvrLm1 and HA-tagged BnMPK9 were co-expressed in N. benthamiana. Proteins were extracted after 48 hr and subjected to immunoprecipitation by HA-magnetic beads. Immunoprecipitated proteins (IPs) were analyzed by immunoblotting and probing with either anti-HA (α-HA) or anti-GFP (α-GFP). Scale bars represent 20 μm.
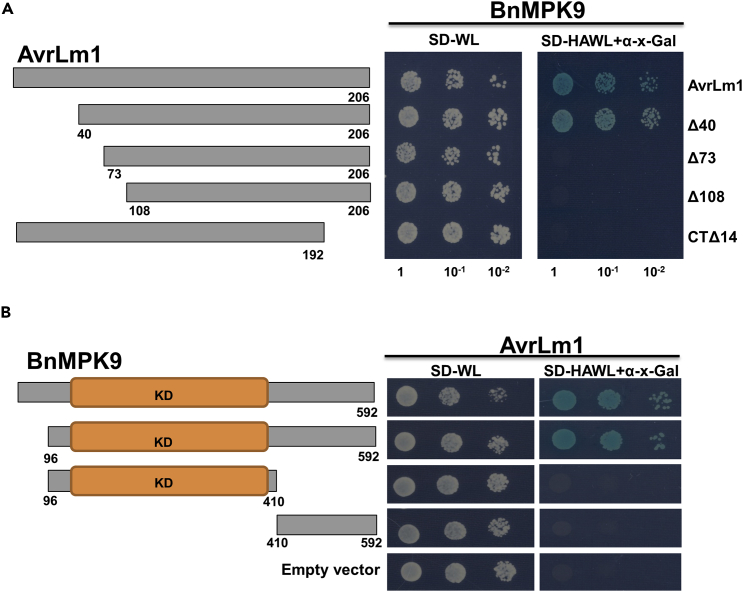
AvrLm1 Specifically Interacts with BnMPK9 and Its Small N-Terminal Region Is Dispensable for the Interaction
It has been reported that Arabidopsis MPK9 and MPK12 positively regulate ABA, SA, and MeJA signaling in Arabidopsis (Jammes et al., 2009, Khokon et al., 2017) and MPK9 and MPK12 are functionally redundant. Two other Arabidopsis MPKs, AtMPK3 and AtMPK6, are well known to be involved in plant immunity (Asai et al., 2002). To assess whether AvrLm1 specifically interacts with BnMPK9, we tested the interaction of AvrLm1 with either AtMPK12 or BnMPK3 in yeast two-hybrid (Y2H) assay. Neither AtMPK12 nor BnMPK3 interacted with AvrLm1, confirming the specificity of interaction between AvrLm1 and BnMPK9 (Figure S7).
To define which region of AvrLm1 is required for the interaction with BnMPK9, three N-terminally and one C-terminally truncated AvrLm1 proteins were generated based on the predicted AvrLm1 secondary structure (Ma and Borhan, 2015). Four variants were constructed: AvrLm1-Δ40, AvrLm1-Δ73, AvrLm1-Δ108, and CTΔ14. Only AvrLm1-Δ40 interacted with BnMPK9 (Figure 5A), indicating that a small (40-amino acid [aa]) N-terminal region of AvrLm1 is not required for the interaction but the remaining region of AvrLm1 is indispensable for its interaction with MPK9. To further define the region of BnMPK9 that is required to interact with AvrLm1, we generated three truncations of BnMPK9 that were used for Y2H with AvrLm1. One of them carrying a small N-terminal deletion region (95 aa) enabled the growth of yeast on selective plates, indicating that deletion of up to 95 N-terminal amino acid from the BnMPK9 protein does not disrupt its interaction with AvrLm1 (Figure 5B).
Figure 5.
N-Terminal Region of AvrLm1 Is Dispensable for the Interaction with BnMPK9
(A) Truncated AvrLm1 constructs were generated including N-terminal deletion of residues 1 to 40 (Δ40), deletion of residues 40 to 73 (Δ73), deletion of residues of 70–108 (Δ108), and C-terminal deletion of residues 192–206 (CTΔ14). Each of the constructs was co-transformed with BnMPK9 into yeast. All transformants are able to grow on synthetic dropout medium without leucine and tryptophan (SD-WL) medium. Yeast colonies that were able to grow on selective medium (SD medium without leucine, tryptophan, histidine, and adenine supplemented with X-α-Gal and Aureobasidin A [SD-HAWL]) and displayed blue coloration confirmed the protein-protein interaction.
(B) Three truncations of BnMPK9 were generated that included a small N-terminal deletion (95 aa), kinase domain of BnMPK9, and the remaining segment. Only BnMPK9 carrying a small N-terminal deletion enabled the growth of yeast on selective plate, while no interactions were detected between AvrLm1 and any of the other two truncated constructs.
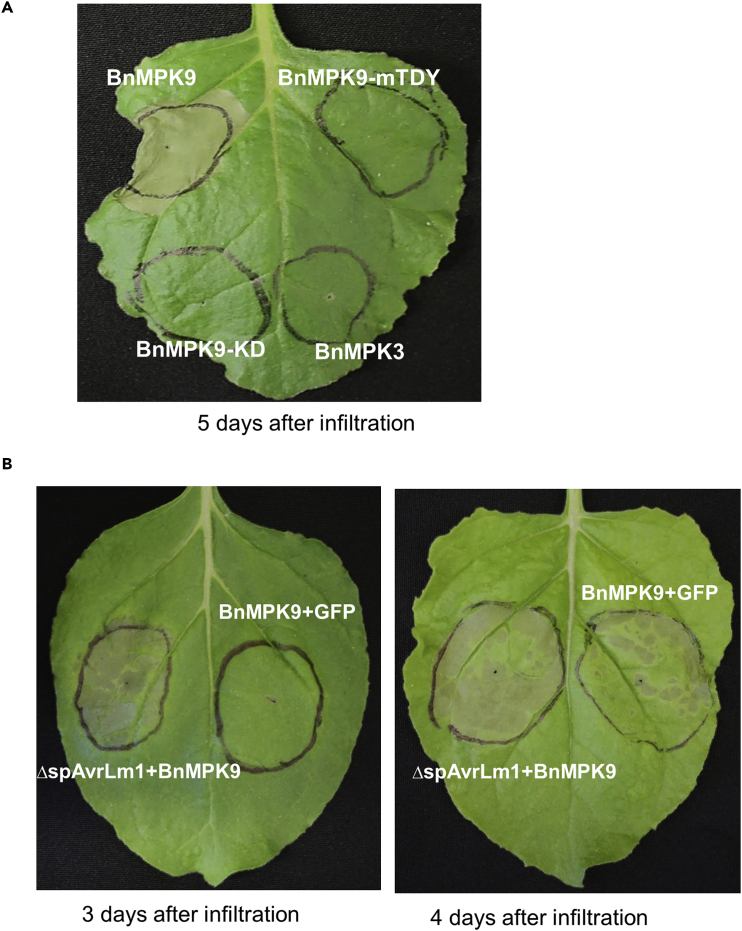
AvrLm1 Enhances BnMPK9-Dependent Cell Death
Transient expression in tobacco leaves has been extensively used to rapidly establish gene function. For instance, transient expression of tomato LeMKK4 and MAPKKKα, N. benthamiana MKK1, and B. napus MAPKKK4, MAPKKK18, and MAPKKK19 in tobacco leaves causes HR-like cell death (del Pozo et al., 2004, Li et al., 2015, Pedley and Martin, 2004, Sun et al., 2014, Takahashi et al., 2007). BnMPK9 was transiently expressed in N. benthamiana leaves via agroinfiltration. HR-like cell death developed extensively by 5 days after BnMPK9 infiltration, whereas no HR was observed with the control BnMPK3 (Figure 6A). To examine whether BnMPK9-dependent cell death is regulated by its phosphorylation activity, a mutated version of BnMPK9 was generated, in which the predicted TDY phosphorylation sites were mutated (BnMPK9-mTDY). As shown in Figure 6A, BnMPK9-mTDY failed to induce cell death when infiltrated in tobacco leaves, indicating that phosphorylation activity is required for the BnMPK9-induced cell death.
Figure 6.
AvrLm1 Enhances the Development of BnMPK9-Dependent Cell Death
(A) BnMPK9 triggers cell death, and the plant-specific TDY phosphorylation motif is required for the induction of cell death. N. benthamiana leaves were infiltrated with Agrobacterium carrying BnMPK9 or truncated BnMPK9 encoding the kinase domain (BnMPK9-KD) or mutated TDY motif (BnMPK9-mTDY). BnMPK3 was included as a control. Representative pictures were taken 5 days post-infiltration.
(B) Co-expression of AvrLm1 and BnMPK9 enhanced the development of BnMPK9-induced cell death. N. benthamiana leaves co-infiltrated with Agrobacterium strains carrying AvrLm1 and BnMPK9 or GFP. The representative pictures were taken 3 and 4 days post-infiltration.
Since AvrLm1 physically interacts with BnMPK9 in vivo, we examined whether AvrLm1 can influence BnMPK9-induced cell death. For this BnMPK9 was co-expressed with ΔspAvrLm1 (AvrLm1 without its native signal peptide). GFP was used as a negative control. Interestingly, co-expression of ΔspAvrLm1 and BnMPK9 enhanced the development of BnMPK9-induced cell death in the infiltrated region (Figure 6B), resulting in earlier development of visible cell death phenotype (as early as 3 days post-infiltration) compared with control BnMPK9 co-infiltrated with GFP, which showed cell death at 4 days post-infiltration.
AvrLm1 Stabilizes Accumulation and Enhances BnMPK9 Phosphorylation
Based on the presented evidences that AvrLm1 interacts with BnMPK9 and enhances BnMPK9-induced cell death, we investigated the stability and level of accumulation of BnMPK9 in the presence of AvrLm1 protein. HA and StrepII tags were added to BnMPK9 and ΔspAvrLm1, respectively. BnMPK9-HA was co-expressed in N. benthamiana with ΔspAvrLm1-StrepII or GFP (negative control) using agroinfiltration. As shown in Figure S8, BnMPK9-HA accumulated to a higher level in the presence of ΔspAvrLm1-StrepII compared with the GFP control. The ubiquitin/26S proteasome pathway is the major proteolysis machinery in eukaryotic cells, removing most abnormal peptides and short-lived cellular regulators (Sadanandom et al., 2012). We examined and compared the stability and accumulation of BnMPK9 in the presence or absence of MG132, a proteasome inhibitor. N. benthamiana leaves co-expressing BnMPK9-HA and ΔspAvrLm1-StrepII or GFP were pre-treated with proteasomal inhibitor MG132. MG132 application resulted in increased accumulation of BnMPK9 in the leaves of control treatment (co-expressing BnMPK9-HA and GFP), to a similar level of accumulation of BnMPK9 that was observed in the leaves co-expressing BnMPK9-HA and ΔspAvrLm1-StrepII (Figure 7A). However, to ensure that overexpression of AvrLm1 does not interfere with the MPK9 expression level, we measured the AtMPK9 expression in wild-type Col and Col-AvrLm1 transgenic lines. AtMPK9 expression profile in Col plants overexpressing AvrLm1 was similar to the Col wild-type (Figure S9), proving that the effect of AvrLm1 on MPk9 is only at the protein level. Taken together, these observations suggest that AvrLm1 stabilizes BnMPK9 by preventing its degradation via the ubiquitin/26S proteasome pathway.
Figure 7.
AvrLm1 Stabilizes the Accumulation of BnMPK9 and Enhances the Phosphorylation of BnMPK9 in planta
(A) Western blots probed with anti-HA and anti-StrepII antibodies showing the stabilization of BnMPK9 in the presence of AvrLm1 or 26S proteasome inhibitor (MG132). HA-tagged BnMPK9 was co-expressed with StrepII-tagged AvrLm1 or GFP in N. benthamiana leaves using agroinfiltration. Dimethyl sulfoxide (DMSO) or MG132 (100 mM) was infiltrated into the leaves at 24 hr post-infiltration. Proteins were extracted after 48 hr post-infiltration and subjected to western blotting. Numbers below the blots represent relative abundances of BnMPK9. ImageJ was employed to quantify the protein bands. ** represents the significant differences (p < 0.01, one-way analysis of variance [ANOVA]). Ponceau S (PS) staining shows equal amount of protein loaded in each well.
(B) Western blot probed with anti-HA and anti-phosphothreonine monoclonal antibody showing the enhanced phosphorylation of BnMPK9 in the presence of Avrlm1. HA-tagged BnMPK9 was co-expressed with StrepII-tagged AvrLm1 or GFP in N. benthamiana leaves using agroinfiltration, and MG132 (100 mM) was infiltrated into the leaves at 24 hr post-infiltration. Proteins were extracted 48 hr post-infiltration and immunoprecipitated with HA magnetic beads. Numbers below the blots represent relative abundances of phosphorylated BnMPK9, and quantification of phosphorylated BnMPK9 was performed using ImageJ by normalizing to total amount of BnMPK9 protein. * represents the significant differences (p < 0.05, one-way ANOVA). Total BnMP9 protein loading is shown with anti-HA antibody. The experiments were repeated three times with consistent results.
It has been demonstrated that the TDY phosphorylation site of BnMPK9 is essential for its cell death function. Thus, we hypothesized that AvrLm1 might affect the phosphorylation of BnMPK9. HA-tagged BnMPK9 was co-expressed with ΔspAvrLm1-StrepII in N. benthamiana leaves. BnMPK9 and GFP co-expression served as a negative control. HA-magnetic beads were used to immunoprecipitate transiently expressed BnMPK9 from the N. benthamiana leaves in the presence of MG132. The level of phosphorylation of BnMPK9 was determined by immunoblot using anti-phosphothreonine monoclonal antibodies. As shown in Figure 7B, the AvrLm1 transgene specifically enhanced the phosphorylation of BnMPK9. BnMPK9 purified from N. benthamiana leaves infiltrated with BnMPK9 and GFP showed a basal level of phosphorylation (Figure 7B), which could be due to MPK9 autophosphorylation as recently reported by Nagy et al. (Nagy et al., 2015).
Overexpression of BnMPK9 Enhances Production of Hydrogen Peroxide in Brassica napus
Since AvrLm1 stabilizes BnMPK9 accumulation and enhances its phosphorylation, we examined the effect of BnMPK9 in response to L. maculans infection. We initially performed RNA interference (RNAi) silencing to knockdown the expression of BnMPK9 in Topas DH16516. Despite several attempts, we failed to generate the transgenic B. napus plant carrying the BnMPK9 RNAi construct. Failure to generate MPK9 knockouts in Arabidopsis has also been reported (Jammes et al., 2009). However, we were successful in generating BnMPK9 overexpression B. napus lines. Three homozygous single-insert B. napus Topas DH16516 lines in which the BnMPK9 expression was driven by the 35S promoter were generated. Overexpression of BnMPK9 (15–20 fold) in these lines was confirmed by qRT-PCR (Figure S10). As described above, transient expression of BnMPK9 induces cell death in N. benthamiana plants (Figure 6); however, transgenic B. napus lines overexpressing BnMPK9 grew normally and did not display any visual cell death symptoms. High accumulation of hydrogen peroxide has been used as an indicator of cell death. To detect the H2O2 production, BnMPK9-overexpressing lines were stained by 3,3-Diaminobenzidine (DAB). Interestingly, we observed pronounced staining for hydrogen peroxide in the cotyledons of BnMPK9-overexpressing lines. By contrast, the cotyledon of control line Topas DH16516 showed only weak staining (Figure 8). To further confirm this observation, we measured the production of hydrogen peroxide in cotyledons infected with L. maculans isolate v23.1.3. The intensively stained area, due to H2O2 production, was much larger in cotyledons overexpressing BnMPK9 than in control plant (Figure 8). Taken together, it could be concluded that overexpression of BnMPK9 enhances the production of hydrogen peroxide, while no cotyledon of transgenic lines shows any macroscopically visible cell deaths.
Figure 8.
DAB Staining of Hydrogen Peroxide in BnMPK9-Overexpresing B. napus Lines
Cotyledons of 7-day-old Topas DH16516 seedlings and three BnMPK9-overexpressing B. napus lines were inoculated on wound sites using a spore suspension of L. maculans isolate v23.1.3. Four day post-inoculation, detached non-inoculated and inoculated cotyledons were used for DAB staining.
Overexpression of BnMPK9 Enhances L. maculans Growth in Brassica napus
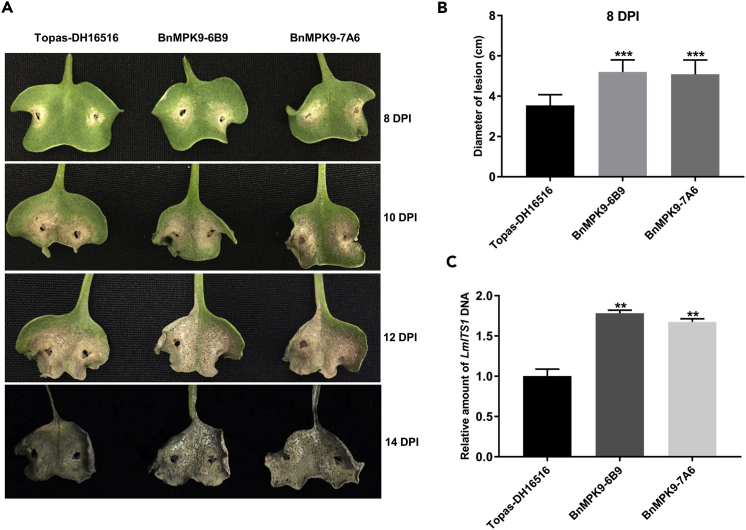
Expression of SA marker genes PR1 and WRKY70, and JA-related genes LOX3 and AOS, was measured by qRT-PCR in Topas DH16516 overexpressing BnMPK9 and the control Topas DH16516 lines. PR1 and WRKY70 expression levels were reduced to 70% and 30% compared with the control, respectively. In contrast, the expression levels of LOX3 and AOS were slightly elevated in the BnMPK9 overexpression lines (Figure 9). Two BnMPK9 transgenic lines were inoculated with the L. maculans isolate v23.1.3. Topas DH16516 that is susceptible to L. maculans was included as a positive control. At 8 dpi enhanced disease development was evident as formation of larger lesion on the cotyledons of Topas:BnMPK9 compared with the control (Figures 10A and 10B). Next, disease development was monitored over time. Asexual spore (pycnidiospore) formation, seen as black dots formed within the lesion, was more prevalent in the BnMPK9 overexpression lines at 10, 12, and 14 dpi (Figure 10A). Furthermore, the amount of fungal DNA in the cotyledons of BnMPK9-overexpresing and wild-type Topas lines was quantified by quantitative PCR (qPCR) analysis of L. maculans' LmITS1 DNA at 8 dpi. Higher levels of L. maculans DNA in the Topas:BnMPK9 provided additional support that overexpression of BnMPK9 enhances the susceptibility of B. napus to L. maculans (Figure 10C).
Figure 9.
Relative Expression of Salicylic Acid (SA) and Jasmonic Acid (JA) Marker Genes in BnMPK9-Overexpressing B. napus Lines
Cotyledons of 7-day-old seedlings of BnMPK9-overexpressing B. napus plants were collected and used for RNA extraction. Expressions of B. napus signaling marker genes including PR1, WRKY70, LOX3, and AOS were determined by quantitative reverse-transcriptase polymerase chain reaction and normalized to B. napus actin. Values represent means ± standard error (SE) from three independent experiments. *, **, and *** represent the significant differences (p < 0.05, p < 0.01, and p < 0.001, respectively; one-way analysis of variance [ANOVA] followed by the Turkey post-test).
Figure 10.
Expression of BnMPK9 Results in Increased Susceptibility to L. maculans
Cotyledons of 7-day-old Topas DH16516 seedlings and two BnMPK9-expressing lines (BnMPk9-6B9 and BnMPK9-7A6) were inoculated on wound sites using a spore suspension of L. maculans isolate v23.1.3.
(A) Representative cotyledons at 8, 10, 12, and 14 dpi were photographed.
(B) The size of the lesions was measured at 8 dpi and statistically analyzed. Values represent means ± standard error (SE) (n > 30). Asterisks indicate statistically significant differences (***, p < 0.001, one-way analysis of variance [ANOVA]). Three independent biological experiments were conducted.
(C) qRT-PCR analysis showing the fungal mass in L. maculans-infected cotyledons of B. napus at 8 dpi. Constitutively expressed LmITS1 gene was used as a marker showing DNA level and pathogen mass. LmITS1 gene expression levels were normalized to expression of B. napus actin. Values are means ± SE of triplicate reactions of three independent biological samples. Significant differences are represented by ** (**, p < 0.01, one-way ANOVA).
Discussion
B. napus resistance at the seedling stage to L. maculans is conferred by race-specific resistance genes. Despite significant advances in defining the genetics of the B. napus-L. maculans interaction, including isolation of two R genes and several Avr genes (Raman et al., 2013), up to now information about the molecular interaction and function of R/Avr proteins for the B. napus-L. maculans pathosystem has been lacking. The study presented here is the first report that describes the virulence function of an L. maculans effector by identifying its target in B. napus. We identified BnMPK9 as the host target of AvrLm1 and determined their interaction both in vitro and in planta. The BnMPK9-AvrLm1 interaction enhances cell death caused by BnMPK9, and mutation within kinase domain of BnMPK9 abolishes cell death. We further showed that AvrLm1 stabilizes the accumulation and facilitates the phosphorylation of BnMPK9, leading to the development of enhanced cell death in tobacco. Furthermore, overexpression of BnMPK9 perturbs hormone signaling pathways and enhances the growth of L. maculans in B. napus. Together, these results uncover a novel role for BnMPK9 in plant defense and its modulation by a fungal effector, causing enhanced plant susceptibility.
L. maculans is an intercellular pathogen, and not surprisingly, the LepR3 protein, the B. napus receptor for AvrLm1, is a membrane-bound RLP. To gain insight into the localization of AvrLm1, we tracked the AvrLm1-mCherry fusion protein expressed in L. maculans during infection of B. napus cotyledon tissues. Although we could not detect mCherry fluorescent signals inside the host plant cells, we frequently observed accumulation of mCherry signals at the focal points of contact between the fungal hyphae and plant host mesophyll cells. In addition, proof of function of AvrLm1 signal peptide in yeast (Figure S11) and enrichment of AvrLm1 protein in the apoplastic fluid of B. napus tissue infected with L. maculans (Figure S3) provided further evidence that L. maculans secretes AvrLm1 into the host apoplast. AvrLm1 encodes a protein with one cysteine residue, which is atypical for the commonly reported apoplastic fungal effector proteins (Fudal et al., 2007), indicating that AvrLm1 may enter into plant cell to avoid the harsh environment of the apoplast. Therefore, lack of discernible red fluorescent signals inside the host cell may be due to the rapid diffusion of AvrLm1-mCherry protein, reducing fluorescent signals to a level below our detection limits. Similar technical limitations have been reported for the translocation studies of fluorescently labeled effectors from other filamentous pathogens sash as the RXLR effectors from oomycetes or effectors from Colletotrichum higginsianum (Jing et al., 2016, Kleemann et al., 2012). Alternative localization techniques reported recently, such as the split fluorescent GFP system, may overcome these technical limitations (Henry et al., 2017, Park et al., 2017).
The physical interaction between AvrLm1 and BnMPK9 results in increased accumulation and enhanced phosphorylation of BnMPK9 (Figure 7). So far, there has been no report of a fungal effector directly targeting the MAPK pathway. The only example from filamentous pathogens is the P. infestans RXLR effector PexRD2, which has been reported to interact with host MAPKKKε and suppress MAPKKKε-dependent cell death to perturb plant immune signaling (King et al., 2014). However, accumulating evidences from studies on a variety of bacterial pathogens support that manipulation of components of MAPK kinase signaling cascade is an important virulence strategy of bacterial pathogens (Bigeard et al., 2015, Feng and Zhou, 2012). Two known MAPK modules, MKK4/MKK5-MPK3/MPK6 and MEKK1-MKK1/MKK2-MPK4 involved in plant defense signaling, are targeted by different bacterial effectors either to inactivate or enhance their kinase activities. For example, HopAI1 inactivates AtMPK3, AtMPK4, and AtMPK6 by irreversibly removing the phosphate group from the threonine residue of the MAPK activation loop (Zhang et al., 2007). P. syringae effector HopF2 targets AtMKK5, and the P. syringae effector AvrB interacts with AtMPK4 and induces the phosphorylation and activation of MPK4 (Cui et al., 2010, Wang et al., 2010). Our results provide the first example that a fungal pathogen also targets MAPK modules to manipulate plant defense response. The RLK proteins, SOBIR1 and BAK1, are components of the LepR3 receptor complex and are required for the recognition of AvrLm1 (Ma and Borhan, 2015), indicating the importance of MAPK signaling cascade downstream of the LepR3 receptor complex.
Previous studies showed that AtMPK9, together with AtMPK12, functions in ABA- and methyl jasmonate-induced stomatal closure in Arabidopsis (Lee et al., 2016, Jammes et al., 2009, Khokon et al., 2015) and that they act redundantly. However, our data show that AvrLm1 does not interact with MPK12, proving the specificity of AvrLm1-MPK9 interaction (Figure S7) and indicating that in the Brassicaceae, MPK12 is dispensable for the virulence function of AvrLm1. Furthermore, transient overexpression of BnMPK9 in N. benthamiana resulted in cell death. However, we did not detect any visible cell death phenotype when BnMPK12 was overexpressed in tobacco (Figure 6). These observations indicated that MPK9's function in inducing cell death and mediating L. maculans virulence in B. napus is independent of MPK12. The importance of MPKs in regulating plant immune response is well documented (Pitzschke et al., 2009, Popescu et al., 2009). Overexpression of the N. benthamiana homologs of AtMPK3 and AtMPK6 and Arabidopsis MKK4, MKK5, MKK7, and MKK9 cause cell death in tobacco (Popescu et al., 2009, Ren et al., 2006, Yang et al., 2001). Also, overexpression of B. napus MAPKKK4 causes up-regulation of genes related to reactive oxygen species accumulation, cell death, and defense response (Li et al., 2015). Plant cell death is an effective mechanism of containing the growth of biotrophic pathogens, whereas induction of cell death is a virulence mechanism employed by hemibiotrophic and necrotrophic fungi to more readily absorb nutrients from the host plant (Ding et al., 2011, Greenberg and Yao, 2004). Interestingly, previous study of the B. napus transcriptome in response to L. maculans infection revealed that the peak expression of AvrLm1 and NLP (necrosis- and ethylene-inducing protein) coincide with the transition from biotrophy to necrotrophy of L. maculans during B. napus cotyledon infection (Haddadi et al., 2016). Taken together, heightened expression of AvrLm1 by L. maculans leading to enhanced induction of cell death through the interaction of AvrLm1 with BnMPK9 could be perceived as a virulence mechanism supporting the transition from biotrophy to necrotrophy to facilitate the acquisition of host nutrients by L. maculans.
Our results show that in the BnMPK9 transgenic B. napus plants, transcription of PR-1 and WYKR70 is significantly reduced but expression levels of LOX3 and AOS are elevated, indicating suppression of the SA-dependent pathway and activation of the JA-dependent pathway. However, expression of LOX2 gene in AvrLm1-overexpressing Arabidopsis is down-regulated. This apparent discrepancy in LOX gene expression could be attributed to the suggested presence of a cytoplasm- and a chloroplast-localized pathway for JA biosynthesis. LOX2 is reported to be targeted to the chloroplast (He et al., 2002). Although LOX3 is possibly targeted to the chloroplast, its expression pattern is not necessarily similar to that of LOX2. For instance, it was reported that LOX3 was up-regulated during the leaf senescence, but expression of LOX2 was significantly down-regulated (He et al., 2002). Importance of SA and JA pathways during the L. maculans-B. napus interaction has also been previously reported (Becker et al., 2017, Haddadi et al., 2016). In B. napus cotyledon tissues infected with L. maculans, SA-related genes were induced at the initial phases of infection (2–4 dpi), but as the infection progressed and at the initiation of necrotrophic stage, genes related to the JA pathway were up-regulated (Haddadi et al., 2016). Thus, we hypothesize that AvrLm1 as a virulence factor facilitates the phosphorylation of BnMPK9, leading to the suppression of SA-dependent pathway and supporting the colonization of L. maculans at the initial infection stage. AvrLm4-7, another L. maculans effector, was recently reported to act as a virulence factor by suppressing the host SA signaling pathway (Nováková et al., 2016). AvrLm1 and AvrLm4-7 also contribute to the aggressiveness of L. maculans during leaf infection (Huang et al., 2006, Huang et al., 2009).
To the best of our knowledge, this is the first study to show that an effector from a fungal plant pathogen targets the MPK pathway to suppress plant host defense. Our results show that AvrLm1 supports the L. maculans infection by stabilizing the accumulation and inducing the phosphorylation of BnMPK9. Owing to technical limitations for genetic manipulation and also the complexity of the B. napus genome, most of the experiments that were carried out in the present study had to be done using the model plants A. thaliana and N. benthamiana. These model plants have been extensively used in plant biology research and proved to be invaluable in the translation of such research to crop species. However, further experiments in B. napus will provide additional support for the role of AvrLm1 and its target BnMPK9 in defense against L. maculans in its the natural host plant.
Until recently the molecular interaction of B. napus with the economically important blackleg pathogen L. maculans remained largely unknown. The research presented here expands our knowledge of the Brassica-Leptosphaeria pathosystem and highlights the importance of the MPK pathway, adding to our recent advances in understanding the perception of L. maculans and induction of downstream signaling in B. napus.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Helen Lui for generating the L. maculans-YFP transgenic line. Funding for this research was provided by AAFC, SaskCanola and Alberta Canola Producers Commission (Grant number: AIP-P032).
Author Contributions
L.M. and M.H.B. designed the experiments and prepared the manuscript. L.M. conducted the majority of the experiments. M. D., H.W., N.J.L., P.H., E.B., and G.G. contributed to the experiments and read the manuscript. N.J.L. edited the manuscript.
Declaration of Interests
The authors declare no conflict of interest.
Published: May 25, 2018
Footnotes
Supplemental Information includes Transparent Methods, 11 figures, and 2 tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.04.015.
Supplemental Information
References
- Asai T., Tena G., Plotnikova J., Willmann M.R., Chiu W.-L., Gomez-Gomez L., Boller T., Ausubel F.M., Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- Becker M.G., Zhang X., Walker P.L., Wan J.C., Millar J.L., Khan D., Granger M.J., Cavers J.D., Chan A.C., Fernando D.W.G. Transcriptome analysis of the Brassica napus–Leptosphaeria maculans pathosystem identifies receptor, signaling and structural genes underlying plant resistance. Plant J. 2017;90:573–586. doi: 10.1111/tpj.13514. [DOI] [PubMed] [Google Scholar]
- Bigeard J., Colcombet J., Hirt H. Signaling mechanisms in pattern-triggered immunity (PTI) Mol. Plant. 2015;8:521–539. doi: 10.1016/j.molp.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Block A., Alfano J.R. Plant targets for Pseudomonas syringae type III effectors: virulence targets or guarded decoys? Curr. Opin. Microbiol. 2011;14:39–46. doi: 10.1016/j.mib.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub B., Denoeud F., Liu S., Parkin I.A.P., Tang H., Wang X., Chiquet J., Belcram H., Tong C., Samans B. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 2014;345:950–953. doi: 10.1126/science.1253435. [DOI] [PubMed] [Google Scholar]
- Cui H., Wang Y., Xue L., Chu J., Yan C., Fu J., Chen M., Innes R.W., Zhou J.-M. Pseudomonas syringae effector protein AvrB perturbs Arabidopsis hormone signaling by activating MAP kinase 4. Cell Host Microbe. 2010;7:164–175. doi: 10.1016/j.chom.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge R., Peter van Esse H., Kombrink A., Shinya T., Desaki Y., Bours R., van der Krol S., Shibuya N., Joosten M.H.A.J., Thomma B.P.H.J. Conserved fungal LysM effector Ecp6 prevents chitin-triggered immunity in plants. Science. 2010;329:953–955. doi: 10.1126/science.1190859. [DOI] [PubMed] [Google Scholar]
- del Pozo O., Pedley K.F., Martin G.B. MAPKKKα is a positive regulator of cell death associated with both plant immunity and disease. EMBO J. 2004;23:3072–3082. doi: 10.1038/sj.emboj.7600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslandes L., Rivas S. Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci. 2012;17:644–655. doi: 10.1016/j.tplants.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Ding L., Xu H., Yi H., Yang L., Kong Z., Zhang L., Xue S., Jia H., Ma Z. Resistance to hemi-biotrophic F. graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways. PLoS One. 2011;6:e19008. doi: 10.1371/journal.pone.0019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Duran J.D., Volko S., Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Feng F., Zhou J.-M. Plant–bacterial pathogen interactions mediated by type III effectors. Curr. Opin. Plant Biol. 2012;15:469–476. doi: 10.1016/j.pbi.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Fisher M.C., Henk D.A., Briggs C.J., Brownstein J.S., Madoff L.C., McCraw S.L., Gurr S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature. 2012;484:186–194. doi: 10.1038/nature10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudal I., Ross S., Gout L., Blaise F., Kuhn M.L., Eckert M.R., Cattolico L., Bernard-Samain S., Balesdent M.H., Rouxel T. Heterochromatin-like regions as ecological niches for Avirulence genes in the Leptosphaeria maculans genome: map-based cloning of AvrLm6. Mol. Plant Microbe Interact. 2007;20:459–470. doi: 10.1094/MPMI-20-4-0459. [DOI] [PubMed] [Google Scholar]
- Gao M., Liu J., Bi D., Zhang Z., Cheng F., Chen S., Zhang Y. MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008;18:1190–1198. doi: 10.1038/cr.2008.300. [DOI] [PubMed] [Google Scholar]
- Gout L., Fudal I., Kuhn M.-L., Blaise F., Eckert M., Cattolico L., Balesdent M.-H., Rouxel T. Lost in the middle of nowhere: the AvrLm1 avirulence gene of the dothideomycete Leptosphaeria maculans. Mol. Microbiol. 2006;60:67–80. doi: 10.1111/j.1365-2958.2006.05076.x. [DOI] [PubMed] [Google Scholar]
- Greenberg J.T., Yao N. The role and regulation of programmed cell death in plant–pathogen interactions. Cell. Microbiol. 2004;6:201–211. doi: 10.1111/j.1462-5822.2004.00361.x. [DOI] [PubMed] [Google Scholar]
- Haddadi P., Ma L., Wang H., Borhan M.H. Genome-wide transcriptomic analyses provide insights into the lifestyle transition and effector repertoire of Leptosphaeria maculans during the colonization of Brassica napus seedlings. Mol. Plant Pathol. 2016;8:1196–1210. doi: 10.1111/mpp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry E., Toruño T.Y., Jauneau A., Deslandes L., Coaker G.L. Direct and indirect visualization of bacterial effector delivery into diverse plant cell types during infection. Plant Cell. 2017;29:1555–1570. doi: 10.1105/tpc.17.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Fukushige H., Hildebrand D.F., Gan S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002;128:876–884. doi: 10.1104/pp.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-J., Balesdent M.-H., Li Z.-Q., Evans N., Rouxel T., Fitt B.D.L. Fitness cost of virulence differs between the AvrLm1 and AvrLm4 loci in Leptosphaeria maculans (phoma stem canker of oilseed rape) Eur. J. Plant Pathol. 2009;126:279. [Google Scholar]
- Huang Y.-J., Li Z.-Q., Evans N., Rouxel T., Fitt B.D.L., Balesdent M.-H. Fitness cost associated with loss of the AvrLm4 avirulence function in Leptosphaeria maculans (phoma stem canker of oilseed rape) Eur. J. Plant Pathol. 2006;114:77–89. [Google Scholar]
- Ichimura K., Shinozaki K., Tena G., Sheen J., Henry Y., Champion A., Kreis M., Zhang S., Hirt H. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7:301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Jammes F., Song C., Shin D., Munemasa S., Takeda K., Gu D., Cho D., Lee S., Giordo R., Sritubtim S. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20520–20525. doi: 10.1073/pnas.0907205106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes F., Yang X., Xiao S., Kwak J.M. Two Arabidopsis guard cell-preferential MAPK genes, MPK9 and MPK12, function in biotic stress response. Plant Signal. Behav. 2011;6:1875–1877. doi: 10.4161/psb.6.11.17933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing M., Guo B., Li H., Yang B., Wang H., Kong G., Zhao Y., Xu H., Wang Y., Ye W. A Phytophthora sojae effector suppresses endoplasmic reticulum stress-mediated immunity by stabilizing plant binding immunoglobulin proteins. Nat. Commun. 2016;7:11685. doi: 10.1038/ncomms11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J.D.G., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Khokon M.A.R., Salam M.A., Jammes F., Ye W., Hossain M.A., Okuma E., Nakamura Y., Mori I.C., Kwak J.M., Murata Y. MPK9 and MPK12 function in SA-induced stomatal closure in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2017;81:1394–1400. doi: 10.1080/09168451.2017.1308244. [DOI] [PubMed] [Google Scholar]
- Khokon M.A.R., Salam M.A., Jammes F., Ye W., Hossain M.A., Uraji M., Nakamura Y., Mori I.C., Kwak J.M., Murata Y. Two guard cell mitogen-activated protein kinases, MPK9 and MPK12, function in methyl jasmonate-induced stomatal closure in Arabidopsis thaliana. Plant Biol. (Stuttg.) 2015;17:946–952. doi: 10.1111/plb.12321. [DOI] [PubMed] [Google Scholar]
- King S.R.F., McLellan H., Boevink P.C., Armstrong M.R., Bukharova T., Sukarta O., Win J., Kamoun S., Birch P.R.J., Banfield M.J. Phytophthora infestans RXLR effector PexRD2 interacts with host MAPKKKε to suppress plant immune signaling. Plant Cell. 2014;26:1345–1359. doi: 10.1105/tpc.113.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemann J., Rincon-Rivera L.J., Takahara H., Neumann U., Ver Loren van Themaat E., van der Does H.C., Hacquard S., Stuber K., Will I., Schmalenbach W. Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012;8:e1002643. doi: 10.1371/journal.ppat.1002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevernic I.V., Stafford M.J., Morrice N., Peggie M., Morton S., Cohen P. Characterization of the reversible phosphorylation and activation of ERK8. Biochem. J. 2006;394:365–373. doi: 10.1042/BJ20051288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkan N.J., Lydiate D.J., Parkin I.A.P., Nelson M.N., Epp D.J., Cowling W.A., Rimmer S.R., Borhan M.H. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. New Phytol. 2013;197:595–605. doi: 10.1111/nph.12043. [DOI] [PubMed] [Google Scholar]
- Larkan N.J., Ma L., Borhan M.H. The Brassica napus receptor-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotechnol. J. 2015;13:983–992. doi: 10.1111/pbi.12341. [DOI] [PubMed] [Google Scholar]
- Larkan N.J., Yu F., Lydiate D.J., Rimmer S.R., Borhan M.H. Single R gene introgression lines for accurate dissection of the Brassica - Leptosphaeria pathosystem. Front. Plant Sci. 2016;7:1771. doi: 10.3389/fpls.2016.01771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim Y.J., Kim M.-H., Kwak J.M. MAPK cascades in guard cell signal transduction. Front. Plant Sci. 2016;7:80. doi: 10.3389/fpls.2016.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ye C., Zhao R., Li X., Liu W.-Z., Wu F., Yan J., Jiang Y.-Q., Yang B. Mitogen-activated protein kinase kinase kinase (MAPKKK) 4 from rapeseed (Brassica napus L.) is a novel member inducing ROS accumulation and cell death. Biochem. Biophys. Res. Commun. 2015;467:792–797. doi: 10.1016/j.bbrc.2015.10.063. [DOI] [PubMed] [Google Scholar]
- Ma L., Borhan H. The receptor-like kinase SOBIR1 interacts with Brassica napus LepR3 and is required for Leptosphaeria maculans AvrLm1-triggered immunity. Front. Plant Sci. 2015;6:933. doi: 10.3389/fpls.2015.00933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Zhang S. MAPK cascades in plant disease resistance signaling. Annu. Rev. Phytopathol. 2013;51:245–266. doi: 10.1146/annurev-phyto-082712-102314. [DOI] [PubMed] [Google Scholar]
- Montillet J.-L., Leonhardt N., Mondy S., Tranchimand S., Rumeau D., Boudsocq M., Garcia A.V., Douki T., Bigeard J., Laurière C. An abscisic acid-independent oxylipin pathway controls stomatal closure and immune defense in Arabidopsis. PLoS Biol. 2013;11:e1001513. doi: 10.1371/journal.pbio.1001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy S.K., Darula Z., Kállai B.M., Bögre L., Bánhegyi G., Medzihradszky K.F., Horváth G.V., Mészáros T. Activation of AtMPK9 through autophosphorylation that makes it independent of the canonical MAPK cascades. Biochem. J. 2015;467:167–175. doi: 10.1042/BJ20141176. [DOI] [PubMed] [Google Scholar]
- Nováková M., Šašek V., Trdá L., Krutinová H., Mongin T., Valentová O., Balesdent M.-H., Rouxel T., Burketová L. Leptosphaeria maculans effector AvrLm4-7 affects salicylic acid (SA) and ethylene (ET) signalling and hydrogen peroxide (H2O2) accumulation in Brassica napus. Mol. Plant Pathol. 2016;17:818–831. doi: 10.1111/mpp.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Lee H.-Y., Woo J., Choi D., Dinesh-Kumar S.P. Spatiotemporal monitoring of Pseudomonas syringae effectors via type III secretion using split fluorescent protein fragments. Plant Cell. 2017;29:1571–1584. doi: 10.1105/tpc.17.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley K.F., Martin G.B. Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. J. Biol. Chem. 2004;279:49229–49235. doi: 10.1074/jbc.M410323200. [DOI] [PubMed] [Google Scholar]
- Pitzschke A., Schikora A., Hirt H. MAPK cascade signalling networks in plant defence. Curr. Opin. Plant Biol. 2009;12:421–426. doi: 10.1016/j.pbi.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Popescu S., Popescu G., Bachan S., Zhang Z., Gerstein M., Snyder M., Dinesh-Kumar S. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti L.L., Lanver D., Schweizer G., Tanaka S., Liang L., Tollot M., Zuccaro A., Reissmann S., Kahmann R. Fungal effectors and plant susceptibility. Annu. Rev. Plant Biol. 2015;66:513–545. doi: 10.1146/annurev-arplant-043014-114623. [DOI] [PubMed] [Google Scholar]
- Raman H., Raman R., Larkan N. Genetic dissection of blackleg resistance loci in rapeseed (Brassica napus L. In: Andersen S.B., editor. Plant Breeding from Laboratories to Fields. InTech; 2013. Ch. 04. [Google Scholar]
- Rasmussen M.W., Roux M., Petersen M., Mundy J. MAP kinase cascades in Arabidopsis innate immunity. Front. Plant Sci. 2012;3:169. doi: 10.3389/fpls.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Yang K.-Y., Li G.-J., Liu Y., Zhang S. Activation of Ntf4, a tobacco mitogen-activated protein kinase, during plant defense response and its involvement in hypersensitive response-like cell death. Plant Physiol. 2006;141:1482–1493. doi: 10.1104/pp.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandom A., Bailey M., Ewan R., Lee J., Nelis S. The ubiquitin–proteasome system: central modifier of plant signalling. New Phytol. 2012;196:13–28. doi: 10.1111/j.1469-8137.2012.04266.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vallet A., Saleem-Batcha R., Kombrink A., Hansen G., Valkenburg D.-J., Thomma B.P.H.J., Mesters J.R. Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. Elife. 2013;2:e00790. doi: 10.7554/eLife.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiopoulos I., de Wit P.J. Fungal effector proteins. Annu. Rev. Phytopathol. 2009;47:233–263. doi: 10.1146/annurev.phyto.112408.132637. [DOI] [PubMed] [Google Scholar]
- Sun Y., Wang C., Yang B., Wu F., Hao X., Liang W., Niu F., Yan J., Zhang H., Wang B. Identification and functional analysis of mitogen-activated protein kinase kinase kinase (MAPKKK) genes in canola (Brassica napus L.) J. Exp. Bot. 2014;65:2171–2188. doi: 10.1093/jxb/eru092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Nasir K.H.B., Ito A., Kanzaki H., Matsumura H., Saitoh H., Fujisawa S., Kamoun S., Terauchi R. A high-throughput screen of cell-death-inducing factors in Nicotiana benthamiana identifies a novel MAPKK that mediates INF1-induced cell death signaling and non-host resistance to Pseudomonas cichorii. Plant J. 2007;49:1030–1040. doi: 10.1111/j.1365-313X.2006.03022.x. [DOI] [PubMed] [Google Scholar]
- van den Burg H.A., Westerink N., Francoijs K.-J., Roth R., Woestenenk E., Boeren S., de Wit P.J.G.M., Joosten M.H.A.J., Vervoort J. Natural disulfide bond-disrupted mutants of AVR4 of the tomato pathogen Cladosporium fulvum are sensitive to proteolysis, circumvent Cf-4-mediated resistance, but retain their chitin binding ability. J. Biol. Chem. 2003;278:27340–27346. doi: 10.1074/jbc.M212196200. [DOI] [PubMed] [Google Scholar]
- Wang Y., Li J., Hou S., Wang X., Li Y., Ren D., Chen S., Tang X., Zhou J.-M. A Pseudomonas syringae ADP-ribosyltransferase inhibits Arabidopsis mitogen-activated protein kinase kinases. Plant Cell. 2010;22:2033–2044. doi: 10.1105/tpc.110.075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J.S., Kharbanda P.D., Barbetti M.J., Fitt B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001;50:10–27. [Google Scholar]
- Win J., Chaparro-Garcia A., Belhaj K., Saunders D.G.O., Yoshida K., Dong S., Schornack S., Zipfel C., Robatzek S., Hogenhout S.A. Effector biology of plant-associated organisms: concepts and perspectives. Cold Spring Harb. Symp. Quant. Biol. 2012;77:235–247. doi: 10.1101/sqb.2012.77.015933. [DOI] [PubMed] [Google Scholar]
- Yang K.-Y., Liu Y., Zhang S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA. 2001;98:741–746. doi: 10.1073/pnas.98.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Shao F., Li Y., Cui H., Chen L., Li H., Zou Y., Long C., Lan L., Chai J. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe. 2007;1:175–185. doi: 10.1016/j.chom.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Zipfel C., Kunze G., Chinchilla D., Caniard A., Jones J.D.G., Boller T., Felix G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–760. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.